Introduction
Gliomas are the most common primary tumors in the
central nervous system. Glioblastoma multiforme (GBM, grade IV
according to WHO classification) is one of the most lethal human
tumors, accounting for almost half of malignant brain tumors.
Glioblastoma is highly invasive and usually recurs even after
aggressive resection, radiation or chemotherapy (1). Since the prognosis for patients with
high-grade glioma, including anaplastic astrocytoma and GBM,
remains dismal, novel therapeutic approaches are required.
MicroRNAs (miRNAs) are single stranded 21–25
nucleotide non-coding RNAs, which post-transcriptionally regulate
the expression of protein-coding genes through perfect or imperfect
base pairing with the 3′-untranslated region (3′UTR) of target
mRNAs (2). The role of miRNAs in
tumors has been studied extensively and they are considered to be
novel therapeutic targets. miRNAs are frequently located in genomic
regions which are amplified, deleted or suffer loss of
heterozygosity in cancer (3) and
miRNAs can regulate the expression of tumor-associated genes in
multiple tumor types, including gliomas (4–7).
The brain-enriched miR-128 is an important miRNA in
several tumors, with a varied expression pattern (8). miR-128 is strongly downregulated in
gliomas, compared to normal brain tissues (6,9).
Previous studies have shown that miR-128 is involved in cell
death/survival processes via targeting the truncated isoform of
NTRK3 in SH-SY5Y neuroblastoma cells (10) and the Bax protein in human embryonic
kidney cells (11); it can inhibit
glioma cell proliferation via downregulation of the transcription
factor E2F3a in glioma cells (12,13),
it can reduce glioma self-renewal by inhibition of Bmi-1 expression
(13) and it can also participate
in neuroblastoma cell motility and invasiveness by inhibiting
Reelin and DCX (14).
The erythropoietin-producing hepatocellular
receptors (Eph) are the largest sub-family of receptor tyrosine
kinases (RTKs), and their ligands, the ephrins, compose a major
repulsive signaling system. The erythropoietin-producing
hepatocellular receptor B2 (EphB2), a member of the Eph receptor
tyrosine kinase family, has been shown to regulate cytoskeleton
organization and cell migration in various cell types (15). The EphB2 expression has been shown
to be upregulated in gliomas as compared to normal brain tissues
(16), and overexpression of EphB2
reduces cell adhesion and increases cell invasion in glioma tissues
and cells (16,17).
In this study, we report a novel function of miR-128
in glioma cells. We have demonstrated that overexpression of
miR-128 in glioma cells increased cell-cell adhesion and inhibited
cell migration. Furthermore, we have identified the
erythropoietin-producing hepatocellular receptor B1 (EphB1) and
EphB2 as novel targets of miR-128, and have shown that miR-128
promotes cell-cell adhesion by regulating the EphB2. The results of
this study provide new insight into the mechanism by which miR-128
inhibits glioma cell migration into, and invasion of, the
surrounding tissues.
Materials and methods
Cell culture
The human U87 glioma cell line and HEK-293T (293T)
cell line were purchased from the American Type Culture Collection
(ATCC, Gaithersburg, MD, USA). Both cell lines were cultured in
Dulbecco’s Modified Eagle’s Medium (DMEM)/High Glucose medium
(Thermo Scientific) supplemented with 10% fetal bovine serum (FBS)
and 100 U/ml penicillin/streptomycin.
Vector construction
For expression of miR-128, a 287 bp genomic fragment
containing the human miR-128-2 precursor and flanking sequences was
amplified using the primers listed in Table I and cloned into the modified pll3.7
vector under the control of the human U6 promoter. This construct
was termed pmiR-128. To construct luciferase reporter vectors, the
3′UTR fragments containing the putative miR-128 binding sequences
of human EphB1 (655 bp) and EphB2 (856 bp) were amplified and
cloned downstream of Renilla luciferase in the modified psiCheck2
vector. For overexpression of EphB2, the full length EphB2 coding
sequence without the 3′UTR was amplified from U87 cell total RNA by
RT-PCR and cloned into pcDNA3.1 (Invitrogen).
 | Table IPrimers used for vector construction
and quantitative Reverse Transcription PCR (qRT-PCR). |
Table I
Primers used for vector construction
and quantitative Reverse Transcription PCR (qRT-PCR).
| Gene | Primer (5′→3′) |
|---|
| miR-128
precursor | F:
CACAAGTCGACACAGATTATGGCTTAGGACAGT |
| R:
AAGGATCCTTCCCATTACTAATTCTGCTTC |
| EphB1–3′UTR | F:
CACAACTCGAGAACTCTTGTTTCTTGGGGAAGGAG |
| R:
AAAGATCTATGCAAACAAAAGAAAAACGAGGT |
| EphB2–3′UTR | F:
CACAACTCGAGGATCCTGCATCTGGGTTTGTTTAC |
| R:
AAGGATCCTTCACTAACTGATTGCTCTGCTTG |
|
EphB1–3′UTR-MUT | F:
GAGGGAAAAGGACCAGGGTCATGGCGCTGGAGACCAAGACGTGACACGAATGTACTG |
| R:
CCAGTCTCTCCAGTACATTCGTGTCACGTCTTGGTCTCCAGCGCCATGACCCTGGTC |
|
EphB2–3′UTR-MUT | F:
GGCCAGGACCCGGATCAAAGTGACACTTACCCTGCCCTCCAGAGG |
| R:
CCTCTGGAGGGCAGGGTAAGTGTCACTTTGATCCGGGTCCTGGCCT |
| EphB2
expression | F:
CACAAGTCGACGAAGCGCAACCATGGCTCTGCGGAG |
| R:
AAGGATCCAGTGTCACTTTGATCGGGGACTCAAA |
| miR-128 | RT:
CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAAAGAGAC |
| F:
CCAGCTGGGTCACAGTGAACCGGT |
The mutant luciferase reporter constructs
psiCheck2-EphB1–3′UTR-MUT or psiCheck2-EphB2–3′UTR-MUT carrying
mutations in the sequence of the complementary seed region miR-128
site were generated by fusion PCR. The 655 and 856 bp amplified
3′UTRs were divided into two fragments in which the mutated
sequence was introduced into the overlapping regions. Then, the two
purified PCR products were mixed and amplified using primers for
the whole 655 or 856 bp 3′UTR fragment. All the primers used are
listed in Table I.
Lentivirus production and establishment
of stable cell lines
VSV-G pseudotyped lentiviruses were produced by
co-transfection of 293T cells with the transfer vector and three
packaging vectors: pMDLg/pRRE, pRSV-REV and pCMV-VSVG. Then, the
production medium containing lentivirus was harvested, centrifuged
to remove cell debris and viral supernatant was used for
infection.
U87 cells were seeded at 30% confluence in a 25
cm2 dish in preparation for lentiviral infection and 24
h later, 8 μg/ml polybrene (Sigma) was added to the media, the
cells were infected with lentivirus and 48 h later 0.25 μg/ml
puromycin (Enzo Life Sciences, Farmingdale, USA) was added to the
culture media to select U87 infected cells.
RNA extraction and quantitative
reverse-transcription PCR (qRT-PCR)
Total RNA was extracted using TRIzol (Invitrogen)
and quantification of mature miRNA using qRT-PCR was performed
using primers listed in Table I.
Briefly, total RNA was used to synthesize cDNA with the Reverse
Transcriptase (RT)-PCR kit (Toyobo, Japan) and a stem-loop-like RT
primer containing a miRNA specific region in the 3′ end (Gene
Science and Health, China). qRT-PCR was performed using the
StepOne™ Real-Time PCR system (Applied Biosystems) and the miRNA
qPCR Quantitation kit (Gene Science and Health) according to the
manufacturer’s instructions. The qRT-PCR cycle conditions were:
denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for
15 sec and 60°C for 1 min. Each sample, including the no template
control, was run in triplicate and Ct values were
determined for the target transcripts using fixed threshold
settings. Cellular miRNA expression was normalized to U6 snRNA.
Cell-cell adhesion assay
Cells were plated in DMEM containing 10% FBS. Images
of cell-cell contact sites were captured under an inverted
fluorescence microscope (DIC optics; model TE2000; Nikon, Japan)
with a ×40 air Fluor objective using a 12-bit CCD camera and Image
Pro software (Nikon). The frequency of adhesions between cells
expressing miR-128, shRNA2 or control cells was analyzed. Cell-cell
adhesion was defined as a cell-cell contact area of >50%. At
least 100 contact sites were randomly selected, and three
independent replicates were performed.
Dual-luciferase reporter assay
The dual-luciferase reporter assay was carried out
as previously described (18).
Briefly, 2.5×104 293T cells in 100 μl growth medium were
plated in 96-well plates, 2×104 U87 cells in 200 μl
growth medium were plated in 48-well plates. The next day, the
cells were transfected with 100 ng psiCheck2-EphB1–3′UTR,
psiCheck2-EphB2–3′UTR or psiCheck2-EphB2–3′UTR-MUT and 300 ng
pmiR-128 or pmiR-CTRL using Lipofectamine™ 2000 (Invitrogen) or
FuGENE (Roche Applied Science). The cells were harvested 48 h after
transfection and assayed using the Dual-Luciferase Reporter Assay
kit (Promega) according to the manufacturer’s instructions. Each
transfection was repeated in triplicate.
Immunoblotting analysis
Cells were solubilized in lysis buffer at 100°C for
30 min and centrifuged at 12,000 × g for 10 min at 4°C to obtain
whole-cell protein extract supernatants. The protein samples were
resolved by SDS-polyacrylamide gel electrophoresis (PAGE),
transferred to Immobilon-P membranes (Millipore), incubated in TBS
containing 0.2% Tween-20 and 5% skimmed milk to block non-specific
binding overnight at 4°C, then incubated for 2 h at room
temperature with a primary rabbit polyclonal antibody against human
EphB2 (0.2 μg/ml dilution; R&D Systems) or mouse monoclonal
antibody against human β-actin (1:2,000 dilution; Sigma). The
membranes were washed three times for 10 min in TBS containing 0.2%
Tween-20, incubated for 1 h with goat anti-rabbit IgG (H+L)
(1:30,000; Jackson ImmunoResearch) or goat anti-mouse IgG (H+L)
(1:10,000 dilution; Jackson ImmunoResearch) secondary antibodies,
washed thoroughly and the bound antibodies were detected using
enhanced chemiluminescence.
Wound-healing assay
Cell culture conditions were optimized to ensure
homogeneous and viable cell monolayers prior to wounding. When the
cell confluence reached ~90%, a homogenous artificial wound was
created using a sterile plastic 1,000 μl micropipette tip and cell
debris was removed by washing the cells in serum-free medium.
Following incubation for 12 and 24 h, cell migration into the
wounded area was photographed using an inverted microscope
(magnification, ×100).
Cell migration assay
Cells (5×104) in 200 μl DMEM without FBS
were seeded on a polycarbonate membrane which was inserted in a
transwell apparatus (Costar, Cambridge, MA, USA). DMEM (600 μl)
with 10% FBS was added as a chemoattractant in the lower chamber.
The insert was washed with PBS after the cells were incubated for 4
h at 37°C in a 5% CO2 atmosphere, and cells on the top
surface of the insert were removed with a cotton swab. Cells
adhering to the lower surface were fixed with 4% paraformaldehyde,
stained with crystal violet solution and counted under a microscope
in five random fields.
Statistical analysis
Data are presented as the means ± SD of at least
three separate experiments. All data are analyzed using the
Student’s t-test. Differences were considered statistically
significant at P<0.05 (*P<0.05;
**P<0.01; ***P<0.001).
Results
miR-128 promotes cell-cell adhesion in
U87 glioma cells
Expression of miR-128 is significantly decreased and
negatively correlates with tumor grade in gliomas (12,19).
Functional studies indicate that miR-128 plays an important role in
glioma tumorigenesis, including cell proliferation, self-renewal
and invasion (12–14); however, the role of miR-128 in
cell-cell adhesion and other functions has received little
attention. In order to evaluate the role of miR-128 in glioma
cell-cell adhesion, we constructed a U87 cell line overexpressing
miR-128 (U87-miR-128 cells) using lentivirus infection and
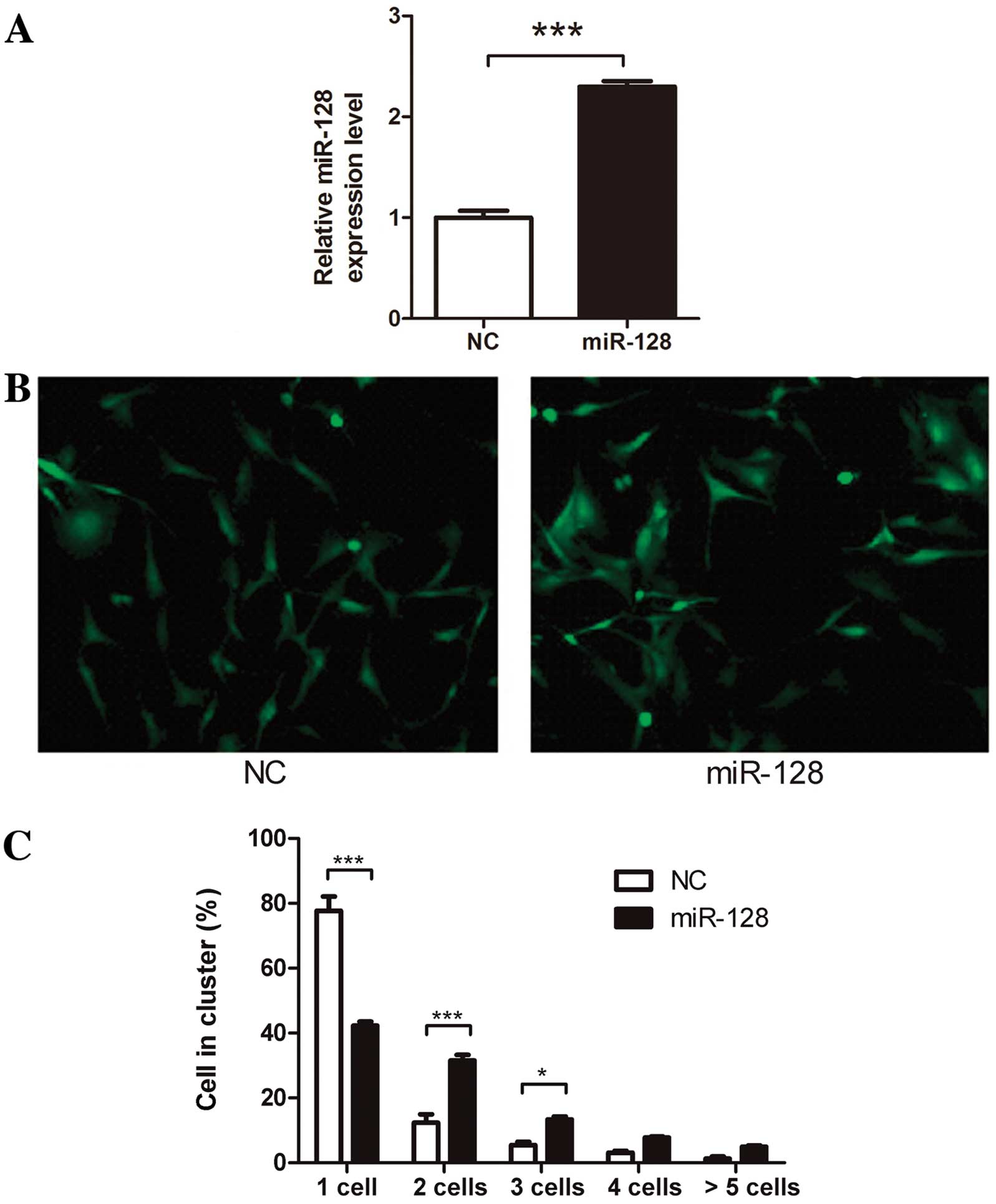
puromycin selection. qRT-PCR indicated a 2.3-fold increase in
miR-128 expression in U87-miR-128 cells (Fig. 1A). U87-miR-128 cells clustered
together more strongly in cell-cell adhesion assays (Fig. 1B and C) suggesting that miR-128 may
promote cell-cell adhesion in U87 cells.
miR-128 regulates EphB2 expression
through the 3′UTR binding site
miRNAs regulate downstream gene expression via
binding to the 3′UTRs of target mRNAs. In order to find miR-128
target genes, we used the target prediction database miRanda
(http://cbio.mskcc.org/mirnaviewer/)
(20). A list of 744 putative
target genes were generated and ranked by the binding energy, which
estimates the thermodynamic properties of a predicted duplex and
represents the likelihood of an interaction between a miRNA and
mRNA. The top ten predicted targets, ranked according to the
binding energy, are listed in Table
II. Notably, two members of the Eph family, EphB1 and EphB2,
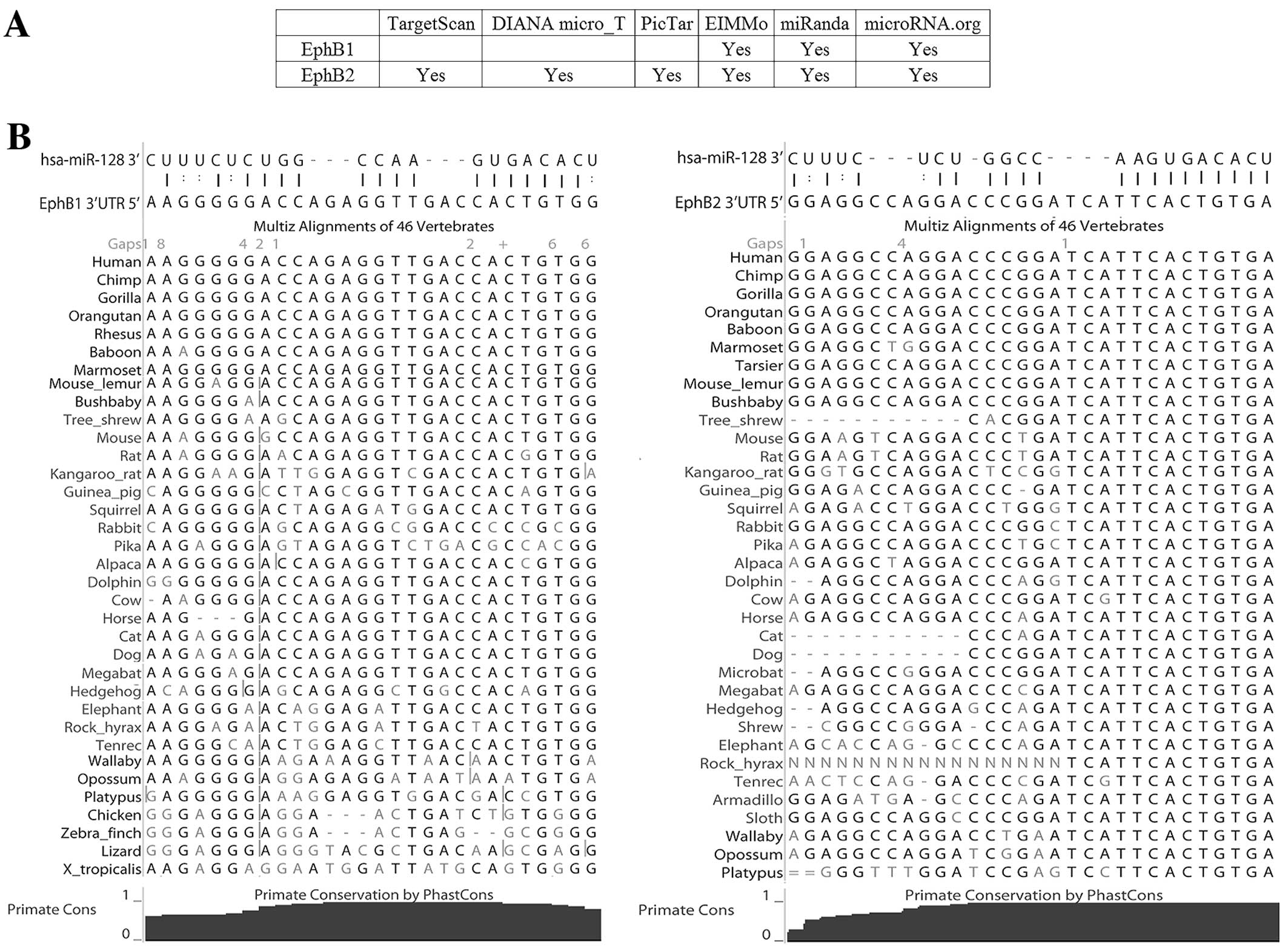
were potential miR-128 targets. To improve the robustness of
prediction, five other target prediction algorithms were used to
predict the interaction between miR-128 and EphB1 and EphB2. EphB2
was predicted to be a mir-128 target gene by all five algorithms
and EphB1 was predicted by two algorithms (Fig. 2A). In addition, no other Eph family
members were predicted by any of the five algorithms. The Eph
family is of particular interest as: i) previous studies have
demonstrated that they are related to cell repulsion and adhesion
(21–23); ii) the expression levels of some Eph
family members are increased in gliomas (12), in contrast to reduced miR-128
expression (6,9); iii) the EphB1 and EphB2 3′UTRs both
contain a complementary site for the miR-128 seed region (2–8 nt)
and are both predicted to form classic duplexes with miR-128
(Fig. 2B) (24); and iv) both the putative target
sites are conservative, shown by 46-way vertebrate alignments
(Fig. 2B).
 | Table IIThe top 10 targets of miR-128
predicted by the miRanda algorithm. |
Table II
The top 10 targets of miR-128
predicted by the miRanda algorithm.
| Gene | Ensembl gene
ID | Binding energy
(kCal/mol) | miRanda score |
|---|
| LRRC1 |
ENSG00000137269 | −34.1 | 164 |
| KPNB1 |
ENSG00000108424 | −30.5 | 152 |
| TMEM1 |
ENSG00000160218 | −29.9 | 170 |
| HMGB3 |
ENSG00000029993 | −29.5 | 165 |
| FRMD4 |
ENSG00000151474 | −29.4 | 175 |
| TTN |
ENSG00000155657 | −28.1 | 171 |
| EphB1 |
ENSG00000154928 | −28.0 | 172 |
| EphB2 |
ENSG00000133216 | −27.0 | 172 |
| SEC61A1 |
ENSG00000058262 | −26.8 | 170 |
| SLC1A2 |
ENSG00000110436 | −26.8 | 150 |
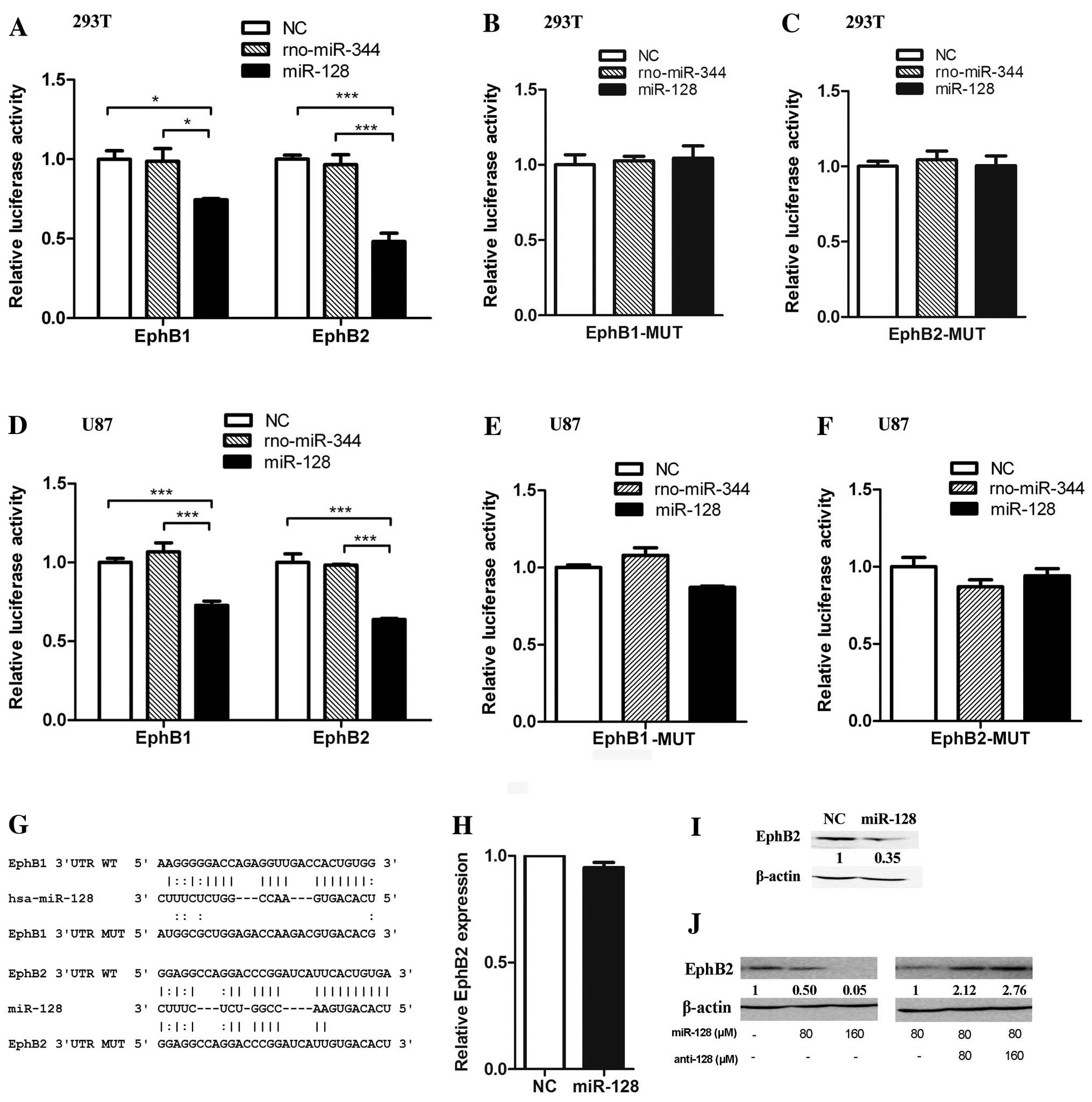
To confirm whether miR-128 binds directly to the
3′UTRs, we constructed two luciferase reporter vectors containing
the predicted miR-128 binding site fragments from the human EphB1
and EphB2 3′UTRs. The vectors were co-transfected with miR-128 into
293T cells. Ectopic expression of miR-128 reduced the activity of
the luciferase reporter plasmids containing the EphB1 and EphB2
3′UTR miR-128 binding sites (Fig.
3A). Mutating the reporter vectors containing the EphB1 or
EphB2 3′UTR miR-128-binding sites using a fusion PCR method and
co-transfecting with miR-128, miR-128 had no effect on the
luciferase activity of either mutant (Fig. 3B, C and G). The results of
experiments were confirmed in U87 cells (Fig. 3D–F). These results suggest that
miR-128 regulates luciferase activity via the predicted binding
sites in the 3′UTRs EphB1 and EphB2 mRNAs. As EphB1 and EphB2
belong to the same Eph sub-family and miR-128 reduced the
luciferase activity of the reporter vector containing the EphB2
3′UTR more significantly than EphB1, we selected EphB2 for further
investigation.
To further determine the function of miR-128 as a
regulator of EphB2, we investigated the effect of miR-128 on
endogenous EphB2 expression. The qRT-PCR quantification of
endogenous EphB2 mRNA indicated that ectopic stable expression of
miR-128 in U87 glioma cells did not affect the mRNA level of EphB2
(Fig. 3H). However, immunoblotting
showed that ectopic stable expression of miR-128 in U87 glioma
cells reduces EphB2 expression at the protein level (Fig. 3I). Consistently, the increase of
miR-128 mimics in U87 cells resulted in the decrease of EphB2
protein (Fig. 3J, left panel).
Furthermore, introduction of miR-128 to U87 cells by transfecting
miR-128 mimics and then increasing miR-128 antisense RNA resulted
in the increase of EphB2 protein (Fig.
3J, right panel). These results further indicated that miR-128
suppressed EphB2 expression via the miR-128-binding site in the
EphB2 mRNA 3′UTR.
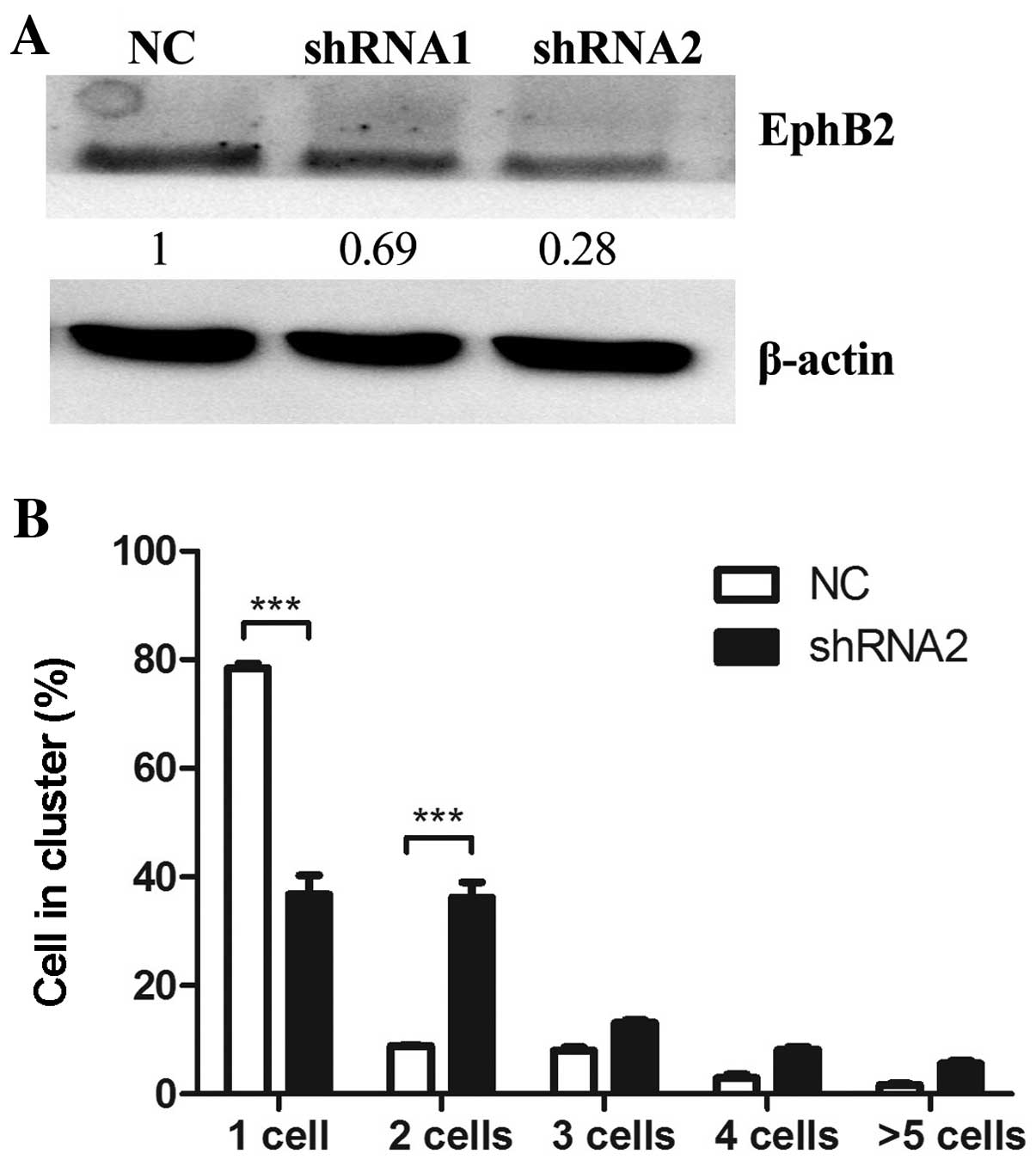
To determine whether reduced EphB2 expression mimics
the effect of miR-128 overexpression on cell-cell adhesion, two
EphB2 shRNAs were constructed and transiently transfected into U87
cells. The expression level of endogenous EphB2 protein was
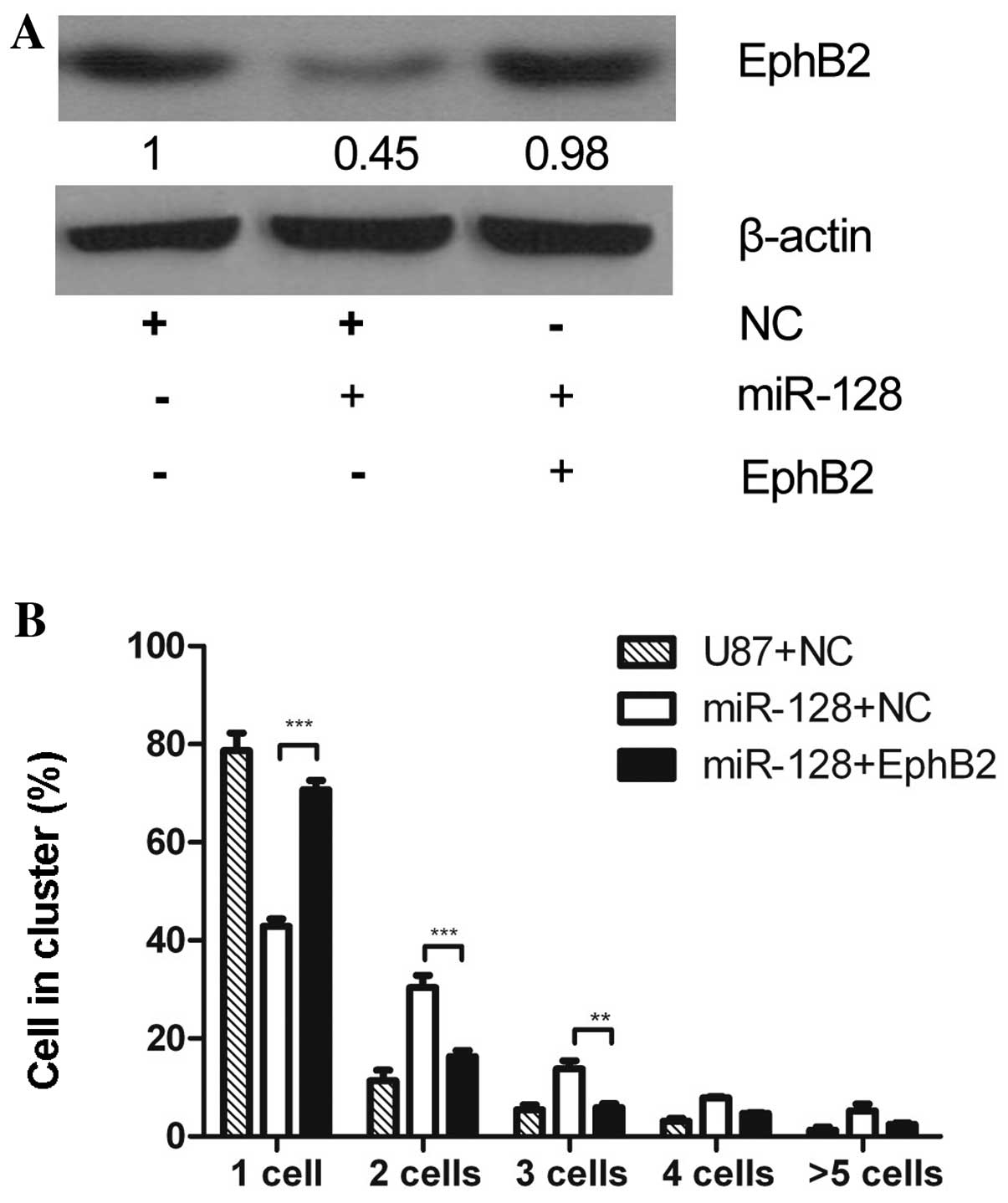
inhibited more prominently by shRNA2 than shRNA1 (Fig. 4A), thus, shRNA2 was selected for
further experiments. The cell-cell adhesion assay was performed in
U87 glioma cells transfected with EphB2 shRNA2. EphB2
shRNA2-transfected cells displayed increased cell clustering
compared to empty vector-transfected control cells. Notably, the
effect of EphB2 knockdown on cell-cell adhesion was similar to
miR-128 overexpression (Fig.
4B).
EphB2 suppresses miR-128-mediated
increase of cell-cell adhesion
To further investigate the hypothesis that the
phenotype resulting from miR-128 overexpression was due to
downregulation of EphB2, miR-128-overexpressing U87-miR-128 cells
were transfected with the EphB2 expressing vector pEphB2. As the
pEphB2 expression vector contains only the EphB2 coding sequences,
but not the 3′UTR, miR-128 cannot affect the expression of EphB2 by
pEphB2. Immunoblotting indicated that EphB2 expression increased in
U87-miR-128 cells transfected with pEphB2, compared to the negative
control-transfected U87-miR-128 cells or empty vector (Fig. 5A). Additionally, pEphB2 transfection
reduced the number of clustering cells in the cell-cell adhesion
assay, compared to controls of U87-miR-128 cells or U87 cells
transfected with empty vector (Fig.
5B). These results indicate that overexpression of EphB2 can
rescue the cell-cell adhesion effect induced by miR-128
overexpression, suggesting that EphB2 mediates miR-128-regulated
cell-cell adhesion.
miR-128 inhibits glioma cell migration
via EphB2
To evaluate the effect of miR-128 on the regulation
of EphB2 in glioma cell migration, a transwell assay and a
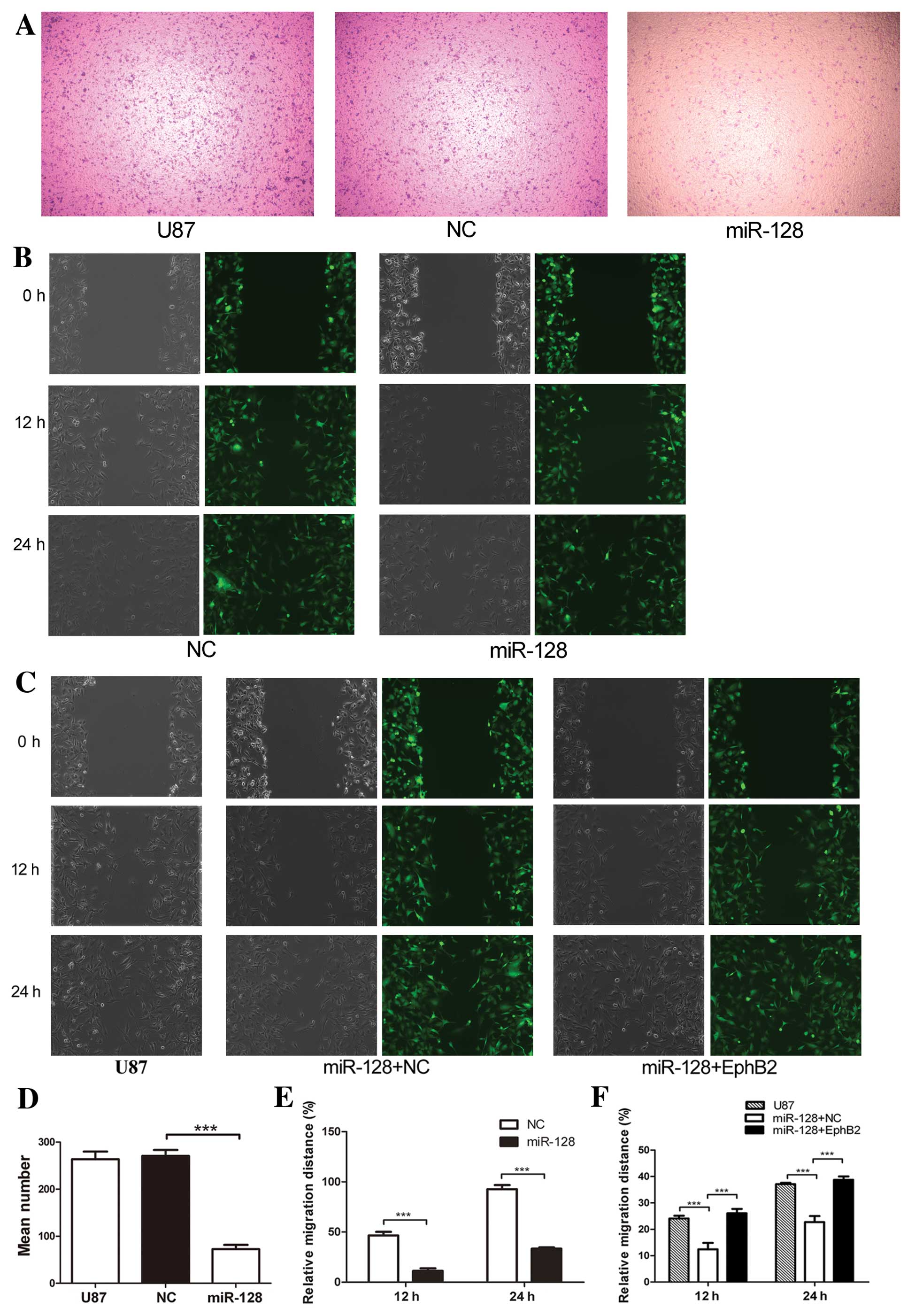
wound-healing assay were performed. The overexpression of miR-128
decreased the cell migration using transwell assay as shown in
Fig. 6A and D. An artificial wound
was made 24 h after U87-miR-128, negative control and U87 cells
were plated, both phase-contrast photomicrograph and fluorescent
photomicrograph were captured. Migration into the wound was
measured at 12 and 24 h. Migration was significantly decreased in
U87-miR-128 cells (Fig. 6B and E);
however, the effect of miR-128 was inhibited in cells co-expressing
EphB2 (Fig. 6C and F). These
findings indicate that EphB2 overexpression antagonizes the effect
of miR-128 inhibition on U87 cell migration. Collectively, these
results provide evidence that miR-128 promotes cell-cell adhesion
and suppresses cell migration in glioma cells in a mechanism
dependent on EphB2 expression.
Discussion
Brain-enriched miR-128 has been shown to be
downregulated in glioma tissues and cell lines (12) and it has also been shown to regulate
cell death/survival and invasion by various types of cancer.
miR-128 could target different genes in a certain tissue or cell
lines and have different functions. Two important studies have
shown that miR-128 can suppress glioma cell proliferation by
targeting E2F3a and reduce glioma self-renewal by targeting Bmi-1
or RTK signaling (12,13). miR-128 inhibits tumor growth and
angiogenesis by targeting p70S6K1 (12). Additionally, miR-128 has been linked
to neuroblastoma cell motility and invasiveness by targeting Reelin
and DCX (14). In the present
study, we reported for the first time that miR-128 promotes
cell-cell adhesion and inhibits cell migration in glioma cells
through a novel target EphB2, a gene relative to cell-cell
adhesion. Overexpression of EphB2 could rescue the function of
miR-128.
miRNAs play a critical role in gene regulation;
however, the regulation of Eph family members by miRNAs is poorly
characterized. Recently, miR-26b was found to directly regulate
EphA2 expression via a specific binding site in the 3′UTR region of
EphA2 mRNA (25). To our knowledge,
brain-enriched miR-128 is the second miRNA found to regulate Eph
family members. Our study indicates that EphB1 and EphB2 are novel
targets of miR-128, and several lines of evidence support a direct
interaction between miR-128 and the EphB1 and EphB2 3′UTRs.
Firstly, the human EphB1 and EphB2 3′UTRs both contain a putative
miR-128 binding site with a prominent seed match (Fig. 2B). Secondly, miR-128 suppresses the
activity of luciferase reporter genes fused to the 3′UTR of either
EphB1 or EphB2 mRNA. Thirdly, miR-128 suppresses endogenous
expression of human EphB2 at the protein level. We observed that
cell-cell adhesion increased and cell migration decreased when
miR-128 was overexpressed in U87 glioma cells, which suggests that
miR-128 affects cell-cell adhesion and cell migration in glioma
cells via downregulation of EphB2. This hypothesis was confirmed by
the rescue experiments. Overexpression of EphB2 without the 3′UTR,
which the miR-128 has no effect on, inhibited the ability of
miR-128 to promote cell-cell adhesion and suppress cell migration
in U87 cells. This study provides the first identification and
demonstration of a miRNA which directly regulates EphB1 and EphB2
and this, in turn, enhances our understanding of the function and
regulation of EphB2 in glioma cells.
Several Ephs and ephrin ligands are expressed at
high levels in multiple types of cancer where they demonstrate
mostly tumor promoting activities (reviewed in ref. 22). High expression levels of EphB2 have
been reported in a variety of tumors such as gliomas (16,17),
synovial sarcomas (26), liver
cancer (27), gastric cancer
(28), colon carcinomas (29), lung cancer (30) and breast cancer (31). In human brain tumors, EphB2
expression negatively correlates with tumor grade (16). EphB2 is involved in malignant
progression in glioma tissues, as more migratory glioma cells
express higher levels of EphB2, and ectopic overexpression of EphB2
promotes glioma cell migration and invasion. In agreement with
these observations, blocking EphB2 expression in glioma cells
significantly inhibits migration and invasion (16). The molecular mechanisms which
regulate the high levels of EphB2 expression in glioma tissues and
cell lines are not known. In this study, we demonstrated that
miR-128 binds the 3′UTR of EphB2 mRNA to post-transcriptionally
regulate EphB2 expression. miR-128 is downregulated in gliomas
(6,9), which, in turn, could lead to increased
expression levels of EphB2. Furthermore, our observation that
miR-128-induced downregulation of EphB2 inhibits glioma cell
migration is consistent with previous studies which have reported
that low expression levels of EphB2 can block a variety of
malignant processes in tumor cells (16,17).
EphB2 mediates repulsion and adhesion of nerve cells
(15,21,23)
and once cell-cell contact occurs, separation of interacting cells
is difficult due to high expression of EphB receptors on the cell
surface and the high affinity of EphB-EphrinB binding. To separate
two interacting cells, the connection between EphB and EphrinB at
contact sites has to be destroyed, and two mechanisms have been
proposed: clearing of the receptor or ligand by a transmembrane
protease or removal of the EphB-EphrinB complex interaction by
endocytosis and trans-endocytosis (22). Increased expression of EphB
receptors can reduce cell-cell contact and promote cell-cell
repulsion via endocytosis of activated ephrinB-EphB receptors,
leading to a reduced number of contact sites between
receptor-expressing and ligand-expressing cells (32). As regards U87 glioma cells, we
propose that high levels of EphB2 expression are mediated by
miR-128 downregulation, leading to increased endocytosis of the
EphB-EphrinB complex, which reduces cell-cell adhesion and promotes
glioma cell migration. Conversely, overexpression of miR-128
reduces EphB2 expression and promotes cell-cell adhesion.
In summary, our findings suggest that the aberrant
expression of miR-128 in gliomas plays a critical role in the
regulation of cell-cell adhesion and cell migration. The function
of miR-128 in cell-cell adhesion and cell migration is dependent on
regulation of the EphB2 receptor by direct targeting of EphB2 mRNA
via binding the 3′UTR. The present study provides evidence for a
novel function of miR-128 in the regulation of EphB2 in glioma
cells.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 81272773 and
81101960); the Scientific and Technological Planning of Guangzhou
(grant no. 2012J4100082); the Fundamental Research Funds for the
Central Universities (grant no. 10lgpy23).
References
|
1
|
Surawicz TS, Davis F, Freels S, Laws ER Jr
and Menck HR: Brain tumor survival: results from the National
Cancer Data Base. J Neurooncol. 40:151–160. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Calin GA, Sevignani C, Dumitru CD, et al:
Human microRNA genes are frequently located at fragile sites and
genomic regions involved in cancers. Proc Natl Acad Sci USA.
101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen CZ: MicroRNAs as oncogenes and tumor
suppressors. N Engl J Med. 353:1768–1771. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Croce CM and Calin GA: miRNAs, cancer, and
stem cell division. Cell. 122:6–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ciafre SA, Galardi S, Mangiola A, et al:
Extensive modulation of a set of microRNAs in primary glioblastoma.
Biochem Biophys Res Commun. 334:1351–1358. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Silber J, Lim DA, Petritsch C, et al:
miR-124 and miR-137 inhibit proliferation of glioblastoma
multiforme cells and induce differentiation of brain tumor stem
cells. BMC Med. 6:142008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Monteys AM, Spengler RM, Wan J, et al:
Structure and activity of putative intronic miRNA promoters. RNA.
16:495–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lages E, Guttin A, El Atifi M, et al:
MicroRNA and target protein patterns reveal physiopathological
features of glioma subtypes. PLoS One. 6:e206002011. View Article : Google Scholar
|
|
10
|
Guidi M, Muinos-Gimeno M, Kagerbauer B,
Marti E, Estivill X and Espinosa-Parrilla Y: Overexpression of
miR-128 specifically inhibits the truncated isoform of NTRK3 and
upregulates BCL2 in SH-SY5Y neuroblastoma cells. BMC Mol Biol.
11:952010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adlakha YK and Saini N: MicroRNA-128
downregulates Bax and induces apoptosis in human embryonic kidney
cells. Cell Mol Life Sci. 68:1415–1428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Chao T, Li R, et al: MicroRNA-128
inhibits glioma cells proliferation by targeting transcription
factor E2F3a. J Mol Med. 87:43–51. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Godlewski J, Nowicki MO, Bronisz A, et al:
Targeting of the Bmi-1 oncogene/stem cell renewal factor by
microRNA-128 inhibits glioma proliferation and self-renewal. Cancer
Res. 68:9125–9130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Evangelisti C, Florian MC, Massimi I, et
al: MiR-128 up-regulation inhibits Reelin and DCX expression and
reduces neuroblastoma cell motility and invasiveness. FASEB J.
23:4276–4287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Halloran MC and Wolman MA: Repulsion or
adhesion: receptors make the call. Curr Opin Cell Biol. 18:533–540.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakada M, Niska JA, Miyamori H, et al: The
phosphorylation of EphB2 receptor regulates migration and invasion
of human glioma cells. Cancer Res. 64:3179–3185. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakada M, Niska JA, Tran NL, McDonough WS
and Berens ME: EphB2/R-Ras signaling regulates glioma cell
adhesion, growth, and invasion. Am J Pathol. 167:565–576. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen X, Fang J, Lv X, et al: Heparin
impairs angiogenesis through inhibition of microRNA-10b. J Biol
Chem. 286:26616–26627. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smirnova L, Grafe A, Seiler A, Schumacher
S, Nitsch R and Wulczyn FG: Regulation of miRNA expression during
neural cell specification. Eur J Neurosci. 21:1469–1477. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Human MicroRNA targets. PLoS Biol.
2:e3632004. View Article : Google Scholar
|
|
21
|
Kullander K and Klein R: Mechanisms and
functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol.
3:475–486. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Himanen JP, Saha N and Nikolov DB:
Cell-cell signaling via Eph receptors and ephrins. Curr Opin Cell
Biol. 19:534–542. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Flanagan JG and Vanderhaeghen P: The
ephrins and Eph receptors in neural development. Annu Rev Neurosci.
21:309–345. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu N, Zhao X, Liu M, et al: Role of
microRNA-26b in glioma development and its mediated regulation on
EphA2. PLoS One. 6:e162642011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barco R, Hunt LB, Frump AL, et al: The
synovial sarcoma SYT-SSX2 oncogene remodels the cytoskeleton
through activation of the ephrin pathway. Mol Biol Cell.
18:4003–4012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hafner C, Schmitz G, Meyer S, et al:
Differential gene expression of Eph receptors and ephrins in benign
human tissues and cancers. Clin Chem. 50:490–499. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kataoka H, Tanaka M, Kanamori M, et al:
Expression profile of EFNB1, EFNB2, two ligands of EPHB2 in human
gastric cancer. J Cancer Res Clin Oncol. 128:343–348. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu W, Ahmad SA, Jung YD, et al:
Coexpression of ephrin-Bs and their receptors in colon carcinoma.
Cancer. 94:934–939. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang XX, Brodeur GM, Campling BG and
Ikegaki N: Coexpression of transcripts encoding EPHB receptor
protein tyrosine kinases and their ephrin-B ligands in human small
cell lung carcinoma. Clin Cancer Res. 5:455–460. 1999.PubMed/NCBI
|
|
31
|
Wu Q, Suo Z, Risberg B, Karlsson MG,
Villman K and Nesland JM: Expression of Ephb2 and Ephb4 in breast
carcinoma. Pathol Oncol Res. 10:26–33. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Marston DJ, Dickinson S and Nobes CD:
Rac-dependent trans-endocytosis of ephrinBs regulates Eph-ephrin
contact repulsion. Nat Cell Biol. 5:879–888. 2003. View Article : Google Scholar : PubMed/NCBI
|