Introduction
Bladder cancer is one of the most common
genitourinary malignant diseases worldwide. More than 90% of
bladder cancer cases are bladder urothelial carcinoma (BUC)
(1). At the time of diagnosis,
75–85% of the patients harbored superficial bladder cancer
(non-muscle-invasive bladder cancer; NMIBC) (2). Despite transurethral resection of
bladder tumor (TURBT) and intravesical therapy, 1–45% of cases
progress to invasive bladder cancer (muscle-invasive bladder
cancer; MIBC) within 5 years (3).
Approximately 50% patients with MIBC develop metastatic disease,
which is almost invariably lethal (4). The exact mechanisms of tumor formation
and progression are not yet completely understood. Genetic and
molecular factors may both play a role in the process (5).
Transforming growth factor-β (TGF-β) superfamily
signaling pathways play opposing roles in human cancer, functioning
both as tumor suppressors and as tumor promoters (6,7). TGF-β
superfamily ligands exert these biological effects by directly or
indirectly binding to three cell surface receptors, type I
(TGFBR1), type II (TGFBR2) and type III (TGFBR3) transforming
growth factor-β receptor. Ligands binding to TGFBR2 cause TGFBR2 to
phosphorylate TGFBR1; the activated TGFBR1 phosphorylates and in
turn activates Smad transcription factors, which move into the
nucleus and regulate target genes by interacting with other
transcription factors (8). In the
TGF-β superfamily signaling pathways, TGFBR3, as a co-receptor,
plays an important role in mediating ligand-dependent TGF-β
superfamily signaling and possesses important ligand-independent
functions (7).
Loss or reduced expression of TGFBR3 has been
demonstrated in multiple types of human cancer including breast,
prostate, ovarian, pancreatic, non-small cell lung cancer and renal
cell carcinoma, whereas restoring TGFBR3 expression results in
decreased migration and invasion. These results indicate that
TGFBR3 may play a suppressive role in cancer progression (9–14).
However, Gatza et al(15)
reported that TGFBR3 expression was actually enhanced in human
colon cancer and may even function to promote colon cancer
progression. These data suggest that TGFBR3 may play a dual role in
cancer progression. However, little is known about TGFBR3 in
bladder cancer. In the present study, we investigated the
expression of TGFBR3 in BUC tissues and cells. We also silenced the
TGFBR3 gene by chemically synthesizing siRNA in bladder cancer cell
line T24 and determined the growth, motility and invasion of the
cells after the gene knockdown. Our results suggest that TGFBR3 may
play diverse roles in bladder cancer, functioning to suppress
tumorigenesis in early stages and promoting cancer progression in
later stages.
Materials and methods
Specimens
Fresh tumor and the corresponding paracarcinoma
tissue specimens were collected from 56 BUC patients who
participated in the study at The Second Affiliated Hospital of
Soochow University. Of the 56 patients, 27 had undergone
transurethral resection of bladder tumor (TURBT), 25 had undergone
radical cystectomy and 4 had undergone partial cystectomy. Tumors
were pathologically staged according to the 2010 American Joint
Committee on Cancer TNM staging system, and tumors were graded
using the 1973 World Health Organization (WHO) classification
(Table I). The present study was
approved by The Academic and Ethics Advisory Board of the Second
Affiliated Hospital of Soochow University. None of these patients
had received radiotherapy or chemotherapy prior to surgery. These
specimens were snap-frozen and stored in liquid nitrogen
immediately after the operation and until analysis.
 | Table IClinical characteristics of the 56
patients who participated in this study. |
Table I
Clinical characteristics of the 56
patients who participated in this study.
| Pathological
parameters | Tumorsa | Gendera | Age (years) |
|---|
|
|---|
| Male | Female |
|---|
| pT stage |
| Ta/T1 | 30 | 24 | 6 | 28–78 |
| T2 | 16 | 16 | 0 | 42–87 |
| T3 | 7 | 6 | 1 | 61–72 |
| T4 | 3 | 2 | 1 | 46–74 |
| pN stage |
| N0 | 44 | 39 | 5 | 28–79 |
| N+ | 12 | 9 | 3 | 64–87 |
| pM stage |
| M0 | 56 | 48 | 8 | 28–87 |
| M1 | 0 | 0 | 0 | - |
| Grade |
| 1 | 25 | 21 | 4 | 33–87 |
| 2 | 20 | 17 | 3 | 28–86 |
| 3 | 11 | 10 | 1 | 46–79 |
Cell culture and transfection
The human bladder cancer cell lines 5637 and T24 and
the human normal urothelial cell line SV-HUC-1 were obtained from
the Shanghai Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). All cell lines were cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS), 100 U/ml of
penicillin and 100 mg/l of streptomycin in a humidified atmosphere
of 95% air maintained at 37°C. The siRNAs were designed and
chemically synthesized by Shanghai GenePharma Co., Ltd. (Shanghai,
China). Then, 5×105 cells/well were planted in a 6-well
plate. A 30–50% confluence was achieved after 24 h. Transfections
were performed using Entranster-R siRNA transfection reagent
(Entranster, China) according to the manufacturer’s instructions.
Control for non-specific effects caused by the transfection
procedure is referred to as mock-transfection control (mock). The
sequences of negative control siRNA (siRNA-NC), the sequences of
specific TGFBR3 siRNAs and their targeting sites are listed in
Table II.
 | Table IIThe sequences of siRNAs and their
targeting sites. |
Table II
The sequences of siRNAs and their
targeting sites.
| siRNA | Sequences | Targeting sites |
|---|
| siRNA-1 | Sense: GGU CACA CUUC
ACCU GAAUTT | 761–779 |
| Antisense: AUU CAGG
UGAA GUGU GACCTT | |
| siRNA-2 | Sense: GGA UCUU GAAG
UGGU CAAATT | 1298–1316 |
| Antisense: UUU GACC
ACUU CAAG AUCCTT | |
| siRNA-3 | Sense: GGU GUGGU
CUACU AUAACUTT | 2061–2079 |
| Antisense: AGU UAUAG
UAGA CCACACCTT | |
| siRNA-4 | Sense: GGGCC AUGAUG
GAGA AUAATT | 2731–2749 |
| Antisense: UUAUU
CUGCAU CAU GGCCCTT | |
| siRNA-NC | Sense: UUC UCC GAA
CGU GUC ACGUTT | No |
| Antisense: ACG UGA
CAC GUU CGG AGAATT | |
RNA isolation and quantitative RT-PCR
(qRT-PCR)
RNAiso Plus reagent (Takara Bio Inc., Shiga, Japan)
was used to isolate total RNA according to the manufacturer’s
protocol. RNA (~150 ng) was reverse transcribed in a 20 μl reaction
according to the manufacturer’s instructions. The duplex qRT-PCR
was performed in a 25 μl reaction system using the ABI PRISM 7500
Real-Time PCR system (Applied Biosystems, Foster City, CA, USA)
with SYBR-Green qPCR SuperMix-UDG (Invitrogen, Carlsbad, CA, USA).
All Ct values were equilibrated to the GAPDH control. All reactions
were performed in triplicate. PCR primers for specific genes and
GAPDH are shown in Table III
(from PrimerBank http://pga.mgh.harvard.edu/primerbank/).
 | Table IIIPrimers used for qRT-PCR. |
Table III
Primers used for qRT-PCR.
| Gene | Sequences | Size of PCR product
(bp) |
|---|
| TGFBR3 | Sense:
TGGGGTCTCCAGACTGTTTTT | 149 |
| Antisense:
CTGCTCCATACTCTTTTCGGG | |
| GAPDH | Sense:
ACAACTTTGGTATCGTGGAAGG | 101 |
| Antisense:
GCCATCACGCCACAGTTTC | |
Western blotting
Cell lysate was prepared by extracting proteins with
RIPA buffer (Fermentas, Ontario, Canada) supplemented with 1%
protease inhibitors (Sigma). The cells or tissues were lysed in
boiling sample buffer and resolved by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and immunoblotted for
the proteins of interest. TGFBR3 antibody was purchased from
R&D Systems (Minneapolis, MN, USA), and a 1:1,000 dilution was
used for immunoblot analysis. Secondary antibody was purchased from
Santa Cruz Biotechnology, and a 1:5,000 dilution was used for
immunoblot analysis. Membranes were blocked with 0.25% gelatin in
TBST, followed by incubation with primary antibodies overnight at
4°C. Membranes were washed three times in TBST, followed by
incubation with secondary antibody for 2 h at room temperature.
Membranes were washed three times in TBST and developed with
enhanced chemiluminescence (Fermentas).
Water-soluble tetrazolium salt (WST-1)
assay
The viability of the cells was determined by the
WST-1 assay according to the manufacturer’s protocol. T24 cells
(5×103/well) were seeded into 96-well flat-bottom
plates. Twenty four hours later, the cells were transfected with
siRNAs. Following transfection for 0, 24, 48 and 72 h, cell
viability was determined. One and a half hours before termination
of the experiment, 10 μl of WST (Beyotime Institute of
Biotechnology, Jiangsu, China) was added to each 100 μl well.
Analysis was performed with a microplate reader (Molecular Devices,
Sunnyvale, CA, USA) at OD450 and OD620 nm.
Colony formation assay
T24 cells were seeded in 6-well plates. The
following day, they were transfected as previously described
according to the manufacturer’s instructions. Forty-eight hours
after transfection, the cells were harvested and plated in a 6-well
plate at a density of 200 cells/well. The cells were cultured in an
incubator at 37°C for 14 days. On day 15, cells were washed with
PBS, fixed with absolute methanol, and stained with Giemsa. The
colonies with >50 cells were counted. All assays were repeated
at least three times, and the data are presented as the means ±
SD.
Analysis of DNA contents by propidium
iodide (PI) staining
PI staining reagent was purchased from Beyotime
Institute of Biotechnology. PI staining was performed according to
the manufacturer’s protocol. Cells (106) were briefly
fixed with 70% alcohol (v/v) for at least 24 h. After washing with
PBS, the cells were incubated with PI staining reagent at 37°C for
30 min. This analysis was performed with FC500 flow cytometry
(Beckman Coulter, Brea, CA, USA). Cells with subdiploid DNA content
were considered apoptotic cells. Cell cycle distributions were
analyzed by the cell fit software package.
Monolayer wound healing
T24 cells (1×105) were plated in each
well of a 6-well plate. Following overnight incubation, the cells
were treated with siRNAs (50 nM siControl, 50 nM siRNA-TGFBR3) for
48 h. Confluent sheets of cells were wounded by scraping with a
pipette tip, rinsed with PBS to remove dislodged cells two times,
and added to a fresh medium. At 0 and 24 h after the scratching,
images were captured with an Olympus inverted microscope (Olympus,
Tokyo, Japan) at a magnification of ×40. Cells were maintained at
37°C and 5% CO2 during incubation.
Transwell motility assay and Matrigel
invasion assay
The motility and invasive activity of T24 cells were
analyzed in a transwell cell culture chamber (Costar 3422,
polycarbonate membrane, 24-well format, 8-μm pore size; Corning,
Inc., New York, NY, USA). T24 cells were plated in each well of a
6-well plate at a density of 8×104 cells/well. After
overnight incubation, the cells were treated with siRNAs (50 nM
siControl, 50 nM siRNA-TGFBR3) for 48 h. T24 cells
(1×105/well for motility assay, 5×104/well
for invasion assay) were then applied to the upper chamber in a 200
μl RPMI-1640 medium supplemented with 5% FBS. RPMI-1640 medium
supplemented with 20% FBS was added to the lower chamber to act as
a chemoattractant. Cells were then allowed to seep out of the
Matrigel, across the membrane, at 37°C for 24 h. Non-invasive cells
were then removed from the top compartment of the transwell with a
cotton swab. The filters were fixed with methanol and stained with
0.1% crystal violet. The cells that had invaded through Matrigel
and filter to the lower surface were manually counted under a
microscope in five predetermined fields at a magnification of
×100.
Statistical analysis
The chi-square test was used to determine whether
increased TGFBR3 expression was associated with muscle-invasion.
The one-way analysis of variance (ANOVA) was used to compare
statistical significance between treatment groups and controls. The
two-tailed equal variance Student’s t-test was used to compare the
average number of migrated cells to the invaded cells of the five
fields. All statistical analyses were performed using SPSS
statistical software version 17.0. P≤0.05 was considered to
indicate a statistically significant difference.
Results
TGFBR3 expression in human bladder cancer
tissues
To explore the possible role of TGFBR3 in bladder
tumorigenesis, we detected the protein expression of TGFBR3 in 56
pairs of BUCs and corresponding paracarcinoma tissues using western
blotting. TGFBR3 protein expression was significantly altered in
50/56 (89.26%) tumor samples when compared to paracarcinoma
tissues. In 24 altered pairs of MIBC, TGFBR3 was increased in 19 of
the tumor samples, and decreased in 5 patients. Of the 26 altered
pairs of NMIBC, 8 patients experienced increased TGFBR3 in tumor
focuses, and 18 patients showed decreased TGFBR3 in the tumors
(Table IV). Thus, it appears that
the increased TGFBR3 expression in the bladder cancer tissues was
associated with muscle-invasion (MIBC).
 | Table IVSummary of TGFBR3 expression in
patients with bladder cancer. |
Table IV
Summary of TGFBR3 expression in
patients with bladder cancer.
| TGFBR3
expressiona |
|---|
|
|
|---|
| Bladder
cancerb | Increased | Decreased |
|---|
| MIBC | 19 | 5 |
| NMIBC | 8 | 18 |
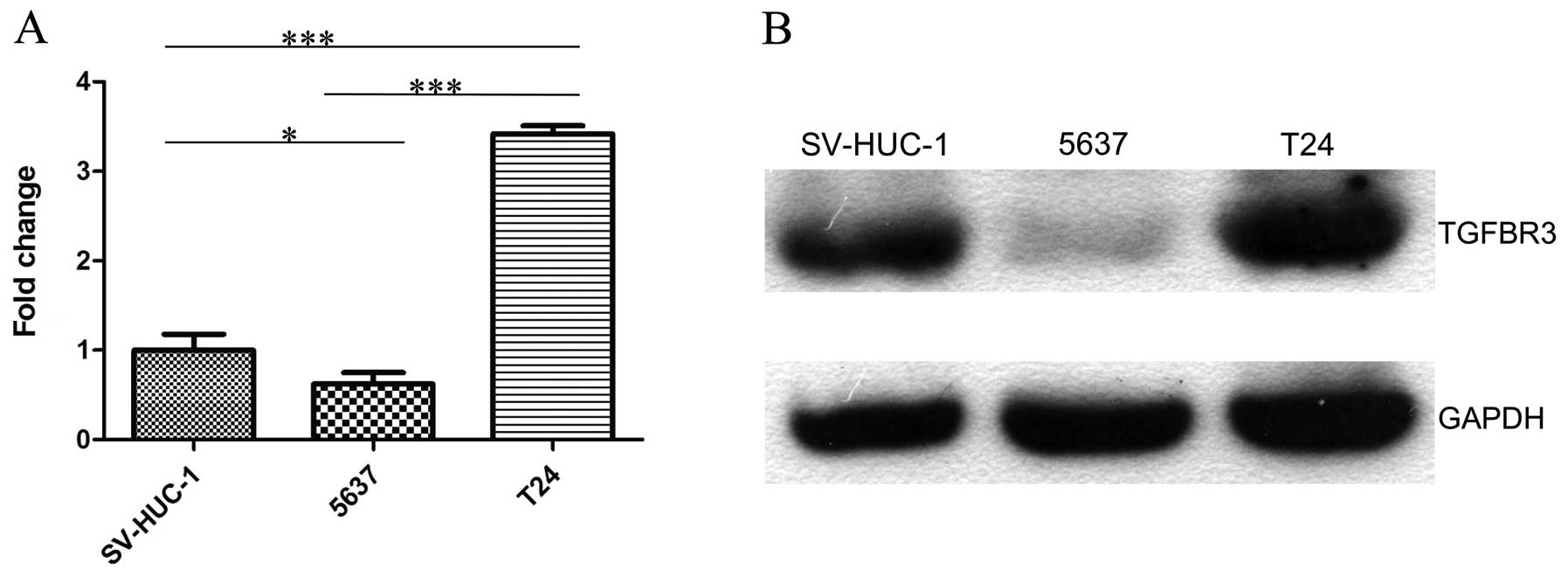
TGFBR3 expression in normal bladder
epithelium and bladder cancer cell lines
To identify TGFBR3 expression in bladder cancer
cells, we detected TGFBR3 expression in normal bladder epithelium
cell line SV-HUC-1, superficially derived bladder cell line 5637,
and invasive cell line T24 by qRT-PCR via western blotting. We
found that both mRNA and protein expression of TGFBR3 was decreased
in 5637 cells when compared to SV-HUC-1 cells, whereas the
expression of TGFBR3 significantly increased in T24 cells (Fig. 1).
Knockdown of TGFBR3 inhibits cell
viability and colony formation, but not cell cycle arrest in T24
cells
In order to achieve efficient and specific TGFBR3
depletion, T24 cells were transfected with 50 nM of siRNA-NC,
siRNA-1, siRNA-2, siRNA-3 and siRNA-4, respectively. We found that
the expression of TGFBR3 was decreased by up to 80–85% when
transfected with siRNA-1, siRNA-2 and siRNA-3 (data not shown).
Thus, we mixed them together equally into a siRNA pool and called
it siRNA-TGFBR3. The expression of TGFBR3 in T24 cells that were
separately transfected with siRNA-TGFBR3 at various concentrations
was detected by qRT-PCR. Consequently, 50 nM siRNA-TGFBR3 achieved
an effective knockdown; therefore, 50 nM siRNA-TGFBR3 was used as
the final concentration in the following experiments to investigate
the effects of silencing TGFBR3 on T24 cells at 48 h
post-transfection.
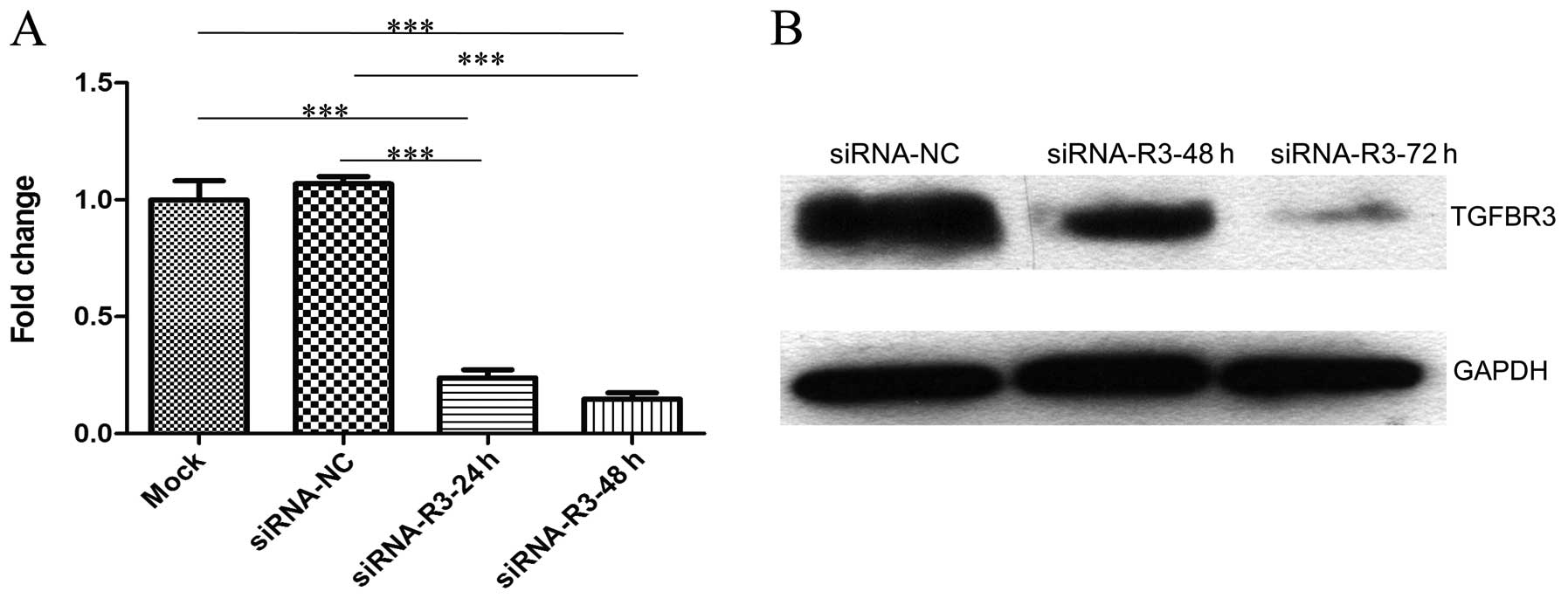
T24 cells were transfected with 50 nM siRNA-TGFBR3
or siRNA-NC. The TGFBR3 mRNA expression was detected by qRT-PCR at
24 and 48 h (Fig. 2A) and TGFBR3
protein expression was detected by western blotting at 48 and 72 h
(Fig. 2B) post-transfection,
respectively. Compared to the mock and siRNA-NC, TGFBR3 expression
in siRNA-TGFBR3 group decreased significantly.
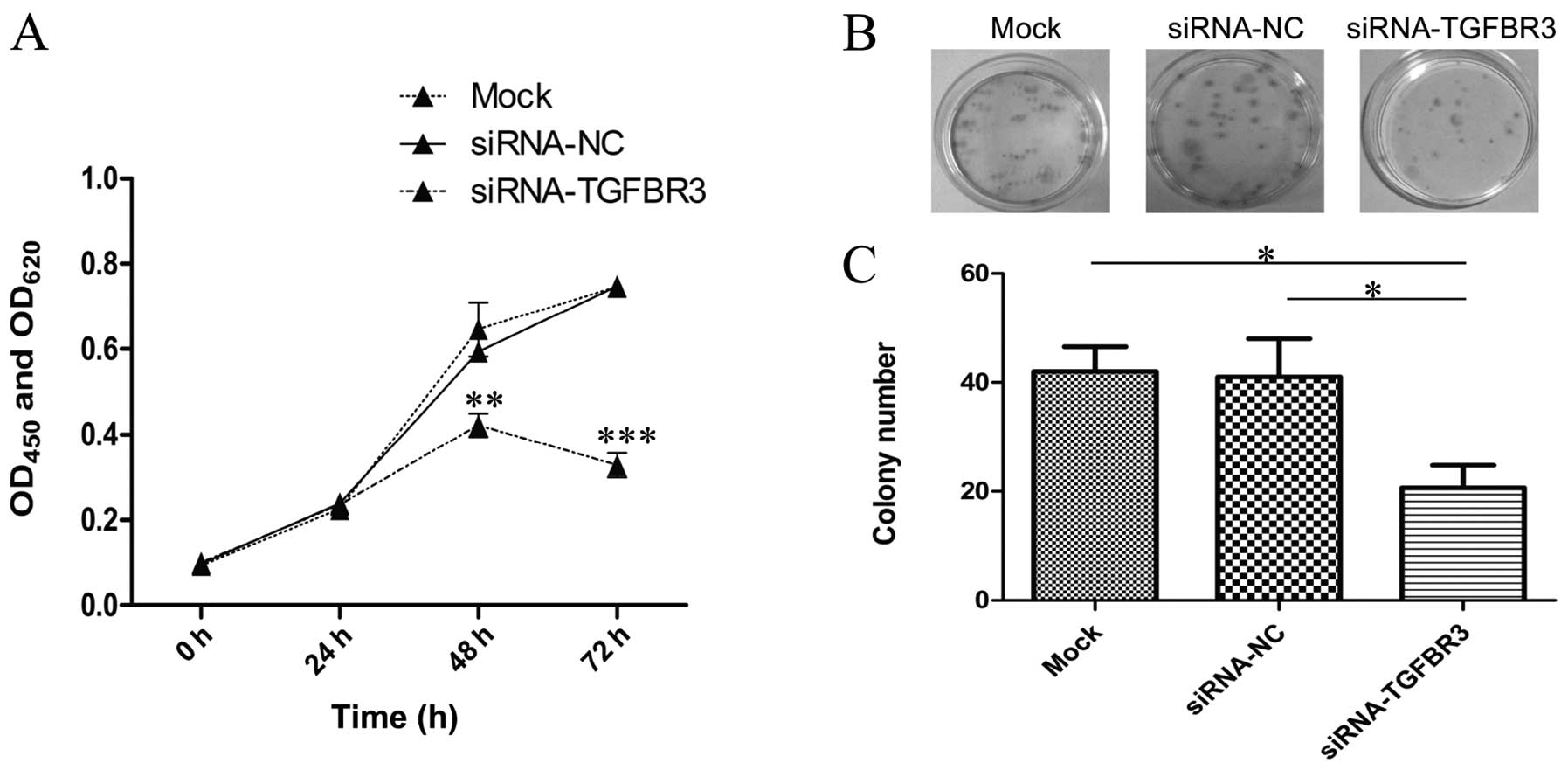
To determine whether TGFBR3 expression affects cell
proliferation and growth, we knocked down TGFBR3 in T24 cells and
analyzed cell viability using a WST-1 assay. In the experiment,
there was no clear difference in cell proliferation among the
blank, mock and siRNA-NC groups. The proliferation of cells
transfected with siRNA-TGFBR3, however, was notably inhibited from
48 to 72 h as compared to the siRNA-NC group (Fig. 3A).
Similarly, the colony formation assay showed that
the total colony number of the siRNA-TGFBR3 group was less than
that of the siRNA-NC group, and that there was no evident
difference in colony formation among the blank and siRNA-NC groups
(Fig. 3B and C).
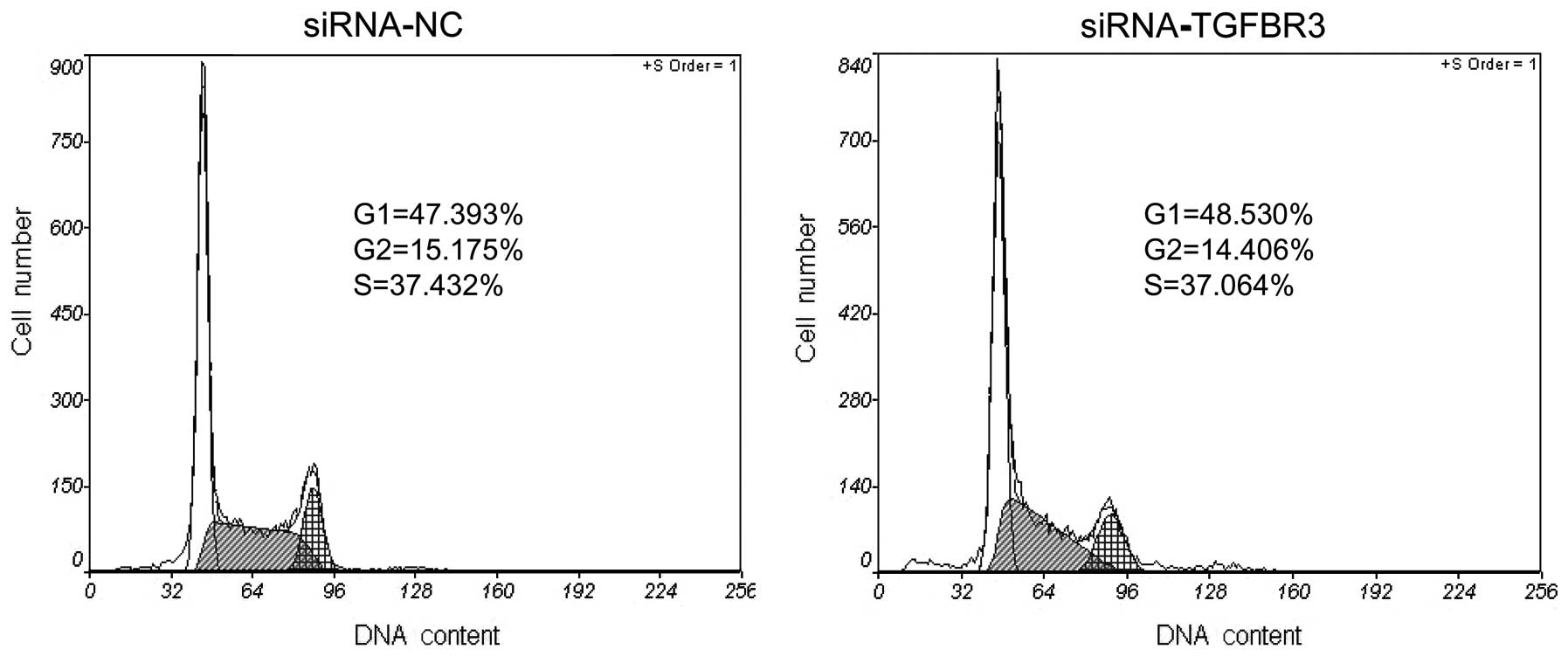
To determine whether the inhibition of the cell
viability and colony formation is related to cell cycle arrest, the
phase distribution of the cell cycle was analyzed by flow
cytometry. Compared to the siRNA-NC group, there was no marked
change of cell cycle distribution in the siRNA-TGFBR3 group
(Fig. 4).
Since the cell viability is ~2/3 in the siRNA cells
at 48 h (Fig. 3A) and the cell
cycle distribution is the same as the control cells (Fig. 4), and the cell death seems to be the
same from sub-G1/G0 cell numbers in this figure, the explanation
for the low viability is the slow cell growth and the presence of
the same cell cycle pattern.
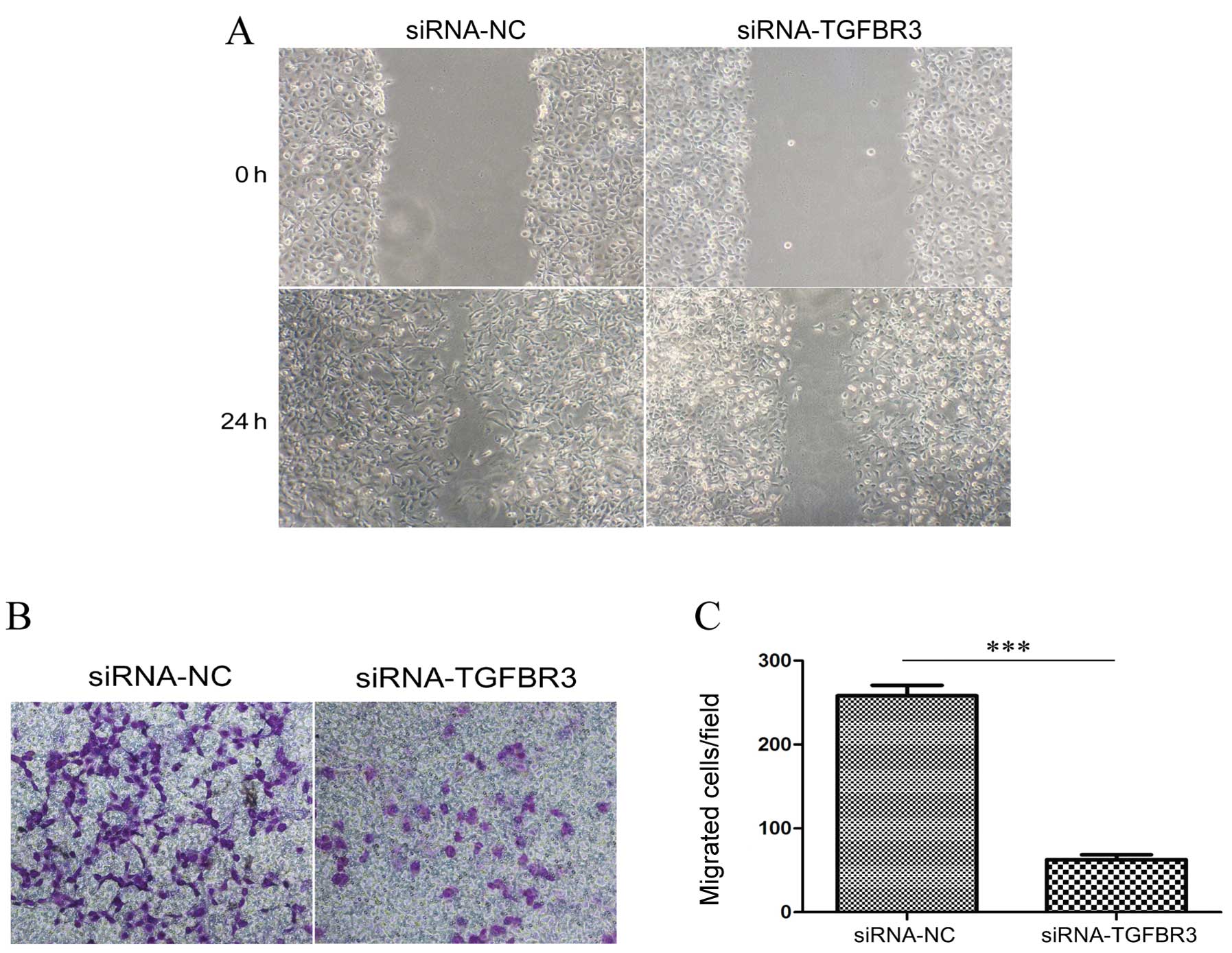
Knockdown of TGFBR3 decreases the
migration and invasion of T24 cells
To determine whether TGFR3 expression is associated
with cellular migration and invasion, we detected cell migration
ability in TGFR3-knockdown T24 cells using a monolayer wound
healing assay and a transwell migration assay. The siRNA-NC-treated
cells consistently migrated faster than the siRNA-TGFBR3-treated
cells in the monolayer wound healing assay (Fig. 5A), while the transwell migration
assay demonstrated a trend toward a bigger increase in the basal
migration of siRNA-TGFBR3-treated cells than in the
siRNA-NC-treated cells (Fig. 5B and
C).
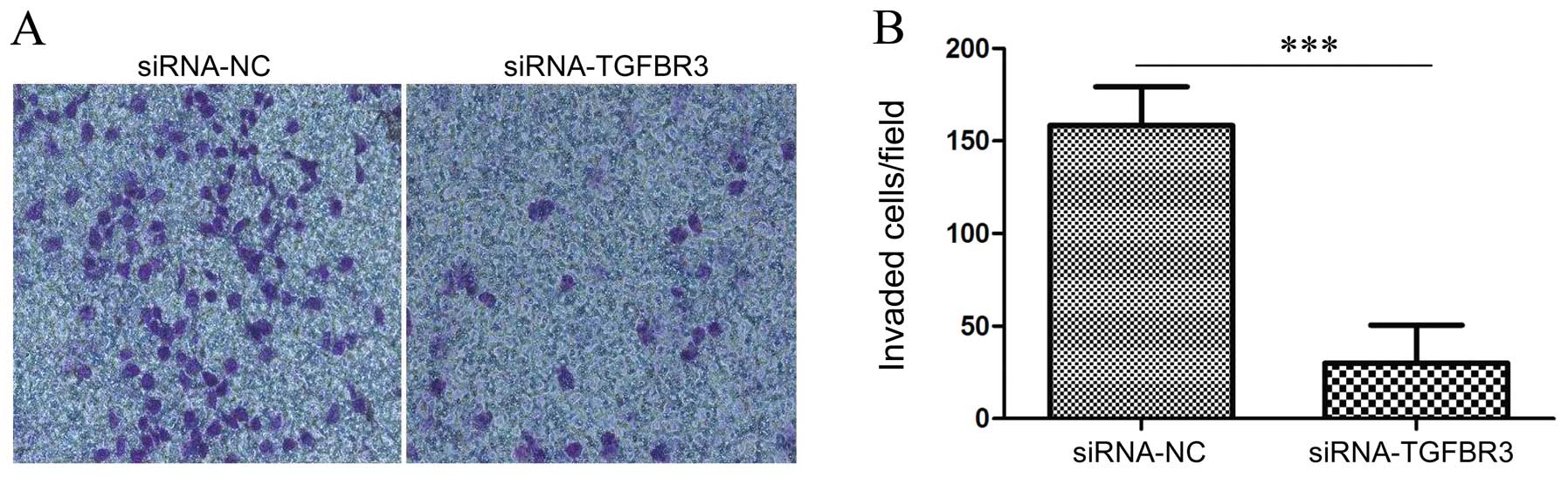
We used the reconstituted extracellular matrix,
Matrigel, to mimic the basement membrane and examined the role of
TGFBR3 on T24 cancer cell invasion in vitro compared with
the cells treated with siRNA-NC, the T24 cells treated with
siRNA-TGFBR3 significantly inhibited their ability to invade
Matrigel (Fig. 6A and B).
Discussion
Bladder cancer is the most common tumor of the
urinary tract. The global age standardised incidence rate is 10.1
per 100,000 males and 2.5 per 100,000 females (16). A report estimated that in 2008,
386,000 cases of the cancer were diagnosed, out of which 150,200
patients succumbed to the disease worldwide (17). Therefore, the search for new
treatment strategies is urgent.
It has been reported that the loss of TGFBR3
expression directly mitigated the effects of TGFBR3 on regulating
cell migration, invasion, proliferation and angiogenesis. This
suggests that TGFBR3 acts as a suppressor of cancer progression
and/or as a metastasis suppressor, directly influencing breast,
prostate, ovarian, pancreatic, renal, non-small cell lung cancer
and endometrial carcinoma (7).
In the present study, we identified that TGFBR3
expression was reduced in most superficial BUCs compared to the
corresponding normal tissues, while the expression of TGFBR3
typically increased in invasive samples. In addition to identifying
TGFBR3 expression in bladder cancer tissues, we also observed
TGFBR3 expression in 3 different cells: human normal bladder
epithelium cell line SV-HUC-1, superficial derived bladder cancer
cell line 5637 and invasive bladder cancer cell line T24. In the
present study, too, TGFBR3 expression was suppressed in 5637 cells
and heightened in T24 cells. These data are different from previous
reports on TGRBR3 and suggest that TGFBR3 may have a different role
in BUCs, functioning to suppress tumor progression in early stages
but promoting tumor progression during invasion.
In order to further characterize the role of TGFBR3
in invasive bladder cancer cells, we used specific siRNAs to knock
down TGFBR3 expression in T24 cells and evaluated the biological
effects. We demonstrated that silencing TGFBR3 expression could
notably deregulate cell growth, motility and invasion in
vitro. This result indicates that TGFBR3 may play a tumor
promoter role in invasive bladder cancer cells.
In fact, Criswell et al(18) reported an oncogenic role for TGFBR3
in human and murine breast cancer cells. Knockdown of TGFBR3
resulted in decreased growth, motility and invasion, which are
dependent on NF-κB signaling. Recently, Gatza et al(15) reported that TGFBR3 expression is
maintained and enhanced in human colon cancer and functions to
promote colon cancer progression through the promotion of
proliferation, migration, anchorage-independent growth and
resistance to apoptosis.
Given that TGF-β has numerous and often opposite
cellular effects, serving as a tumor promoter and a tumor
suppressor, and as an inhibitor and stimulator of cellular
proliferation, apoptosis and angiogenesis (19,20),
it is possible that, similar to TGF-β, TGFBR3 also plays different
roles in different stages, possibly dependent upon the activation
or inhibition of other oncogenes or tumor suppressor genes.
Collectively, in the present study we observed the
loss of TGFBR3 expression in superficial bladder cancer, but an
increase of TGFBR3 expression in invasive bladder cancer.
Furthermore, knockdown of TGFBR3 in invasive bladder cancer cells
resulted in decreased growth, motility and invasion. These data
revealed that TGFBR3 may act as a suppressor in early stages and as
a promoter in later stages of BUCs. We consider that targeting
TGFBR3 in the future may become a promising anticancer strategy
against bladder cancer. However, further studies are required to
determine TGFBR3 status and its potential impact on TGFBR3-based
therapy.
Acknowledgements
The authors acknowledge the participation and
cooperation of patients with bladder urothelial carcinoma. The
present study was in part supported by grants from the National
Natural Science Foundation of China (81171894 to H.T.Z.), the
Program for New Century Excellent Talents in University
(NCET-09-0165 to H.T.Z.), the ‘333’ Project of Jiangsu Province
Government (to H.T.Z.), the Soochow Scholar Project of Soochow
University (to H.T.Z), the Suzhou Key Laboratory for Molecular
Cancer Genetics (SZS201209 to H.T.Z), the Suzhou Science and
Education Youth Health Foundation (KJXW2012014 to X.L.L.) and the
Scientific Innovation Research of College Graduate of Jiangsu
Province (CXZZ12_0844 to X.L.L.).
References
|
1
|
Fleshner NE, Herr HW, Stewart AK, Murphy
GP, Mettlin C and Menck HR: The National Cancer Data Base report on
bladder carcinoma. The American College of Surgeons Commission on
Cancer and the American Cancer Society. Cancer. 78:1505–1513. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Babjuk M, Oosterlinck W, Sylvester R,
Kaasinen E, Bohle A, Palou-Redorta J and Roupret M: EAU guidelines
on non-muscle-invasive urothelial carcinoma of the bladder, the
2011 update. Actas Urol Esp. 36:389–402. 2012.(In Spanish).
|
|
3
|
Di Stasi SM, Valenti M, Verri C, Liberati
E, Giurioli A, Leprini G, Masedu F, Ricci AR, Micali F and
Vespasiani G: Electromotive instillation of mitomycin immediately
before transurethral resection for patients with primary urothelial
non-muscle invasive bladder cancer: a randomised controlled trial.
Lancet Oncol. 12:871–879. 2011.
|
|
4
|
DeGraff DJ, Clark PE, Cates JM, Yamashita
H, Robinson VL, Yu X, Smolkin ME, Chang SS, Cookson MS, Herrick MK,
Shariat SF, Steinberg GD, Frierson HF, Wu XR, Theodorescu D and
Matusik RJ: Loss of the urothelial differentiation marker FOXA1 is
associated with high grade, late stage bladder cancer and increased
tumor proliferation. PLoS One. 7:e366692012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaufman DS, Shipley WU and Feldman AS:
Bladder cancer. Lancet. 374:239–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Massague J: TGFβ in cancer. Cell.
134:215–230. 2008.
|
|
7
|
Gatza CE, Oh SY and Blobe GC: Roles for
the type III TGF-β receptor in human cancer. Cell Signal.
22:1163–1174. 2010.
|
|
8
|
Siegel PM and Massague J: Cytostatic and
apoptotic actions of TGF-β in homeostasis and cancer. Nat Rev
Cancer. 3:807–821. 2003.
|
|
9
|
Dong M, How T, Kirkbride KC, Gordon KJ,
Lee JD, Hempel N, Kelly P, Moeller BJ, Marks JR and Blobe GC: The
type III TGF-β receptor suppresses breast cancer progression. J
Clin Invest. 117:206–217. 2007.
|
|
10
|
Turley RS, Finger EC, Hempel N, How T,
Fields TA and Blobe GC: The type III transforming growth factor-β
receptor as a novel tumor suppressor gene in prostate cancer.
Cancer Res. 67:1090–1098. 2007.
|
|
11
|
Hempel N, How T, Dong M, Murphy SK, Fields
TA and Blobe GC: Loss of betaglycan expression in ovarian cancer:
role in motility and invasion. Cancer Res. 67:5231–5238. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gordon KJ, Dong M, Chislock EM, Fields TA
and Blobe GC: Loss of type III transforming growth factor β
receptor expression increases motility and invasiveness associated
with epithelial to mesenchymal transition during pancreatic cancer
progression. Carcinogenesis. 29:252–262. 2008.
|
|
13
|
Finger EC, Turley RS, Dong M, How T,
Fields TA and Blobe GC: TβRIII suppresses non-small cell lung
cancer invasiveness and tumorigenicity. Carcinogenesis. 29:528–535.
2008.
|
|
14
|
Cooper SJ, Zou H, Legrand SN, Marlow LA,
von Roemeling CA, Radisky DC, Wu KJ, Hempel N, Margulis V, Tun HW,
Blobe GC, Wood CG and Copland JA: Loss of type III transforming
growth factor-β receptor expression is due to methylation silencing
of the transcription factor GATA3 in renal cell carcinoma.
Oncogene. 29:2905–2915. 2010.
|
|
15
|
Gatza CE, Holtzhausen A, Kirkbride KC,
Morton A, Gatza ML, Datto MB and Blobe GC: Type III TGF-β receptor
enhances colon cancer cell migration and anchorage-independent
growth. Neoplasia. 13:758–770. 2011.
|
|
16
|
Ploeg M, Aben KK and Kiemeney LA: The
present and future burden of urinary bladder cancer in the world.
World J Urol. 27:289–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
18
|
Criswell TL, Dumont N, Barnett JV and
Arteaga CL: Knockdown of the transforming growth factor-β type III
receptor impairs motility and invasion of metastatic cancer cells.
Cancer Res. 68:7304–7312. 2008.
|
|
19
|
Elliott RL and Blobe GC: Role of
transforming growth factor β in human cancer. J Clin Oncol.
23:2078–2093. 2005.
|
|
20
|
Pardali K and Moustakas A: Actions of
TGF-β as tumor suppressor and pro-metastatic factor in human
cancer. Biochim Biophys Acta. 1775:21–62. 2007.
|