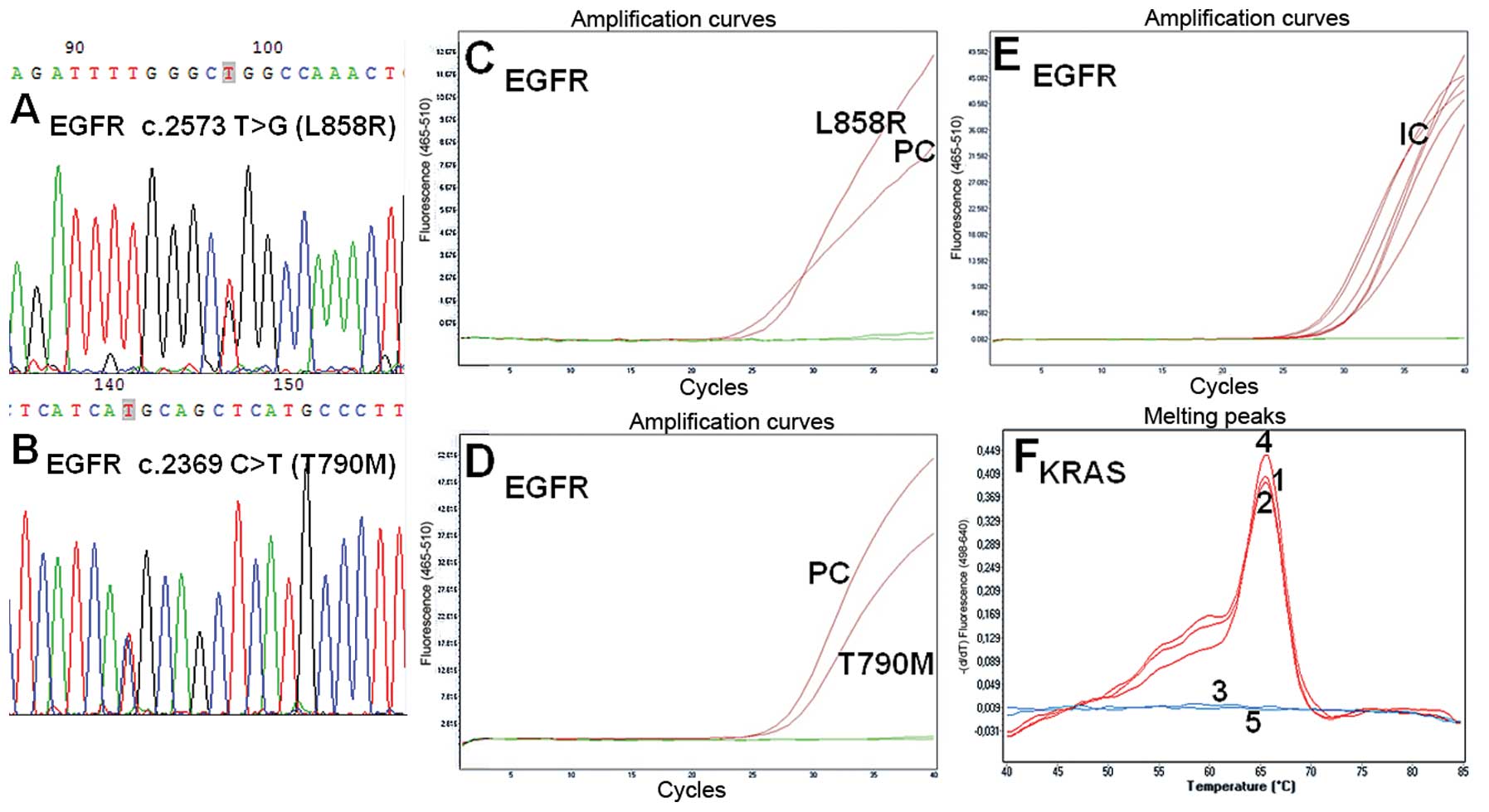
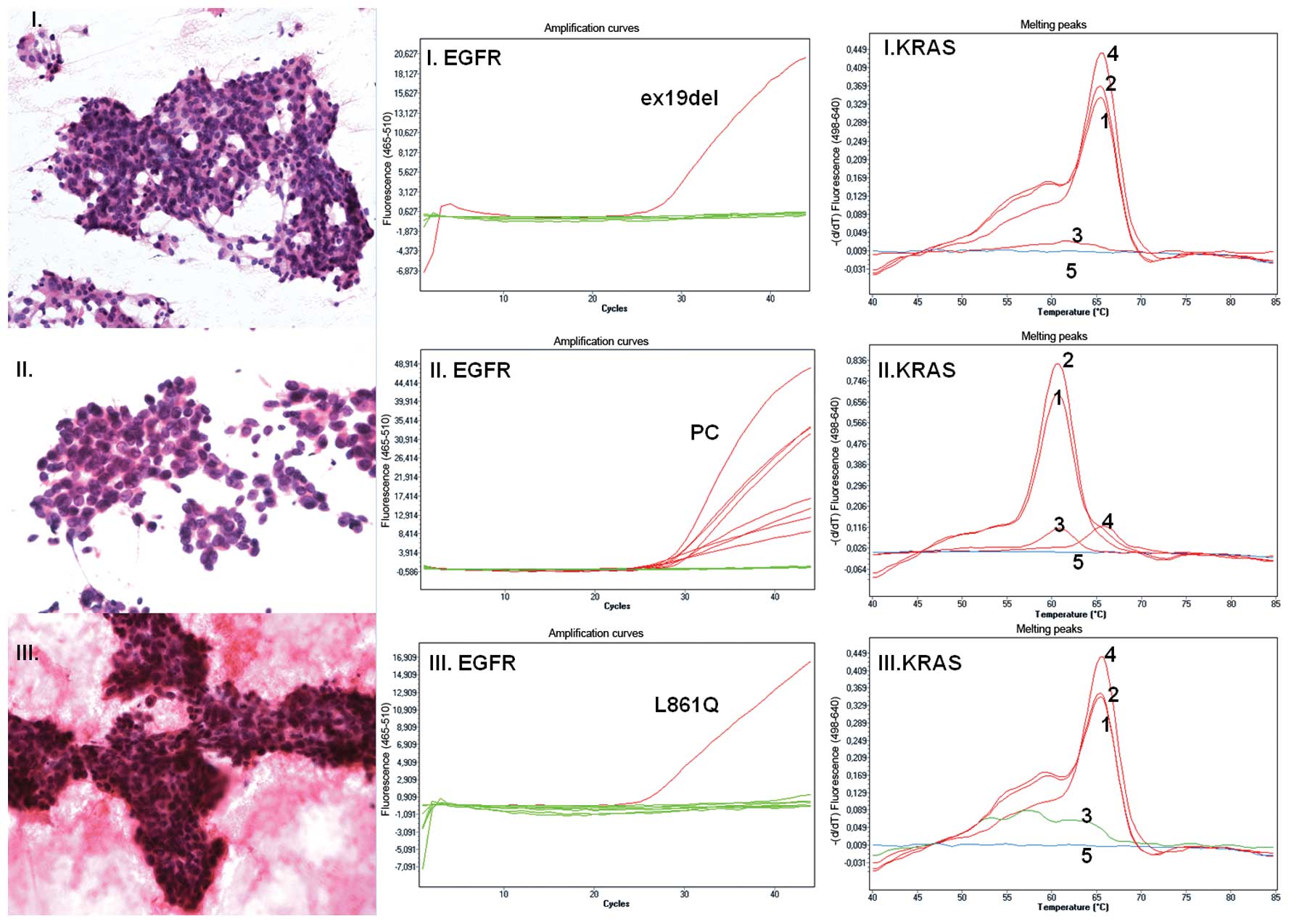
|
1
|
Milella M, Nuzzo C, Bria E, Sperduti I,
Visca P, Buttitta F, Antoniani B, Merola R, Gelibter A, Cuppone F,
D’Alicandro V, Ceribelli A, Rinaldi M, Cianciulli A, Felicioni L,
Malatesta S, Marchetti A, Mottolese M and Cognetti F: EGFR
molecular profiling in advanced NSCLC: a prospective phase II study
in molecularly/clinically selected patients pretreated with
chemotherapy. J Thorac Oncol. 7:672–680. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsao MS, Sakurada A, Cutz JC, Zhu CQ,
Kamel-Reid S, Squire J, Lorimer I, Zhang T, Liu N, Daneshmand M,
Marrano P, da Cunha Santos G, Lagarde A, Richardson F, Seymour L,
Whitehead M, Ding K, Pater J and Shepherd FA: Erlotinib in lung
cancer - molecular and clinical predictors of outcome. N Eng J Med.
353:133–144. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malapelle U, Bellevicine C, Zeppa P,
Palombini L and Troncone G: Cytology-based gene mutation tests to
predict response to anti-epidermal growth factor receptor therapy:
a review. Diagn Cytopathol. 39:703–710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bean J, Brennan C, Shih JY, Riely G, Viale
A, Wang L, Chitale D, Motoi N, Szoke J, Broderick S, Balak M, Chang
WC, Yu CJ, Gazdar A, Pass H, Rusch V, Gerald W, Huang SF, Yang PC,
Miller V, Ladanyi M, Yang CH and Pao W: MET amplification occurs
with or without T790M mutations in EGFR mutant lung tumors with
acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci
USA. 104:20932–20937. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bean J, Riely GJ, Balak M, Marks JL,
Ladanyi M, Miller VA and Pao W: Acquired resistance to epidermal
growth factor receptor kinase inhibitors associated with a novel
T854A mutation in a patient with EGFR-mutant lung adenocarcinoma.
Clin Cancer Res. 14:7519–7525. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu SG, Shih JY, Yu CJ and Yang PC: Lung
adenocarcinoma with good response to erlotinib after gefitinib
treatment failure and acquired T790M mutation. J Thorac Oncol.
3:451–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garcia-Olivé I, Monsó E, Andreo F,
Sanz-Santos J, Taron M, Molina-Vila MA, Llatjós M, Castellà E,
Moran T, Bertran-Alamillo J, Mayo-de-Las-Casas C, Queralt C and
Rosell R: Endobronchial ultrasound-guided transbronchial needle
aspiration for identifying EGFR mutations. Eur Respir J.
35:391–395. 2010.PubMed/NCBI
|
|
8
|
Lewandowska MA, JóŸwicki W, Starzynski J
and Kowalewski J: Analysis of EGFR mutation frequency and
coexistence of KRAS and EGFR mutations using RT-PCR in lung
adenocarcinoma: may a clinical and pathological model of a
patient’s qualification for targeted therapy have an impact on time
to obtain genetic results? Pol J Cardiothorac Surg. 9:443–451.
2012.
|
|
9
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J and
Haber DA: Activating mutations in the epidermal growth factor
receptor underlying responsiveness of non-small-cell lung cancer to
gefitinib. N Eng J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawahara A, Azuma K, Sumi A, Taira T,
Nakashima K, Aikawa E, Abe H, Yamaguchi T, Takamori S, Akiba J and
Kage M: Identification of non-small-cell lung cancer with
activating EGFR mutations in malignant effusion and cerebrospinal
fluid: rapid and sensitive detection of exon 19 deletion E746-A750
and exon 21 L858R mutation by immunocytochemistry. Lung Cancer.
74:35–40. 2011. View Article : Google Scholar
|
|
11
|
Yung TK, Chan KC, Mok TS, Tong J, To KF
and Lo YM: Single-molecule detection of epidermal growth factor
receptor mutations in plasma by microfluidics digital PCR in
non-small cell lung cancer patients. Clin Cancer Res. 15:2076–2084.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marotti JD, Schwab MC, McNulty NJ, Rigas
JR, Delong PA, Memoli VA, Tsongalis GJ and Padmanabhan V:
Cytomorphologic features of advanced lung adenocarcinomas tested
for EGFR and KRAS mutations: a retrospective review of 50 cases.
Diagn Cytopathol. 41:15–21. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dacic S, Shuai Y, Yousem S, Ohori P and
Nikiforova M: Clinicopathological predictors of EGFR/KRAS
mutational status in primary lung adenocarcinomas. Mod Pathol.
23:159–168. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pennycuick A, Simpson T, Crawley D, Lal R,
Santis G, Cane P, Tobal K and Spicer J: Routine EGFR and KRAS
mutation analysis using COLD-PCR in non-small cell lung cancer. Int
J Clin Pract. 66:748–752. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Smits AJ, Kummer JA, Hinrichs JW, Herder
GJ, Scheidel-Jacobse KC, Jiwa NM, Ruijter TE, Nooijen PT,
Looijen-Salamon MG, Ligtenberg MJ, Thunnissen FB, Heideman DA, de
Weger RA and Vink A: EGFR and KRAS mutations in lung carcinomas in
the Dutch population: increased EGFR mutation frequency in
malignant pleural effusion of lung adenocarcinoma. Cell Oncol.
35:189–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Whitehall V, Tran K, Umapathy A, Grieu F,
Hewitt C, Evans TJ, Ismail T, Li WQ, Collins P, Ravetto P, Leggett
B, Salto-Tellez M, Soong R, Fox S, Scott RJ, Dobrovic A and
Iacopetta B: A multicenter blinded study to evaluate KRAS mutation
testing methodologies in the clinical setting. J Mol Diagn.
11:543–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smouse JH, Cibas ES, Janne PA, Joshi VA,
Zou KH and Lindeman NI: EGFR mutations are detected comparably in
cytologic and surgical pathology specimens of nonsmall cell lung
cancer. Cancer. 117:67–72. 2009.PubMed/NCBI
|
|
18
|
Nomoto K, Tsuta K, Takano T, Fukui T,
Yokozawa K, Sakamoto H, Yoshida T, Maeshima AM, Shibata T, Furuta
K, Ohe Y and Matsuno Y: Detection of EGFR mutations in archived
cytologic specimens of non-small cell lung cancer using
high-resolution melting analysis. Am J Clin Pathol. 126:608–615.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pao W, Wang TY, Riely GJ, Miller VA, Pan
Q, Ladanyi M, Zakowski MF, Heelan RT, Kris MG and Varmus HE: KRAS
mutations and primary resistance of lung adenocarcinomas to
gefitinib or erlotinib. PLoS Med. 2:e172005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sharma SV, Bell DW, Settleman J and Haber
DA: Epidermal growth factor receptor mutations in lung cancer. Nat
Rev Cancer. 7:169–181. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Greulich H, Chen TH, Feng W, Jänne PA,
Alvarez JV, Zappaterra M, Bulmer SE, Frank DA, Hahn WC, Sellers WR
and Meyerson M: Oncogenic transformation by inhibitor-sensitive and
-resistant EGFR mutants. PLoS Med. 2:e3132005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stamos J, Sliwkowski MX and Eigenbrot C:
Structure of the epidermal growth factor receptor kinase domain
alone and in complex with a 4-anilinoquinazoline inhibitor. J Biol
Chem. 277:46265–46272. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yun CH, Boggon TJ, Li Y, Woo MS, Greulich
H, Meyerson M and Eck MJ: Structures of lung cancer-derived EGFR
mutants and inhibitor complexes: mechanism of activation and
insights into differential inhibitor sensitivity. Cancer Cell.
11:217–227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kwak EL, Sordella R, Bell DW,
Godin-Heymann N, Okimoto RA, Brannigan BW, Harris PL, Driscoll DR,
Fidias P, Lynch TJ, Rabindran SK, McGinnis JP, Wissner A, Sharma
SV, Isselbacher KJ, Settleman J and Haber DA: Irreversible
inhibitors of the EGF receptor may circumvent acquired resistance
to gefitinib. Proc Natl Acad Sci USA. 102:7665–7670. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kobayashi S, Boggon TJ, Dayaram T, Janne
PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG and Halmos
B: EGFR mutation and resistance of non-small-cell lung cancer to
gefitinib. N Eng J Med. 352:786–792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin WH, Song JS, Lien TW, Chang CY, Wu SH,
Huang YW, Chang TY, Fang MY, Yen KJ, Chen CH, Chu CY, Hsieh HP,
Chen YR, Chao YS and Hsu JT: A high-throughput cell-based screening
for L858R/T790M mutant epidermal growth factor receptor inhibitors.
Anticancer Res. 32:147–151. 2012.PubMed/NCBI
|
|
27
|
Taube E, Jokinen E, Koivunen P and
Koivunen JP: A novel treatment strategy for EGFR mutant NSCLC with
T790M-mediated acquired resistance. Int J Cancer. 131:970–979.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li D, Ambrogio L, Shimamura T, Kubo S,
Takahashi M, Chirieac LR, Padera RF, Shapiro GI, Baum A,
Himmelsbach F, Rettig WJ, Meyerson M, Solca F, Greulich H and Wong
KK: BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective
in preclinical lung cancer models. Oncogene. 27:4702–4711. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gately K, O’Flaherty J, Cappuzzo F, Pirker
R, Kerr K and O’Byrne K: The role of the molecular footprint of
EGFR in tailoring treatment decisions in NSCLC. J Clin Pathol.
65:1–7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lewandowska MA, JóŸwicki W and Zurawski B:
KRAS and BRAF mutation analysis in colorectal adenocarcinoma
specimens with a low percentage of tumor cells. Mol Diagn Ther.
17:193–203. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martelli MP, Sozzi G, Hernandez L,
Pettirossi V, Navarro A, Conte D, Gasparini P, Perrone F, Modena P,
Pastorino U, Carbone A, Fabbri A, Sidoni A, Nakamura S, Gambacorta
M, Fernández PL, Ramirez J, Chan JKC, Grigioni WF, Campo E, Pileri
SA and Falini B: EML4-ALK rearrangement in non-small cell lung
cancer and non-tumor lung tissues. Am J Pathol. 174:661–670. 2009.
View Article : Google Scholar : PubMed/NCBI
|