Introduction
Hepatocellular carcinoma (HCC), the most common
primary hepatic malignancy, is the third leading cause of
cancer-related deaths worldwide (1). In recent years, the incidence of HCC
has been rising in developing countries and in most developed
countries (2,3). Although some significant advances have
been achieved in HCC treatments, poor prognoses and high recurrence
risks have been a major challenge to researchers. Currently,
surgical resection is the main treatment option for HCC patients;
however, the complexities arising from surgery can reduce the
therapeutic effect and the survival rate of patients (4,5).
Accordingly, it is urgent to find more effective and alternative
therapeutic strategies which may benefit HCC patients.
Signal transducer and activator of transcription 3
(STAT3) is an important transcription factor that plays an
essential role in relaying extracellular signals initiated by
cytokines and growth factors from the cytoplasm to the nucleus
(6–9). Following activation, phosphorylated
STATs dimerize and translocate to the nucleus where they regulate
the expression of numerous critical genes involved in cell cycle
progression, proliferation and survival (10,11).
The constitutive activation of STAT3 is frequently detected in
primary human cancer cells including HCC cells (12). These reports indicate that
constitutive activation of STAT3 is one of the important pathways
which contributes to the oncogenesis of HCC and can serve as an
attractive therapeutic target for HCC.
Natural products have received recent interest as
therapeutic agents for HCC due to their relatively few side effects
and have long been used as alternative remedies for a variety of
diseases including cancer (13,14).
Rubus aleaefolius Poir. is a major genus of the rose family,
Rosaceae, generally used as a folk medicine to treat various types
of hepatic disease including HCC. Our previous studies demonstrated
that total alkaloids of Rubus aleaefolius Poir. (TARAP)
inhibited HCC growth in vivo and in vitro via
activation of mitochondrial-dependent apoptosis (15). However, the precise mechanism of its
anticancer activity remains largely unclear. To further elucidate
its antitumor mechanism of action, in the present study we
evaluated the efficacy of TARAP against tumor growth in
vitro and in vivo and investigated the underlying
molecular mechanisms.
Materials and methods
Materials and reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS), penicillin-streptomycin, Trypsin-EDTA and
TRIzol reagent were purchased from Invitrogen (Carlsbad, CA, USA).
SuperScript II reverse transcriptase was obtained from Promega
(Madison, WI, USA). Antibodies for PCNA, cyclin-dependent kinase
(CDK) 2, cyclinE, CDK4, cyclinD1 and p21 were obtained from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), and the cell cycle
detection kit was obtained from Becton-Dickinson (San Jose, CA,
USA). All other chemicals, unless otherwise stated, were obtained
from Sigma (St. Louis, MO, USA). The roots of Rubus
alceifolius Poir. were collected from Anxi of Fujian Province,
identified and authenticated by experts in Fujian University of
Tradional Chinese Medicine.
Preparation and content of TARAP
The preparation of TARAP was performed as previously
described (15). The roots of
Rubus alceifolius Poir. were collected from Anxi of Fujian
Province, identified and authenticated by experts in our
University, and the alkaloids were extracted.
Cell culture
Human HCC HepG2 cells were obtained from the
American Type Culture Collection (ATCC; Manassas, VA, USA). The
cells were grown in DMEM containing 10% (v/v) FBS, and 100 U/ml
penicillin and 100 μg/ml streptomycin in a 37ºC humidified
incubator with 5% CO2. The cells were subcultured at
80–90% confluency.
Colony formation
HepG2 cells were seeded into 6-well plates at a
density of 1×105 cells/well in 2 ml medium. After
treatment with various concentrations of TARAP for 24 h, the cells
were collected and then reseeded into 6-well plates at a density of
1×103 cells/well. Following incubation for 8 days in a
37ºC humidified incubator with 5% CO2, the formed
colonies were fixed with 10% formaldehyde, stained with 0.01%
crystal violet and counted. Cell survival was calculated by
normalizing the survival of the control cells as 100%.
Cell cycle analysis
The cell cycle analysis was carried out by flow
cytometry using fluorescence activated cell sorting (FACSCalibur;
Becton-Dickinson) and propidium iodide (PI) staining. Subsequent to
treatment with various concentrations of TARAP for 24 h, HepG2
cells were collected and adjusted to a concentration of
1×106 cells/ml, and fixed in 70% ethanol at 4ºC
overnight. The fixed cells were washed twice with cold PBS, and
then incubated for 30 min with RNase (8 μg/ml) and PI (10 μg/ml).
The fluorescent signal was detected through the FL2 channel, and
the proportion of DNA in different phases was analyzed using
ModfitLT v3.0 (Verity Software House, Topsham, MA, USA).
In vivo tumor xenograft study
HepG2 cells were grown in culture and then detached
by trypsinization, washed and resuspended in serum-free DMEM.
Six-week-old athymic BALB/c nu/nu male mice received an s.c.
injection of 4×106 HepG2 cells mixed with Matrigel (1:1)
in the right flank to initiate tumor growth. After 7 days of
xenograft implantation when the tumor size reached 3 mm in
diameter, mice were randomly divided into two groups and gavaged
with the following: i) control group (n=10), physiological saline
(PS); and ii) TARAP group (n=10), 3 g/kg/day dose of TARAP in PS.
All treatments were administered 5 days weekly for 21 days. At the
end of the experiment, tumors were excised, and part of the tumor
was fixed in buffered formalin and the remaining was stored at
−80ºC for molecular analyses.
Immunohistochemical analysis
Tumor samples were fixed with 10% formaldehyde for
12 h and subsequently processed conventionally for
paraffin-embedded tumor slides. The slides were subjected to
antigen retrieval and the endogenous peroxidase activity was
blocked with 3% hydrogen peroxide for 10 min. The sections were
incubated with 1% bovine serum albumin in order to decrease
non-specific staining and reduce endogenous peroxidase activity.
For immunohistochemical staining, the sections were incubated with
antibodies against phosphorylated STAT3 (pSTAT3), PCNA, CDK2,
cyclinE, cyclinD1, CDK4 or p21 (all in 1:200 dilution; Santa Cruz
Biotechnology, Inc.). After washing with PBS, slides were incubated
with biotinylated secondary antibody followed by conjugated
horseradish peroxidase (HRP)-labeled streptavidin (Dako) and then
washed with PBS. The slides were then incubated with
diaminobenzidine (DAB, Sigma) as the chromogen, followed by
counterstaining with diluted Harris’ hematoxylin (Sigma). After
staining, five high-power fields (×400) were randomly selected in
each slide, and the average proportion of positive cells in each
field were counted using the true color multi-functional cell image
analysis management system (Image-Pro Plus, Media Cybernetics,
USA). To rule out any non-specific staining, PBS was used to
replace the primary antibody as a negative control.
RNA extraction and RT-PCR analysis
The expression levels of CDK2, CDK4, cyclinD1,
cyclinE and p21 genes were detected by RT-PCR. Total RNA was
isolated with TRIzol reagent. Oligo(dT)-primed RNA (1 μg) was
reverse-transcribed with SuperScript II reverse transcriptase
according to the manufacturer’s instructions. The obtained cDNA was
used to determine the mRNA amount of CDK2, CDK4, cyclinD1, cyclinE
and p21 by PCR. GAPDH was used as an internal control.
Statistical analysis
All data are the means of three determinations, and
data were analyzed using the SPSS Package for Windows (v11.5).
Statistical analysis of the data was performed with the Student’s
t-test and ANOVA. Differences with P<0.05 were considered to
indicate a statistically significant result.
Results
TARAP inhibits the proliferation of HepG2
cells
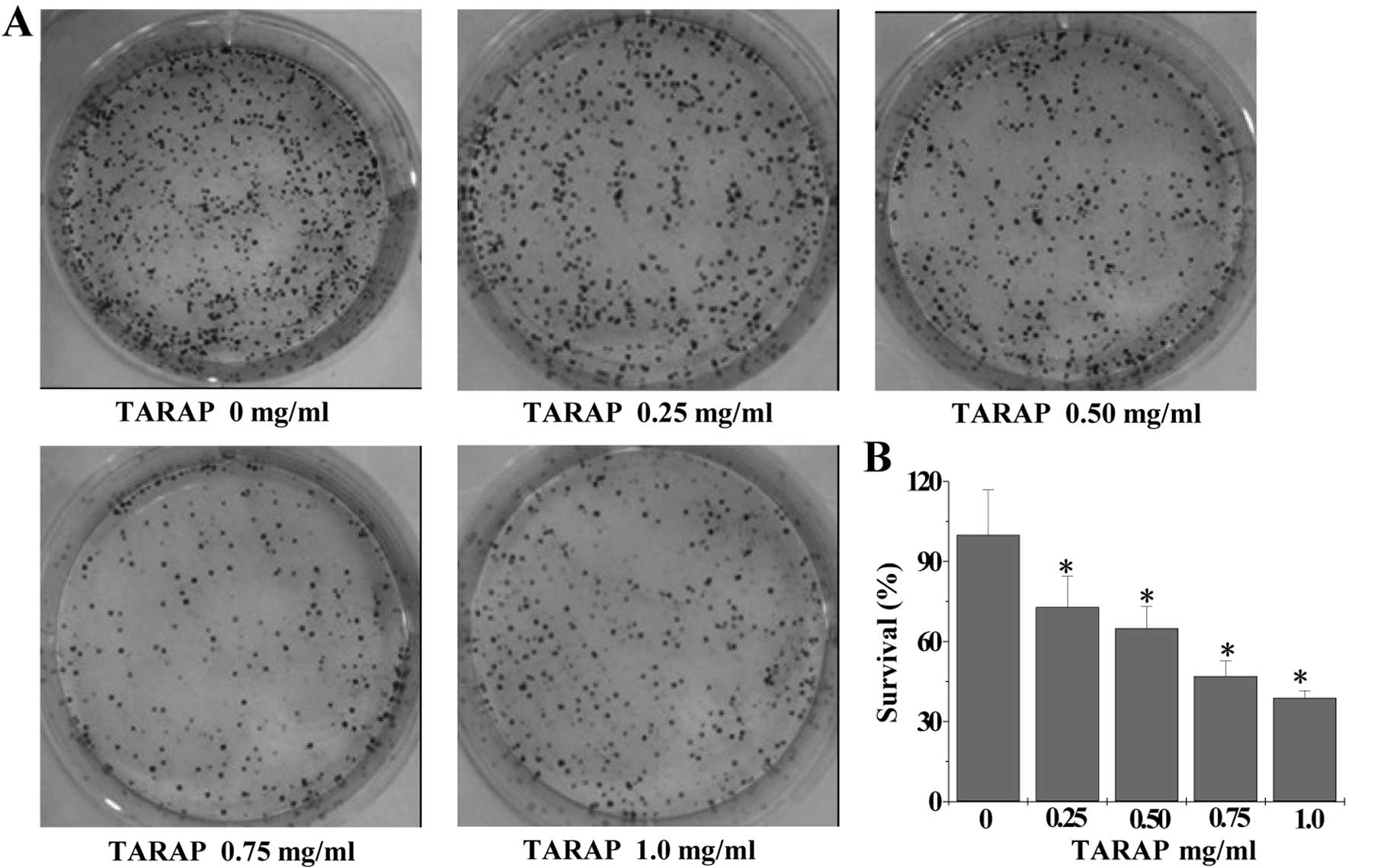
A colony formation assay was used to assess the
proliferation of HepG2 cells. As shown in Fig. 1A and B, TARAP treatment
dose-dependently reduced the cell survival rate by 27–61% as
compared to the untreated control cells (P<0.01).
TARAP blocks the G1/S progression of
HepG2 cells
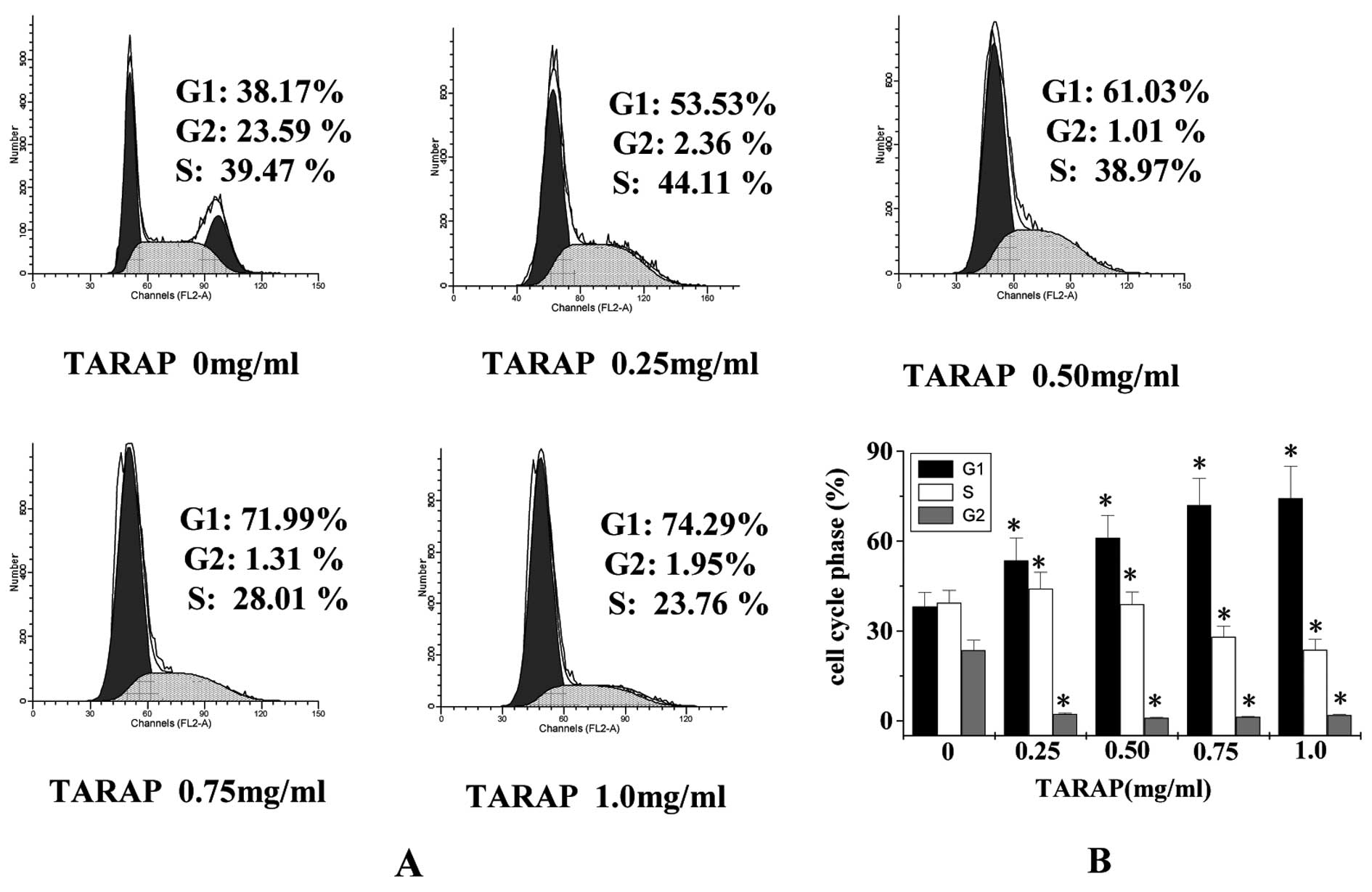
The effect of TARAP on the G1 to S progression in
HepG2 cells was examined via PI staining followed by FACS analysis.
As shown in Fig. 2, the percentage
of cells in the G1 phase following treatment with 0, 0.25, 0.5,
0.75 and 1.0 mg/ml of TARAP was 38.17±4.69, 53.53±7.51, 61.03±7.56,
71.99±8.91 and 74.29±10.57%, respectively (P<0.01), indicating
that TARAP inhibits HepG2 proliferation by arresting the cell cycle
in the G1 phase.
TARAP suppresses STAT3 phosphorylation
and cancer cell proliferatiom in HCC xenograft tumors in mice
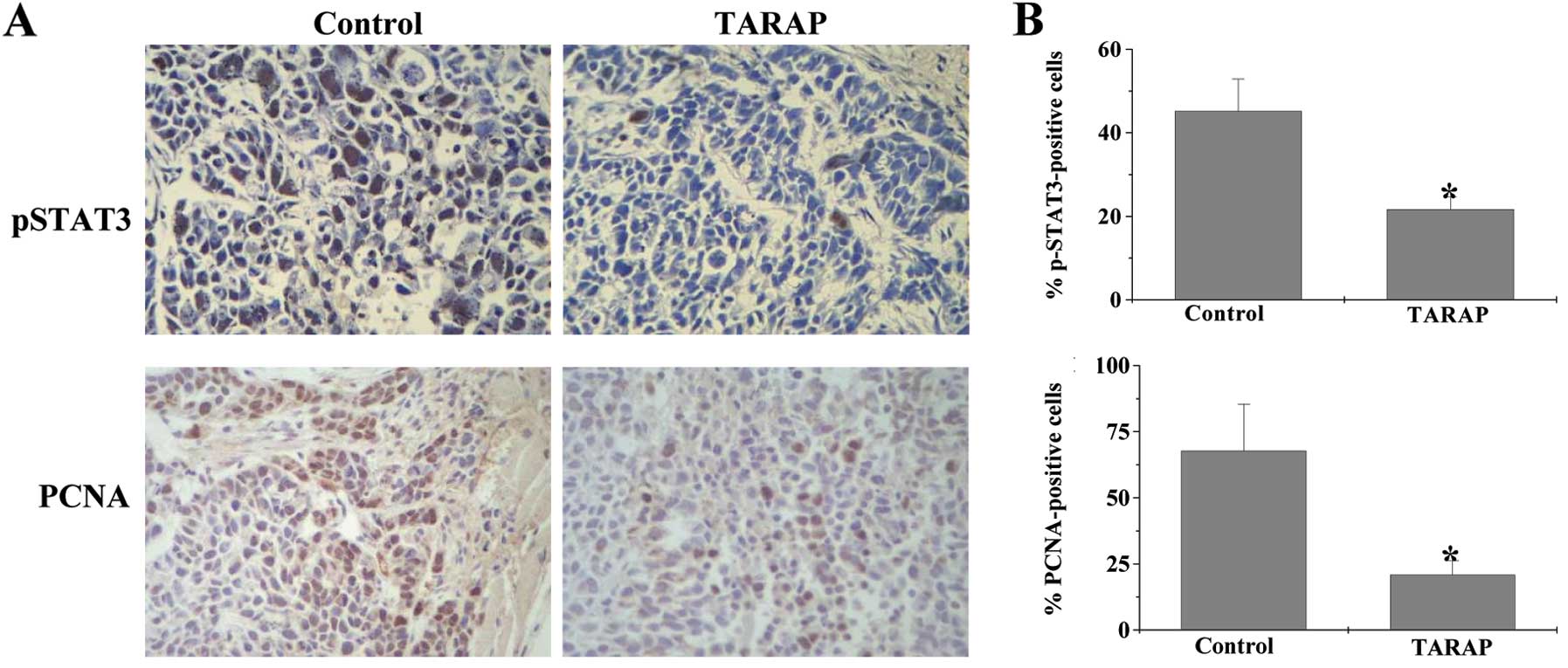
STAT3 plays an important role in cell survival and
proliferation. pSTAT3 leads to promotion of cell proliferation. We
therefore examined the effect of TARAP on STAT3 phosphorylation in
tumor tissues using IHC assay. As shown in Fig. 3, the percentage of pSTAT3-positive
cells in the control and TARAP-treated xenograft tumors was
45.17±7.72 and 21.67±3.01%, respectively (P<0.01), suggesting
that TARAP treatment significantly suppresses the activation of
STAT3 in HCC mice. To determine whether the inhibitory effect of
TARAP on cancer growth is due to cell proliferation, we examined
the expression of PCNA. As shown in Fig. 3, the percentage of PCNA-positive
cells in control or TARAP-treated mice was 67.83±17.61 and
20.83±5.42%, respectively. Taken together, these data suggest that
TARAP inhibits the proliferation of HCC cells via suppression of
pSTAT3.
TARAP regulates the expression of CDK2,
CDK4, cyclinD1, cyclinE, p21 in HCC xenograft tumors in mice
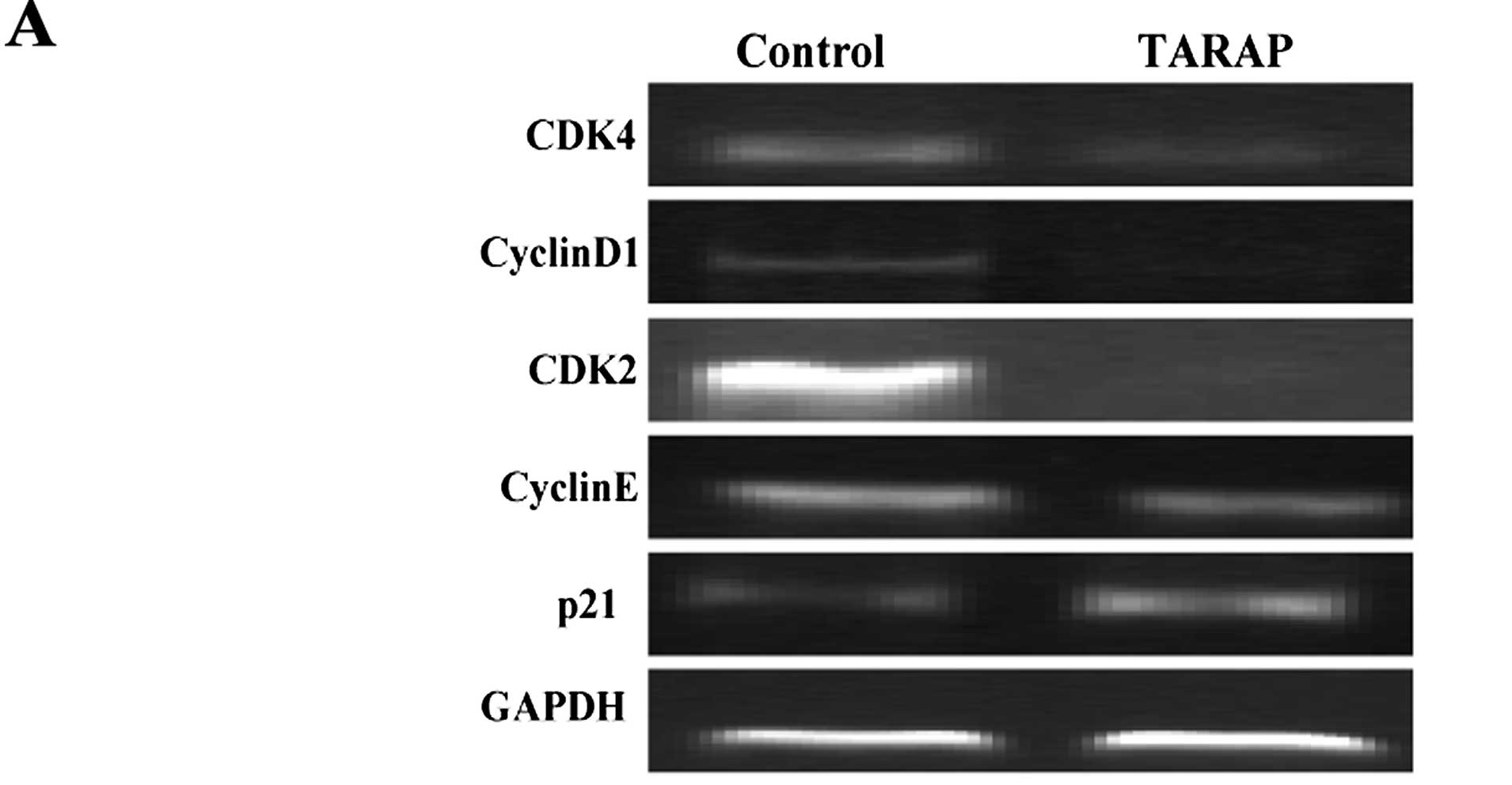
To explore the mechanism of the antiproliferative
activity of TARAP, we performed RT-PCR and IHC assay to
respectively examine the mRNA and protein expression of CDK2, CDK4,
cyclinD1, cyclinE and p21 in HCC tumors in mice. Results of the
RT-PCR showed that TARAP treatment reduced the mRNA expression of
CDK2, CDK4, cyclinD1, cyclinE, in tumors, whereas p21 was increased
(Fig. 4A). Data from IHC assay
indicated that the protein expression patterns of CDK2, CDK4,
cyclinD1, cyclinE and p21 were similar to their respective mRNA
levels. The percentage of CDK2-, CDK4-, cyclinD1-, cyclinE- and
p21-positive cells in the control group was 28.67±4.88,
58.33±10.03, 37.67±5.39, 46.17±6.56 or 32.67±4.81%, whereas that in
TARAP-treated mice was 17.67±2.53, 24.33±3.48, 18.67±3.23,
25.33±3.59 and 89.5±12.44% (Fig.
4B).
Discussion
Although curative therapies such as surgical
resection, liver transplantation and ablative therapies have led to
improvement in the survival of patients with HCC (2,3),
unfortunately, most patients are still diagnosed at advanced stages
and receive only palliative treatments (16,17).
In addition, many currently used anticancer agents possessing
intrinsic cytotoxicity to normal cells limit the effectiveness of
current cancer therapies. Natural products have relatively fewer
side effects and have been used clinically for thousands of years
to treat various types of diseases, including cancer (13,14).
As a natural product, Rubus aleaefolius Poir. has exhibited
strong activity against liver disease including liver cancer.
However, the precise mechanism of its anticancer activity remains
largely unclear.
Cancer cells are characterized by uncontrolled
proliferation (18). Therefore,
inhibiting excessive proliferation of tumor cells is one of the key
approaches for the development of anticancer drugs. Here, using
colony formation assay, we demonstrated that TARAP inhibited the
proliferation of human HCC HepG2 cells in a dose-dependent manner.
The transcription factor STAT3 is essential for cell survival and
proliferation. Constitutive activation of STAT3 is one of the
important pathways which contributes to the oncogenesis in HCC
(12). Our data showed that TARAP
suppressed the activation of STAT3. Eukaryotic cell proliferation
is primarily regulated by the cell cycle. Cell cycle progression is
tightly regulated by cyclins, CDKs, CDK inhibitors (CKIs) and many
other factors. Cyclins associated with CDKs are the primary
regulators of CDK activity. The activation of CDK4 plays an
important role in the passage through the G1 restriction point when
the cell becomes committed to proceed through the cell cycle, while
CDK2 activation plays an essential role in the transition into
S-phase and DNA synthesis. CyclinD1 expression, which is induced
during G1 phase in response to mitogens, complexes with and
activates CDK4. CDK2 is regulated primarily by cyclinE during G1/S
transition and S phase, respectively (19,20).
The KIP protein p21 is generally believed to act as a negative
regulator of cell cycle progression. As a proliferation inhibitor,
p21 protein plays an important role in G1 arrest by binding to and
inhibiting the activity of cyclin-CDK complexes; in contrast, when
bound to PCNA, p21 is degraded more slowly compared with p21
binding to cyclin/CDK, which increases the rate of p21 degradation
(21). Using a HCC mouse xenograft
model, in the present study we found that TARAP decreased the
phosphorylation activation of STAT3, Consequently, the inhibitory
effect of TARAP on STAT3 activation resulted in the suppression of
cell proliferation and cell cycle arrest. Moreover, TARAP treatment
profoundly reduced the expression of PCNA, cyclinE, CDK2, cyclinD1
and CDK4, as well as increased expression of antiproliferative
p21.
In conclusion, for the first time we demonstrated
that TARAP inhibits growth in vivo and in vitro via
inhibition of proliferation and cell cycle arrest, which is
mediated by the suppression of the STAT3 pathway. Our findings
suggest that TARAP may be a potential novel therapeutic agent for
the treatment of cancers with constitutive activation of STAT3.
Acknowledgements
The present study was supported by the Nature
Science Foundation of Fujian Province of China (no. 2010J01191 and
2010J01194); and the project was sponsored by Medical Originality
Foundation of Fujian Province of China (no. 2009-CX-18).
References
|
1
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Ward E, Hao Y and Thun M: Trends
in the leading causes of death in the United States, 1970–2002.
JAMA. 294:1255–1259. 2005.
|
|
3
|
Bosch FX, Ribes J, Díaz M and Cléries R:
Primary liver cancer: worldwide incidence and trends.
Gastroenterology. 127:S5–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patel M, Shariff MI, Ladep NG, et al:
Hepatocellular carcinoma: diagnostics and screening. J Eval Clin
Pract. 18:335–342. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shariff MI, Cox IJ, Gomaa AI, et al:
Hepatocellular carcinoma: current trends in worldwide epidemiology,
risk factors, diagnosis and therapeutics. Expert Rev Gastroenterol
Hepatol. 3:353–367. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Frank DA: STAT3 as a central mediator of
neoplastic cellular transformation. Cancer Lett. 251:199–210. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Germain D and Frank DA: Targeting the
cytoplasmic and nuclear functions of signal transducers and
activators of transcription 3 for cancer therapy. Clin Cancer Res.
13:5665–5669. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Turkson J and Jove R: STAT proteins: novel
molecular targets for cancer drug discovery. Oncogene.
19:6613–6626. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calò VV, Migliavacca M, Bazan V, et al:
STAT proteins: from normal control of cellular events to
tumorigenesis. J Cell Physiol. 197:157–168. 2003.PubMed/NCBI
|
|
10
|
Bromberg J and Darnell JE Jr: The role of
STATs in transcriptional control and their impact on cellular
function. Oncogene. 19:2468–2473. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aggarwal BB, Kunnumakkara AB, Harikumar
KB, et al: Signal transducer and activator of transcription-3,
inflammation, and cancer: how intimate is the relationship? Ann NY
Acad Sci. 1171:59–76. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moran DM, Mattocks MA, Cahill PA, et al:
Interleukin-6 mediates G(0)/G(1) growth arrest in hepatocellular
carcinoma through a STAT3-dependent pathway. J Surg Res. 147:23–33.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ji HF, Li XJ and Zhang HY: Natural
products and drug discovery. Can thousands of years of ancient
medical knowledge lead us to new and powerful drug combinations in
the fight against cancer and dementia? EMBO Rep. 10:194–200.
2009.PubMed/NCBI
|
|
15
|
Zhao JY, Chen XZ, Lin W, et al: Total
alkaloids of Rubus aleaefolius Poir. inhibit hepatocellular
carcinoma growth in vivo and in vitro via activation
of mitochondrial-dependent apoptosis. Int J Oncol. 42:971–978.
2013.
|
|
16
|
Bruix J and Sherman M: Management of
hepatocellular carcinoma. Hepatology. 42:1208–1236. 2005.
View Article : Google Scholar
|
|
17
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar
|
|
18
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morgan DO: Principles of CDK regulation.
Nature. 374:131–134. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sherr CJ and Roberts JM: CDK inhibitors:
positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cayrol C and Ducommun B: Interaction with
cyclin-dependent kinases and PCNA modulates proteasome-dependent
degradation of p21. Oncogene. 17:2437–2444. 1998. View Article : Google Scholar : PubMed/NCBI
|