Introduction
Angiogenesis, the formation of new blood vessels
from the pre-existing vasculatures, plays an important role in a
wide range of biological processes including wound healing,
reproduction and embryonic development. However, deregulation of
this vital process is also essential for cancer progression
(1–4). In the initial stage, tumor cells
obtain oxygen and nutrients from nearby blood vessels by simple
passive diffusion. However, when tumor grows to reach a size larger
than 2 mm3, oxygen delivery by diffusion is no longer
sufficient, which causes tumor cells to induce the sprouting of new
blood vessels to support the continued growth of tumor and provide
an avenue for hematogenous metastasis (5–9).
The process of angiogenesis is highly regulated by
multiple cellular signaling transduction pathways including signal
transducer and activator of transcription 3 (STAT3),
serine-threonine kinase Akt and mitogen-activated protein kinases
(MAPKs) (10–14). Aberrant activation of these pathways
promotes tumor angiogenesis by inducing the expression of numerous
critical angiogenic stimulators (15–17),
including vascular endothelial growth factor A (VEGF-A), basic
fibroblast growth factor (bFGF) and nitric oxide (NO) (18–22).
Due to the essential role of angiogenesis in cancer
progression and metastasis, inhibition of tumor angiogenesis has
become a promising strategy for anticancer chemotherapy. A variety
of anti-angiogenic agents is currently in preclinical development,
with some of them now entering clinical trials. However, the
angiogenesis-related signaling pathways are usually redundant; and
crosstalk between these pathways form a complicated and robust
network that is regulated by compensatory mechanisms. Therefore,
most currently used angiogenesis inhibitors that target only a
single pathway may be insufficient and probably generate drug
resistance (23). These problems
highlight the need for the development of novel anticancer agents.
Natural products, such as traditional Chinese medicine (TCM), have
been used clinically to treat various kinds of diseases including
cancer for thousands of years (24–26).
TCM formula is a complex combination of many natural products, each
of which contains numerous chemical compounds. TCM formulas,
therefore, are considered to be multi-component and multi-target
agents exerting their therapeutic function in a more holistic way.
Pien Tze Huang (PZH) is a well-known traditional Chinese
formulation that was first prescribed 450 years ago by a royal
physician in the Ming Dynasty. The main ingredients of PZH include
Moschus, Calculus Bovis, Snake Gall and Radix Notoginseng.
These products together confer PZH properties of heat-clearing,
detoxification, promotion of blood circulation and removal of blood
stasis (27). Since in the Chinese
medicine system accumulation of toxic dampness and heat is one of
the major causative factors in the pathogenesis of cancers, PZH is
believed to be an effective anticancer agent. In fact, PZH has long
been used as an alternative remedy for cancers in China and
Southeast Asia. Recently, we reported that PZH can inhibit
colorectal cancer growth in vivo and in vitro via
promotion of cancer cell apoptosis and inhibition of cell
proliferation, which is probably mediated by its inhibitory effect
on activation of STAT3 pathway in tumor tissues (28–32).
To further elucidate the mechanism of the tumoricidal activity of
PZH, in the present we used a colorectal cancer mouse xenograft
model to evaluate the effect of PZH on tumor angiogenesis and
investigated the underlying molecular mechanisms.
Materials and methods
Materials and reagents
Pien Tze Huang (PZH) was obtained from and
authenticated by the sole manufacturer Zhangzhou Pien Tze Huang
Pharmaceutical Co., Ltd., China (Chinese FDA approval No:
Z35020242). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine
serum (FBS), penicillin-streptomycin, Trypsin-EDTA, TRIzol reagent,
were purchased from Invitrogen (Grand Island, NY, USA). All
antibodies were purchased from Santa Cruz Biotechnology (Santa
Cruz, CA, USA). Bio-Plex phosphoprotein assay kits were purchased
from Bio-Rad Laboratories (Hercules, CA, USA). All the other
chemicals, unless otherwise stated, were obtained from Sigma
Chemicals (St. Louis, MO, USA).
Cell culture
Human colon carcinoma HT-29 cells were obtained from
the American Type Culture Collection (ATCC, Manassas, VA, USA).
Cells were grown in DMEM containing 10% (v/v) FBS, 100 Units/ml
penicillin and 100 μg/ml streptomycin in a 37°C humidified
incubator with 5% CO2. The cells were subcultured at
80–90% confluency.
Animals
Athymic male nude mice were obtained from the Vital
River Laboratory Animal Technology Co., Ltd. (Beijing, China) and
housed in specific pathogen-free rooms in an environment with
controlled temperature (22°C), humidity, and a 12-h light/dark
cycle. Food and water were given ad libitum throughout the
experiment. All animal treatments were strictly in accordance with
the international ethics guidelines and the National Institutes of
Health Guide concerning the Care and Use of Laboratory Animals, and
the experiments were approved by the Institutional Animal Care and
Use Committee of the Fujian University of Traditional Chinese
Medicine.
In vivo nude mice xenograft study
Cells (1.5×106) mixed with Matrigel (1:1)
were subcutaneously injected in the right flank area of athymic
nude mice to initiate tumor growth. After 3 days of xenograft
implantation, mice were randomized into two groups (n=10) and given
intragastric administration of 234 mg/kg/day dose of PZH or saline
daily, 5 days a week for 16 days. Tumor growth were measured every
two days. Tumor growth was determined by measuring the major (L)
and minor (W) diameter with a caliper. The tumor volume was
calculated according to the following formula: tumor volume = π/6 ×
L × W2. At the end of the experiment, the animals were
anaesthetized and the tumor tissue was removed and weighed.
Immunohistochemstry analysis
Tumor samples were fixed with 10% formaldehyde for
12 h and subsequently processed conventionally for
paraffin-embedded tumor slides. The slides were subjected to
antigen retrieval and the endogenous peroxidase activity was
blocked with 3% hydrogen peroxide in water. For immunohistochemical
staining, slides were incubated with rabbit polyclonal antibodies
against CD31, VEGF-A, VEGFR2, bFGF, bFGFR, iNOS or eNOS (all in
1:200 dilution; Santa Cruz Biotechnology). After washing with PBS,
slides were incubated with biotinylated secondary antibody followed
by conjugated horseradish peroxidase (HRP)-labelled streptavidin
(Dako) and then washed with PBS. The slides were then incubated
with diamino-benzidine (DAB, Sigma) as the chromogen, followed by
counterstaining with diluted Harris hematoxylin (Sigma). After
staining, five high-power fields (×400) were randomly selected in
each slide, and the average proportion of positive cells in each
field were counted using the true color multi-functional cell image
analysis management system (Image-Pro Plus; Media Cybernetics,
Silver Spring, MD, USA). To rule out any non-specific staining, PBS
was used to replace the primary antibody as a negative control.
Bio-Plex phosphoprotein assay
Tumors were homogenized and then lysed using a
commercially available lysis kit (Bio-Rad Laboratories), followed
by centrifugation at 14,000 × g for 15 min. Protein concentrations
of the clarified supernatants were determined by BCA protein assay.
The presence of p-STAT3, p-Akt, p-Erk1/2, p-JNK and p-p38 was
detected using a bead-based multiplex assay for phosphoproteins
(Bio-Plex phosphoprotein assay; Bio-Rad Laboratories) according to
the manufacturer’s protocol. Data were collected and analyzed using
the Bio-Plex 200 suspension array system (Bio-Rad
Laboratories).
Statistical analysis
Data are presented as mean ± SD for the indicated
number of independently performed experiments. Statistical analysis
was carried out with the Student’s t-test. Differences with
P<0.05 were considered to be statistically significant.
Results and Discussion
PZH suppresses colorectal cancer growth
via inhibition of tumor angiogenesis
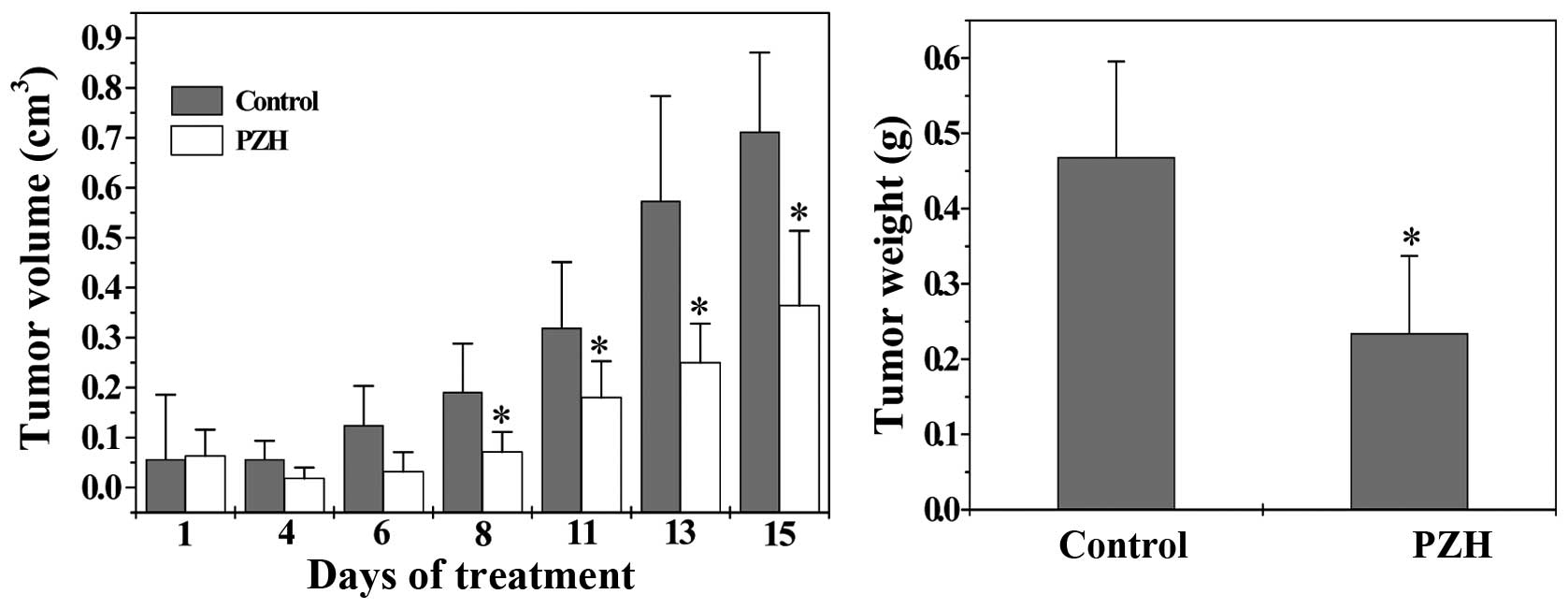
The therapeutic efficacy of PZH against tumor growth
was evaluated through comparison of tumor weight and volume in
treated and control CRC xenograft mice. As shown in Fig. 1, tumor growth was significantly
suppressed by PZH treatment throughout the experiment. The final
tumor volume or tumor weight per mouse in control group was
0.71±0.16 cm3 or 0.47±0.13 g; while that in PZH-treated
group was 0.36±0.15 cm3 or 0.23±0.10 g (P<0.05),
demonstrating that PZH is effective in suppressing colorectal tumor
growth.
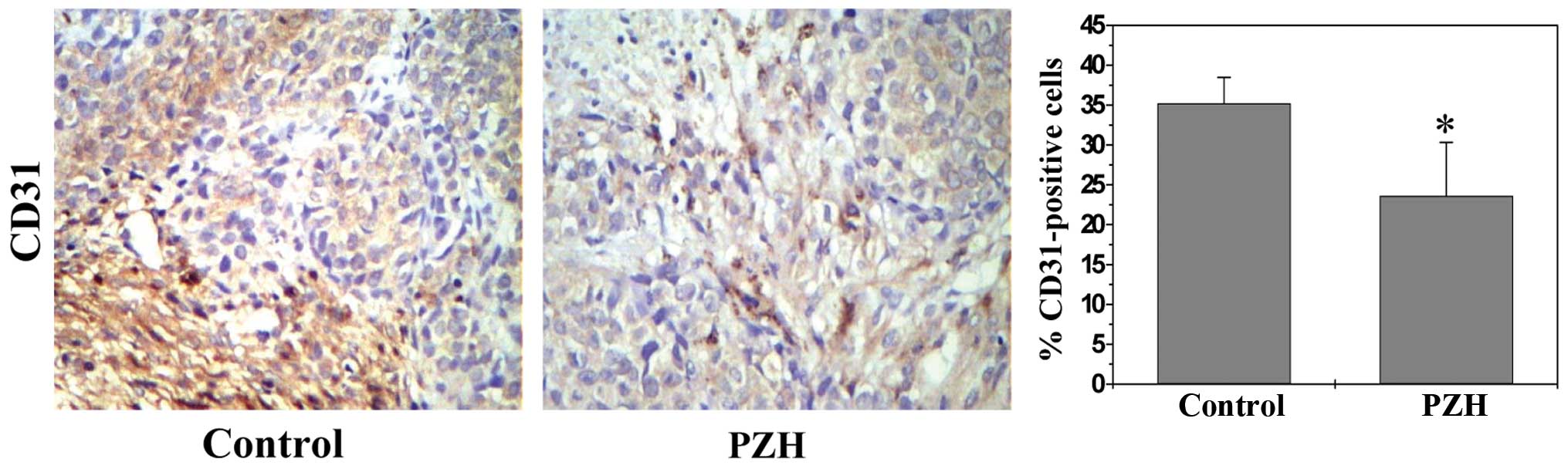
Angiogenesis plays an essential role in cancer
development; we therefore investigated the effect of PZH on
intratumoral microvessel density (MVD) that is an indicator of new
blood vessel growth. Tumors from CRC xenograft mice were evaluated
by immunohistochemical staining (IHS) for the expression of an
endothelial cell-specific marker CD31; and data in Fig. 2 show that the percentage of
CD31-positive cells in control or PZH-treated mice was 35.22±3.26
or 23.60±6.73%, respectively (P<0.05), suggesting that
PZH-caused inhibition of tumor growth is associated with its
anti-angiogenic activity.
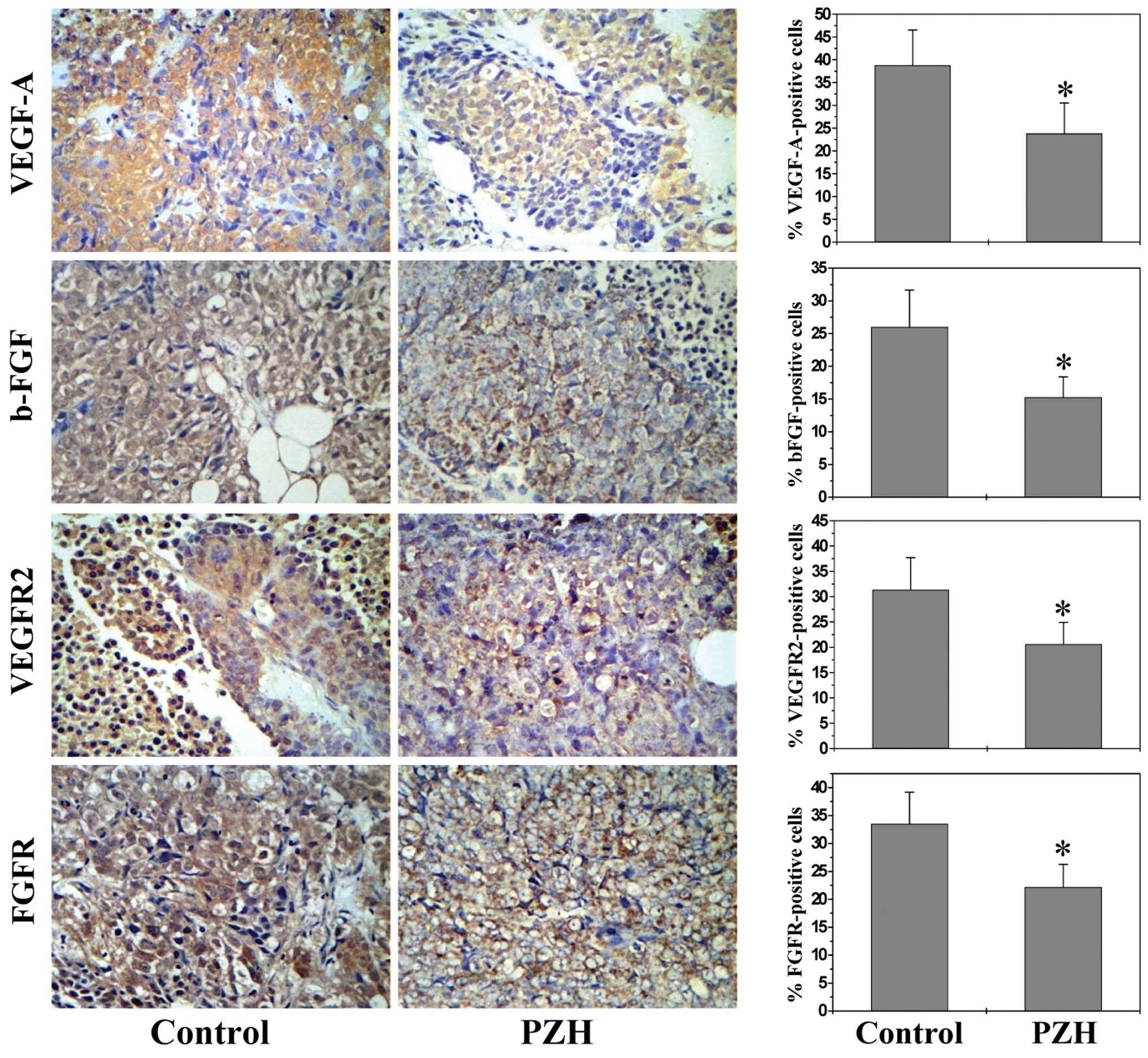
PZH inhibits the expression of VEGF-A,
VEGFR2, bFGF and bFGFR
As most potent angiogenic stimulators, VEGF-A and
bFGF are commonly overexpressed in many kinds of human cancer,
which is correlated with tumor progression, invasion and
metastasis, and poorer survival and prognosis in patients (33–36).
VEGF-A and bFGF exert their biological function primarily through
interaction with their specific receptors located on the surface of
vascular endothelial cells, such as VEGFR-2 and bFGFR. Binding of
VEGF-A and bFGF and their receptors leads to receptor dimerization,
which in turn activates downstream signaling cascades such as the
Akt and Erk pathways, leading to the proliferation, migration,
survival, sprouting and eventually tube formation of endothelial
cells (35–37).
By performing IHS analysis we found that PZH
treatment profoundly inhibited the expression of VEGF-A and bFGF as
well as their receptors in tumor tissues. The percentage of VEGF-A,
VEGFR2, bFGF or bFGFR-positive cells in control group was,
respectively, 38.77±7.76, 31.38±3.36, 23.80±6.73 and 33.50±5.67%,
whereas that in PZH-treated mice was 26.0±5.67, 20.60±4.32,
15.25±3.17 and 22.20±4.07% (Fig. 3;
P<0.05).
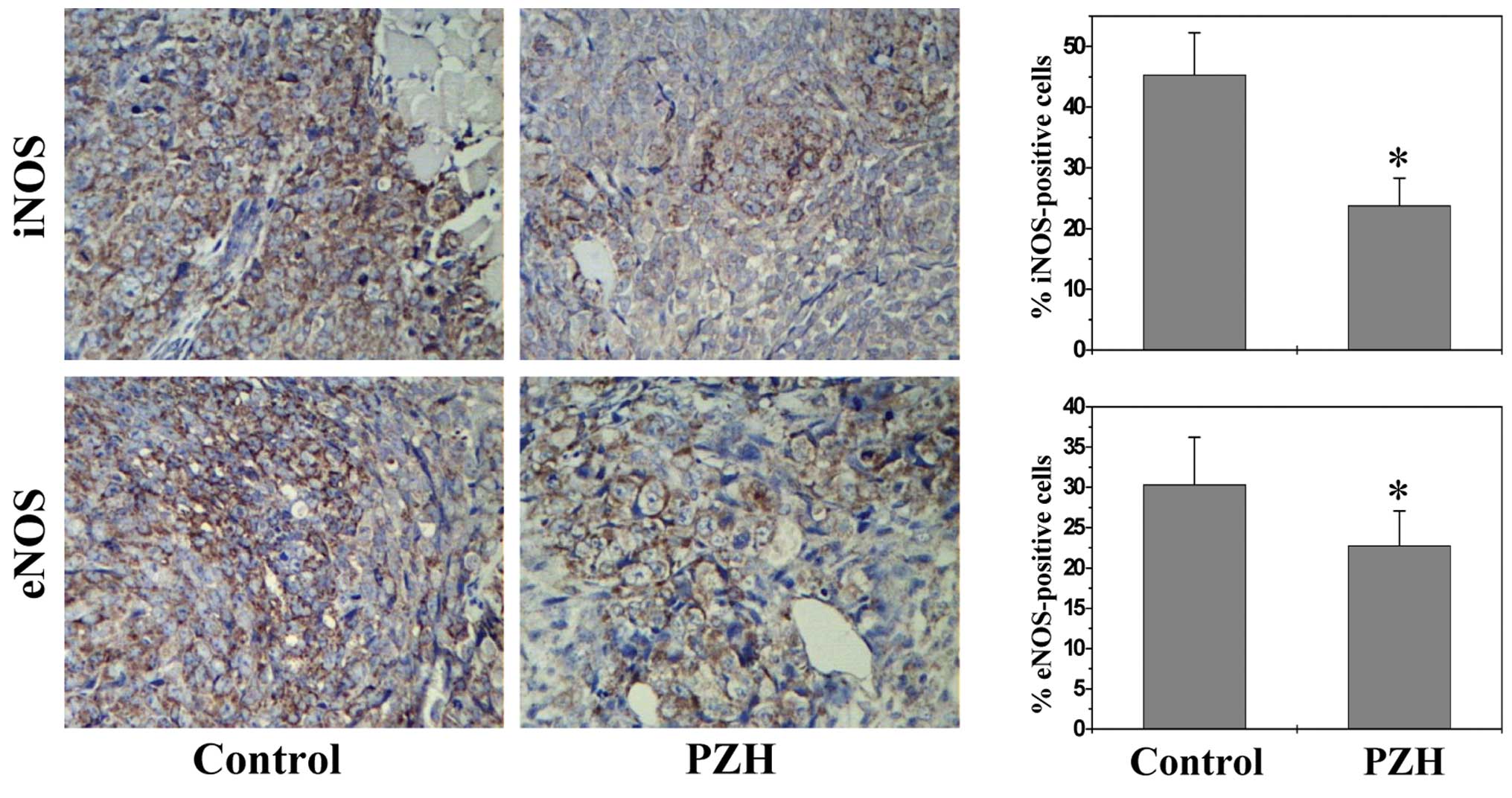
PZH inhibits expression of iNOS and
eNOS
Nitric oxide synthases (NOSs), particularly the
inducible (iNOS) and endothelial (eNOS) isoforms, have been
strongly implicated in tumor angiogenesis (21,22,37–41);
we therefore determined the effect of PZH on NOS expression. As
shown in Fig. 4, the percentage of
iNOS- or eNOS-positive cells in control group was, respectively,
45.33±6.93 and 30.33±5.87%, while that in PZH-treated mice was
23.80±4.52 and 22.75±4.34% (P<0.05), suggesting that iNOS and
eNOS could be potential molecular targets for the antitumor and
anti-angiogenic effects of PZH.
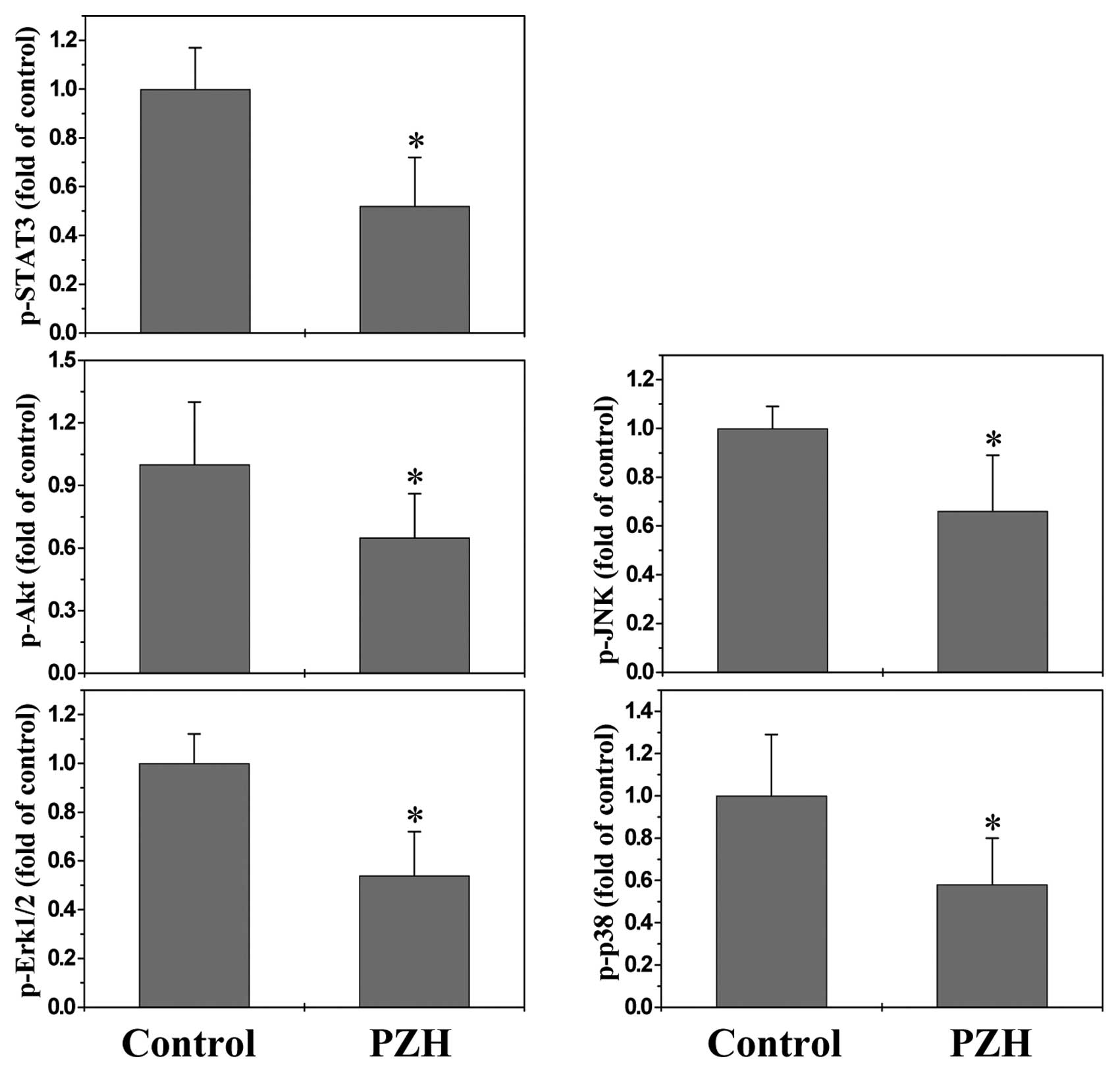
PZH suppresses the activation of multiple
signaling pathways
Cancer development is tightly regulated by multiple
intracellular signaling pathways, including STAT3, Akt, ERK, JNK
and p38. Aberrant activation of these pathways alters the
expression of various critical target genes, such as
above-mentioned angiogenic factors and NOSs, leading to the
promotion of tumor angiogenesis. To further elucidate the
underlying mechanisms of antitumor activity of PZH, we determined
its effect on the activation of STAT3, Akt, ERK, JNK and p38
pathways. The activation (phosphorylation) of STAT3, Akt, Erk1/2,
JNK and p38 in xenograft tumor tissues was determined by Bio-Plex
phosphoprotein assay. As shown in Fig.
5, after PZH treatment the phosphorylation levels of STAT3,
ERK, Akt, JNK and p38 in tumors were decreased as compared to
controls (P<0.05), suggesting that PZH exerts its antitumor
activity probably through affecting multiple intracellular
targets.
In conclusion, in the present study, we demonstrate
for the first time that PZH inhibits colorectal cancer growth via
suppression of multiple intracellular signaling pathways leading to
the inhibition of tumor angiogenesis, which may in part explain its
anticancer activity.
Acknowledgements
The present study was sponsored by the National
Natural Science Foundations of China (81073097 and 81202790), the
Developmental Fund of Chen Keji Integrative Medicine (CKJ 2011001)
and the China Postdoctoral Science Foundation (2012M511437).
Abbreviations:
|
PZH
|
Pien Tze Huang
|
|
TCM
|
traditional Chinese medicine
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
MAPK
|
mitogen-activated protein kinase
|
|
CRC
|
colorectal cancer
|
|
IHS
|
immunohistochemical staining
|
|
MVD
|
microvessel density
|
|
VEGF-A
|
vascular endothelial growth factor
A
|
|
bFGF
|
basic fibroblast growth factor
|
|
NOS
|
nitric oxide synthase
|
References
|
1
|
Folkman J: Tumor angiogenesis: therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Folkman J and Shing Y: Angiogenesis. J
Biol Chem. 267:10931–10934. 1992.
|
|
3
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other diseases. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Folkman J: Angiogenesis. Annu Rev Med.
57:1–18. 2006. View Article : Google Scholar
|
|
5
|
Cook KM and Figg WD: Angiogenesis
inhibitors: current strategies and future prospects. CA Cancer J
Clin. 60:222–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar
|
|
7
|
Whiteside TL: The tumor microenvironment
and its role in promoting tumor growth. Oncogene. 27:5904–5912.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jain RK: Transport of molecules in the
tumor interstitium: a review. Cancer Res. 47:3039–3051.
1987.PubMed/NCBI
|
|
9
|
Folkman J: How is blood vessel growth
regulated in normal and neoplastic tissue? GHA Clowes memorial
award lecture. Cancer Res. 46:467–473. 1986.PubMed/NCBI
|
|
10
|
Robert S and Kerbel RS: Tumor
angiogenesis. N Engl J Med. 358:2039–2049. 2008. View Article : Google Scholar
|
|
11
|
Sun W: Angiogenesis in metastatic
colorectal cancer and the benefits of targeted therapy. J Hematol
Oncol. 5:632012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weis SM and Cheresh DA: Tumor
angiogenesis: molecular pathways and therapeutic targets. Nat Med.
17:1359–1370. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qian WF, Guan WX, Gao Y, Tan JF, Qiao ZM,
Huang H and Xia CL: Inhibition of STAT3 by RNA interference
suppresses angiogenesis in colorectal carcinoma. Braz J Med Biol
Res. 44:1222–1230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berra E, Pagès G and Pouysségur J: MAP
kinases and hypoxia in the control of VEGF expression. Cancer
Metastasis Rev. 19:139–145. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stromblad S and Cheresh DA: Integrins,
angiogenesis and vascular cell survival. Chem Biol. 3:881–885.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Breier G and Risau W: The role of vascular
endothelial growth factor in blood vessel formation. Trends Cell
Biol. 6:454–456. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis-correlation in invasive breast
carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ferrara N: Role of vascular endothelial
growth factor in physiologic and pathologic angiogenesis:
therapeutic implications. Semin Oncol. 29:10–14. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jain RK: Tumor angiogenesis and
accessibility: role of vascular endothelial growth factor. Semin
Oncol. 29:3–9. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Risau W: Mechanisms of angiogenesis.
Nature. 386:671–674. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ziche M and Morbidelli L: Molecular
regulation of tumour angiogenesis by nitric oxide. Eur Cytokine
Netw. 20:164–170. 2009.PubMed/NCBI
|
|
22
|
Cooke JP and Losordo DW: Nitric oxide and
angiogenesis. Circulation. 105:2133–2135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eikesdal HP and Kalluri R: Drug resistance
associated with antiangiogenesis therapy. Semin Cancer Biol.
19:310–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Newman DJ, Cragg GM and Snader KM: The
influence of natural products upon drug discovery. Nat Prod Rep.
17:215–234. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ji HF, Li XJ and Zhang HY: Natural
products and drug discovery. EMBO Rep. 10:194–200. 2009.PubMed/NCBI
|
|
27
|
Chinese Pharmacopoeia Commission.
Pharmacopoeia of the Peoples Republic of China. Chinese Medical
Science and Technology Press; 1. pp. 573–575. 2010
|
|
28
|
Lin JM, Wei LH, Chen YQ, Liu XX, Hong ZF,
Sferra TJ and Peng J: Pien Tze Huang-induced apoptosis in human
colon cancer HT-29 cells is associated with regulation of the Bcl-2
family and activation of caspase 3. Chin J Integr Med. 17:685–690.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhuang QC, Hong F, Shen AL, Zheng LP, Zeng
JW, Lin W, Chen YQ, Sferra TJ, Hong ZF and Peng J: Pien Tze Huang
inhibits tumor cell proliferation and promotes apoptosis via
suppressing the STAT3 pathway in a colorectal cancer mouse model.
Int J Oncol. 40:1569–1574. 2012.PubMed/NCBI
|
|
30
|
Shen AL, Hong F, Liu LY, Lin JM, Zhuang
QC, Hong ZF and Peng J: Effects of Pien Tze Huang on angiogenesis
in vivo and in vitro. Chin J Integr Med. 18:431–436. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shen AL, Hong F, Liu LY, Lin JM, Wei LH,
Cai QY, Hong ZF and Peng J: Pien Tze Huang inhibits the
proliferation of human colon carcinoma cells by arresting G1/S cell
cycle progression. Oncol Lett. 4:767–770. 2012.PubMed/NCBI
|
|
32
|
Shen AL, Chen YQ, Hong F, Lin JM, Wei LH,
Hong ZF, Sferra TJ and Peng J: Pien Tze Huang suppresses
IL-6-inducible STAT3 activation in human colon carcinoma cells
through induction of SOCS3. Oncol Rep. 28:2125–2130.
2012.PubMed/NCBI
|
|
33
|
Kaya M, Wada T, Akatsuka T, Kawaguchi S,
Nagoya S, Shindoh M, Higashino F, Mezawa F, Okada F and Ishii S:
Vascular endothelial growth factor expression in untreated
osteosarcoma is predictive of pulmonary metastasis and poor
prognosis. Clin Cancer Res. 6:572–577. 2000.PubMed/NCBI
|
|
34
|
Maeda K, Chung YS, Ogawa Y, Takatsuka S,
Kang SM, Ogawa M, Sawada T and Sowa M: Prognostic value of vascular
endothelial growth factor expression in gastric carcinoma. Cancer.
77:858–863. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ishigami SI, Arii S, Furutani M, Niwano M,
Harada T, Mizumoto M, Mori A, Onodera H and Imamura M: Predictive
value of vascular endothelial growth factor (VEGF) in metastasis
and prognosis of human colorectal cancer. Br J Cancer.
78:1379–1384. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gille H, Kowalski J, Li B, LeCouter J,
Moffat B, Zioncheck TF, Pelletier N and Ferrara N: Analysis of
biological effects and signaling properties of Flt-1 (VEGFR-1) and
KDR (VEGFR-2). A reassessment using novel receptor-specific
vascular endothelial growth factor mutants. J Biol Chem.
276:3222–3230. 2001. View Article : Google Scholar
|
|
38
|
Fukumura D, Gohongi T, Kadambi A, Izumi Y,
Ang J, Yun CO, Buerk DG, Huang PL and Jain RK: Predominant role of
endothelial nitric oxide synthase in vascular endothelial growth
factor-induced angiogenesis and vascular permeability. Proc Natl
Acad Sci USA. 98:2604–2609. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sessa WC: eNOS at a glance. J Cell Sci.
117:2427–2429. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vakkala M, Kahlos K, Lakari E, Paakko P,
Kinnula V and Soini Y: Inducible nitric oxide synthase expression,
apoptosis, and angiogenesis in situ and invasive breast carcinomas.
Clin Cancer Res. 6:2408–2416. 2000.PubMed/NCBI
|
|
41
|
Aaltomaa SH, Lipponen PK and Kosma VM:
Inducible nitric oxide synthase (iNOS) expression and its
prognostic value in prostate cancer. Anticancer Res. 21:3101–3106.
2001.PubMed/NCBI
|