Introduction
Bladder cancer is the most frequently occurring
urological malignancy. The prognosis for patients with non-invasive
bladder cancers is generally good, and most non-muscle invasive
bladder cancer can be controlled by transurethral resection (TUR)
of the tumor combined with intravesical instillation of cytotoxic
agents or Bacillus Calmette-Guerin (BCG). Patients with invasive
bladder cancer, in contrast, particularly those with
muscle-invasive disease, face the possibility of postoperative
distant metastasis or local recurrence even after radical
cystectomy, and for those patients the recurrence rate is
positively correlated with increased tumor stage (T stage) and
histological grade (1,2). The prognosis is particularly poor for
patients with lymph node metastasis that is histologically
confirmed in radical cystectomy specimens; the 5-year overall
survival rate is only 23–35% (1–3).
Furthermore, patients with distant metastasis have a reduced
survival rate (4).
Although cisplatin-based chemotherapy is effective
for patients with metastatic bladder cancer, patients are rarely
cured by chemotherapy and long-term survival is rare. The 5-year
overall survival of chemotherapy-treated patients with metastatic
disease is reportedly less than 20% (5,6). The
most effective way to improve the prognosis of patients with
bladder cancer is to diagnose the disease early and to operate
immediately. If recurrence after cystectomy could be predicted,
adjuvant chemotherapy could be administered when the lesions are
small. Thus, biomarkers that predict the recurrence of bladder
cancer need to be found.
We previously identified bladder cancer antigens
recognized by the IgG antibody in patients with advanced bladder
cancer using the serological identification of tumor antigens by
cDNA expression cloning (SEREX) method (7). This method has identified tumor
antigens that are recognized by CD8+ T cells and are
possible immunotherapy targets, but it could also be used to
identify antigens that are overexpressed in cancer cells and can be
used as diagnostic or prognostic biomarkers. The SEREX method has
previously identified prognostic biomarkers such as p53, galectin-3
and NY-ESO-1 (8,9). In a previous study (7) we found that the serum of a patient
with metastatic bladder cancer contained IgG antibodies that
recognized α-actinin-4 (ACTN4). We therefore speculated that IgG
antibodies to ACTN4 may be produced in response to the ACTN4
overexpressed in bladder cancer cells.
The α-actinins (ACTNs) are a family of actin-binding
proteins and are involved in cytoskeletal reorganization. Four
isoforms of human ACTN have been identified. ACTN1 and ACTN4 are
nonmuscle proteins thought to cross-link and connect the actin
filaments and connect the actin cytoskeleton to the cell membrane
(10). The two non-muscle ACTNs may
bind different targets. Immunohistochemical analysis found ACTN1 to
be localized at the interface between actin stress fibers and
plasma membrane adherens junctions, whereas ACTN4 appears to
cross-link actin stress fibers (10). ACTN4 is highly concentrated at the
leading edges of motile cells and in cytoplasmic regions with sharp
cell extensions (10). ACTN4 was
found to be preferentially localized in moving structures, such as
the dorsal ruffles of macrophages (11). ACTN4 is reportedly associated with
invasion and metastasis of cancer cells in several malignant
tumors. Its increased expression in the cytoplasm and/or cell
membrane is related to the prognosis of patients with breast cancer
(10,12) and non-small cell lung cancer
(13), and overexpression of ACTN4
in colon cancer cells was found to lead to increased lymph node
metastasis in an animal model (14). ACTN4 has recently been reported to
be involved in the invasive activity of bladder cancer cells
(15). Yet, neither the association
of tissue ACTN4 expression to clinicopathological factors, nor the
impact of ACTN4 expression on recurrence or prognosis has been
fully evaluated in human bladder cancer specimens.
In the present study, we evaluated the expression of
ACTN4 in bladder cancer specimens and evaluated the correlation
between ACTN4 expression level and recurrence and prognosis. We
also examined the effects of ACTN4 knockdown on the invasive
ability and proliferation of cultured bladder cancer cells.
Materials and methods
Cell culture and reagents
The human bladder cancer cell lines used in this
study were T24 (16) and KU19-19
(17). T24 cells were purchased
from ATCC (American Type Culture Collection, Manassas, VA, USA),
and KU19-19 cells were kindly provided by the Urology Department of
Keio University (Shinjuku, Tokyo, Japan). These cell lines were
maintained in RPMI-1640 (Invitrogen Life Technologies, Carlsbad,
CA, USA) supplemented with 10% fetal bovine serum (FBS), 100 IU/ml
penicillin and 100 μg/ml streptomycin (Invitrogen Life
Technologies). Antibodies against ACTN4 (polyclonal; Alexis
Biochemicals, San Diego, CA, USA), phospho-Akt (Ser473), Akt,
phospho-STAT3, STAT3, phospho-ERKs, ERKs (Cell Signaling
Technology, Inc., Boston, MA, USA) and β-actin (Millipore,
Billerica, MA, USA) were used. Horseradish peroxidase-conjugated
secondary antibodies and an enhanced chemiluminescence system
(Amersham Pharmacia Biotech, Piscataway, NJ, USA) were also
used.
Patients
The clinicopathological features of the patients who
underwent surgical intervention (TUR and/or radical cystectomy) for
urothelial carcinoma of the bladder at our institution between 1994
and 2007 were carefully reviewed according to clinical records and
pathological reports. Patients with pure bladder squamous cell
carcinoma and patients with pure adenocarcinoma were excluded from
this study. Patients without sufficient follow-up data and/or
without urothelial carcinoma histology were also excluded. We
evaluated paraffin-embedded sections of surgical specimens obtained
from 95 patients. Among the 95 patients, 49 patients with
superficial bladder cancer who underwent TUR between 1994 and 2000
were randomly selected. In addition, 46 patients who underwent
radical cystectomy at our institute between 1997 and 2007 were
reviewed and enrolled as participants. The 95 participating
patients included 78 men and 17 women whose ages ranged from 29 to
87 years (median age, 68); 42 had invasive bladder cancer and 53
had superficial disease. Forty-six of these 95 patients underwent
radical cystectomy after TUR.
The 49 patients who underwent TUR had only
urothelial carcinoma and one of these patients had some component
of adenocarcinoma in addition to urothelial carcinoma. The
histological diagnoses of all 46 patients who underwent radical
cystectomy were urothelial carcinoma. Five of these patients had
squamous cell carcinoma components and one had an adenocarcinoma
component. The pathological tumor stage and histological grade were
determined according to the 2009 TNM classification system (7th
edition). The predominant histological grade of 21 tumors was grade
1, that of 40 tumors was grade 2, and that of 34 tumors were grade
3. Fifty-two (54.7%) of the tumors had some degree of grade 3
components. Thirty-four of the tumors were pTa, 19 were pT1, 18
were pT2, 20 were pT3, and 4 were pT4. Five of the 46 patients
(10.9%) who underwent radical cystectomy had metastases in
surgically resected lymph nodes, and 35 of the 46 patients (76.1%)
had lymphovascular invasion (LVI).
To monitor intravesical recurrence in the patients
with superficial bladder cancer, the patients were evaluated
postoperatively by cystoscopy every 3 months for the first 2 years
and every 6–12 months thereafter. To monitor the occurrence of
local and/or distant metastases in the patients who underwent
radical cystectomy, they were evaluated postoperatively every 3–6
months for the first 5 years and every 6–12 months thereafter.
Follow-up examinations for the patients after radical cystectomy
consisted of physical examination, chest radiography, abdominal and
chest CT, blood tests, and if indicated, radionuclide bone
scanning. Follow-up intervals were calculated from the date of
surgical intervention to the last recorded follow-up. The median
follow-up interval was 38.4 months (range 1–153 month) for patients
who had superficial bladder cancer and were treated only by TUR,
and the median follow-up interval was 47.7 months (range 3–135
months) for patients who underwent radical cystectomy. Intravesical
recurrence-free survival was evaluated using the date at which
intravesical recurrence was identified, and extravesical
recurrence-free survival was evaluated using the date at which
local recurrence or metastatic disease was identified.
Cause-specific survival was evaluated using the date of death due
to disease progression or the last follow-up date. Disease
progression was defined as evidence of recurrence or metastasis on
radiological examination or physical examinations. This study was
approved by the institutional review board.
Immunohistochemical analysis and tissue
evaluation
Paraffin-embedded sections (5 μm) were mounted on
slides, deparaffinized in xylene, and rehydrated through graded
ethanols. For antigen retrieval, the sections were placed in Dako
Target Retrieval Solution High pH (Dako Corp., Carpinteria, CA,
USA) and heated at 95°C for 50 min. Endogenous peroxidase activity
was quenched with Dako peroxidase blocking reagent (Dako Corp.) for
10 min. Sections were incubated in 10% normal goat serum in
phosphate-buffered saline (PBS) for 60 min at room temperature and
then incubated overnight at 4°C with primary antibody for ACTN4
(rabbit polyclonal; Alexis Biochemicals) at appropriate dilutions
in PBS. They were then stained using the Simple Stain Max PO kit
(Nichirei Corp., Tokyo, Japan) according to the manufacturer’s
instructions. Reaction products were visualized by immersing the
slides in diaminobenzidine for 2 min. After the sections were
counterstained with hematoxylin, they were covered with glass
coverslips. Vascular epithelial cells, which are known to be
abundant in ACTN4 (18), served as
positive internal controls. Immunostaining results for all tumor
sections were evaluated by 2 individuals (H.Y. and K.I.) blinded to
all clinical data. Since the anti-ACTN4 antibody clearly stained
the vascular endothelial cells, the staining intensity of each
tumor section was compared with that of the vascular endothelial
cells in the section. Tumors with a staining intensity equal to
(level 3) or greater than (level 4) that of the vascular
endothelial cells were defined as tumors with high ACTN4
expression, while those with staining intensity less than that of
the vascular endothelial cells were defined as tumors with low
ACTN4 expression (level 1 or 2). Negative ACTN4 staining was
defined as level 1 staining. The percentage of each ACTN4 staining
level in each specimen was determined by microscopically reviewing
the entire slide at ×200 magnification. The predominant level of
ACTN4 expression was determined for each patient and defined as the
ACTN4 expression level for that patient.
Immunocytochemistry for the leading edges
of cultured bladder cancer cells
Cells (T24 and KU19-19) were grown to confluence on
6-well tissue culture plates and a wound was made by scraping in
the middle of the cell monolayer with a cell scraper 1.1 cm wide
(Sumitomo Bakelite Co., Ltd., Tokyo, Japan). After floating cells
were removed by extensive washing with PBS, fresh complete medium
was added. Then cells were incubated for 24 h. Immunocytochemistry
was performed to confirm protein expression of ACTN4 in the bladder
cancer cells. Twenty-four hours after incubation, cells in 6-well
plates were fixed for 5 min in 4% paraformaldehyde in PBS. After
fixation, cells were washed 3 times (5 min/wash) with 0.3 M glycine
and then permeabilized in 0.1% Triton-X in PBS for 7 min. After 3
washes in PBS (5 min/wash), non-specific binding sites were blocked
with 10% normal goat serum (NGS) in PBS for 1 h. Cells were then
incubated with 1 μg/ml anti-ACTN4 antibody and 1% NGS in PBS for 30
min. After 3 washes in PBS (5 min/wash), cells were incubated in a
1:500 dilution of Alexa-Fluor®-594-conjugated goat
anti-rabbit IgG (Invitrogen Life Technologies) for 30 min and then
washed 3 times in PBS (5 min/wash). The leading edge and other
regions of the cultured cells were observed using fluorescence
microscopy (x400).
To compare the protein expression of ACTN4 in the
leading edge of the cultured bladder cancer cells, western blot
analysis was performed. Bladder cancer cells (T24 and KU19-19) were
grown to confluence in 10-cm cell culture plates and were scratched
by a cell scraper (width, 1.1 cm) to create leading edges in the
cell colonies. After a 24-h incubation, cells were obtained by
scraping them from the leading edge and non-leading edge areas of
the colonies on the different plates. These cells were subjected to
western blot analysis.
Western blot analysis
Bladder cancer cells were lysed in RIPA buffer
containing 10 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 5 mM
ethylenediaminetetraacetic acid, 1% sodium deoxycholate, 0.1%
sodium dodecyl sulfate, 1.2% aprotinin, 5 μM leupeptin, 4 μM
antipain, 1 mM phenylmethylsulfonyl fluoride and 0.1 mM
Na3VO4. Equal amounts of the resulting
lysates were separated by using 10% SDS-PAGE and were then
transferred to nitrocellulose membranes. The membranes were blocked
with a solution containing 5% skim milk, and incubated overnight
with primary antibodies at 4°C. They were then incubated with
secondary antibodies coupled to horseradish peroxidase (GE
Healthcare, Buckinghamshire, UK). The reactive proteins were
visualized by enhanced chemiluminescence (Amersham ECL Western
blotting detection reagents and analysis system; GE Healthcare)
according to the manufacturer’s recommendations.
Silencing of ACTN4 by siRNA
transfection
T24 and KU19-19 cells were plated in 24-well culture
plates 1 day before being transfected with 40 nM ACTN4-specific
siRNA (sc-43101) or non-sense siRNA (sc-37007; both from Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA) that had been mixed with
Lipofectamine 2000 (Invitrogen Life Technologies) according to the
manufacturer’s protocol. The cells were then cultured for 48 h and
assayed for ACTN4 expression by western blot analysis, invasion by
Matrigel invasion assay, cell viability by MTS assay, and cell
growth by cell count.
Matrigel invasion assay
The Matrigel invasion assay was performed using
Matrigel-coated invasion chambers (Becton-Dickinson, Franklin
Lakes, NJ, USA) as described previously (19). Briefly, a suspension of
5×104 cells in 500 μl serum-free medium was added to the
insert, and 750 μl RPMI with 10% FBS was added to the bottom of the
well. After the plates were incubated for 22 h at 37°C, the inserts
were fixed in methanol, the filters were stained with 1% toluidine
blue in 1% borax, and the number of cells that invaded through the
Matrigel-coated Transwell inserts was counted at ×40 magnification.
The number of cells was counted in independent triplicate
experiments for at least 5 fields/well.
Cell viability assays and cell
counts
Cell viability was assessed by MTS assay (Promega
Corporation, Madison, WI, USA) according to manufacturer’s
instructions. Briefly, cells treated with ACTN4 siRNA or non-sense
siRNA were incubated for 48 h in 96-well plates (5×103
cells/well). Two hours after adding MTS, the plates were read at a
wavelength of 490 nm in a microplate autoreader. Results are
expressed as the mean optical density of the 6-well set/group,
repeated twice with similar results. For cell counts, cells treated
with ACTN4 siRNA or non-sense siRNA were incubated for 48 h in
24-well plates (1.5×104 cells/well in triplicate). The
total cell number in 3 independent wells/group was counted at the
indicated time using a hemocytometer, and the mean value of 4
fields was recorded.
Statistical analyses
All statistical analyses were performed using the
StatView 5.0 software package for Windows (SAS Institute Inc.,
Cary, NC, USA). Results are presented as the means ± standard
deviation. Variables of different groups were compared using the
Mann-Whitney U test. The independence of fit of categorical data
was analyzed using the Chi-square test. Survival curves were
constructed by the Kaplan-Meier method, and differences between
them were assessed using the log-rank test. In all tests a p-value
≤0.05 was considered to indicate statistical significance.
Results
Expression of ACTN4 in bladder cancer
specimens
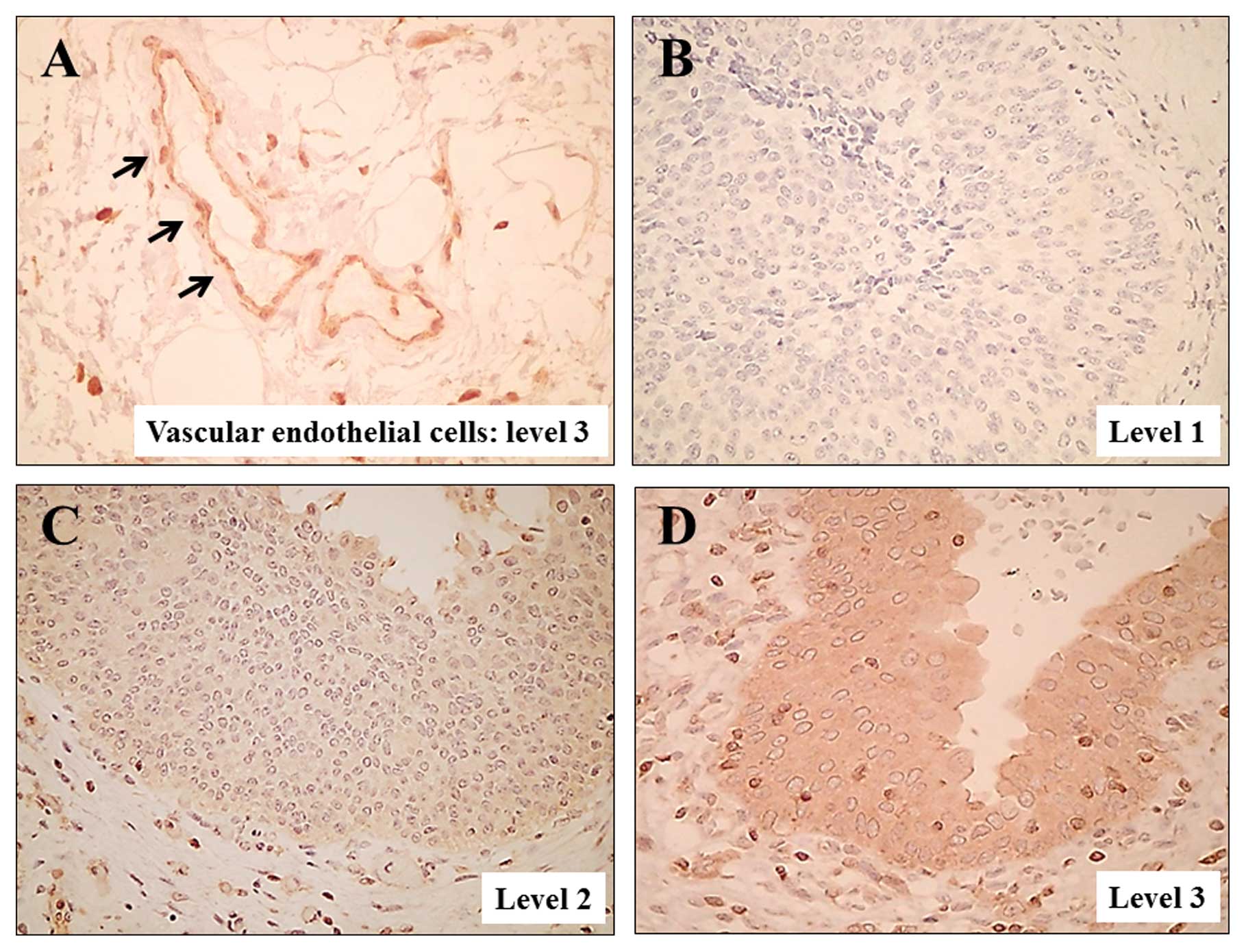
As in a previous study (18), ACTN4 was clearly expressed in
vascular endothelial cells (Fig.
1A; level 3 expression). The expression intensity was level 1
in 9 tumors (Fig. 1B), level 2 in
43 tumors (Fig. 1C), level 3 in 34
tumors (Fig. 1D) and level 4 in 9
tumors. High ACTN4 expression (level 3 or 4) was found in 34 of the
42 (81%) specimens from patients with muscle-invasive bladder
cancer but only in 9 of the 53 (17%) specimens from patients with
superficial bladder cancer. In specimens of patients with
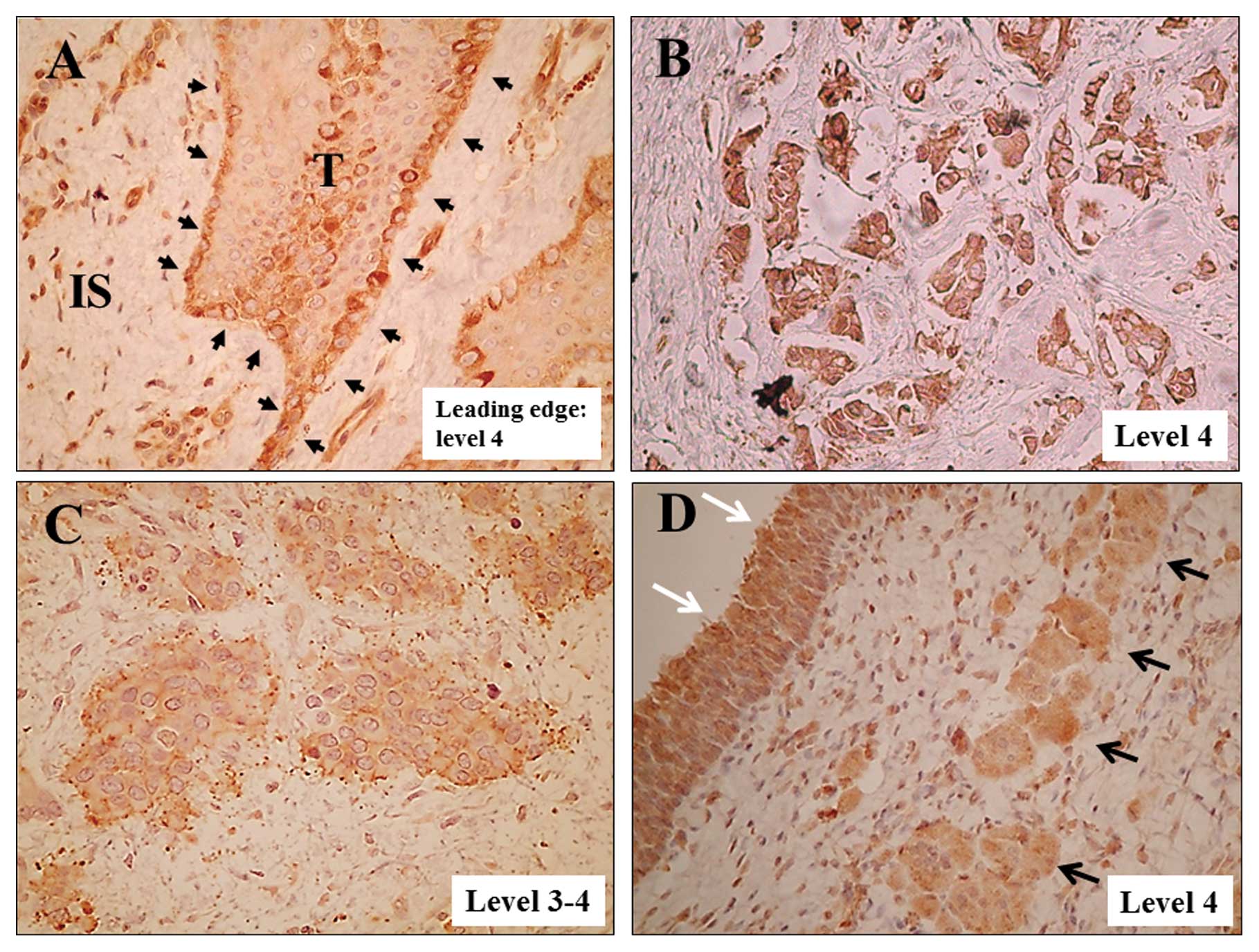
muscle-invasive bladder cancer, cells at the leading edges of the
invasive tumors (Fig. 2A) and
scattered invading cells frequently showed high ACTN4 expression
(Fig. 2B–D). Cells aligned at the
leading edges of invading bladder cancer sometimes showed higher
ACTN4 expression in the cytoplasm than in cells in the non-leading
edge areas (Fig. 2A).
Association of ACTN4 expression level
with clinicopathological factors
Patients with high ACTN4 expression had
significantly higher pathological T stage (p<0.0001) and higher
histological grade (p<0.0001) than those with low ACTN4
expression (Table I). A
significantly higher percentage of patients with muscle-invasive
disease (≥pT2) had high ACTN4 expression when compared with
patients with a pTa or pT1 tumor (81 vs. 17%, p<0.001). The
number of concomitant CIS lesions did not significantly differ
between patients with high ACTN4 expression and those with low
expression. In patients with superficial bladder cancer who
underwent TUR only (n=49) there was no significant difference
between the intravesical recurrence-free survival of patients with
high ACTN4 expression and that of patients with low expression
(p=0.2598). In patients who underwent radical cystectomy (n=46)
neither extravesical recurrence-free survival nor cancer-specific
survival differed significantly between patients with high ACTN4
expression and patients with low ACTN4 expression (p=0.8804 for
extravesical recurrence-free survival and p=0.7529 for
cancer-specific survival) (data not shown).
 | Table ICharacteristics of the 95 patients
with bladder cancer. |
Table I
Characteristics of the 95 patients
with bladder cancer.
| ACTN4 | |
|---|
|
| |
|---|
| Low (n=52) | High (n=43) | P-valuea |
|---|
| Gender, n | | | 0.7087 |
| Male | 42 | 36 | |
| Female | 10 | 7 | |
| Age (years) | | | 0.341 |
| <70 | 34 | 24 | |
| ≥70 | 18 | 19 | |
| T stage, n | | | <0.0001 |
| pTa | 32 | 2 | |
| pT1 | 12 | 7 | |
| pT2 | 3 | 15 | |
| pT3 | 2 | 18 | |
| pT4 | 3 | 1 | |
| Muscle invasion
(≥pT2), n | | | <0.0001 |
| Yes | 8 | 34 | |
| No | 44 | 9 | |
| Tumor grade
(dominant), n | | | <0.0001 |
| G1 | 18 | 3 | |
| G2 | 26 | 14 | |
| G3 | 8 | 26 | |
| Presence of CIS,
n | | | 0.3223 |
| Yes | 4 | 6 | |
| No | 48 | 37 | |
ACTN4 expression in the leading edge of
cultured bladder cancer cells
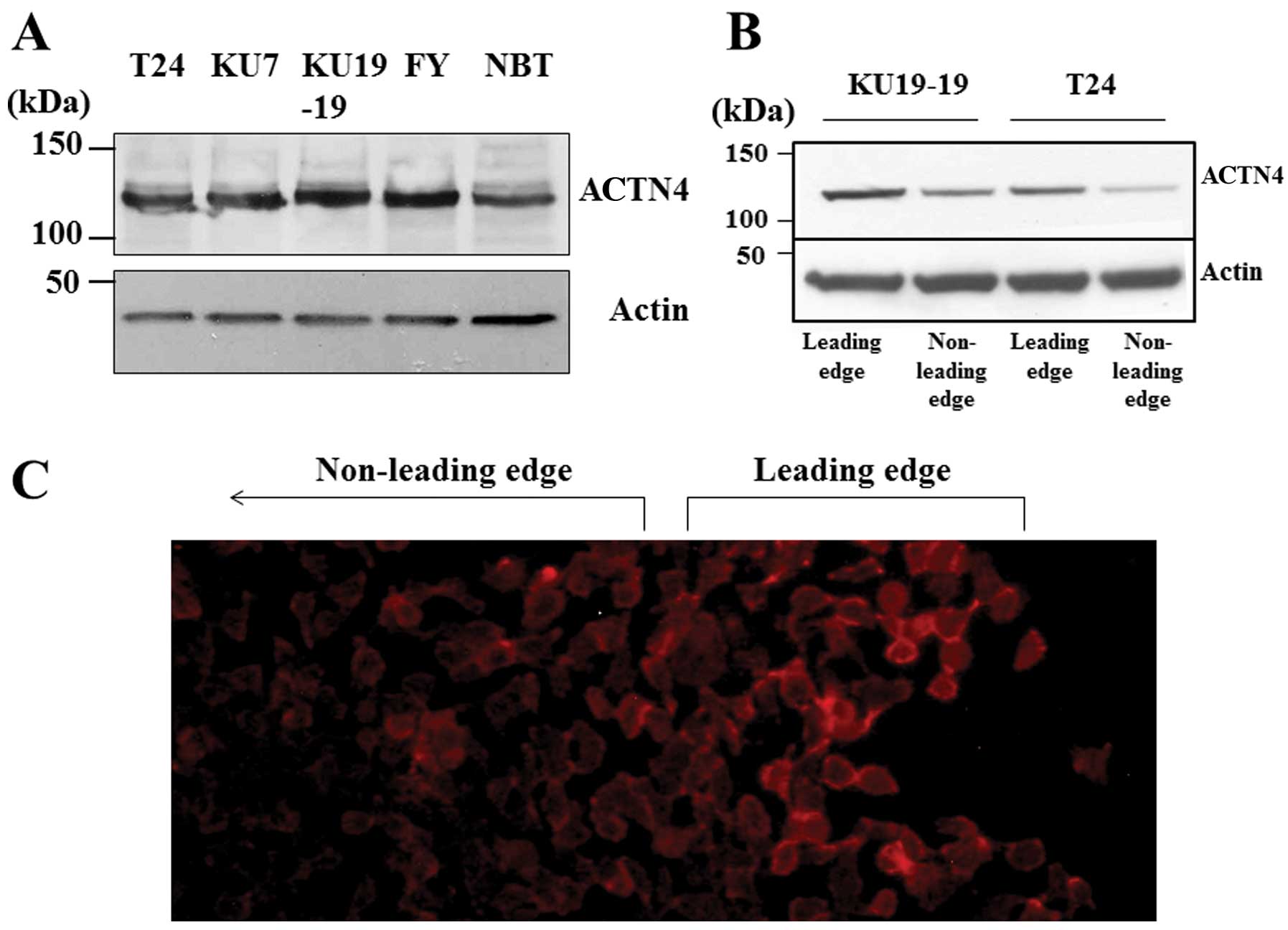
Fig. 3A shows
western blot results for ACTN4 in 5 human bladder cancer cell
lines. All the cell lines clearly expressed ACTN4, and we used T24
and KU19-19 cells for the following experiments.
Immunocytochemistry indicated that in cultured cells there was more
ACTN4 in the leading edge of cells than that in the non-leading
edge of cells (Fig. 3C), and
western blot analysis showed that ACTN4 expression was stronger in
the leading edge of cells (Fig.
3B).
Effect of ACTN4 knockdown on invasive
ability
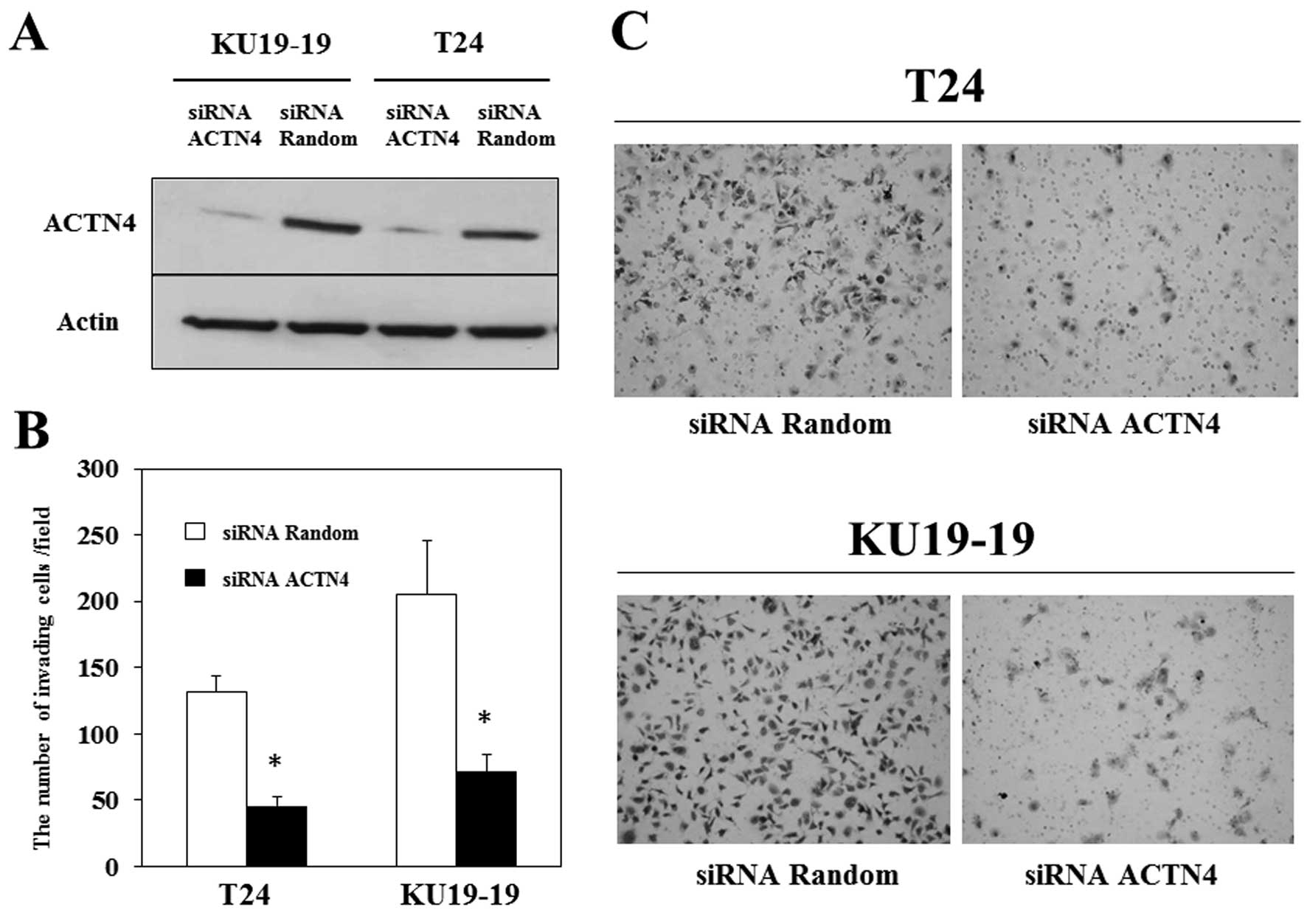
siRNA interference suppressed ACTN4 expression in
T24 and KU19-19 cells (Fig. 4A),
and we assessed the effect of this suppression on the invasiveness
of bladder cancer cells by using Matrigel invasion assays. Matrigel
invasion assay demonstrated that the number of T24 and KU19-19
cells invading through the chamber was significantly decreased by
ACTN4 suppression (Fig. 4B and C),
indicating that ACTN4 may contribute to the invasiveness of bladder
cancer.
Effect of ACTN4 knockdown on bladder
cancer cell viability and proliferation
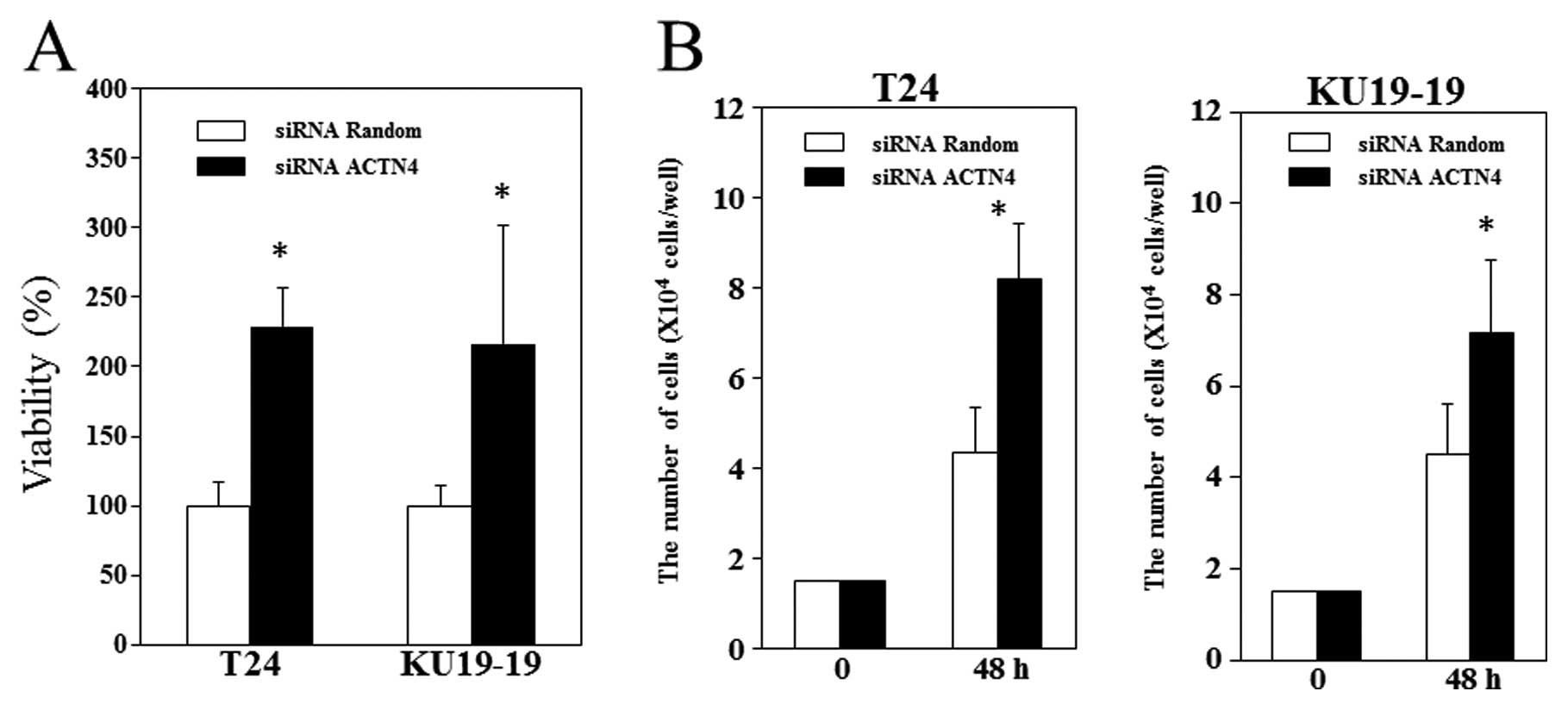
We found that ACTN4 suppression significantly
increased the viability of the 2 bladder cancer cell lines we
investigated (Fig. 5A). We also
found that 2 days after T24 and KU19-19 cells were treated with
control siRNA or ACTN4 siRNA, the total number of
ACTN4-siRNA-treated cells was significantly higher than the number
of control-siRNA-treated cells (Fig. 5B
and C) (p<0.05).
Effects of ACTN4 on intracellular
signaling pathways of bladder cancer cells
The results of the invasion assay, MTS assay, and
cell counts showed that ACTN4 suppression decreased the invasive
ability of bladder cancer cells but increased their proliferation.
Although ACTN4 is reportedly associated with cancer aggressiveness,
the results of the MTS assay and cell counts were unexpected. We,
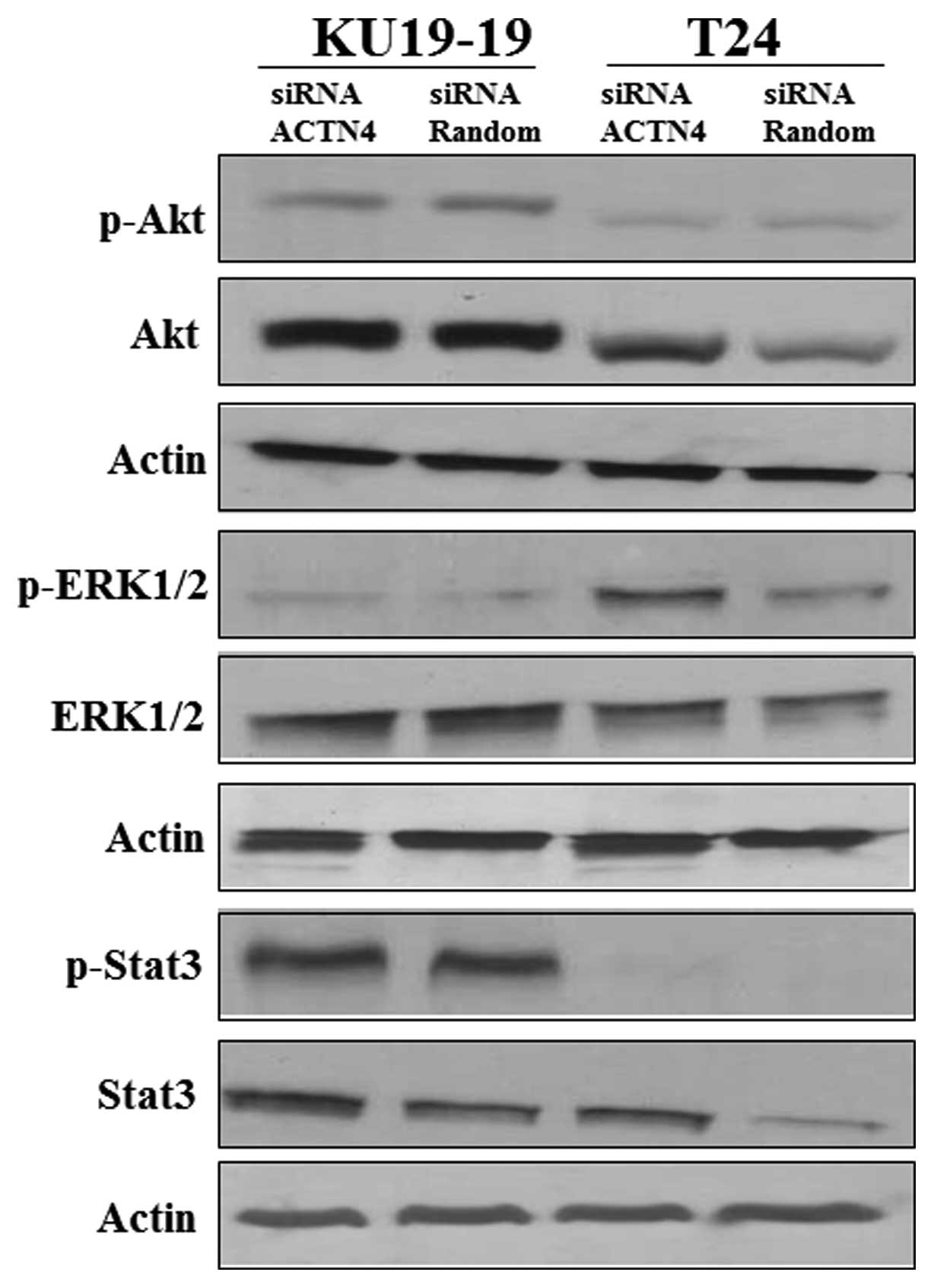
therefore, evaluated changes in the phosphorylation of Akt, ERKs
and STAT3, which are possibly associated with cell proliferation.
The phosphorylation of ERK1/2 in T24 and KU19-19 cells was
increased by ACTN4 suppression, but that of Akt and STAT3 did not
appear to be affected (Fig. 6).
These results suggest that ACTN4 suppression increases bladder
cancer proliferation in part through ERK1/2 phosphorylation.
Discussion
Decreased cell adhesion and increased cell motility
are necessary steps for the initiation of cancer cell metastasis,
and actin stress fiber distribution in cancer cells changes when
their motility increases. ACTN4 was identified as a protein that
was concentrated in the cytoplasm where colon cancer cells were
sharply extended and thus was suggested to be associated with
cancer cell invasion (10). In that
report the increased cytoplasmic expression of ACTN4 was associated
with poor prognosis of patients with breast cancer. Increased
expression of ACTN4 in cancer tissues has been associated with poor
prognosis in breast (10), ovarian
(20), non-small cell lung
(13) and pancreatic cancer
(21). Increased ACTN4 expression
in cancer tissues has been associated with higher percentages of
lymph node metastasis in colon (14) and esophageal cancer (22). Although the above-mentioned studies
indicate that ACTN4 promotes carcinogenesis, inverse findings were
also reported for neuroblastoma (23) and prostate cancer (24,25).
Highly malignant neuroblastoma stem cells showed decreased growth
ability and loss of tumorigenicity after transfection with ACTN4
cDNA (23). ACTN4 expression was
downregulated in prostate cancer cells when compared with normal
prostate epithelial cells, and restoration of ACTN4 expression had
a suppressive effect on the proliferation of prostate cancer cells
(24). The association of ACTN4
expression with cancer aggressiveness thus differs between cancer
types.
In our immunohistochemical analysis results,
increased ACTN4 expression was associated with higher pT stage and
higher histological grade. Although 3 of the 4 patients with pT4
disease had tumors predominantly showing low ACTN4 expression in
the primary lesion, 2 of the 3 cases had cancer cells invading only
into prostatic ducts and not prostatic stroma. Bladder cancer with
only prostatic ductal invasion is considered to be less aggressive
than that with prostatic stromal invasion. The 2 patients with pT4
disease who had only ductal invasion survived more than 5 years
without metastasis. Another patient with pT4 disease who had a
tumor predominantly showing low ACTN4 expression had a small region
that showed high ACTN4 expression. This patient succumbed to
disease within 1 year after radical cystectomy. Although the tumors
in 34 of the 42 patients with invasive bladder cancer (81%) showed
high ACTN4 expression, the tumors in the other 8 cases showed low
ACTN4 expression. Most of these 8 tumors, however, had small
regions showing high ACTN4 expression. In these tumors, ACTN4
expression was particularly increased in tumor budding. Similar
immunohistochemical results have been reported in colon cancer
(14). The increased expression of
ACTN4 was most significant in dedifferentiated cancer cells at the
invasive front.
All bladder cancer cell lines expressed ACTN4
protein (Fig. 3A). Koizumi et
al(15) reported that ACTN4
mRNA and protein expression levels were higher in bladder cancer
cells than in cultured urothelial cells. In the present study the
leading edge of the cultured bladder cancer cells (T24 and KU19-19)
appeared to be stained more strongly for ACTN4 than the non-leading
edge region, and western blot analysis supported these
immunohistochemical results (Fig.
3B). In the immunohistochemical analysis for human bladder
cancer tissues, high expression of ACTN4 was shown in the leading
edges of bladder cancer (Fig. 2A).
Furthermore, ACTN4 knockdown suppressed the invasive ability of
both T24 and KU19-19 cells. The suppression of invasive ability by
ACTN4 knockdown was also noted in J82 invasive bladder cancer cells
(15) and BxPC3 pancreatic cancer
cells (18). These results suggest
that ACTN4 is involved in bladder cancer invasion. Activated forms
of Rac1 and Cdc42 were found to upregulate ACTN4 in NIH3T3 cells,
and Rac1 and Cdc42 may be upstream molecules of ACTN4 in cancer
cell invasion (26). The upstream
molecules that regulate ACTN4 in bladder cancer invasion need to be
determined.
Notably, the proliferation of bladder cancer cells
was increased by ACTN4 knockdown. Koizumi et al(15) reported that ACTN4 suppression
increased the proliferation of J82 bladder cancer cells, and colony
formation was inversely correlated with the amount of ACTN4 protein
in A172 neuroblastoma cells that were ACTN4 stable transfectants
(24). We, therefore, examined
changes in the phosphorylation of major signaling molecules that
can influence cell proliferation and found that ACTN4 suppression
increased the phosphorylation of ERKs but not that of AKT or STAT3,
suggesting that the increased proliferation of bladder cancer cells
that was caused by ACTN4 suppression was at least partly mediated
by the ERK pathway. Koizumi et al(15) suggested that Wnt signaling may also
be associated with bladder cancer cell proliferation elicited by
ACTN4 suppression. The signaling pathways by which ACTN4
suppression increases proliferation warrant further
investigation.
In the present study, we examined whether high ACTN4
expression in resected tumors predicted poor prognosis in patients
who underwent radical cystectomy and found that it was not an
independent predictor of prognosis. A high percentage (81%) of the
patients with muscle-invasive bladder cancer showed high ACTN4
expression, and most of the patients (7 of 8) who had invasive
bladder cancer but with low ACTN4 expression had minor components
with high ACTN4 expression. In the present study we used the
predominant ACTN4 expression level in each patient as that
patient’s ACTN4 expression level, and some patients whose bladder
cancer showed predominantly low ACTN4 expression had components
with high ACTN4 expression. The predominant ACTN4 level therefore
did not always reflect the aggressiveness of each tumor. Because
almost all patients with muscle-invasive bladder cancer (97.6%) had
variable amounts of bladder cancer tissues with increased ACTN4
expression, increased ACTN4 expression appeared to be an essential
change in bladder cancer invasion. If increased ACTN4 expression is
a basic change necessary for invasion, it is reasonable that high
ACTN4 expression was not an independent predictor of prognosis in
patients with invasive bladder cancer who underwent radical
cystectomy. In addition, the results of our cell viability assay
showed the possibility that ACTN4 expression suppresses cell
proliferation in bladder cancer. In the 22RV1 prostate cancer cell
line cell proliferation was inhibited by ACTN4 overexpression
(24). If ACTN4 expression
suppresses cell proliferation in bladder cancer, it may have a
positive impact on patient prognosis even though it stimulates
invasion.
In conclusion, in the present study, high ACTN4
expression was associated with a higher pathological stage and a
higher histological grade but was not an independent predictor of
prognosis in patients with invasive bladder cancer. Our
immunohistochemical and in vitro results indicate that ACTN4
may play a fundamental role in bladder cancer invasion. ACTN4
expression stimulates the invasion of bladder cancer cells but may
suppress their proliferation and produce conditions in which they
invade easily. Further study will be needed to clarify the role of
ACTN4 in bladder cancer invasion.
References
|
1
|
Ghoneim MA, el-Mekresh MM, el-Baz MA,
el-Attar IA and Ashamallah A: Radical cystectomy for carcinoma of
the bladder: critical evaluation of the results in 1,026 cases. J
Urol. 158:393–399. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stein JP, Lieskovsky G, Cote R, et al:
Radical cystectomy in the treatment of invasive bladder cancer:
long-term results in 1,054 patients. J Clin Oncol. 19:666–675.
2001.PubMed/NCBI
|
|
3
|
Takahashi A, Tsukamoto T, Tobisu K, et al:
Radical cystectomy for invasive bladder cancer: results of
multi-institutional pooled analysis. Jpn J Clin Oncol. 34:14–19.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosenberg JE, Carroll PR and Small EJ:
Update on chemotherapy for advanced bladder cancer. J Urol.
174:14–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
von der Maase H, Hansen SW, Roberts JT, et
al: Gemcitabine and cisplatin versus methotrexate, vinblastine,
doxorubicin, and cisplatin in advanced or metastatic bladder
cancer: results of a large, randomized, multinational, multicenter,
phase III study. J Clin Oncol. 18:3068–3077. 2000.
|
|
6
|
von der Maase H, Sengelov L, Roberts JT,
et al: Long-term survival results of a randomized trial comparing
gemcitabine plus cisplatin, with methotrexate, vinblastine,
doxorubicin, plus cisplatin in patients with bladder cancer. J Clin
Oncol. 23:4602–4608. 2005.
|
|
7
|
Ito K, Fujita T, Akada M, et al:
Identification of bladder cancer antigens recognized by IgG
antibodies of a patient with metastatic bladder cancer. Int J
Cancer. 108:712–724. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen YT, Scanlan MJ, Sahin U, et al: A
testicular antigen aberrantly expressed in human cancers detected
by autologous antibody screening. Proc Natl Acad Sci USA.
94:1914–1918. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scanlan MJ, Chen YT, Williamson B, et al:
Characterization of human colon cancer antigens recognized by
autologous antibodies. Int J Cancer. 76:652–658. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Honda K, Yamada T, Endo R, et al:
Actinin-4, a novel actin-bundling protein associated with cell
motility and cancer invasion. J Cell Biol. 140:1383–1393. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Araki N, Hatae T, Yamada T and Hirohashi
S: Actinin-4 is preferentially involved in circular ruffling and
macropinocytosis in mouse macrophages: analysis by fluorescence
ratio imaging. J Cell Sci. 113:3329–3340. 2000.PubMed/NCBI
|
|
12
|
Wang SE, Shin I, Wu FY, Friedman DB and
Arteaga CL: HER2/Neu (ErbB2) signaling to Rac1-Pak1 is temporally
and spatially modulated by transforming growth factor β. Cancer
Res. 66:9591–9600. 2006.PubMed/NCBI
|
|
13
|
Yamagata N, Shyr Y, Yanagisawa K, et al: A
training-testing approach to the molecular classification of
resected non-small cell lung cancer. Clin Cancer Res. 9:4695–4704.
2003.PubMed/NCBI
|
|
14
|
Honda K, Yamada T, Hayashida Y, et al:
Actinin-4 increases cell motility and promotes lymph node
metastasis of colorectal cancer. Gastroenterology. 128:51–62. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koizumi T, Nakatsuji H, Fukawa T, et al:
The role of actinin-4 in bladder cancer invasion. Urology.
75:357–364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bubeník J, Baresová M, Viklický V, et al:
Established cell line of urinary bladder carcinoma (T24) containing
tumour-specific antigen. Int J Cancer. 11:765–773. 1973.PubMed/NCBI
|
|
17
|
Tachibana M, Miyakawa A, Nakashima J, et
al: Constitutive production of multiple cytokines and a human
chorionic gonadotrophin beta-subunit by a human bladder cancer cell
line (KU-19-19): possible demonstration of totipotential
differentiation. Br J Cancer. 76:163–174. 1997. View Article : Google Scholar
|
|
18
|
Kikuchi S, Honda K, Tsuda H, et al:
Expression and gene amplification of actinin-4 in invasive ductal
carcinoma of the pancreas. Clin Cancer Res. 14:5348–5356. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Horiguchi A, Sumitomo M, Asakuma J, et al:
Leptin promotes invasiveness of murine renal cancer cells via
extracellular signal-regulated kinases and rho dependent pathway. J
Urol. 176:1636–1641. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamamoto S, Tsuda H, Honda K, et al:
Actinin-4 gene amplification in ovarian cancer: a candidate
oncogene associated with poor patient prognosis and tumor
chemoresistance. Mod Pathol. 22:499–507. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hayashida Y, Honda K, Idogawa M, et al:
E-cadherin regulates the association between β-catenin and
actinin-4. Cancer Res. 65:8836–8845. 2005.
|
|
22
|
Hatakeyama H, Kondo T, Fujii K, et al:
Protein clusters associated with carcinogenesis, histological
differentiation and nodal metastasis in esophageal cancer.
Proteomics. 6:6300–6316. 2006. View Article : Google Scholar
|
|
23
|
Nikolopoulos SN, Spengler BA, Kisselbach
K, Evans AE, Biedler JL and Ross RA: The human non-muscle α-actinin
protein encoded by the ACTN4 gene suppresses tumorigenicity
of human neuroblastoma cells. Oncogene. 19:380–386. 2000.
|
|
24
|
Hara T, Honda K, Shitashige M, et al: Mass
spectrometry analysis of the native protein complex containing
actinin-4 in prostate cancer cells. Mol Cell Proteomics. 6:479–491.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jasavala R, Martinez H, Thumar J, et al:
Identification of putative androgen receptor interaction protein
modules: cytoskeleton and endosomes modulate androgen receptor
signaling in prostate cancer cells. Mol Cell Proteomics. 6:252–271.
2007. View Article : Google Scholar
|
|
26
|
Teramoto H, Malek RL, Behbahani B,
Castellone MD, Lee NH and Gutkind JS: Identification of H-Ras,
RhoA, Rac1 and Cdc42 responsive genes. Oncogene. 22:2689–2697.
2003. View Article : Google Scholar : PubMed/NCBI
|