Introduction
Nuclear pore complexes (NPCs) connect the nuclear
interior with the cytoplasm and control the exchange between the
two compartments. They are built from nucleoporins (1,2) and
are equipped with a sieve-like barrier that is freely permeable for
small molecules, but becomes increasingly restrictive as inert
mobile species approach or exceed 5 nm in diameter (3). This limit corresponds to a mass of ≥30
kDa for spherical proteins whose transportation needs nuclear
transport receptors (NTRs). Serving as the NTRs, the importin α and
β target hundreds of proteins to NPCs and facilitate their
translocation across the nuclear envelope. Importin α binds to
classical nuclear localization signal (NLS)-containing proteins and
links them to importin β, the karyopherin that ferries the ternary
complex through the NPCs (4).
RhoA, with molecular mass of 21 kDa, is the most
extensively studied member of the Rho GTPase family which belongs
to the Ras super-family of small G proteins. It has been reported
to regulate many biological activities including gene transcription
(5) and tumor progression (6). RhoA activation is generally associated
with invasive growth and metastasis. RhoA overexpression is found
in many human cancers, likely contributing to the loss of growth
control and the invasive phenotype of cancer cells, whereas RhoA
inhibition decreases tumor cell invasion and metastasis.
Recent studies (7–9) have
indicated that the distribution of RhoA was not only in the
membrane and in the cytoplasm but also in the nucleus. Further
investigations in our research group showed that the nucleus
localization of RhoA was affected by many factors, such as
inflammatory factors and antimicrotubule agents (10).
RhoA has a close relationship with NF-κB, a focal
point of many signal transduction pathways (11). NF-κB has double distribution in the
cytoplasm and nucleus in many cell lines, and highly expressed at
inflammation. It is composed of protein dimers, such as the
heterodimer p50/p65. Classical NLSs are found in both p50 and p65
(12). In the nucleus, RhoA may
directly bind with NF-κB, Stat3, Stat5a, c-Myc and be involved in
regulation and control role of genetic transcription (13,14).
It has been reported that statins exert a positive or negative
modulation on NF-κB through the involvement of RhoA, but the exact
mechanism has not yet been clarified (15). Results from Chang and coworkers
(16) provide evidence that
Ras-induced RhoA and NF-κB activation was involved in the
invasion/migration of bladder cancer. Furthermore, LPS-induced
nuclear translocation of RhoA is found to be dependent on NF-κB in
rat lung epithelial cancer cells (17). The underlying mechanism(s) for RhoA
entering the nucleus is still unclear and warrants further
investigation.
The biochemical characteristic of RhoA might incur
the thought that RhoA enters the nucleus through simple diffusion,
because it is a small molecule protein and does not have any
classical NLS. However, based on our experiments, we questioned
whether RhoA might enter the nucleus via active transportation with
the help of NTRs (importin α/β) through composing complex with
other protein(s) containing NLS. Thus, the mechanism(s) on RhoA
nuclear translocation requires further elucidation. In the present
study, we took the NF-κB as the entry point to explore the manner
in which RhoA enters the cell nucleus in the human gastric cancer
cell line AGS.
Materials and methods
Cell lines and cell culture
Human gastric cancer cell line AGS was obtained from
the Institution of Cell Biology of the Chinese Academy of Sciences
(Shanghai, China). The cells were maintained in DMEM supplemented
with 10% FBS at 37°C in a humidified atmosphere of 5%
CO2. The medium was changed every two days and the cells
were kept at subconfluence.
Reagents
Dulbecco’s modified Eagle’s media (DMEM) and fetal
bovine serum (FBS) were purchased from Gibco (Grand Island, NY,
USA). Antibodies against RhoA (cat. no. sc-418) and NF-κB P50 (cat.
no. sc-114) were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). Monoclonal anti-importin α antibody (no.
I1784) and nuclear fluorochrome Hoechst 33342 were purchased from
Sigma (St. Louis, MO, USA). Leptomycin B (LMB) was purchased from
Beyotime Institute of Biotechnology (Shanghai, China). Nuclear and
Cytoplasmic Extract kit (cat. no. KC-435) and the antibody against
GAPDH (glyceraldehyde phosphate dehydrogenase) were purchased from
Kangcheng Bio-tech (Hangzhou, China). FITC, TRITC and horseradish
peroxidase (HRP)-conjugated secondary antibodies were purchased
from Jackson ImmunoResearch Laboratories (West Grove, PA, USA).
Electrochemiluminescence (ECL) reagents were bought from Amersham
Biosciences (Buckinghamshire, UK).
Preparation of cytoplasmic and nuclear
protein samples
The cytoplasmic and nuclear proteins were extracted
according to the instructions of the manufacturer of the Nuclear
and Cytoplasmic Extract kit (Kangcheng Bio-tech).
Immunofluorescence microscopy
AGS cells grown on cover slips were fixed with
freshly prepared paraformaldehyde (40 g/l in PBS) for 30 min. After
penetration with 30 ml/l Triton X-100 and blocking with 30 g/l
bovine serum albumin (BSA), the cells were incubated with the
primary antibody at 4°C overnight (o/n) followed by an another
incubation with fluorescein isothiocyanate (FITC) and/or
tetrarhodamine isothiocyanate (TRITC)-conjugated second antibody
for 1 h at room temperature (RT). Hoechst 33342 (0.2 mM) was
employed in the last incubation of 10 min to reveal the cell
nuclei. AGS cells were washed three times after each incubation.
The distribution of target protein of the cells was analyzed by
fluorescence microscopy.
Western blotting and quantification
The sample proteins were run on 10/12.5%
SDS-polyacrylamide gels, and were transferred onto polyvinyl
difluoride (PVDF) membranes (Bio-Rad Laboratories, Hercules, CA,
USA). The PVDF membranes were firstly blocked with 5% milk in TBS-T
(NaCl 80 g/l, KCl 2 g/l, Tris-HCl 30 g/l, Tween-20 0.1%, pH 7.4)
for 1 h at room temperature and then incubated with the primary
antibodies at 4°C overnight. After the incubation of membranes with
the horseradish peroxidase-conjugated secondary antibodies for 1 h
at room temperature, ECL reagents were used to show the positive
bands on the membrane. The bands were detected by Typhoon 9400 (GE
Healthcare, Piscataway, NJ, USA). To quantify the protein amount of
RhoA, western blot results were further analyzed by SPSS 13.0 Tool
software (SPSS Inc.) and the volume ratio of RhoA/GAPDH input was
calculated and presented.
Co-immunoprecipitation assay
Immunoprecipitated AGS cells were lysed in TNEN
buffer (50 mM Tris-HCl, pH 7.2, 100 mM NaCl, 2.5 mM EDTA, 0.5%
NP-40). The antibodies against RhoA, importin α, NF-κB and isotype
matched IgG were used for immunoprecipitation, respectively.
Immunoprecipitates were analyzed by western blotting as described
above, using anti-importin α, anti-NF-κB or anti-RhoA antibody for
corresponding protein. For analyzing proteins in cell fractions,
cells were first lysed in TNEN buffer, and then extracted for
nuclear and cytoplasmic proteins as described above. Proteins in
cell fractions were further immunoprecipitated with an antibody
against RhoA and detected by western blotting, using anti-NF-κB
antibody.
Results
The nuclear localization of RhoA in the
human gastric cancer cell line AGS
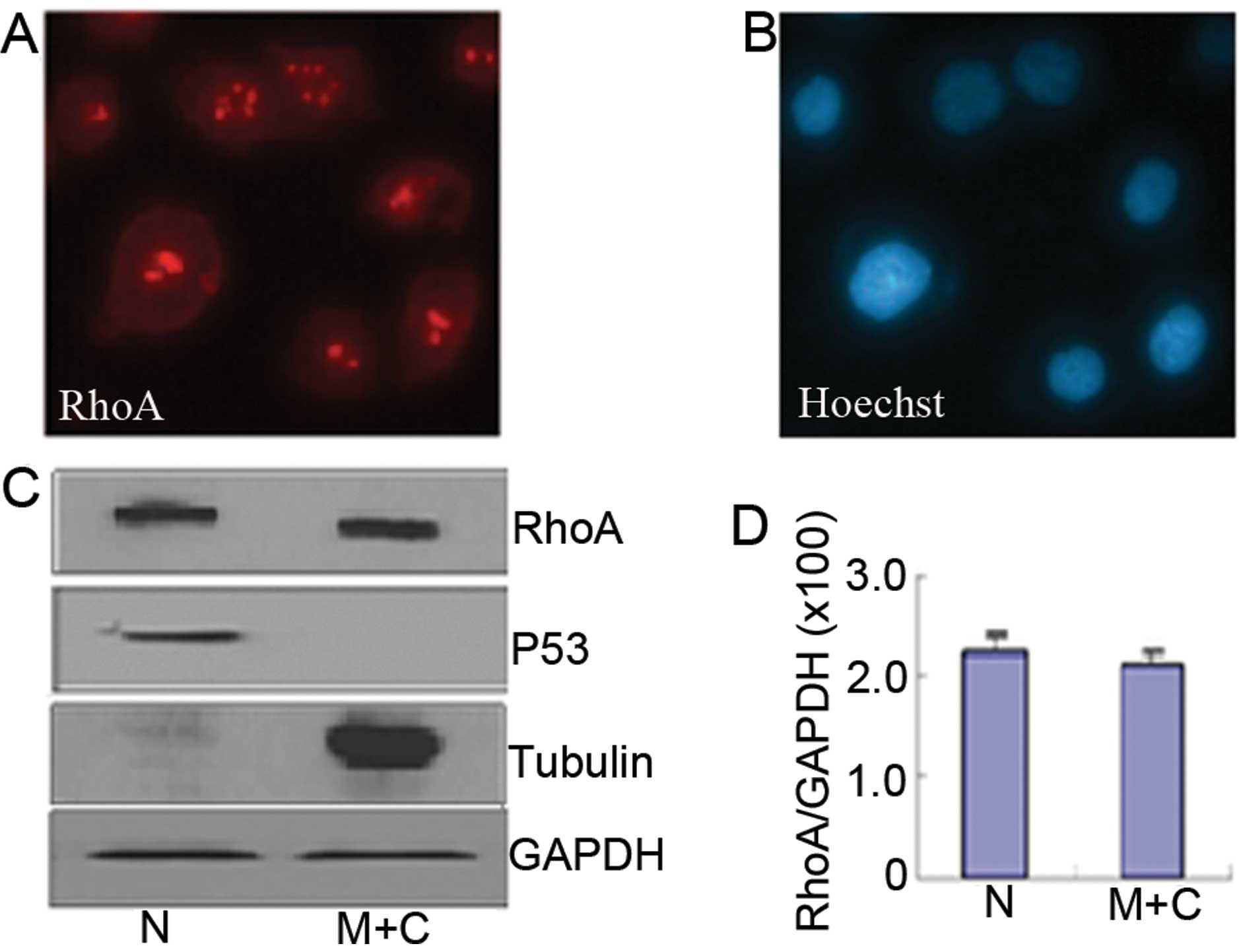
Immunofluorescence microscopy showed that in the AGS
cells, RhoA protein was localized on the membrane, in the cytoplasm
and in the cell nucleus. Within the nucleus its predominant
localization was in the nucleolus (Fig.
1A and B). Western blotting revealed similar subcellular
distribution of RhoA (Fig. 1C).
Quantification of protein amount (Fig.
1D) confirmed that RhoA concentrated in the nucleus of AGS
cells.
Intracellular colocalization of RhoA with
importin α and NF-κB P50
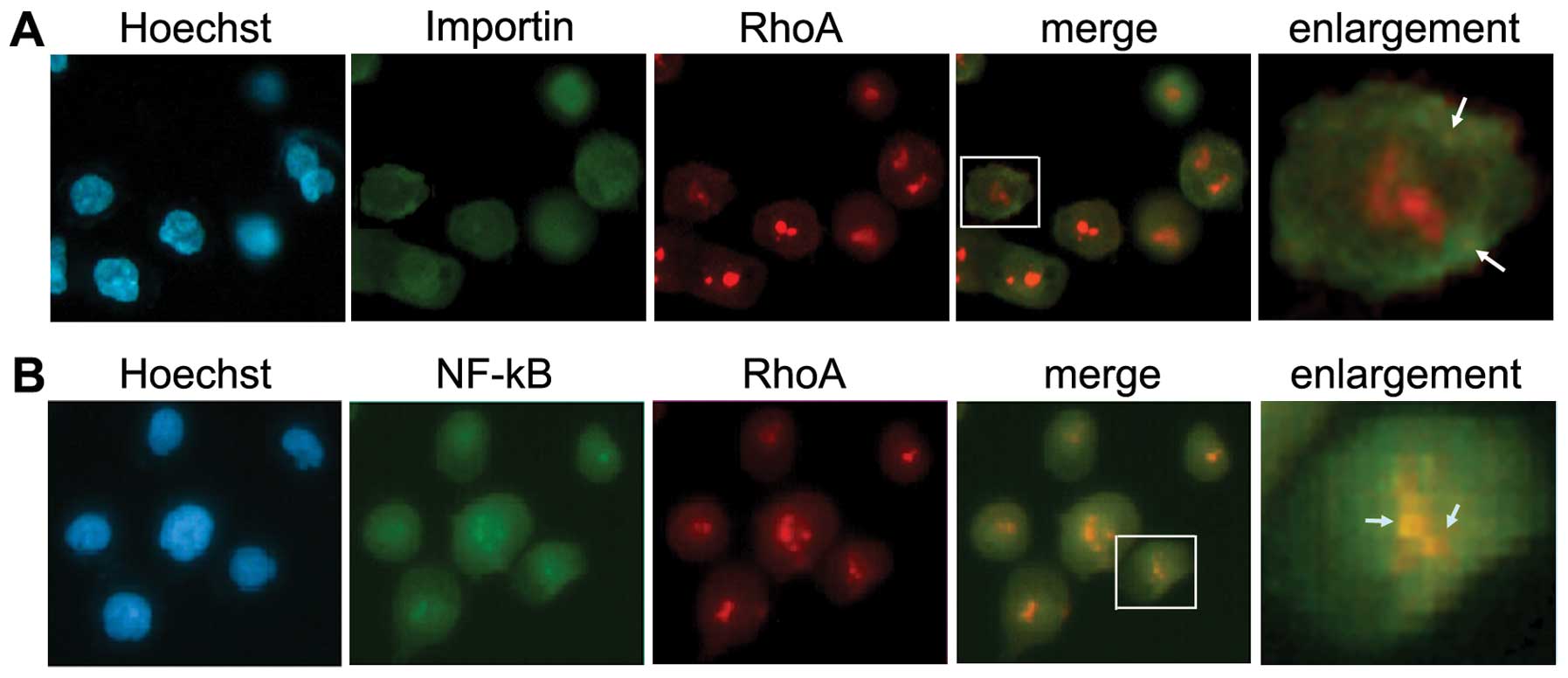
Importin α recruits cargo at low RanGTP levels in
the cytoplasm, releases the cargo upon RanGTP binding into the
nucleus, and returns RanGTP bound to the cytoplasm. There, GTP
hydrolysis triggers dissociation of the importin-RanGTP complex and
allows the importin α to bind a next cargo molecule (18). Thus, importin α has subcelluar
distribution in both cytoplasm and nucleus. To determine whether
importin was involved in the nulear translocation of RhoA,
double-labeling immunofluorescence was performed. A partial
colocalization of RhoA with importin α was observed, mainly
surrounding and on the nuclear membrane in AGS cells (Fig. 2A, arrows). Importantly, an intensive
colocalization of RhoA with NF-κB P50 was found in both cytoplasm
and nucleus, particularly in the cell nucleoli (Fig. 2B, arrows).
Co-immunoprecipitation of RhoA with
importin α and NF-κB P50
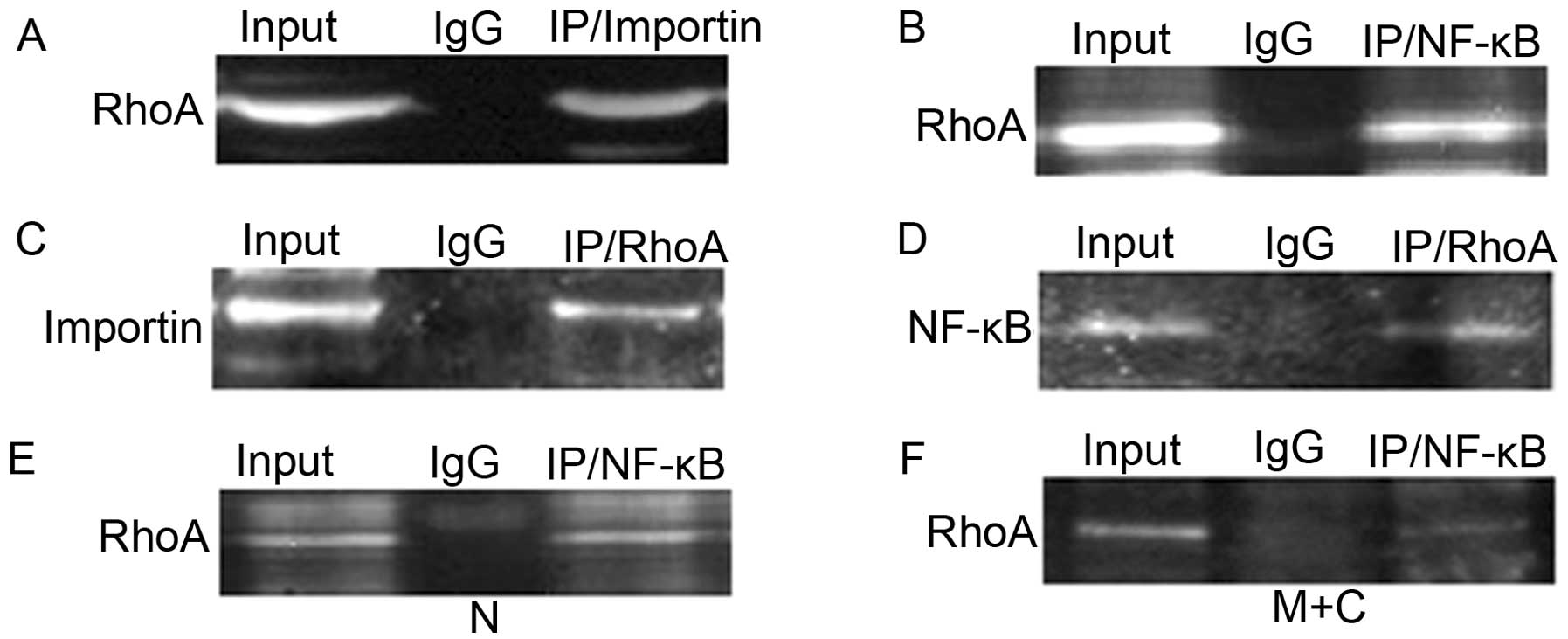
To verify the findings from double labeling
immunofluorescence, co-immunoprecipitation (Co-IP) assay was
performed for protein interactions. Consistent with our
colocalization results, there was a strong association between RhoA
and importin α (Fig. 3A and C) as
well as RhoA and NF-κB P50 (Fig. 3B and
D) in AGS cells. Further investigation on cellular
fractionation showed that RhoA bound NF-κB P50 in the cytoplasmic
and membrane fraction (Fig. 3F) and
more profound in the nuclear fraction of AGS cells (Fig. 3E).
Distribution of RhoA within the nucleus
upon nuclear export inhibition
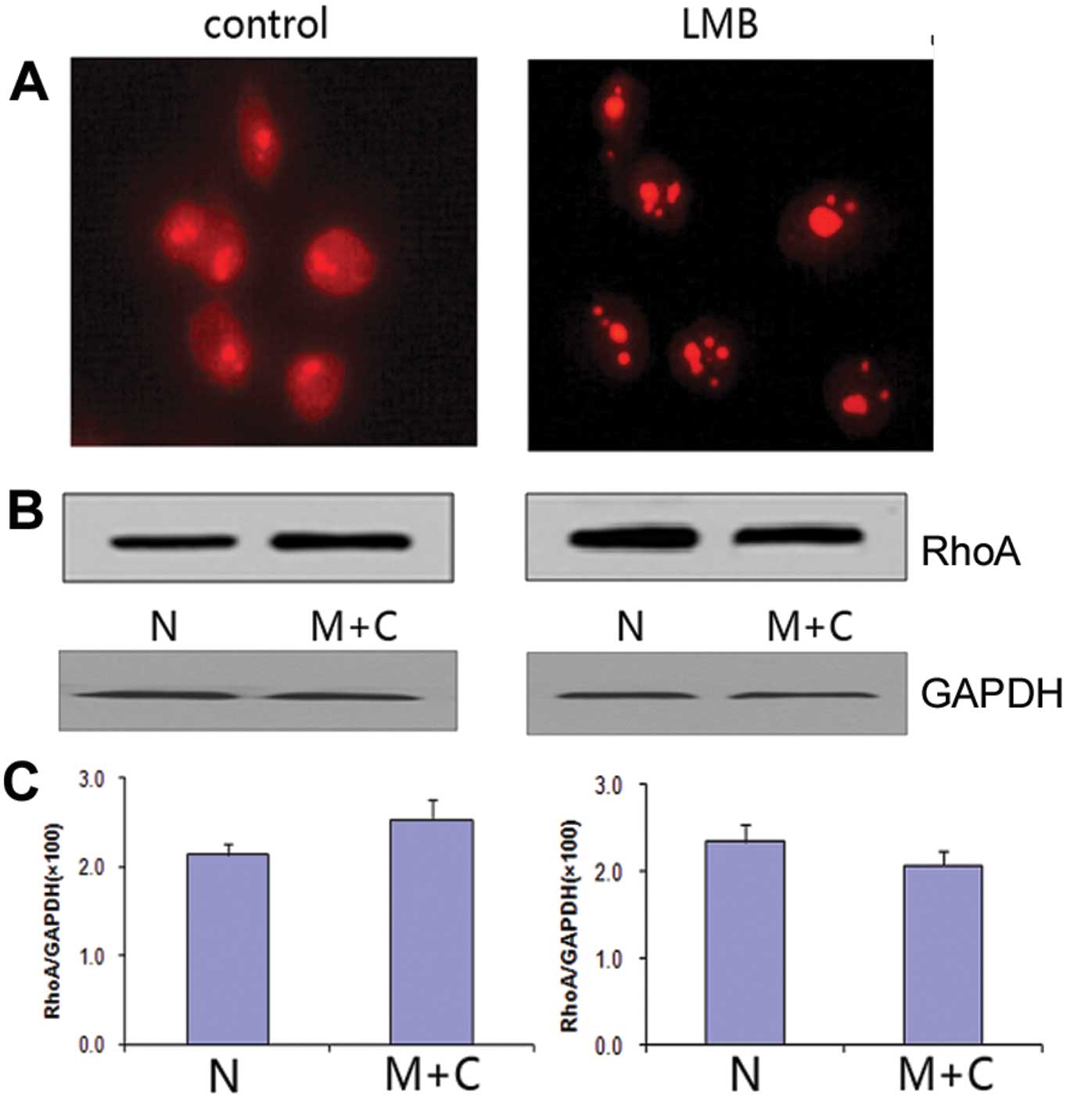
To acquire further evidence for the molecular
mechanism of RhoA nuclear transportation, AGS cells were treated
with the nuclear export inhibitor, leptomycin B (LMB) for 12 h.
Immunofluorescence revealed a reduction of RhoA signal in the
cytoplasm but a strong accumulation of RhoA in the nucleoli of
LMB-treated cells (Fig. 4A).
Western blot assay confirmed that the protein amount of RhoA was
highly increased in the nucleus of LMB-treated AGS cells (Fig. 4B and C).
Discussion
In the classical nuclear import pathway (19), a protein containing a classical
basic nuclear localization signal (NLS) is imported by a
heterodimeric import receptor consisting of the β-karyopherin
importin β, which mediates interactions with the nuclear pore
complex, and the adaptor protein importin α, which directly binds
the classical NLS. Over the past twenty years, the universe of
basic transport sequences has been greatly expanded leading to the
definition of classical NLSs and, more recently, non-classical
NLSs, which can be more complex in sequence, length and amino acid
composition (20).
In the small G protein family only a small part of
members has NLSs, e.g., Rac1 and R-Ras. In spite of RhoA being a
small molecule protein and lacking NLSs, it is hard to conclude
that nuclear translocation of RhoA is simply through diffusion,
because RhoA content is higher in the nucleus than in the cytoplasm
in some cancer cells (7,10) and its nuclear translocation has the
intimate connection with the inflammation and cancer.
Serving as a focal point of many signal transduction
pathways, NF-κB regulates the expression of genes involved in
inflammation, cellular proliferation and apoptosis (21). Activation of NF-κB is followed by
its rapid translocation into the nucleus where it activates the
transcription of numerous genes including those encoding for
cytokines and cell adhesion molecules. NF-κB contains classical
NLSs and enters the nucleus through active transportation with the
help of importin α (12).
Consistent with the previous reports, the present
research results confirmed the subcellular localization of RhoA on
the membrane and in the cytoplasm and cell nucleus of human gastric
cancer AGS cells. Furthermore, RhoA colocalized partially with
importin α on and surrounding the nuclear membrane and intensively
with NF-κB P50 in both cytoplasm and nucleus, particularly in the
cell nucleoli. A strong association between RhoA and importin α as
well as RhoA and NF-κB P50 was verified by co-immunoprecipitation
and western blotting, indicating RhoA, NF-κB and importin α bound
together in the cytoplasm and nucleus. Since RhoA cannot be
recognized and bound directly by importin α, a possible explanation
is that RhoA might form a complex with NF-κB, probably also other
proteins, and thereby indirectly binding to importin α. These
results suggest that the RhoA entry to the nucleus is via active
transportation with the help of NTR importin α through composing
complex with NF-κB in human gastric cancer AGS cells.
Cancer cells utilize the normal processes of
nuclear-cytoplasmic transport through the nuclear pore complex of a
cell to effectively evade anti-neoplastic mechanisms (22). Karyopherins include both importins
and exportins (23). Leptomycin B
(LMB), the first identified small molecule inhibitor of CRM1 (also
known as exportin-1), has greatly facilitated investigation to
uncouple nuclear export from import and to analyze shuttling
proteins via nucleocytoplasmic transport (24). However, no small molecule inhibitor
of nuclear import has been described up to now (25). A significant increase of nuclear
RhoA in AGS cells after LMB treatment implies that the molecular
mechanism of RhoA protein transport out of the nucleus is also
through the active transportation pathway.
The intracellular location of a protein is crucial
to its functioning in a cell. As nuclear translocation of RhoA
promotes the cancer progression, elucidating the nuclear
functioning of RhoA will be the future task and may provide a new
approach to treating cancer.
Acknowledgements
The present study was supported by the Specialized
Research Fund for Senior Personnel Program of Jiangsu University
(no. 11JDG129), the Postdoctoral Foundation of China (no.
2012M521018) and the Jiangsu province (no. 1201025B) to Y.L. and by
the Zhenjiang Social Development Project (no. SH2010004) to
X.Y.
References
|
1
|
Labokha AA, Gradmann S, Frey S, Hülsmann
BB, Urlaub H, Baldus M and Görlich D: Systematic analysis of
barrier-forming FG hydrogels from Xenopus nuclear pore complexes.
EMBO J. 23:204–218. 2013.PubMed/NCBI
|
|
2
|
Hetzer MW and Wente SR: Border control at
the nucleus: biogenesis and organization of the nuclear membrane
and pore complexes. Dev Cell. 17:606–616. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mohr D, Frey S, Fischer T, Güttler T and
Görlich D: Characterisation of the passive permeability barrier of
nuclear pore complexes. EMBO J. 28:2541–2553. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldfarb DS, Corbett AH, Mason DA,
Harreman MT and Adam SA: Importin α: a multipurpose
nuclear-transport receptor. Trends Cell Biol. 14:505–514. 2004.
|
|
5
|
Sotiropoulos A, Gineitis D, Copeland J and
Treisman R: Signal-regulated activation of serum response factor is
mediated by changes in actin dynamics. Cell. 98:159–169. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kamai T, Yamanishi T, Shirataki H, Takagi
K, Asami H, Ito Y and Yoshida K: Overexpression of RhoA, Rac1, and
Cdc42 GTPases is associated with progression in testicular cancer.
Clin Cancer Res. 10:4799–4805. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Chen Y, Tao Y, Xu J and Chen M: RhoA
protein is generally distributed in the nuclei of cancer cells.
Oncol Rep. 24:1005–1009. 2010.PubMed/NCBI
|
|
8
|
Dubash AD, Guilluy C, Srougi MC, Boulter
E, Burridge K and García-Mata R: The small GTPase RhoA localizes to
the nucleus and is activated by Net1 and DNA damage signals. PloS
One. 6:e173802011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guilluy C, Dubash AD and García-Mata R:
Analysis of RhoA and Rho GEF activity in whole cells and the cell
nucleus. Nat Protoc. 6:2050–2060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Chen Y and Xu J: Factors influencing
RhoA protein distribution in the nucleus. Mol Med Rep. 4:1115–1119.
2011.PubMed/NCBI
|
|
11
|
Okamoto T, Sanda T and Asamitsu K: NF-κB
signaling and carcinogenesis. Curr Pharm Des. 13:447–462. 2007.
|
|
12
|
Fagerlund R, Kinnunen L, Köhler M,
Julkunen I and Melén K: NF-{kappa}B is transported into the nucleus
by importin {alpha}3 and importin {alpha}4. J Biol Chem.
280:15942–15951. 2005.
|
|
13
|
Tonozuka Y, Minoshima Y, Bao YC, et al: A
GTPase-activating protein binds STAT3 and is required for
IL-6-induced STAT3 activation and for differentiation of a leukemic
cell line. Blood. 104:3550–3557. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benitah SA, Valerón PF, van Aelst L,
Marshall CJ and Lacal JC: Rho GTPases in human cancer: an
unresolved link to upstream and downstream transcriptional
regulation. Biochim Biophys Acta. 1705:121–132. 2004.PubMed/NCBI
|
|
15
|
Riganti C, Doublier S, Costamagna C,
Aldieri E, Pescarmona G, Ghigo D and Bosia A: Activation of nuclear
factor-kappa B pathway by simvastatin and RhoA silencing increases
doxorubicin cytotoxicity in human colon cancer HT29 cells. Mol
Pharmacol. 74:476–484. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang HR, Huang HP, Kao YL, et al: The
suppressive effect of Rho kinase inhibitor, Y-27632, on oncogenic
Ras/RhoA induced invasion/migration of human bladder cancer TSGH
cells. Chem Biol Interact. 183:172–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tao Y, Chen YC, Lan T, Qian H, Wang Y and
Jiang L: LPS-induced nuclear translocation of RhoA is dependent on
NF-κB in the human lung cancer cell line A549. Oncol Lett.
3:1283–1287. 2012.
|
|
18
|
Izaurralde E, Kutay U, von Kobbe C, Mattaj
IW and Görlich D: The asymmetric distribution of the constituents
of the Ran system is essential for transport into and out of the
nucleus. EMBO J. 16:6535–6547. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lange A, Mills RE, Lange CJ, Stewart M,
Devine SE and Corbett AH: Classical nuclear localization signals:
definition, function, and interaction with importin alpha. J Biol
Chem. 282:5101–5105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sessler RJ and Noy N: A ligand-activated
nuclear localization signal in cellular retinoic acid binding
protein-II. Mol Cell. 18:343–353. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Greten FR and Karin M: The IKK/NF-κB
activation pathway - a target for prevention and treatment of
cancer. Cancer Lett. 206:193–199. 2004.
|
|
22
|
Turner JG, Dawson J and Sullivan DM:
Nuclear export of proteins and drug resistance in cancer. Biochem
Pharmacol. 83:1021–1032. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fried H and Kutay U: Nucleocytoplasmic
transport: taking an inventory. Cell Mol Life Sci. 60:1659–1688.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wolff B, Sanglier JJ and Wang Y:
Leptomycin B is an inhibitor of nuclear export: inhibition of
nucleo-cytoplasmic translocation of the human immunodeficiency
virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chem Biol.
4:139–1347. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ambrus G, Whitby LR, Singer EL, Trott O,
Choi E, Olson AJ, Boger DL and Gerace L: Small molecule
peptidomimetic inhibitors of importin α/β mediated nuclear
transport. Bioorg Med Chem. 18:7611–7620. 2010.PubMed/NCBI
|