Introduction
Fluorescent and luminescent tools are commonly used
to study the dynamics of cancer progression and metastases in
real-time. They are widely used to determine the spatiotemporal
dynamics of intracellular molecules, organelles, and whole cells as
they can provide high resolution images with great sensitivity and
without causing significant cytotoxicity (1). Fluorescence-labeled cells, in
particular, can be visualized using fluorescence microscopy, flow
cytometry, and whole-body imaging techniques (2,3).
Fluorescent probes have therefore become an essential tool to study
biological events in living cells, tissues and animals (4,5).
An important example of the utility of these
techniques is the generation of genetically engineered cell lines
that stably express natural fluorescent proteins such as GFP
(6,7) introduced using retroviral vectors.
A series of new amphiphilic fluorophores known as
POLARIC™ has recently been reported (8,9). This
series of fluorophores is now commercially available. These
fluorophores provide advantages over other fluorescent dyes, such
as strong fluorescence in lipid bilayers, and possess molecular
structures that are readily modified using the Suzuki-Miyaura
cross-coupling reaction. Thus, appropriate chemical modifications
of substituent groups can improve target-site specificity, reduce
cytotoxicity, and prolong emission. These fluorophores, therefore,
show promise for monitoring long-term biological processes for
prolonged periods of time compared with commonly used fluorescent
probes such as PKH26 (10,11). In the present study, we described
long-term observations of tumor growth and metastasis using a
POLARIC derivative.
Materials and methods
Cell line and culture conditions
The super-metastatic human malignant melanoma cell
line, A375-SM, was kindly provided by Prof. I.J. Fidler (M.D.
Anderson Cancer Center, Houston, TX, USA). The cells were cultured
in Minimum Essential Medium (Gibco, Grand Island, NY, USA)
supplemented with 10% FBS at 37°C in a humidified atmosphere
containing 5% CO2 and 95% air.
Fluorescence staining techniques and
plasmid transfection
Cells were labelled using POLARIC (POLARIC-500c6F;
Goryo Chemical, Inc., Sapporo, Japan) and a PKH26 Red Fluorescent
Cell Linker kit (Sigma-Aldrich, St. Louis, MO, USA) according to
the manufacturer’s instructions. The expression vectors for
CS-RfA-CMV-m red fluorescent protein 1 (mRFP1) were obtained from
Dr Roger Tsien (UCSD, CA, USA). The packaging vector pCAG-HIVgp and
the VSV-G- and REV-expressing construct pCMV-VSV-G-RSV-REV were
obtained from Dr H. Miyoshi (RIKEN, Tsukuba, Japan). For
transfection, expression plasmids were incubated with FuGene HD
(Roche, Basel, Switzerland) according to the manufacturer’s
recommendations. Lentivirus-mediated gene transfer was performed as
previously described (12).
Microscopy
Images were acquired using an FV-10i confocal
microscope (Olympus, Tokyo, Japan). The 572-nm (PKH) and 520-nm
(for POLARIC) emission filter sets were used. To assess bleaching
rate, each stained cell was exposed to a 473-nm laser beam at a
power setting of 100% output (11.9 mW). Fluorescent intensity was
calculated using ImageJ Software (NIH, Bethesda, MD, USA).
Flow cytometry
To assess fluorescence intensity, the cells were
trypsinized at 1, 2 and 3 weeks after staining. To evaluate
fluorescence intensity of primary tumors or to detect lung
metastases, excised tissues were minced and digested with
collagenase II (Gibco). Red blood cells were removed by lysing them
with Lysis Buffer (BD Biosciences, Franklin Lakes, NJ, USA).
Fluorescence intensity was measured using a FACS Aria II (BD
Biosciences) with emission at 488 nm as well as 572-nm or 650-nm
filter sets. Dead cells were distinguished from viable cells using
DAPI (100 ng/ml).
Animal model
The local animal research authorities approved all
procedures for animal experimentation. The protocol for animal care
was in accordance with institutional guidelines. Six-week-old
female nude mice (BALB/c Slc-nu/nu) were obtained from Sankyo Labo
Service Corporation (Tokyo, Japan). The A375-SM human melanoma
xenograft model was generated by subcutaneous injection of
1×106 fluorophore-labeled A375-SM cells.
In vivo fluorescence imaging
In vivo fluorescence imaging was performed
using an IVIS Spectrum (Caliper Life Science, Hopkinton, MA, USA)
on the indicated days. Mice were anesthetized with 2% isoflurane
and placed onto the warmed stage inside of the IVIS light-tight
chamber and anesthesia was maintained with 1.5% isoflurane. Mice
were imaged in ventral positions. Fluorescence signals were
acquired using the respective band pass excitation and emission
filter sets as follows: POLARIC, 500 nm (30-nm bandwidth) and 620
nm (20-nm bandwidth); PKH, 535 and 580 nm; and mRFP1, 570 and 620
nm. Photon acquisition time was 1 sec, small binning, and F/Stop 4.
After the mice were sacrificed, primary tumors and lungs were
surgically resected. To examine spontaneous lung metastasis, lungs
were subjected to ex vivo fluorescent imaging. Fluorescence
signals were quantified using Living Image 3.0 (Caliper Life
Science). Signal intensity data are expressed as the total
photons/s within a uniform region of interest positioned over
specific tumor sites.
Statistical analysis
Differences among experimental groups were evaluated
using the Mann-Whitney U test and P<0.01 was considered to
indicate a statistically significant difference.
Results
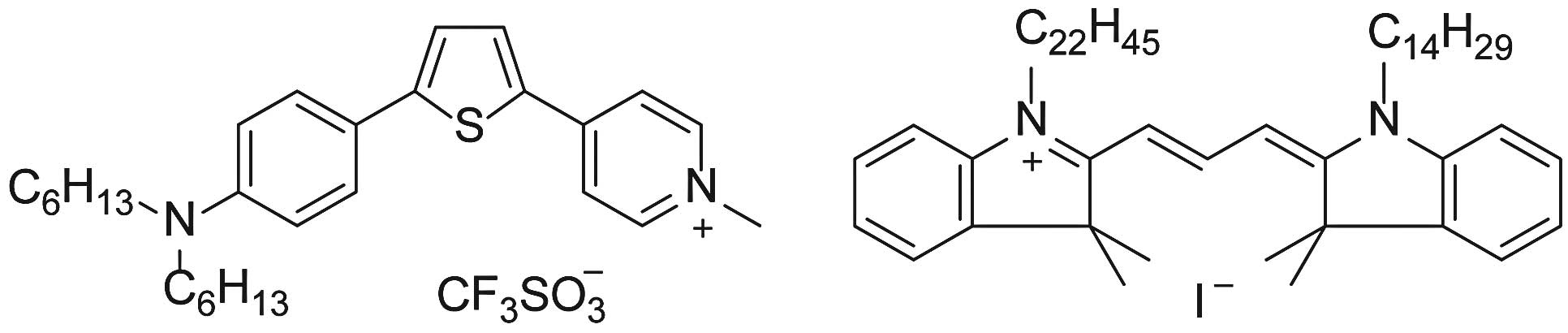
Selection of a POLARIC derivative
suitable for studying tumor cells
The central aromatic moiety of POLARIC derivatives
absorbs and emits light. The terminal alkyl chains have essentially
no effect on optical properties such as absorption and emission
wavelengths. However, the alkyl chains strongly influence the
fluorescence intensities in the lipid membrane due to the
dispersion of dye (13). We first
stained tumor cells using POLARIC derivatives with different alkyl
chains and used the brightest derivative, which had two hexyl
groups (Fig. 1). Since the
dispersed fluorophore suppresses the fluorescence quenching and
decomposition reactions, the dihexyl derivative is expected to be
suitable for long-term monitoring. We compared the characteristics
of the POLARIC probe with those of PKH26 (10,11),
which is commercially available from Sigma-Aldrich, and mRFP1,
which was introduced using a lentivirus vector as described
above.
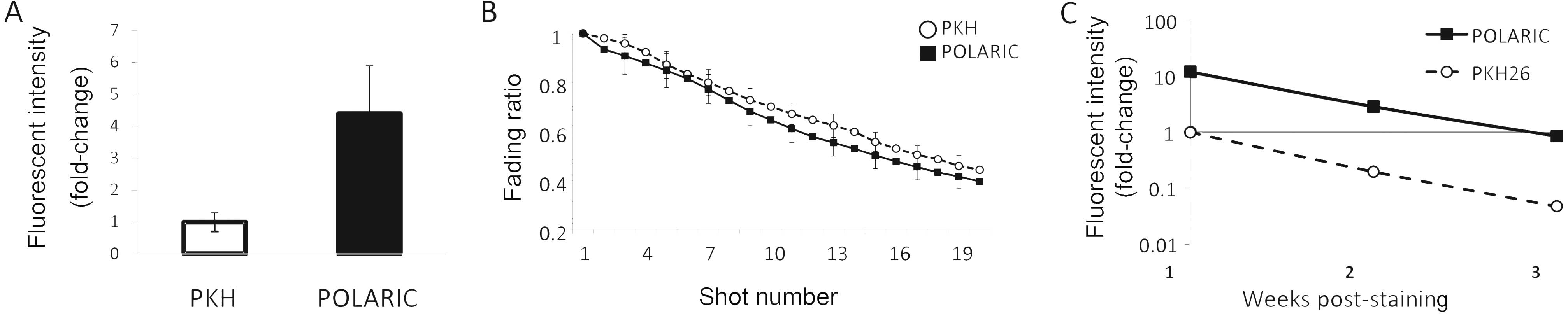
In vitro characterization of POLARIC and
PKH probes
We found that the fluorescence intensity of cells
labeled for 12 h with POLARIC was higher than that of cells labeled
with PKH after staining for 12 h (Fig.
2A). Next, the stained cells were exposed to a 473-nm laser
beam and it was found that the fluorescence decay depended on the
frequency of the number of pulses and that the fluorescent decay
rate of POLARIC was almost equal to that of PKH (Fig. 2B). We next quantified the
fluorescence intensity for each dye every two days in cultures
grown to sub-confluence for three weeks (Fig. 2C).
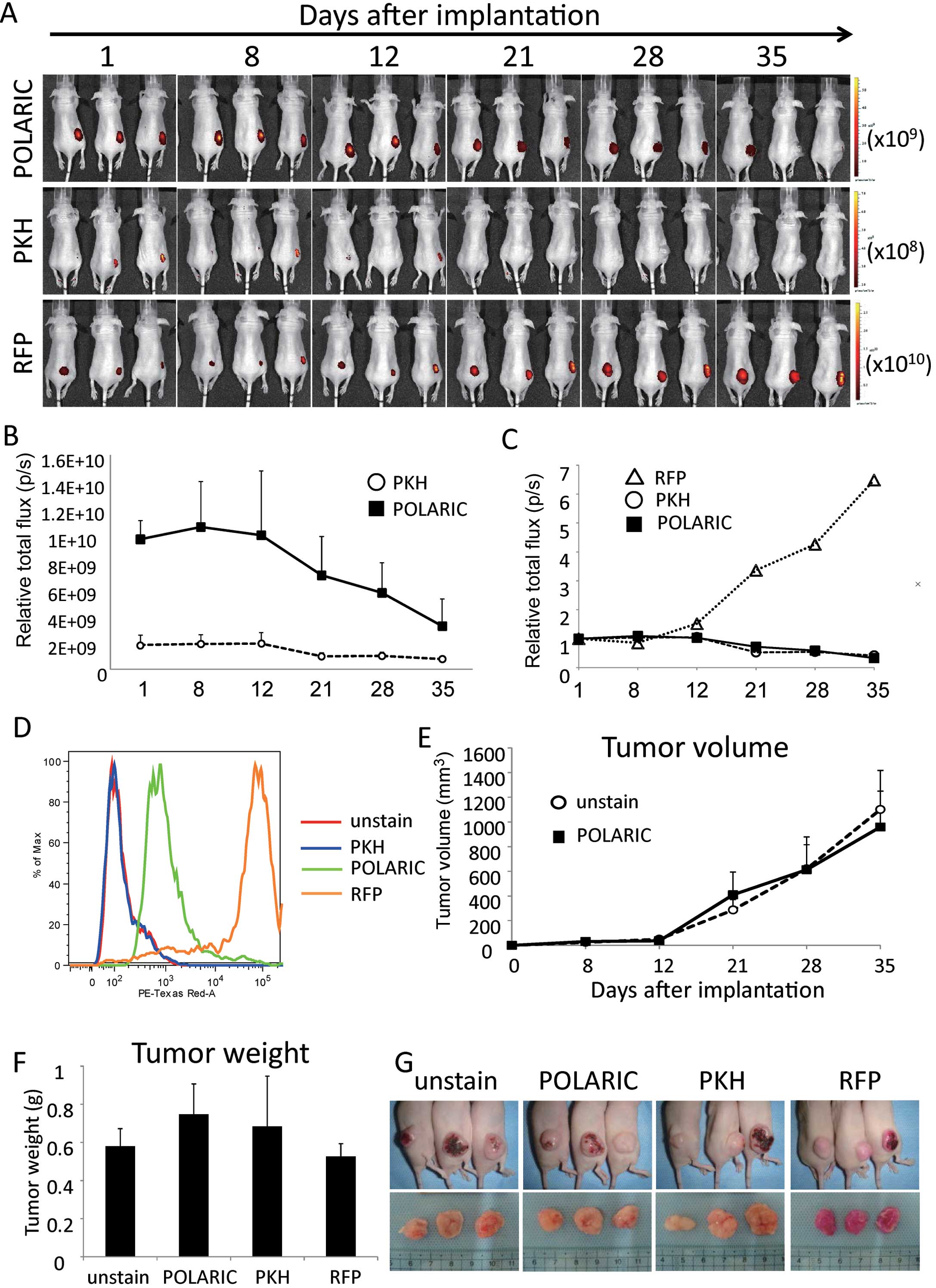
Whole-body imaging of tumors
A375-SM tumor cells labeled with POLARIC or PKH were
engrafted subcutaneously into nude mice. As a positive control,
cells transfected with RFP inserted into rentivirus were used.
Primary tumor lesions labeled with each dye were externally imaged
using an IVIS Spectrum on the indicated days after implantation
(Fig. 3A). PKH-labeled tumors
showed low intensity from the beginning and diminished over time,
while fluorescence signals emitted by RFP-labeled tumors increased,
even when the tumors grew progressively larger.
The fluorescence intensity of tumors labeled with
POLARIC also gradually decreased due to cell division; however, the
signal intensity was stronger than that of PKH-labeled tumors as
the initial signal was much brighter than that of the PKH-labeled
tumors. Therefore, POLARIC-labeled cells could be detected >5
weeks after injection (Fig. 3A and
B). The rates of decay in fluorescence were comparable between
the POLARIC- and PKH-labeled tumors (Fig. 3C), and this finding was consistent
with the in vitro data (Fig.
2C).
Next, we determined the fluorescence intensities of
tumors using flow cytometry. The fluorescence of tumors labeled
with POLARIC was readily detected in contrast to the fluorescence
of tumors labeled with PKH (Fig.
3D). The fluorescent indicators did not affect tumor size
(Fig. 3E) or tumor weight (Fig. 3F), suggesting that POLARIC did not
inhibit tumor growth or cell proliferation, similar to other
fluorescent proteins. POLARIC did not induce significant toxicity
in this mouse model. Therefore, each of the fluorescent dyes
studied here can be used for in vivo experiments without any
harmful effects on mice or their tumors (Fig. 3G).
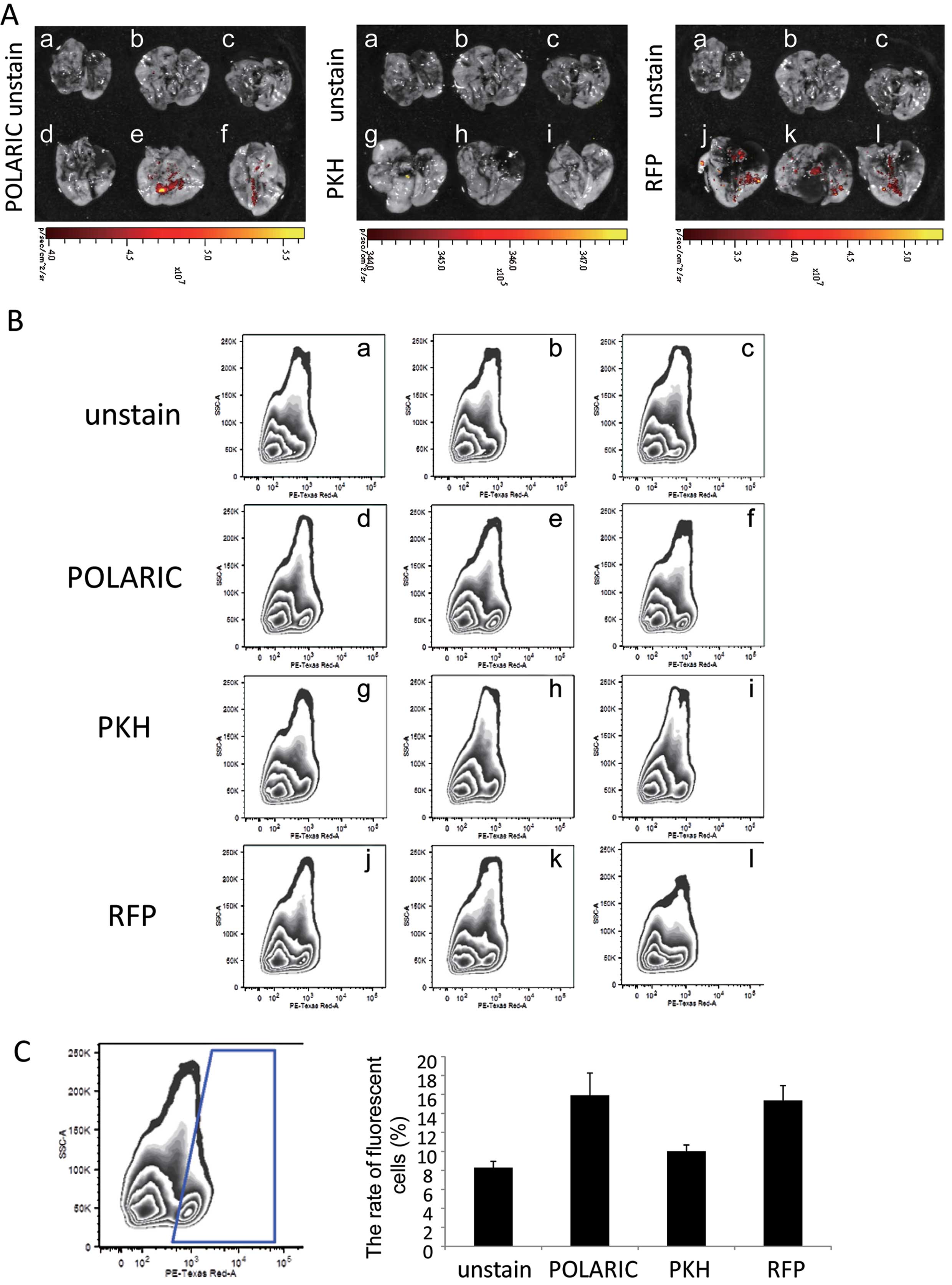
POLARIC-stained tumor cells are useful
for in vivo detection of lung metastases
We found that A375-SM cells metastasized to the
lungs at a high frequency when they were engrafted in
immunocompromised mice (14–16).
Ex vivo evaluation of lungs excised from engrafted mice
revealed metastasis in 2/3, 0/3 and 3/3 lungs excised from mice
engrafted with POLARIC-, PKH-labeled, or mRFP1-expressing tumors,
respectively (Fig. 4A). To confirm
these imaging data, lung tissues were homogenized and analyzed by
flow cytometry to quantify the rate of fluorescent metastatic tumor
cells (Fig. 4B and C). The
percentages of fluorescent cells in dissociated lung cells were
high in POLARIC-labeled and mRFP1-expressing tumor-bearing mice,
whereas those in the lungs of PKH-labeled tumor-bearing mice were
hardly detectable (Fig. 4B and
C).
Discussion
In the present study, we investigated the utility of
the novel fluorescent POLARIC probes for in vivo imaging
studies and compared the results with those of other fluorescent
probes used most frequently for cancer research (17). The fluorescence intensity of A375-SM
cells labeled with POLARIC was higher than that of A375-SM cells
labeled with PKH, and this intensity could be detected even after
three weeks of culture. Dilution of the probes by cell division
in vitro caused the loss of fluorescence intensity (10,18,19);
however, there was no detectable difference between cells labeled
with either POLARIC or PKH. Furthermore, the rate of division of
cells labeled with POLARIC was almost the same as that of cells
labeled with PKH or unlabeled control cells, suggesting that
POLARIC did not affect cell proliferation. Several fluorescent
indicators can only be used for relatively limited periods, and the
duration of the signal emitted by POLARIC-labeled cells represents
a significant advancement in this technique. Although the
fluorescence emitted by PKH persisted for as long as that emitted
by POLARIC, the initially greater intensity of the POLARIC signal
made its detection much easier than that of the PKH signal.
POLARIC labeling was evaluated in the orthotopic
melanoma-xenograft mouse model. Image analysis of the mice showed
that POLARIC-labeled primary tumors were clearly visible, even as
long as five weeks after injection. Ex vivo imaging
demonstrated that labeling with POLARIC also facilitates the
visualization of metastatic nodules in the lungs. In addition,
POLARIC showed no harmful effects on tumor progression and
metastasis. However, the emission maximum of POLARIC at 592 nm is
relatively low for the IVIS Spectrum (3). Therefore, fluorescent probes that
absorb red light more efficiently must be developed to detect
signals emitted by deeper organs. Since the absorption and emission
wavelengths of POLARIC fluorophores are altered by replacing the
central aromatic moiety (8,9), the proper probes may be designed and
synthesized in a manner similar to the derivatives of POLARIC using
a more extended aromatic moiety.
Cell lines that stably express fluorescent proteins
are commonly used for in vivo experiments (17). However, this requires sophisticated
molecular genetic manipulation and laboratory facilities.
Furthermore, these techniques are not always adequate for primary
cells, which are notoriously difficult to transfect (20). Therefore, DNA transfection is likely
to be unsuitable for labeling primary cells soon after harvesting
from animals. Using cultured cells can introduce artifacts as their
phenotypes differ from their cells of origin (21). Our present study suggests that
labeling cells with POLARIC fluorophores can be easily accomplished
in a short time without using advanced techniques and facilities.
Therefore, it should be possible to adapt this technique to a
variety of applications.
Acknowledgements
The authors thank Prof. I.J. Fidler for providing
A375-SM, the super-metastatic human malignant melanoma cell line
used in the present study; S.H. Son for selecting a suitable
POLARIC probe; Dr Roger Tsien (UCSD, CA, USA) for providing the
expression vectors for CS-RfA-CMV-mRFP1; Dr H. Miyoshi (RIKEN,
Tsukuba, Japan) for the packaging vector pCAG-HIVgp and the VSV-G-
and REV-expressing construct pCMV-VSV-G-RSV-REV; and Dr Kondoh, Dr
Ohmura, Mr. Sadamoto and Ms. Suzuki for their technical assistance
with the experiments.
References
|
1
|
Lukyanov KA, Chudakov DM, Lukyanov S and
Verkhusha VV: Innovation: Photoactivatable fluorescent proteins.
Nat Rev Mol Cell Biol. 6:885–891. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fernández-Suárez M, Chen TS and Ting AY:
Protein-protein interaction detection in vitro and in cells by
proximity biotinylation. J Am Chem Soc. 130:9251–9253.
2008.PubMed/NCBI
|
|
3
|
Shcherbo D, Shemiakina II, Ryabova AV, et
al: Near-infrared fluorescent proteins. Nat Methods. 7:827–829.
2010. View Article : Google Scholar
|
|
4
|
Haugland RA, Varma M, Sivaganesan M, Kelty
C, Peed L and Shanks OC: Evaluation of genetic markers from the 16S
rRNA gene V2 region for use in quantitative detection of selected
Bacteroidales species and human fecal waste by qPCR. Syst
Appl Microbiol. 33:348–357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wolfbeis OS: Fiber-optic chemical sensors
and biosensors. Anal Chem. 80:4269–4283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shimomura O, Johnson FH and Saiga Y:
Extraction, purification and properties of aequorin, a
bioluminescent protein from luminous hydromedusan, Aequorea. J Cell
Compar Physl. 59:223–239. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shimomura O: The discovery of aequorin and
green fluorescent protein. J Microsc. 217:1–15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Son SH, Abe Y, Yuasa M, et al: A
systematic analysis of aromatic heterocyclic rings in
solvatochromic fluorophores. Chem Lett. 40:378–380. 2011.
View Article : Google Scholar
|
|
9
|
Son SH, Yamagishi Y, Tani M, Yuasa M and
Yamada K: Spectral shifts of the environment-sensitive fluorophore
POLARIC (TM) in heterogeneous interfaces. Chem Lett. 40:989–991.
2011. View Article : Google Scholar
|
|
10
|
Horan PK, Melnicoff MJ, Jensen BD and
Slezak SE: Fluorescent cell labeling for in vivo and in vitro cell
tracking. Methods Cell Biol. 33:469–490. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wallace PK, Tario JD Jr, Fisher JL,
Wallace SS, Ernstoff MS and Muirhead KA: Tracking antigen-driven
responses by flow cytometry: monitoring proliferation by dye
dilution. Cytometry A. 73:1019–1034. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyoshi H: Gene delivery to hematopoietic
stem cells using lentiviral vectors. Methods Mol Biol. 246:429–438.
2004.PubMed/NCBI
|
|
13
|
Evtodienko VY, Kovbasnjuk ON, Antonenko YN
and Yaguzhinsky LS: Effect of the alkyl chain length of
monocarboxylic acid on the permeation through bilayer lipid
membranes. Biochim Biophys Acta. 1281:245–251. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kozlowski JM, Hart IR, Fidler IJ and Hanna
N: A human melanoma line heterogeneous with respect to metastatic
capacity in athymic nude mice. J Natl Cancer Inst. 72:913–917.
1984.PubMed/NCBI
|
|
15
|
Clark EA, Golub TR, Lander ES and Hynes
RO: Genomic analysis of metastasis reveals an essential role for
RhoC. Nature. 406:532–535. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ohga N, Ishikawa S, Maishi N, et al:
Heterogeneity of tumor endothelial cells: comparison between tumor
endothelial cells isolated from high- and low-metastatic tumors. Am
J Pathol. 180:1294–1307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hoffman RM: The multiple uses of
fluorescent proteins to visualize cancer in vivo. Nat Rev Cancer.
5:796–806. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bantly AD, Gray BD, Breslin E, et al:
CellVue Claret, a new far-red dye, facilitates polychromatic
assessment of immune cell proliferation. Immunol Invest.
36:581–605. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wallace PK and Muirhead KA: Cell tracking
2007: a proliferation of probes and applications. Immunol Invest.
36:527–561. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maurisse R, De Semir D, Emamekhoo H, et
al: Comparative transfection of DNA into primary and transformed
mammalian cells from different lineages. BMC Biotechnol. 10:92010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seeberger KL, Eshpeter A, Rajotte RV and
Korbutt GS: Epithelial cells within the human pancreas do not
coexpress mesenchymal antigens: epithelial-mesenchymal transition
is an artifact of cell culture. Lab Invest. 89:110–121. 2009.
View Article : Google Scholar
|