Introduction
Osteosarcoma accounts for ~15% of all primary bone
tumor cases (1). Approximately 95%
of the patients who died of metastatic disease exhibited metastases
in the lungs, as indicated in autopsy (2). The mechanisms underlying the
development, progression and metastasis of osteosarcoma remain
elusive. We previously established the osteosarcoma cell line,
9607-F5M2 (F5M2), which has a high tumorigenic ability and
potential for spontaneous pulmonary metastasis (3).
Non-hematopoietic mesenchymal stem cells (MSCs) in
the bone marrow are characterized by clonal, plastic adherent cells
capable of differentiating into osteoblasts, adipocytes and
chondrocytes (4). MSCs are also
stromal cells, structural components of the bone marrow that
support in vitro hematopoiesis by providing extracellular
matrix components, cytokines and growth factors (5–7). MSCs
have been shown to target microscopic tumors and subsequently
proliferate and differentiate, contributing to the formation of a
significant portion of the tumor stroma and promote breast cancer
metastasis (8). These observations
indicate the complexity of the relationship between MSCs and
tumors. There is, however, little data concerning the relationship
between MSCs and osteosarcoma. Chemokines are small soluble
molecules that are best known for their potent ability to induce
cellular migration. Many types of cancer cells express chemokines
and chemokine receptors (CXCR), such as CXCR4 (9). CXCR4 expression on tumor cells is
upregulated by hypoxia and angiogenic factors, such as vascular
endothelial growth factor (VEGF). Patients with CXCR4/VEGF positive
tumors have a significantly shorter survival, suggesting that the
CXCR4/VEGF could be a predictor of potential metastatic development
(10).
In order to study the interaction and the underlying
mechanism between MSCs and osteosarcoma, we co-injected MSCs and
osteosarcoma cells into nude mice and monitored tumor development
and progression, namely growth and pulmonary metastasis in response
to MSCs. Finally, we performed in vitro experiments
including Transwell assays, MTT, ELISA and protein expression
analysis to study the possible mechanisms, with particular focus on
the CXCR4/VEGF signaling pathway and found that CXCR4-mediated
osteosarcoma growth and pulmonary metastasis were promoted by MSCs
through VEGF.
Materials and methods
Isolation and culture of human MSCs and
F5M2
MSCs were isolated and expanded according to a
modification of a previously described protocol (11,12).
We identified MSCs by CD90 and CD45, in which >95% of the
population was positive for CD90 expression and >98% negative
for CD45 surface molecules. The donor of the cells was a healthy
individual without metabolic or inherited disorders or other
diseases that might affect the current study. A signed consent was
obtained and the study was approved by the Institutional Review
Board of the Fourth Military Medical University. In brief, human
MSCs were isolated by density-gradient centrifugation and cultured
in low-glucose Dulbecco’s modified Eagle’s medium (DMEM, Gibco
Laboratories, USA) supplemented with 10% fetal bovine serum (FBS)
(Hyclone), 100 U/ml penicillin and 100 μg/ml streptomycin at
37°C, in a humidified atmosphere containing 5% carbon dioxide
(CO2). Culture medium was replaced by fresh medium twice
a week. Confluent cells were harvested for passage 3–6 with 0.25%
trypsin. The human osteosarcoma cell line 9607-F5M2 (F5M2) was
grown in DMEM supplemented with 10% FBS (Hyclone) (3). Conditioned medium (CM) was derived by
adding 1% FBS medium to cells at 80% confluence for 24 h.
Tumor xenografts in nude mice
Study protocols involving mice were approved by the
Animal Ethics Committee of the Fourth Military Medical University.
BALB/c nu/nu (nude mice) from the animal center of the Fourth
Military Medical University, Xi’an, Shaanxi, China (approval ID:
2009043) (four weeks of age, male) were divided into four groups
(n=6 animals per group): control group, MSCs group, F5M2 group and
MSCs+F5M2 group. MSCs and F5M2 cells were resuspended in PBS at a
final concentration of 1×107 cells/ml. MSCs were labeled
with 4 μg/ml chloromethyl-dialkylcarbocyanine (CM-Dil, Dil)
(Invitrogen) in pre-warmed PBS for 15 min at 37°C followed by an
incubation for 15 min at 4°C. Cells were washed with PBS and
resuspended in PBS before they were used. MSCs and/or F5M2 cells
were injected into the caudal vein of nude mice at a dose of
2×106 cells per mouse. After 6 weeks, mice were
sacrificed with excess pentobarbital. The number of pulmonary
matastatic tumor nodules was counted under a low-powered dissecting
stereomicroscope. The lung, liver, spleen, kidney and serum were
harvested for further analysis (11).
Co-culture and Transwell assays
In direct co-culture studies, MSCs were labeled with
Dil (4 μg/ml). Then F5M2 cells and MSCs (1×105
cells) were seeded and cultured in left and right area separately
and the cells had adhered after 12 h. Cells were grown in DMEM
supplemented with 1% FBS (Hyclone), 100 U/ml penicillin and 100
μg/ml streptomycin at 37°C, in a humidified atmosphere with
5% CO2 for 5 days. Then the F5M2 cells and MSCs labeled
with 5 μg/ml Hoechst (Sigma) in pre-warmed PBS for 10 min at
37°C. The cells were washed with PBS three times, then were fixed
in buffered isotonic formaldehyde (100 ml of 37% formaldehyde
solution, 900 ml distilled water, 4 g monobasic sodium phosphate
and 6.5 g dibasic sodium phosphate).
The invasive potential of the cells was measured in
6.5-mm transwells with an 8-μm pore polycarbonate membrane
insert (Corning, NY), according to the manufacturer’s instructions.
The top chamber filter was coated with 50 μl of diluted
Matrigel following the standard procedure and incubated at 37°C for
2 h. The lower chamber was filled with 600 μl of DMEM
containing 1% FBS as a chemoattractant. Cells were
serum-free-starved overnight and then harvested and resuspended in
migration medium (DMEM with 0.5% BSA). A suspension of 5,000 cells
in 100 μl migration medium was then added into each top
chamber. After incubation for 16 h, the non-invading cells that
remained on the upper surface were removed with a cotton swab. The
invasive cells on the lower surface of the membrane insert were
fixed with 4% paraformaldehyde for 30 min, permeabilized with 0.2%
Triton X-100 at room temperature for 15 min and then stained with
0.1% crystal violet for 5 min. The number of cells on the lower
surface, which had invaded through the membrane, was counted under
a light microscope in five random fields at a magnification of
×100. Data were obtained from three independent experiments. The
procedure for the Transwell migration assay was the same as for the
Transwell invasion assay, except that the filter of the top chamber
was not coated with Matrigel.
Co-cultured cells were also analyzed in 36-mm
transwells with 3-μm pore polycarbonate membrane inserts
(Corning, NY) according to the manufacturer’s instructions. The
upper and lower cultures were separated by a 3-μm pore size
polyvinylpyrolidone-free polycarbonate filter. Briefly, the lower
chambers were filled with 3 ml of DMEM containing 1% FBS and
5×106 cells/ml in 200 μl DMEM/1% FBS were added
to the upper or lower compartment. Chambers were incubated in a
humidified 37°C atmosphere with 5% CO2 for 48 h. Cells
were then lysed and the proteins were extracted. VEGF and CXCR4
protein expression was detected as detailed below. Supernatants
were collected for ELISA assay (3).
Each experiment was performed in triplicates.
For CXCR4 blocking, the CXCR4 inhibitor AMD3100
(Sigma-Aldrich), was incubated at a concentration of 10
μg/ml 1 h before and during the migration and co-culture
assays. SDF-1 neutralizing antibody (SDF-1 Ab, 0.6 μg/ml)
was used to specify neutralize SDF-1 (R&D Systems).
MTT assay
For assessment of cell proliferation, the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was performed according to the manufacturer’s instructions
(Sigma-Aldrich). The experiments were repeated three times
independently. Anti-human VEGF neutralizing antibody (VEGF Ab, 0.1
μg/ml) was used to specify neutralized VEGF (Abcam,
USA).
Immunohistochemistry
Cell slices were treated with 3% hydrogen peroxide
in methanol for 10 min, in order to inactivate endogenous
peroxidases, and were then incubated with primary antibodies
against VEGF or CXCR4 (Abcam) overnight at 4°C. After rinsing with
PBS, cells on the slices were treated for 20 min with pre-diluted
biotin-conjugated broad-spectrum IgG secondary antibody (Gene Tech,
China) and then visualized using streptavidin-conjugated
horseradish peroxidase provided with the Real Envision detection
kit (Gene Tech), following the manufacturer’s instructions. The
experiments were repeated three times independently.
ELISA assay
To determine the levels of VEGF secreted in the CM
from F5M2 cells or MSCs, cells were plated in medium containing 1%
FBS. After cells reached confluence, supernatants were collected
according to the manufacturer’s instructions. Media were analyzed
by a commercially available sandwich VEGF ELISA kit (Invitrogen),
according to the manufacturer’s instructions. Assays were performed
in quadruplicates and the results were normalized for the number of
producing cells and reported as picograms (pg) of VEGF per
1×106 cells per 48 h.
Western blotting
VEGF and CXCR4 protein expression was detected by
western blotting (13). As
mentioned above, cells were lysed and proteins were extracted.
Protein samples were subjected to sodium dodecyl sulfate
polyacrylamide gel eletrophoresis (SDS-PAGE) and transferred to
polyvinylidene difluoride membranes by a semi-dry transfer cell
system (Bio-Rad, Hercules, CA, USA). The membrane was probed with
mouse monoclonal antibodies against VEGF, CXCR4 and β-actin
(internal control) (Abcam).
Statistical analysis
Data were obtained from at least three independent
experiments and presented as means ± SD. Comparisons between two
groups were performed with the Student’s t-test, while statistical
significance of mean differences among multiple groups were
obtained by the analysis of variance (ANOVA) followed by Dunnett’s
post-hoc test. A P-value <0.05 was considered as statistically
significant.
Results
MSCs promote osteosarcoma pulmonary
metastasis
To study the osteosarcoma interaction with MSCs on
pulmonary metastasis, we monitored the development of osteosarcoma
pulmonary metastasis in response to MSCs. Six weeks after injection
of F5M2 cells, with or without MSCs, into the caudal vein, mice
were sacrificed and the total number of osteosarcoma pulmonary
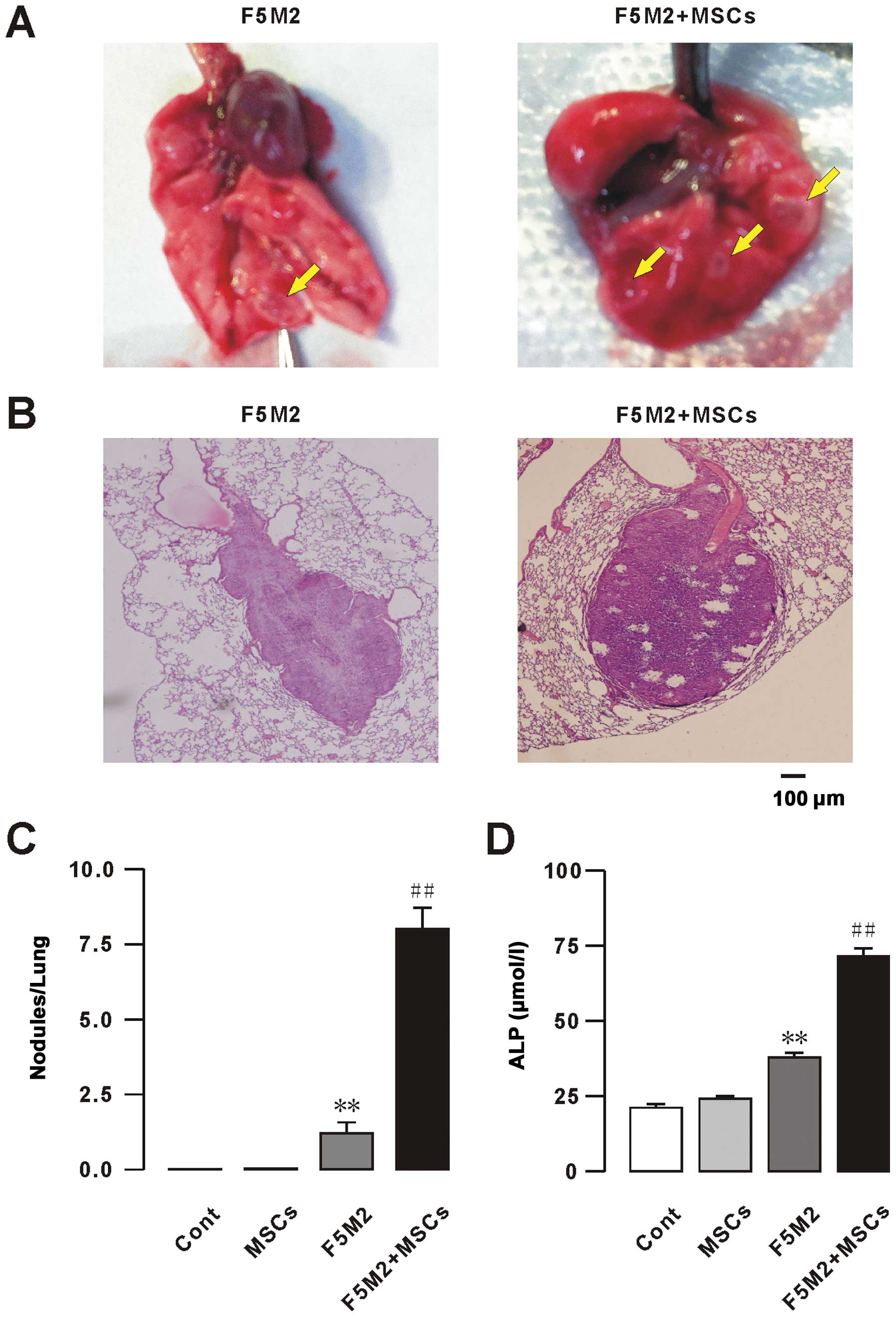
metastatic nodules per lung was calculated. A remarkably higher
number of tumor nodules was observed after co-injection of F5M2
cells with MSCs compared to F5M2 alone, as confirmed by
histological examination of the tumors (Fig. 1A–C). No metastases were found in the
liver, spleen or kidney (data not shown). Levels of alkaline
phosphatase (ALP) in blood serum were measured at week 6 to
determine the progression of osteosarcoma metastasis (2). A statistically significant increase of
ALP levels was observed in response to exogenous MSCs compared to
the osteosarcoma group (Fig. 1D).
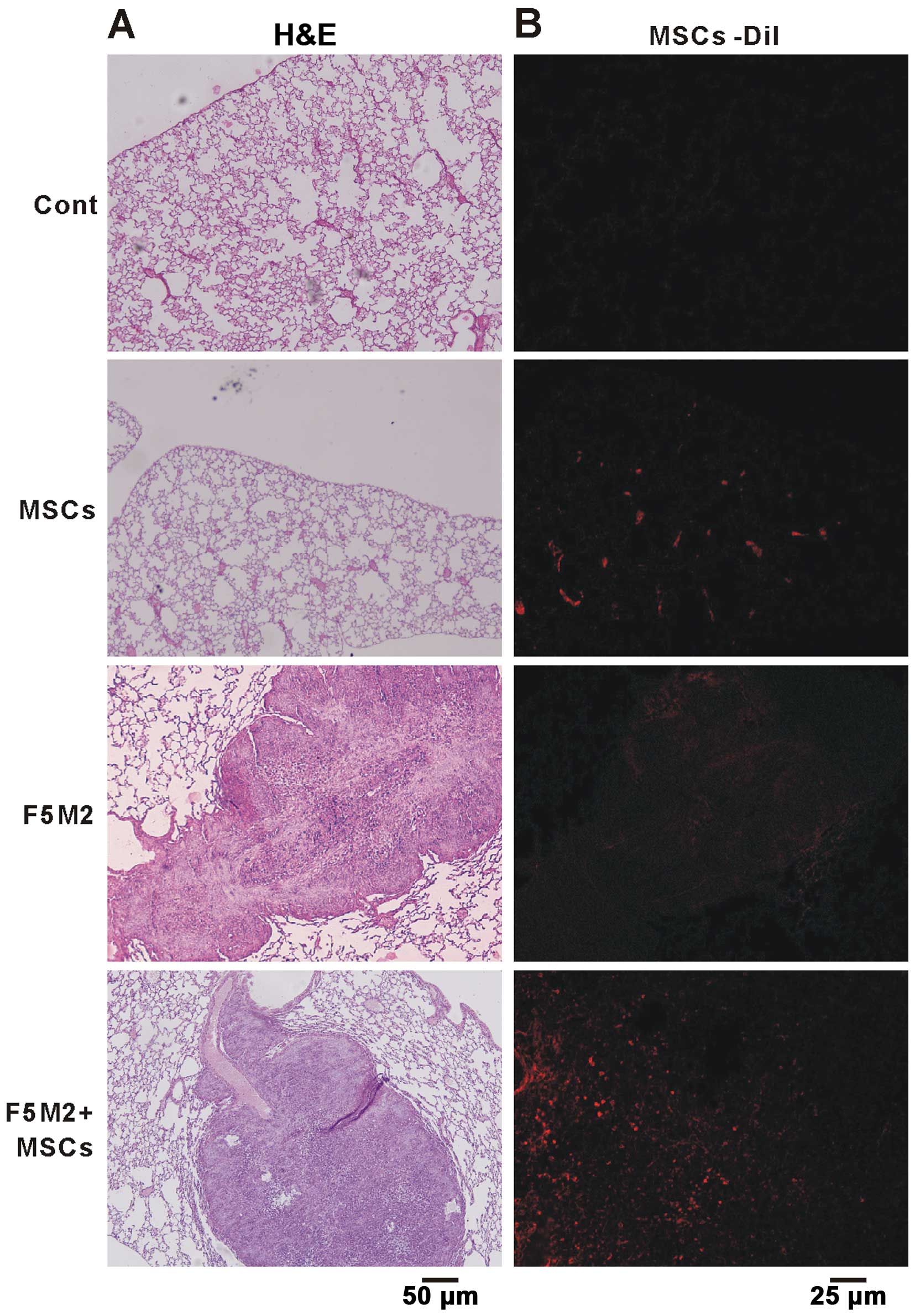
Six weeks after injection of Dil-labeled MSCs, we found a large
number of MSCs in the lung, which correlated with an enhanced
osteosarcoma pulmonary metastasis (Fig.
2). We postulate that F5M2 cells were induced to undergo
pulmonary metastasis by components secreted by MSCs or F5M2 cells,
which could chemoattract each other, leading to an intricate
interaction between them and ultimately to pulmonary
metastasis.
MSCs enhance F5M2 cell migration in a
co-culture model system
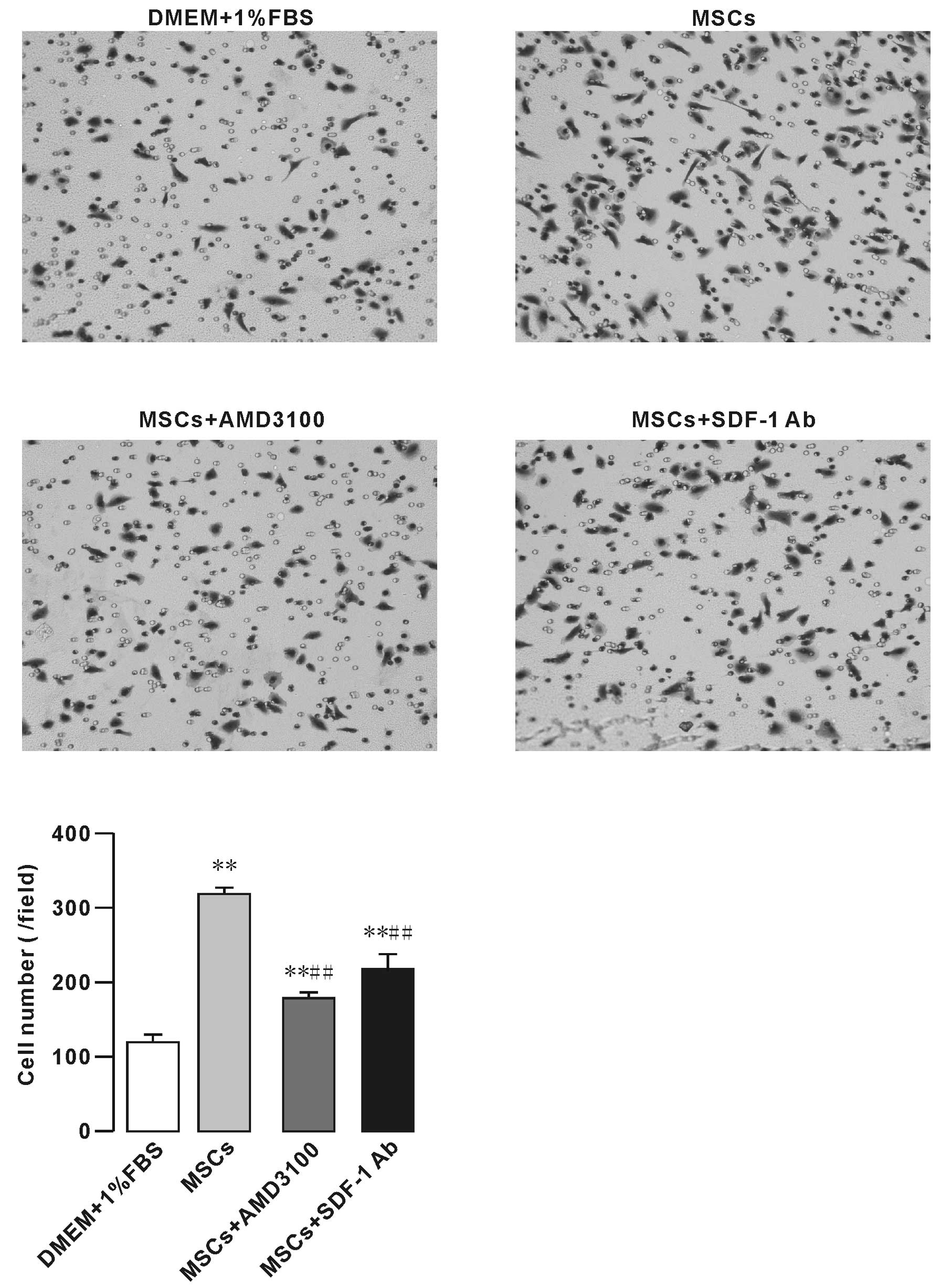
To test whether MSCs have an impact on osteosarcoma
cell migration, we initially performed migration and co-culture
assays in which MSCs were plated in the bottom chamber of a Corning
chamber system. Our results showed that the effect of MSCs on F5M2
cell migration was significantly higher than that of 1% FBS. In
addition, CXCR4 receptor inhibitor AMD3100 (10 μg/ml)
decreased the effect of MSCs on F5M2 cell migration (Fig. 3). SDF1-neutralizing antibody (SDF-1
Ab, 0.6 mg/ml) also significantly inhibited the F5M2 cell migration
(Fig. 3).
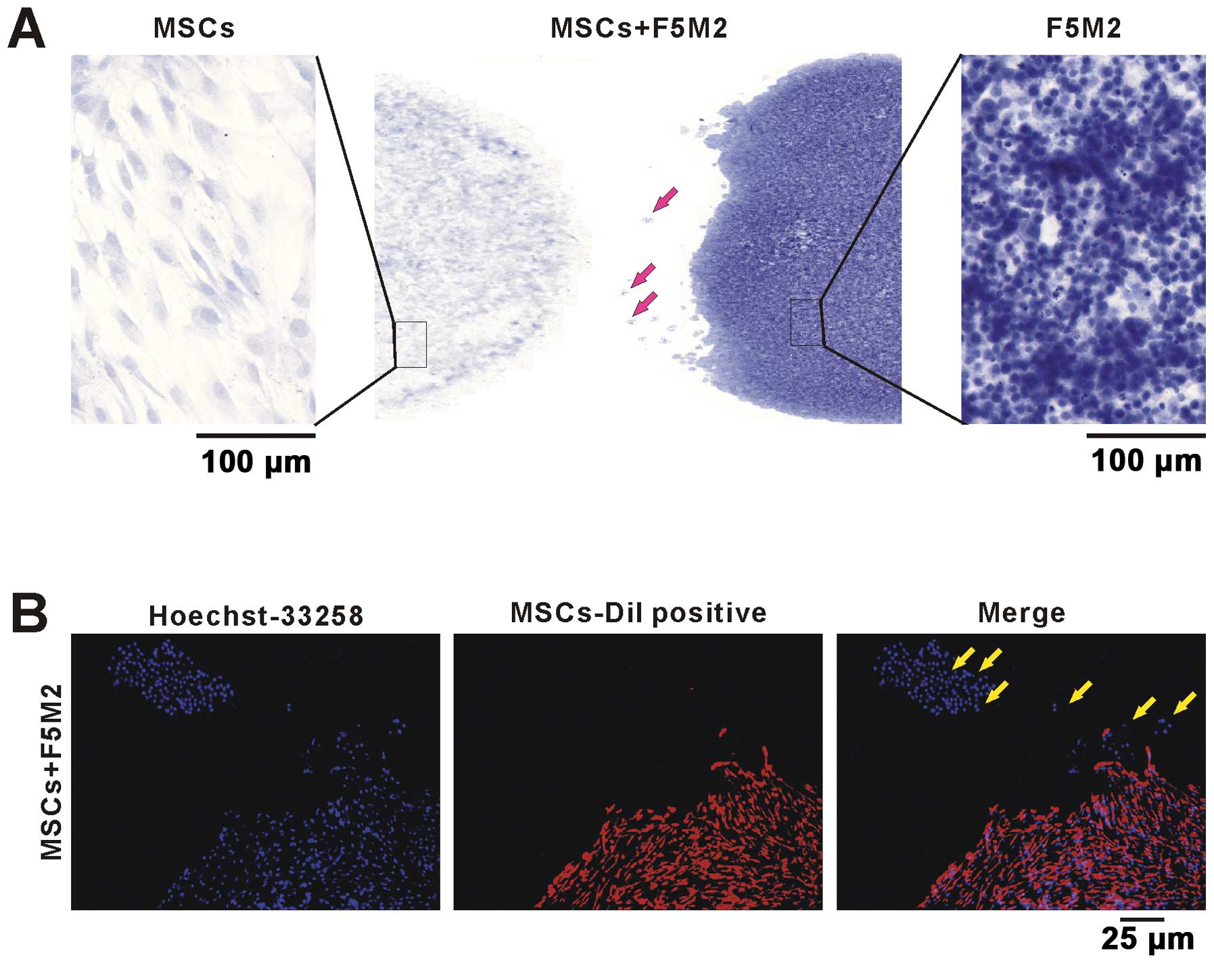
F5M2 and MSCs cells were co-cultured as showed in
Fig. 4A. In the left and right
panel of Fig. 4A, the MSCs or F5M2
cells showed different cell shape and nucleus size stained by
hematoxylin. In Fig. 4B,
Dil-labeled MSCs were co-cultured with F5M2 cells. The F5M2 single
cell or cell clusters migrated from the F5M2 cell cluster to the
MSCs (Fig. 4B). Both MSCs and F5M2
cell nucleus stained by Hoechst 33258 showed blue cell nucleus,
while the membrane of Dil-labeled MSCs were red. The bright arrow
indicates the migrating F5M2 cells from F5M2 cell cluster to MSC
cluster (Fig. 4A and B). These
results showed that F5M2 cells were chemoattracted by MSCs and
migrated towards them.
Enhanced VEGF expression by MSCs in the
F5M2-MSCs co-culture system
To study the possible mechanisms underlying the
interaction between F5M2 and MSCs, we focused our attention on the
CXCR4/VEGF signaling pathway, which is involved in the progression
of many cancers (14–17). We analyzed the expression of VEGF
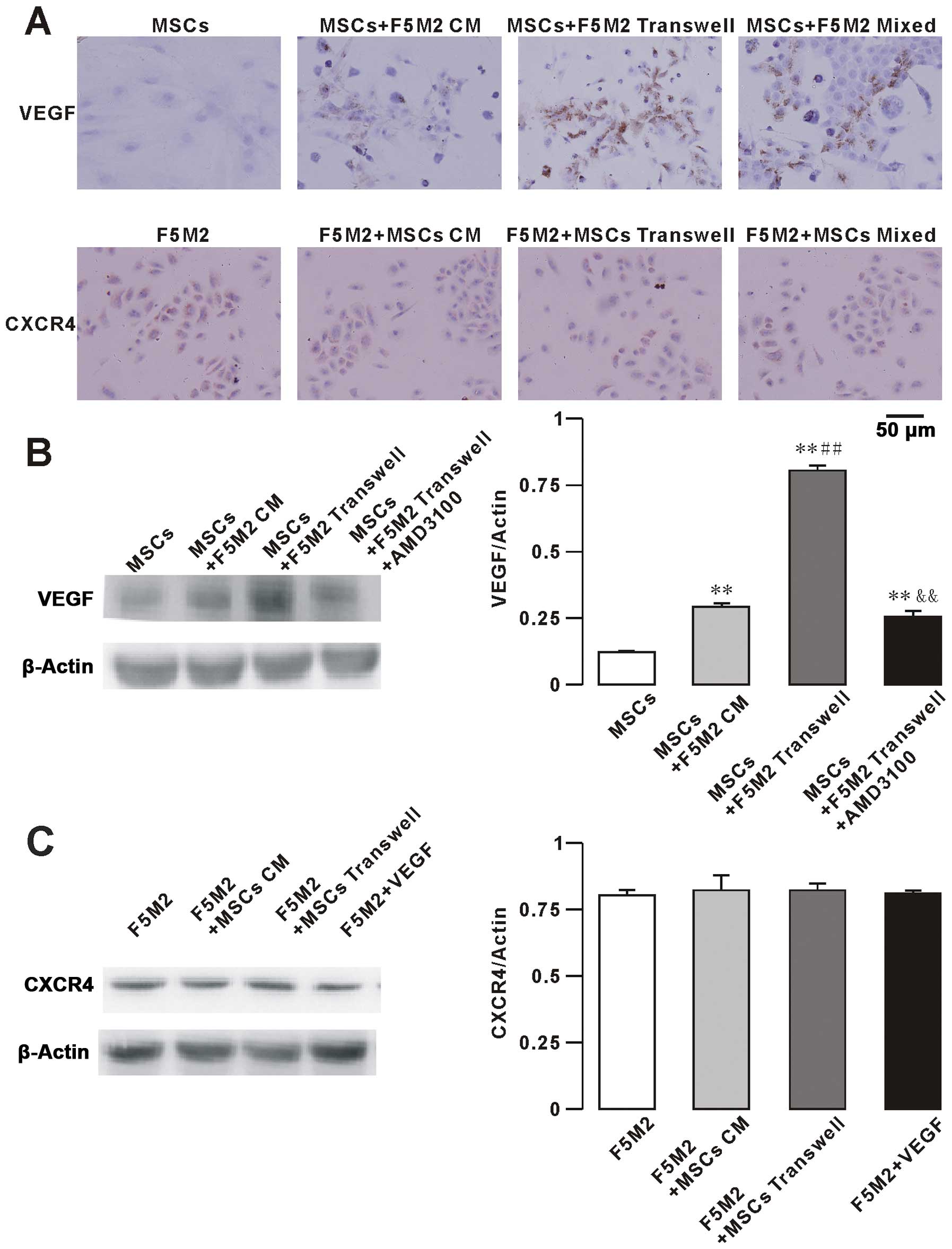
and CXCR4 in F5M2 cells and MSCs by immunohistochemistry (Fig. 5A) and western blotting (Fig. 5B and C). To assess the role of VEGF
and CXCR4 in the migration of osteosarcoma to MSC sites, we
performed Transwell assays using MSCs and CM from F5M2 cells in
combination with the CXCR4 receptor inhibitor AMD3100 (10
μg/ml). In the F5M2-MSC co-culture model system, positive
immunohistochemical stainings for VEGF and CXCR4 are showed in
Fig. 5A. VEGF protein was detected
in the cytoplasm and membrane of MSCs. We observed that the low
basal VEGF expression of MSCs increased when MSCs were co-cultured
with F5M2 CM, using either Transwell chambers or direct contact
with F5M2 cells for 48 h. We also found that the expression of VEGF
was alleviated by the use of the CXCR4 receptor inhibitor AMD3100
(10 μg/ml) in the MSCs plus F5M2 group. The VEGF expression
of F5M2 was not different in the three groups (F5M2, Transwell and
direct co-culture groups, data not shown).
CXCR4 expression was detected in the nucleus as well
as in the cytoplasm of F5M2 cells. CXCR4 expression of F5M2 cells
was not significantly increased in the F5M2+MSCs group compared to
the F5M2 group and in the presence of exogenous VEGF (100 ng/ml).
In addition, the CXCR4 expression of MSCs was not different in the
three groups (MSCs, Transwell and direct co-culture groups, data
not shown).
VEGF secretion from MSCs is modulated by
F5M2
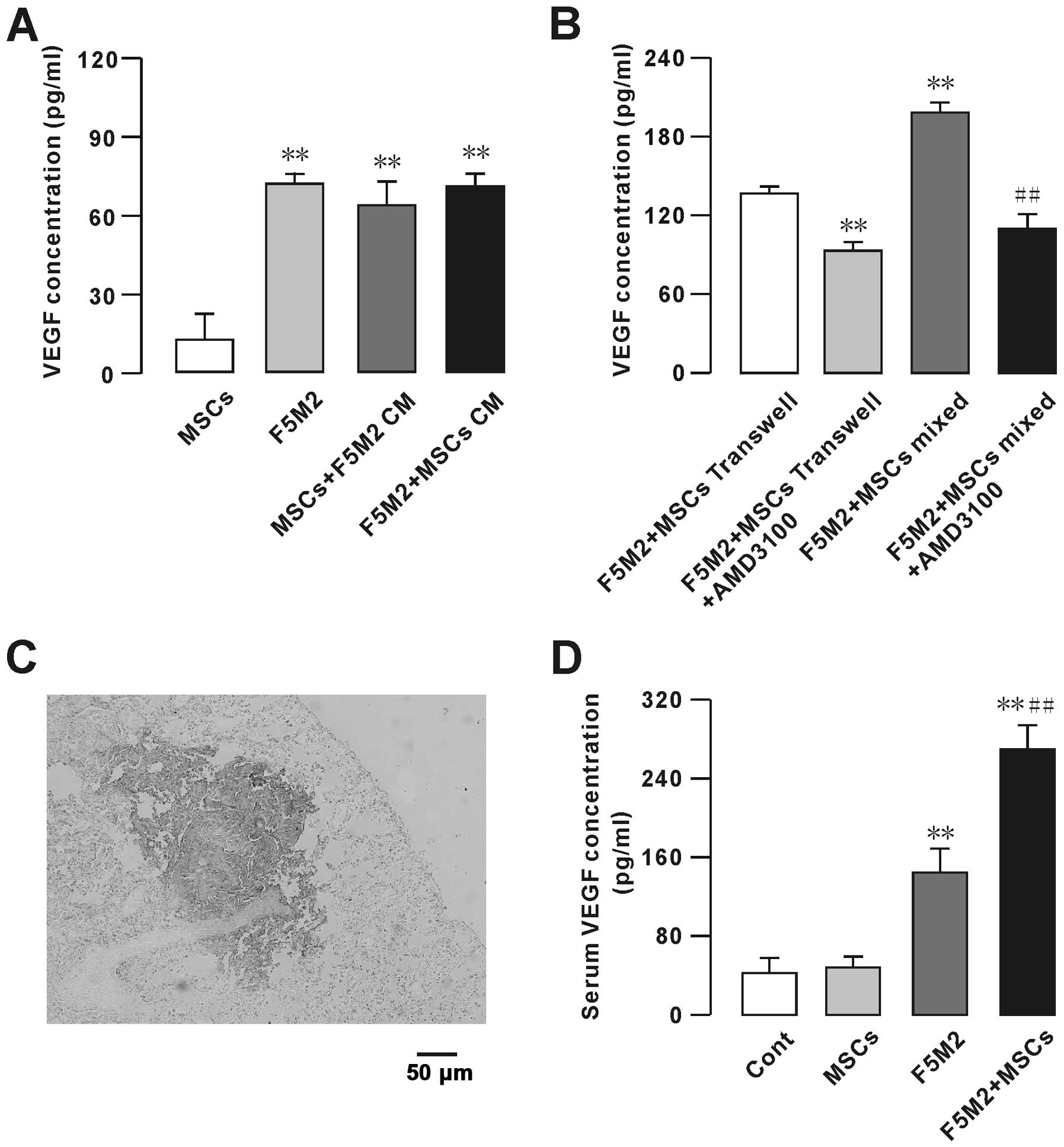
We measured the levels of VEGF secreted by MSCs and
F5M2 by ELISA (Fig. 6A and B) in
the following groups: F5M2 cells (1.0×106 cells) alone,
MSCs (1.0×106 cells) alone, co-culture of F5M2 cells and
MSCs (0.5×106 cells each) and F5M2 cells treated with
the CXCR4 receptor inhibitor AMD3100. MSCs secreted very low levels
of VEGF (Fig. 6A). We subtracted
from the amount of VEGF, in the final supernatant of MSCs cultured
in the conditioned medium. However, MSCs co-cultured with F5M2
secreted higher levels of VEGF than the F5M2 or MSC groups
(Fig. 6A). On the other hand, the
F5M2 plus MSC Transwell, or mixed group, secreted higher VEGF
levels, which were dramatically decreased upon treatment with
AMD3100 (10 μg/ml) (Fig.
6B).
We further studied the VEGF expression on the
metastatic tumor in vivo, positive immunohistochemical
stainings for VEGF was found in the plumonary metastasis sites of
the nude mice. We found VEGF higher expressed in the tumor site as
showed in Fig. 6C, and the
VEGF-positive staining was not statistically different between the
F5M2 and F5M2+MSCs groups. We also checked the serum VEGF
concentration in the control, MSCs, F5M2 and F5M2+MSCs groups, and
found the VEGF concentration was higher in the F5M2 and F5M2+MSCs
groups compared with the control group, especially in the F5M2+MSCs
group the VEGF concentration was significantly higher than the F5M2
group as showed in Fig. 6D.
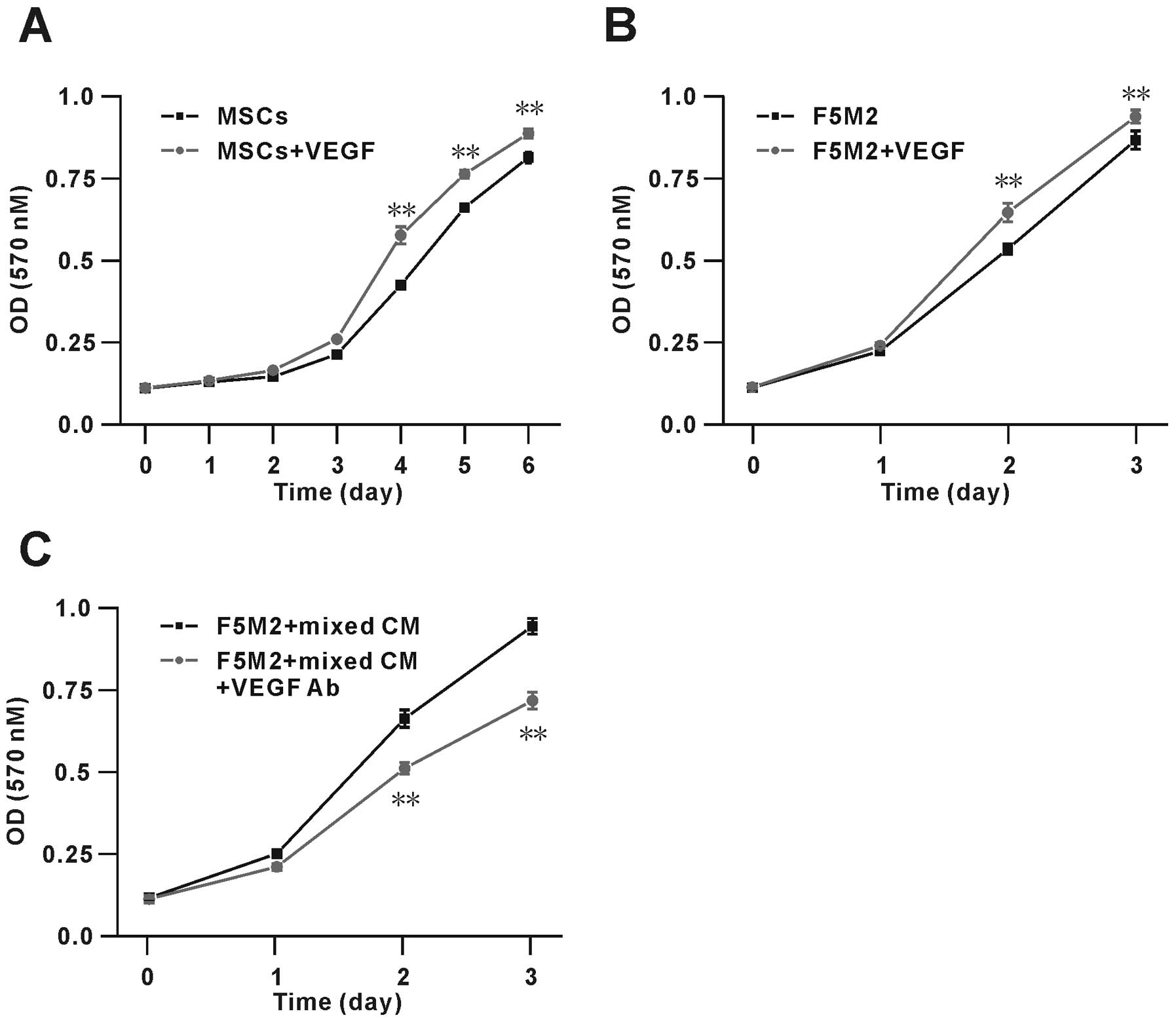
VEGF promotes proliferation of F5M2 cells
and MSCs
To assess the possible role of VEGF on F5M2 and MSC
growth, we performed MTT assays on cells stimulated with exogenous
VEGF (100 ng/ml). The results showed that VEGF significantly
increased the proliferation of F5M2 cells and MSCs (Fig. 7A and B). After 3 days, the number of
MSCs in the presence of VEGF was ~20% higher than that in its
absence. Similarly, after 2 days, the number of F5M2 cells in the
presence of VEGF was ~10% higher than that in its absence. These
data show that VEGF modulates the proliferation of both F5M2 and
MSCs. Furthermore, we found the proliferation of F5M2 cells were
actually promoted in the co-culture condition, moreover we also
used the VEGF neutralizing antibody (VEGF Ab, 0.1 μg/ml) to
verify the VEGF effect on the OS cell proliferation ability, and
found the VEGF Ab could significantly inhibit the F5M2 cell
proliferation by ~30% as showed in Fig.
7C.
Discussion
Osteosarcoma is the most frequent primary bone tumor
in young adults and adolescents. Pulmonary metastasis occurs in
~40–50% of the osteosarcoma patients (18), who present an overall 5-year
survival rate of only 28% (14).
CXCR4 and its ligand, stromal cell-derived factor 1 (SDF-1), has
been found highly expressed in various cancer and play important
roles in tumorigenicity, proliferation, metastasis and angiogenesis
(19–24). It has been shown that CXCR4 also
plays an important role in the migration of hematopoietic stem
cells and downstream progenitors from, and their retention within,
the bone marrow. On the other hand, CXCR4 inhibitors may be used to
inhibit tumor growth and metastasis and also to mobilize
hematopoietic stem cells from the bone marrow into circulation
(7).
However, the underlying mechanisms under which MSCs
target osteosarcoma sites and promote osteosarcoma cell growth and
pulmonary metastasis are still unclear. It has been demonstrated
that MSCs within tumor stroma promote metastasis (8). In our study, we found that MSCs were
involved in the emergence and growth of osteosarcoma pulmonary
metastasis (Fig. 2). An enhanced
tumor cell number was observed in response to the injection of MSCs
and F5M2 compared to F5M2 alone. In our experiments, F5M2 single
cell or F5M2 cell clusters migrated from the F5M2 cell cluster to
the MSCs cluster (Fig. 4A and B).
Tumor-targeting properties of stem cells have been previously
reported (25) and also in studies
of tumor angiogenesis with hematopoietic stem cells (26). Xu et al showed the human
mesenchymal stem cells (hMSCs) target osteosarcoma and promote its
growth and pulmonary metastasis (27). The study of Xu et al(27) indicated SDF-1 and CCL5 played
essential roles in the interaction between hMSCs and OS. Our data
showed that MSCs promote osteosarcoma pulmonary metastasis. The
mechanisms involved in VEGF from MSCs could promote the OS growth.
In addition, CXCR4 plays an important role in regulating the VEGF
of MSCs. A remarkable accumulation of MSCs in pulmonary metastatic
sites was observed after injection with F5M2 cells through the
caudal vein of mice. Furthermore, we also provide evidence that the
migration of F5M2 was promoted in response to MSCs in the
co-culture model system, which was decreased upon treatment with
AMD3100 (Fig. 3).
The expression of CXCR4 has been shown to be
regulated by VEGF (28). VEGF is
also a known inducer of osteosarcoma angiogenesis which could
determine the vascular endothelial cell activation, proliferation
and migration (29). VEGF induced
the expression of CXCR4 via an autocrine pathway (13). The study also showed that CXCR4
inhibition could reduce angiogenesis by inhibiting osteosarcoma
neovessel formation as well as cell migration and invasion by
decreasing the expression of conventional proangiogenic VEGFA165
(30,31). Patients whose tumors exhibited a
positive VEGF165 expression presented poor prognosis (11). The study also found a significant
correlation between the expression of CXCR4 and VEGF in
osteosarcoma samples (32), which
suggested that the CXCR4/VEGF could be a predictor of potential
metastatic development in osteosarcoma (10). Our data revealed that MSCs secrete
very low levels of VEGF, but with F5M2 conditioned medium the MSCs
secreted higher levels of VEGF (Fig.
6A). On the other hand, F5M2 plus MSCs Transwell or mixed group
secreted higher VEGF levels than the individual cell groups, which
were dramatically decreased upon treatment with the CXCR4 inhibitor
AMD3100 (10 μg/ml) (Fig.
6B). These results showed that CXCR4 modulates the production
of VEGF in MSCs. Co-cultured with F5M2 or treated with
F5M2-conditioned medium, MSCs expressed and secreted higher VEGF
levels. CXCR4 expression on F5M2 cells was, however, not
significantly increased in the co-culture model (Fig. 5B).
CXCR plays a central role in determining the target
organ where tumor cells metastasize (1,33), as
well as the blood supply via angiogenesis (30,34).
CXCLs released from the endothelium of target tissues (i.e., lung
and bone) bind to their receptors, CXCRs on tumor cells, which
enables the metastasis to the specific target tissues. Studies have
indicated that CXCR4 plays a role in regulating metastasis of many
solid tumors and their neovasculature (10,35–38).
Moreover, there is a correlation between the expression of
CXCR4/VEGF and blood-borne metastasis (35). Our data show that MSCs secreted
higher VEGF levels when co-cultured with F5M2 cells or transplanted
with F5M2 cells in vivo (Fig.
6). In vitro study we also found VEGF could increase the
growth of both F5M2 cells and MSCs.
Taken together, our data showed that MSCs interacted
with osteosarcoma cells and promoted osteosarcoma pulmonary
metastasis, in which the expression of CXCR4 by osteosarcoma cells
modulated the expression and secretion of VEGF by MSCs. It
suggested that CXCR4 mediated osteosarcoma growth and pulmonary
metastasis were promoted by MSCs through VEGF.
Acknowledgements
This study was supported by grants from the National
Nature Science Foundation of China (no. 30800432 to L.D. and no.
81072194 to B.-A.M.). We would like to thank Dong Shuhua, the
English editor, from Guangdong University of Foreign Studies.
Abbreviations:
|
CM
|
conditioned medium
|
|
CM-Dil
|
Dil
chloro-methyl-dialkylcarbocyanine
|
|
CXCR
|
chemokines and chemokine receptors
|
|
MSCs
|
mesenchymal stem cells
|
|
VEGF
|
vascular endothelial growth factor
|
|
VEGF Ab
|
anti-VEGF antibody
|
|
SDF-1
|
stromal cell-derived factor 1
|
|
SDF-1 Ab
|
human SDF-1 antibody
|
References
|
1
|
Murphey MD, Robbin MR, McRae GA, Flemming
DJ, Temple HT and Kransdorf MJ: The many faces of osteosarcoma.
Radiographics. 17:1205–1231. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Link MP, Goorin AM, Miser AW, et al: The
effect of adjuvant chemotherapy on relapse-free survival in
patients with osteosarcoma of the extremity. N Engl J Med.
314:1600–1606. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen X, Yang TT, Wang W, et al:
Establishment and characterization of human osteosarcoma cell lines
with different pulmonary metastatic potentials. Cytotechnology.
61:37–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Friedenstein AJ, Chailakhyan RK, Latsinik
NV, Panasyuk AF and Keiliss-Borok IV: Stromal cells responsible for
transferring the microenvironment of the hemopoietic tissues.
Cloning in vitro and retransplantation in vivo. Transplantation.
17:331–340. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Austin TW, Solar GP, Ziegler FC, Liem L
and Matthews W: A role for the Wnt gene family in hematopoiesis:
expansion of multilineage progenitor cells. Blood. 89:3624–3635.
1997.PubMed/NCBI
|
|
6
|
Dexter TM, Spooncer E, Schofield R, Lord
BI and Simmons P: Haemopoietic stem cells and the problem of
self-renewal. Blood Cells. 10:315–339. 1984.PubMed/NCBI
|
|
7
|
Reya T, Duncan AW, Ailles L, et al: A role
for Wnt signalling in self-renewal of haematopoietic stem cells.
Nature. 423:409–414. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karnoub AE, Dash AB, Vo AP, et al:
Mesenchymal stem cells within tumour stroma promote breast cancer
metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oda Y, Yamamoto H, Tamiya S, et al: CXCR4
and VEGF expression in the primary site and the metastatic site of
human osteosarcoma: analysis within a group of patients, all of
whom developed lung metastasis. Mod Pathol. 19:738–745. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li D, Dai K and Tang T: Effects of dextran
on proliferation and osteogenic differentiation of human bone
marrow-derived mesenchymal stromal cells. Cytotherapy. 10:587–596.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pittenger MF, Mackay AM, Beck SC, et al:
Multilineage potential of adult human mesenchymal stem cells.
Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang Z, Wu T, Lou H, et al: Inhibition of
breast cancer metastasis by selective synthetic polypeptide against
CXCR4. Cancer Res. 64:4302–4308. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kempf-Bielack B, Bielack SS, Jurgens H, et
al: Osteosarcoma relapse after combined modality therapy: an
analysis of unselected patients in the Cooperative Osteosarcoma
Study Group (COSS). J Clin Oncol. 23:559–568. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim J, Takeuchi H, Lam ST, et al:
Chemokine receptor CXCR4 expression in colorectal cancer patients
increases the risk for recurrence and for poor survival. J Clin
Oncol. 23:2744–2753. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Retz MM, Sidhu SS, Blaveri E, et al: CXCR4
expression reflects tumor progression and regulates motility of
bladder cancer cells. Int J Cancer. 114:182–189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Russell HV, Hicks J, Okcu MF and Nuchtern
JG: CXCR4 expression in neuroblastoma primary tumors is associated
with clinical presentation of bone and bone marrow metastases. J
Pediatr Surg. 39:1506–1511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wada T, Isu K, Takeda N, Usui M, Ishii S
and Yamawaki S: A preliminary report of neoadjuvant chemotherapy
NSH-7 study in osteosarcoma: preoperative salvage chemotherapy
based on clinical tumor response and the use of granulocyte
colony-stimulating factor. Oncology. 53:221–227. 1996. View Article : Google Scholar
|
|
19
|
Beckermann BM, Kallifatidis G, Groth A, et
al: VEGF expression by mesenchymal stem cells contributes to
angiogenesis in pancreatic carcinoma. Br J Cancer. 99:622–631.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Djouad F, Plence P, Bony C, et al:
Immunosuppressive effect of mesenchymal stem cells favors tumor
growth in allogeneic animals. Blood. 102:3837–3844. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gunn WG, Conley A, Deininger L, Olson SD,
Prockop DJ and Gregory CA: A crosstalk between myeloma cells and
marrow stromal cells stimulates production of DKK1 and
interleukin-6: a potential role in the development of lytic bone
disease and tumor progression in multiple myeloma. Stem Cells.
24:986–991. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Otsu K, Das S, Houser SD, Quadri SK,
Bhattacharya S and Bhattacharya J: Concentration-dependent
inhibition of angiogenesis by mesenchymal stem cells. Blood.
113:4197–4205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Potier E, Ferreira E, Andriamanalijaona R,
et al: Hypoxia affects mesenchymal stromal cell osteogenic
differentiation and angiogenic factor expression. Bone.
40:1078–1087. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Romieu-Mourez R, Francois M, Boivin MN,
Bouchentouf M, Spaner DE and Galipeau J: Cytokine modulation of TLR
expression and activation in mesenchymal stromal cells leads to a
proinflammatory phenotype. J Immunol. 182:7963–7973. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aboody KS, Brown A, Rainov NG, et al:
Neural stem cells display extensive tropism for pathology in adult
brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA.
97:12846–12851. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Palma M, Venneri MA, Roca C and Naldini
L: Targeting exogenous genes to tumor angiogenesis by
transplantation of genetically modified hematopoietic stem cells.
Nat Med. 9:789–795. 2003.PubMed/NCBI
|
|
27
|
Xu WT, Bian ZY, Fan QM, Li G and Tang TT:
Human mesenchymal stem cells (hMSCs) target osteosarcoma and
promote its growth and pulmonary metastasis. Cancer Lett.
281:32–41. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bachelder RE, Wendt MA and Mercurio AM:
Vascular endothelial growth factor promotes breast carcinoma
invasion in an autocrine manner by regulating the chemokine
receptor CXCR4. Cancer Res. 62:7203–7206. 2002.
|
|
29
|
Plate K: From angiogenesis to
lymphangiogenesis. Nat Med. 7:151–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Nigris F, Balestrieri ML,
Williams-Ignarro S, et al: Therapeutic effects of autologous bone
marrow cells and metabolic intervention in the ischemic hindlimb of
spontaneously hypertensive rats involve reduced cell senescence and
CXCR4/Akt/eNOS pathways. J Cardiovasc Pharmacol. 50:424–433.
2007.PubMed/NCBI
|
|
31
|
de Nigris F, Crudele V, Giovane A, et al:
CXCR4/YY1 inhibition impairs VEGF network and angiogenesis during
malignancy. Proc Natl Acad Sci USA. 107:14484–14489.
2010.PubMed/NCBI
|
|
32
|
Lin F, Zheng SE, Shen Z, et al:
Relationships between levels of CXCR4 and VEGF and blood-borne
metastasis and survival in patients with osteosarcoma. Med Oncol.
28:649–653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Balkwill F: The significance of cancer
cell expression of the chemokine receptor CXCR4. Semin Cancer Biol.
14:171–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Busillo JM and Benovic JL: Regulation of
CXCR4 signaling. Biochim Biophys Acta. 1768:952–963. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Laverdiere C, Hoang BH, Yang R, et al:
Messenger RNA expression levels of CXCR4 correlate with metastatic
behavior and outcome in patients with osteosarcoma. Clin Cancer
Res. 11:2561–2567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee YH, Tokunaga T, Oshika Y, et al:
Cell-retained isoforms of vascular endothelial growth factor (VEGF)
are correlated with poor prognosis in osteosarcoma. Eur J Cancer.
35:1089–1093. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Murakami T, Maki W, Cardones AR, et al:
Expression of CXC chemokine receptor-4 enhances the pulmonary
metastatic potential of murine B16 melanoma cells. Cancer Res.
62:7328–7334. 2002.PubMed/NCBI
|
|
38
|
Perissinotto E, Cavalloni G, Leone F, et
al: Involvement of chemokine receptor 4/stromal cell-derived factor
1 system during osteosarcoma tumor progression. Clin Cancer Res.
11:490–497. 2005.PubMed/NCBI
|