Introduction
Breast cancer is the most common type of malignancy
in women and is the second leading cause of cancer-related
mortality in women worldwide (1).
Although a variety of therapeutic strategies have been developed to
fight breast cancers in recent years, more than 40% of patients
with breast cancer experience tumor recurrence after comprehensive
antitumor treatments (2). Breast
cancer stem cells are a small group of tumor cells with the
capacity to self-renew and a strong ability to form solid breast
tumors. This small group of tumor cells can also differentiate to a
relatively quiescent primitive group of cancer cells that are
considered the underlying factor for tumor recurrence and the main
reason breast cancers resist therapy (3,4).
With a better understanding of cancer stem cell
theory, stem cell-related genes in malignant tumors have gained
increased research attention. In 2003, Al-Hajj et
al(5) identified and isolated
tumorigenic cells named
CD44+/CD24−/low/Lineage− in 8 of 9
patients using a model in which human breast cancer cells were
grown in immunocompromised mice. CD44 is a cell-surface
glycoprotein involved in cell-cell interactions, cell adhesion, and
migration and has been considered to be a promising potential
target that may lead to more effective therapies (6).
Recent studies have reported that Ezrin may be a
prognostic factor and may predict potential lung metastasis in
osteosarcoma and could also possibly be used as a new therapeutic
target (7,8). Boldrini et al(9) reported, however, that neither CD44 nor
Ezrin immunoexpression predicts the prognosis of patients with
osteosarcoma. Data from in vitro experiments revealed the
direct molecular interactions of Ezrin with molecules related to
metastatic functions such as CD44, merlin and Lamp-1. This is
consistent with its role in forming phagocytic vacuoles, vesicular
sorting, and the migration capacities of melanoma cells. Currently,
the relationship between Ezrin and CD44 expression in breast cancer
and the clinical implications in breast cancer are still unclear.
In the present study, we sorted and identified breast cancer stem
cells (CSCs) and investigated the expression status of Ezrin and
CD44 in breast CSCs. We then examined the clinical implications of
our inquiry in breast cancer in order to lay a foundation for
managing breast cancer.
Materials and methods
Patients and tissue specimens
A total of 726 patients with histologically
confirmed breast cancer who underwent radical operations at the
First Affiliated Hospital of Harbin Medical University, between
January 2001 and January 2006 were enrolled for immunohistochemical
and immunofluorescence double staining and prognostic analysis. The
First Affiliated Hospital of Harbin Medical University approved the
study protocol.
Cell culture and separation of CSCs from
non-stem cancer cells (NSCCs)
Breast cancer cell line MDA-MB-231 was grown in
Dulbecco’s modified Eagle’s medium (DMEM), 10% fetal bovine serum
(FBS), and penicillin-streptomycin. To separate CSCs from NSCCs,
flow cytometric cell sorting was performed on single-cell
suspensions that were stained with the CD44 antibody
(FITC-conjugated) (555478) and with the CD24 antibody
(PE-conjugated) (555428) (both from BD Biosciences, Franklin Lakes,
NJ, USA). As used throughout this study, CSCs are defined by the
minority CD44high/CD24low population, whereas
NSCCs are defined by the majority
CD44low/CD24high. Mammospheres were generated
by placing CSCs in suspension (1,000 cells/ml) in serum-free
DMEM/F12 media, supplemented with B27 (1:50; Invitrogen Life
Technologies), 0.4% BSA, 20 ng/ml EGF, and 4 mg/ml insulin
(10). After 6 days of incubation,
mammospheres were typically >75 mM in size with ~97% being
CD44high/CD24low. For serial passaging,
6-day-old mammospheres were harvested using a 70-μm cell strainer,
whereupon they were dissociated to single cells with trypsin, and
then regrown in suspension for 6 days.
Cell transfection
For siRNA experiments, CSCs or 6-day-old
mammospheres were transfected with 100 nM of siRNAs (Ambion, Inc.)
against Ezrin, and negative control (AM4611), using siPORT NeoFX
transfection agent. The resulting cells were assayed 24 or 48 h
post transfection.
Treatments with chemotherapeutic agents
and assessment of cell viability
The sensitivities of the above cells to three
chemotherapeutic drugs were examined using the Cell Counting Kit-8
(CCK-8) technique. Cells were plated at a density of
5×104/ml cells/well into ultra-low adhering 96-well
plates containing 100 μl Complete MammoCult medium and treated with
concentrations of cisplatin (DDP), epirubicin (EPI) and docetaxel
(DTX) as follows: 0, 0.05, 0.1, 0.25, 0.3, 0.35 and 0.4 mM.
Forty-eight hours after treatment, CCK-8 reagent was added to each
well and incubated for 2 h before reading at a wavelength of 450
nm.
Co-immunoprecipitation and western
blotting
Cell lysate preparation, immunoprecipitation, and
western blotting were performed as described previously (11). For inhibition of proteasomal
degradation, cells were treated with 10 μM of MG132 (Sigma-Aldrich,
St. Louis, MO, USA) for 4 h. Briefly, cells were lysed with a
buffer of 0.1% SDS, 50 mmol/l Tris-HCl (pH 7.6), 1% NP-40, 150
mmol/l NaCl, 2 mg/ml aprotinin, 2 mg/ml leupeptin and 7 mg/ml PMSF.
Protein concentrations were determined using the BCA protein assay
kit (Pierce Biotechnology, Inc., Rockford, IL, USA). Thirty
micrograms of protein was separated on 10% SDS-PAGE gels and
transferred to a PVDF membrane. After blocking, the membrane was
incubated with the primary antibody at 4°C overnight (Ezrin,
ab40839; CD44, ab51037; Abcam). After washing, the membrane was
incubated with a secondary antibody at a dilution 1:2,000 at room
temperature for 1 h. Proteins were detected using an ECL kit
(Varsal Instruments, Beijing, China), and anti-β-actin antibody
(Sigma-Aldrich) was used as the loading control. Densitometry was
performed by Gel-Pro Analyzer software (Media Cybernetics, Silver
Spring, MD, USA).
Immunohistochemistry experimental
procedures
Thin slices of tumor tissue from all cases received
at our histopathology unit were fixed in 4% formaldehyde solution
(pH 7.0) for periods not exceeding 24 h. The tissues were processed
routinely for paraffin embedding, and 4-μm sections were cut and
placed on glass slides coated with (3-aminopropyl)-triethoxysilane
for immunohistochemistry. Tissue samples were stained with
hematoxylin and eosin to determine the histological type and the
grade of the tumors.
Briefly, breast tumor tissues were cut at a
thickness of 4 μm using a cryostat. The sections were mounted on
microscope slides, air-dried, and then fixed in a mixture of 50%
acetone and 50% methanol. The sections were then de-waxed with
xylene, gradually hydrated with gradient alcohol, and washed with
PBS. Sections were incubated for 60 min with the primary antibody
(Ezrin, ab40839; CD44, ab51037). Following washings with PBS,
sections were incubated for 30 min in the secondary biotinylated
antibody (Multilink swine anti-goat/mouse/rabbit immunoglobulin;
Dako, Inc.). Following washings, avidin-biotin complex (1:1,000
dilution; Vector Laboratories, Burlingame, CA, USA) was then
applied to the sections for 30–60 min at room temperature. The
immunoreactive products were visualized by catalysis of
3,3′-diaminobenzidine (DAB) using horseradish peroxidase in the
presence of H2O2, following extensive
washings. Sections were then counterstained in Gill’s hematoxylin
and dehydrated in ascending grades of methanol before clearing in
xylene and mounting under a coverslip.
Evaluation of immunohistochemical
reaction intensities
The intensities of the immunohistochemical reactions
were estimated independently by 2 pathologists. In order to
evaluate the expression of the proteins analyzed, a
semi-quantitative scale of the immunoreactive score (IRS), with the
authors’ own modifications, was applied (12) in which the intensity of the color
reaction (no reaction, 0; weak color reaction, 1; moderate
intensity, 2; intense reaction, 3) and the percentage of positive
cells (no positive cells, 0; <25% positive cells, 1; 25–50%
positive cells, 2; 51–75% positive cells, 3; >75% positive
cells, 4) were both taken into account. The final, integrated
scores ranged from 0 to 12. Cases with expression scores ranging
between 0 and 2 in the IRS scale were considered negative.
Double immunofluorescence staining
Surgical resected breast cancer tissues were fixed
and cut at a thickness of 4 μm. The specimens were stained with
control IgG, mouse anti-human Ezrin (1:50 dilution), rabbit
anti-human CD44 polyclonal antibodies (1:50 dilution) (both from BD
Biosciences) at 4°C for 12 h. After being washed, the cells were
incubated with biotinylated goat anti-mouse IgG secondary
antibodies (1:1,000 dilution) in PBS for 30 min at room
temperature. The bound antibodies were detected with PE and FITC,
followed by mounting with DAPI medium (Vector Laboratories). The
expression of CD44 and Ezrin was examined under a fluorescence
microscope. The cell staining was observed under fluorescence
microscopy.
Statistical analysis
All data were analyzed with SPSS Statistics software
(version 13.0; Chicago, IL, USA). The relationships between Ezrin
and other parameters were studied using the Chi-square test,
Fisher’s exact test, or independent t-tests. Disease-specific
survival was analyzed using the Kaplan-Meier method. The log-rank
test was used to analyze differences in survival. Multivariate
analysis was performed using the Cox proportional hazards model
selected in forward stepwise. A P-value of <0.05 was considered
to indicate a statistically significant result.
Results
Ezrin is co-expressed with CD44 in
CSCs
CSCs (106) were sorted from the
MDA-MB-231 cell line, and the serum-free suspension was cultured.
After 6 days of culture, single-cell suspensions of CSCs separated
from the cell line produced viable mammospheres (20–100 μm), which
could be passaged further. Double immunofluorescence staining
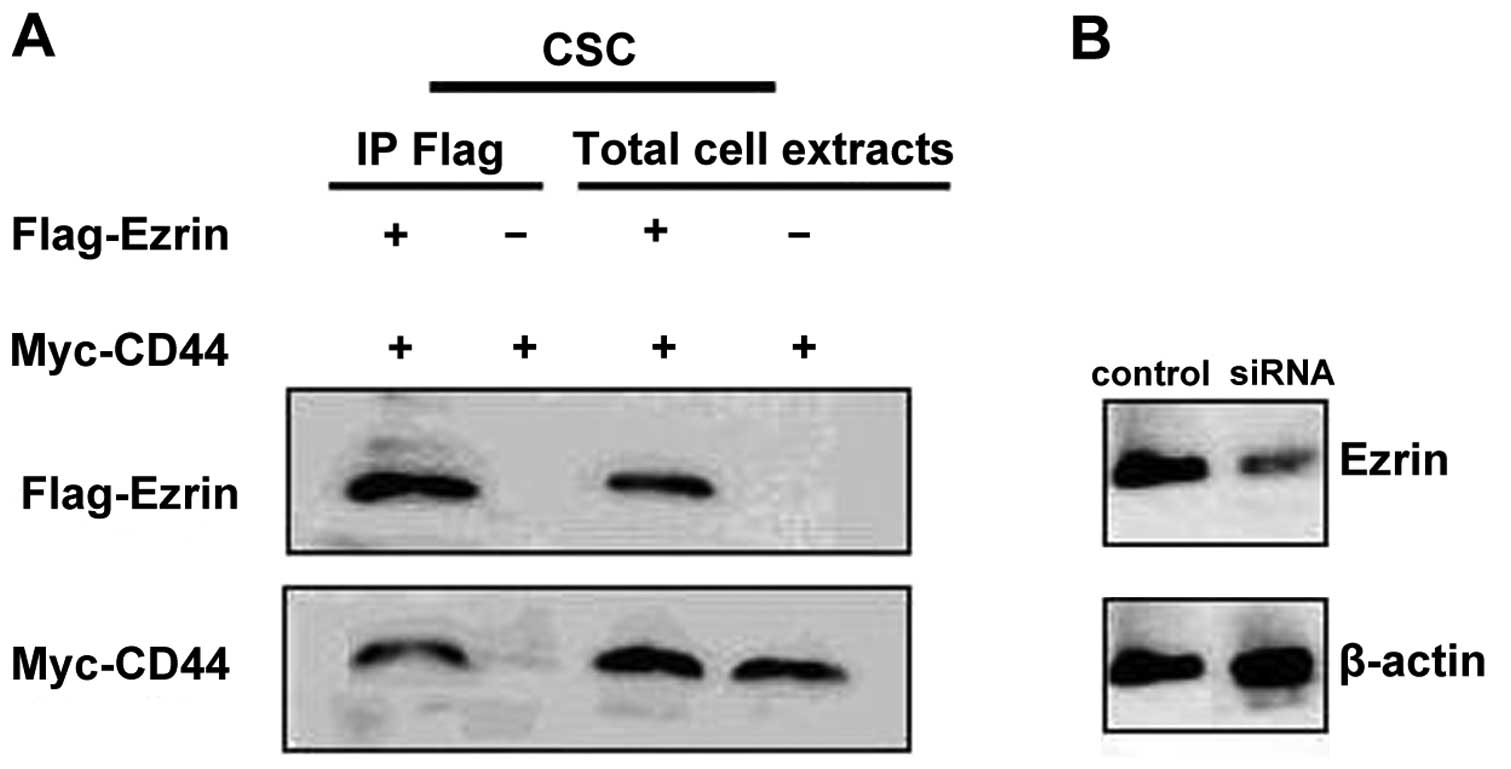
showed that the Ezrin protein was co-expressed with CD44 in CSCs.
Moreover, the co-immunoprecipitation test also showed that the
Ezrin protein was co-precipitated with the CD44 protein (Fig. 1).
Downregulation of Ezrin protein level by
siRNA
By replacing the serum culture medium at 6 h after
siRNA transfection, we observed that the successfully transfected
CSCs appeared as green fluorescence using fluorescence microscopy.
The positive rate of transfected cells was 66.12±18.46% in the
CSCs. The expression of Ezrin was examined by western blotting at
48 h after siRNA transfection (Fig.
1). Efficiency reached >85% at the protein level in
CSCs.
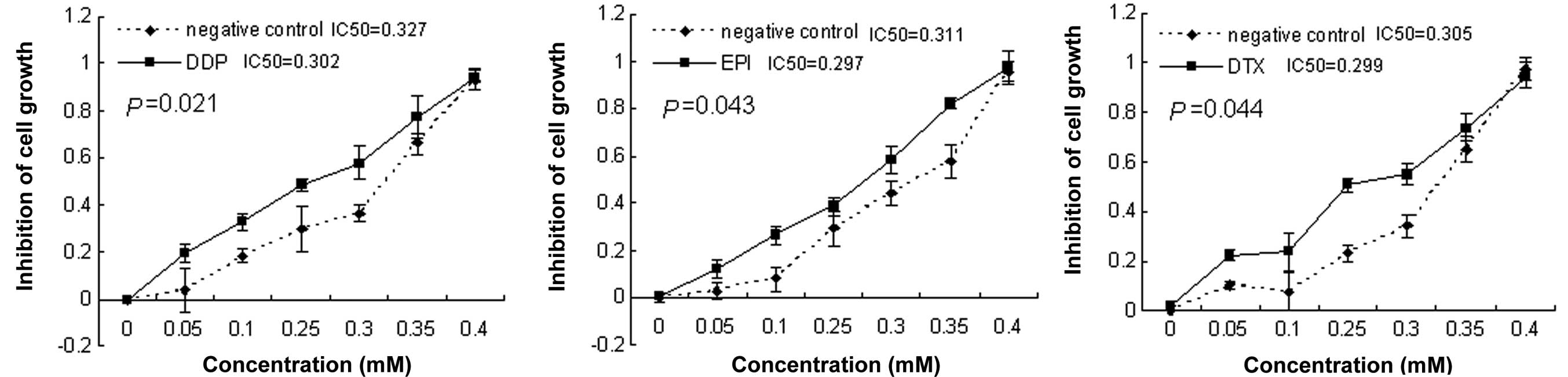
Ezrin downregulation sensitizes CSCs to
chemotherapy drugs
To investigate whether downregulation of Ezrin
expression has the potential to sensitize CSCs to chemotherapy, a
combination treatment of Ezrin-specific siRNA with chemotherapy
drugs was performed. Twenty-four hours after transfection with
siRNA, cells were treated with DDP, EPI and DTX at concentrations
of 0, 0.05, 0.1, 0.25, 0.3, 0.35 and 0.4 mM for 48 h. The
IC50 value was determined by CCK-8 assay. The cells
exposed to the Ezrin siRNA showed a significant decrease in
IC50 for the 3 drugs compared with the control siRNA
(P<0.05, Fig. 2).
Expression status of Ezrin and CD44 and
the relationship between their co-expression and
clinicopathological characteristics
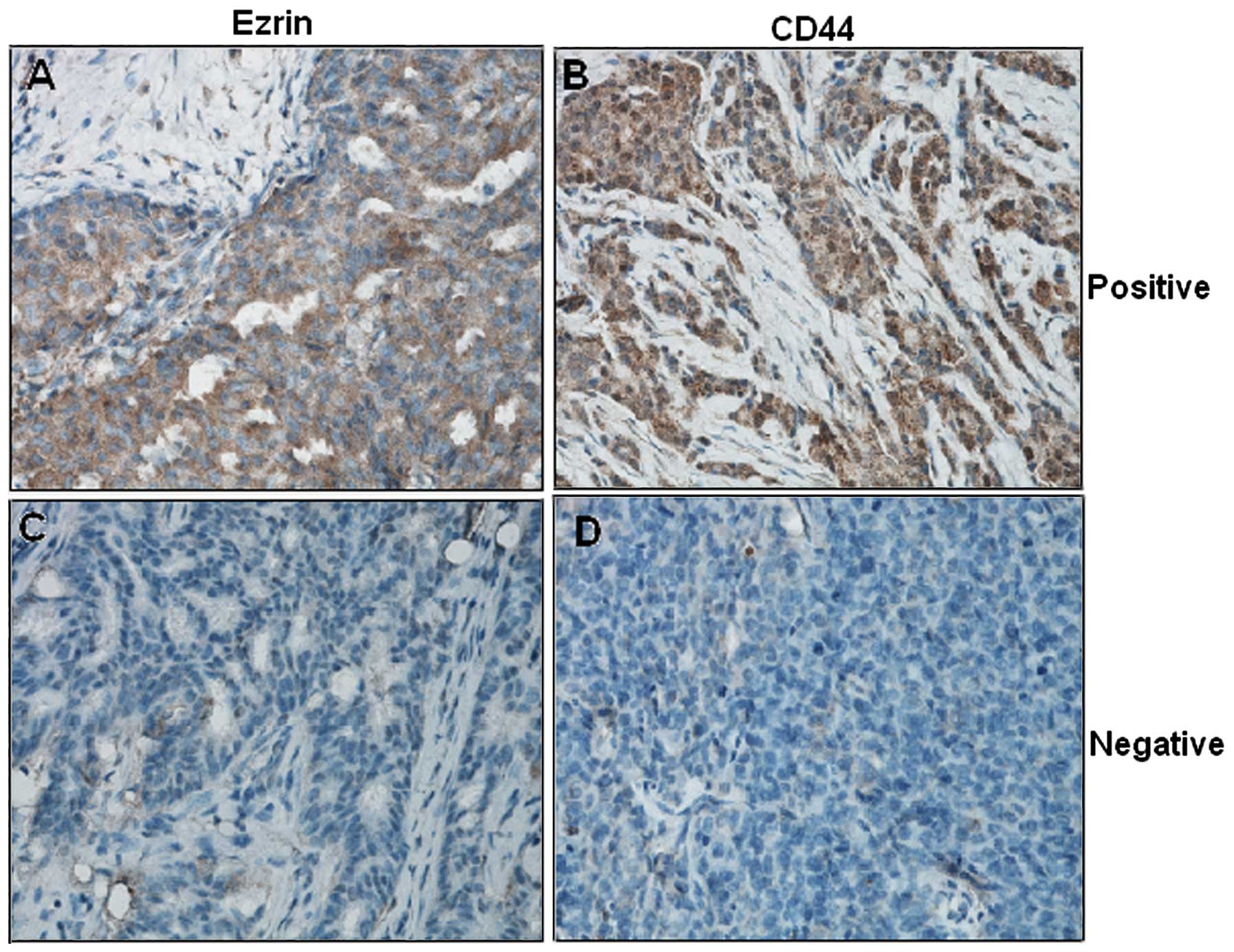
Immunohistochemical examination showed that Ezrin
and CD44 were localized in the membrane ruffles and cytoplasm
(Fig. 3). The majority of patients
(71.35%, 518/726) were Ezrin-positive in the cytoplasm and
membrane; half of the patients (52.20%, 379/726) were
CD44-positive, predominantly in the cytoplasm. It was observed that
the Ezrin protein expression was correlated with CD44 expression
(r=0.285, P<0.001).
Double immunofluorescence staining showed that Ezrin
and CD44 were co-expressed in several cases (Fig. 4). Of the 726 enrolled cases, 235
(32.37%) were determined as co-expressing Ezrin and CD44 protein
(Table I). Univariate analysis
showed that tumor size, histological type, lymph node metastasis,
triple-negative breast cancer, TNM stage, and distant metastasis
were observed to be related to Ezrin and CD44 protein co-expression
(P=0.001, 0.001, 0.001, 0.001, 0.027 and 0.001, respectively;
Table I). Spearman’s correlation
analysis showed that Ezrin and CD44 protein co-expression had a
linear correlation with tumor size, histological type, lymph node
metastasis, triple-negative breast cancer, TNM stage, and distant
metastasis (P=0.001, 0.001, 0.001, 0.001, 0.010 and 0.001,
respectively; Table II). Finally,
multivariate analysis showed that histological type, lymph node
metastasis, triple-negative breast cancer, TNM stage and distant
metastasis were related to Ezrin and CD44 co-expression (P=0.011,
0.006, 0.001, 0.011 and 0.001, respectively; Table III).
 | Table IRelationship between Ezrin and CD44
co-expression and clinicopathological factors (n=726). |
Table I
Relationship between Ezrin and CD44
co-expression and clinicopathological factors (n=726).
| Characteristics | n |
Ezrin+/CD44+ | χ2 | P-value |
|---|
| Age (years) | | | 0.012 | 0.914 |
| ≤35 | 122 | 40 | | |
| >35 | 604 | 195 | | |
| Tumor size | | | 18.881 | 0.001 |
| T1 | 131 | 61 | | |
| T2 | 550 | 167 | | |
| T3 | 45 | 7 | | |
| Histological
type | | | 33.848 | 0.001 |
| I | 50 | 10 | | |
| II | 484 | 131 | | |
| III | 192 | 94 | | |
| Lymph node
metastasis | | | 33.992 | 0.001 |
| Positive | 353 | 151 | | |
| Negative | 373 | 84 | | |
| Triple-negative
breast cancer | | | 17.855 | 0.001 |
| Yes | 182 | 82 | | |
| No | 544 | 153 | | |
| TNM stage | | | 7.221 | 0.027 |
| I | 48 | 13 | | |
| II | 517 | 156 | | |
| III | 161 | 66 | | |
| Distant
metastasis | | | 174.430 | 0.001 |
| Negative | 524 | 95 | | |
| Positive | 202 | 140 | | |
 | Table IISpearman’s correlation analysis
between clinicopathological features, and Ezrin and CD44 protein
co-expression. |
Table II
Spearman’s correlation analysis
between clinicopathological features, and Ezrin and CD44 protein
co-expression.
| Clinicopathological
features | Ezrin and CD44
co-expression P-value (Spearman’s correlation) |
|---|
| Age | 0.914 (0.004) |
| Tumor size | 0.001 (0.161) |
| Histological
type | 0.001 (0.211) |
| Lymph node
metastasis | 0.001 (0.216) |
| Triple-negative
breast cancer | 0.001 (0.096) |
| TNM stage | 0.010 (0.074) |
| Distant
metastasis | 0.001 (0.490) |
 | Table IIIMultivariate analysis of the factors
related to Ezrin and CD44 co-expression. |
Table III
Multivariate analysis of the factors
related to Ezrin and CD44 co-expression.
| Characteristics | OR | 95% CI for OR | P-value |
|---|
| Age | 1.449 | 0.861–2.438 | 0.163 |
| Tumor size | 0.684 | 0.425–1.102 | 0.494 |
| Histological
type | 1.603 | 1.114–2.307 | 0.011 |
| Lymph node
metastasis | 1.664 | 1.009–2.744 | 0.006 |
| Triple-negative
breast cancer | 0.367 | 0.229–0.587 | 0.001 |
| TNM stage | 0.743 | 0.440–1.253 | 0.011 |
| Distant
metastasis | 13.023 | 7.935–21.371 | 0.001 |
Association between Ezrin and CD44
protein co-expression and chemotherapeutic resistance
In the present study, patients exhibiting Ezrin and
CD44 protein co-expression exhibited a significantly higher distant
metastasis rate. Of the 235 cases with Ezrin and CD44 protein
co-expression, 115 (48.94%) developed 5-year, post-operative
distant metastasis, whereas only 88 (17.92%) of cases without Ezrin
and CD44 protein co-expression developed 5-year post-operative
distant metastasis (P=0.001).
We further studied the relationship between Ezrin
and CD44 protein co-expression and chemotherapeutic sensitivity in
102 breast cancer cases with neoadjuvant chemotherapy. Ezrin and
CD44 protein were co-expressed in 16.67, 27.66, 37.50 and 63.16% of
patients with complete response, partial response, stable disease,
and progressive disease (P=0.024), respectively (Table IV).
 | Table IVCorrelations between Ezrin and CD44
protein co-expression and chemotherapeutic resistance of breast
cancers [n=102; n (%)]. |
Table IV
Correlations between Ezrin and CD44
protein co-expression and chemotherapeutic resistance of breast
cancers [n=102; n (%)].
| Response | n |
Ezrin+/CD44+ | χ2
value | P-value |
|---|
| Complete
response | 12 | 2 (16.67) | 9.533 | 0.024 |
| Partial
response | 47 | 13 (27.66) | | |
| Stable disease | 24 | 9 (37.50) | | |
| Progressive
disease | 19 | 12 (63.16) | | |
Prognostic analysis
In the prognostic analysis, Ezrin and CD44 protein
co-expression along with age, histological type, lymph node
metastasis, TNM stage, triple-negative breast cancer, and distant
metastasis were shown to be associated with a poor disease-specific
survival (P=0.001, 0.035, 0.001, 0.010, 0.001, 0.001 and 0.001,
respectively; Fig. 5). In the Cox
regression test, age, tumor size, lymph node metastasis,
triple-negative breast cancer, TNM stage, distant metastasis, and
Ezrin, and CD44 co-expression was detected as independent
prognostic factors (P=0.001, 0.030, 0.006, 0.010, 0.011, 0.007 and
0.027, respectively; Table V).
 | Figure 5Age, histological type, lymph node
metastasis, tumor size, TNM stage, triple-negative breast cancer,
distant metastasis, and Ezrin/CD44 co-expression were shown to be
correlated with patient prognosis (P=0.001, 0.035, 0.001, 0.010,
0.001, 0.001 and 0.001, respectively). |
 | Table VCox model regression analysis of the
breast cancer prognostic factors. |
Table V
Cox model regression analysis of the
breast cancer prognostic factors.
|
Characteristics | OR | 95% CI for OR | P-value |
|---|
| Age | 0.512 | 0.353–0.741 | 0.001 |
| Tumor size | 0.659 | 0.452–0.960 | 0.030 |
| Histological
type | 0.737 | 0.513–1.059 | 0.099 |
| Lymph node
metastasis | 2.007 | 1.215–3.314 | 0.006 |
| Triple-negative
breast cancer | 1.315 | 1.117–1.622 | 0.010 |
| TNM stage | 1.866 | 1.156–3.011 | 0.011 |
| Distant
metastasis | 1.962 | 1.357–2.601 | 0.007 |
| Ezrin and CD44
co-expression | 0.680 | 0.484–0.958 | 0.027 |
Discussion
Recently, studies have confirmed that tumor stem
cells are the cells that initiate tumors and cause malignant tumor
drug resistance, relapse and metastasis (13,14).
Hence, it is important to explore the key factors that regulate the
self-renewal and multipotentiality of tumor cells, and show that
unregulated cell growth is due to disruptions in the regulatory
mechanism of stem cell renewal. Currently, the studies addressing
the function and specific mechanism of stem cell-related genes in
the biological behavior of breast cancer are still sparse (15). In 2003, Al-Hajj et
al(5) first sorted and
identified
CD44+/CD24−/low/Lineage− tumor
cells, which showed higher tumorigenicity and cell microsphere
ability than other groups of cells. This greatly encouraged us to
further explore the characteristics of breast cancer stem cells to
discover new methods of clinical treatment. Little is known,
however, about the relationship between the expression of stem
cell-related genes and the clinicopathological features of breast
cancer.
Ezrin is a protein that is encoded by the EZR gene
in humans (16). As a member of the
ERM protein family, Ezrin works as an intermediary between the
plasma membrane and the actin cytoskeleton. CD44, the breast cancer
CSC marker, receives its anchor function by connecting to the
cytoskeleton via the Ezrin-Radixin-Moesin protein family (17). It has been reported to play a key
role in cell surface structure adhesion, migration and
organization. Ezrin has been shown to interact with CD44 in
osteosarcoma, salivary gland adenoid cystic carcinoma, and in
hematological neoplasias (18,19).
The status of Ezrin protein expression in breast CSCs and breast
cancer, however, is currently unknown. Moreover, the clinical
implications of Ezrin and CD44 co-expression in breast cancer are
also unclear.
In the present study, we selected and identified
breast cancer stem cells based on clinical specimens. It was found
that Ezrin and CD44 were co-expressed in CSC tumor cells and were
related to CSC chemotherapy resistance. We further investigated the
clinical implications of Ezrin and CD44 expression in breast
cancer. Of the 726 breast cancer cases examined in the present
study, Ezrin and CD44 co-expression was observed in 32.37% of
cases. After universal and multivariate analysis, histological
type, lymph node metastasis, triple-negative breast cancer, TNM
stage, and distant metastasis were verified as correlated with
Ezrin and CD44 co-expression. Survival analysis revealed that Ezrin
and CD44 co-expression was also associated with poorer prognosis.
Finally, with Cox regression, Ezrin and CD44 co-expression was
shown to be independent prognostic factors of breast cancer.
In conclusion, Ezrin and CD44 co-expression was
highly expressed in CSCs and may be a potential biomarker for
initiation, progression and differentiation of breast cancer
tumors. The underlying genetic mechanism of Ezrin and CD44
co-expression in breast cancer stem cells, however, remains
unclear. Hence, the relationship between Ezrin gene expression and
the biological behavior of breast cancer stem cells requires
further investigation.
Acknowledgements
The study was funded by the China National Natural
Science Foundation.
References
|
1
|
Dowling EC, Klabunde C, Patnick J and
Ballard-Barbash R; International Cancer Screening Network (ICSN).
Breast and cervical cancer screening programme implementation in 16
countries. J Med Screen. 17:139–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsuji W, Teramukai S, Ueno M, Toi M and
Inamoto T: Prognostic factors for survival after first recurrence
in breast cancer: a retrospective analysis of 252 recurrent cases
at a single institution. Breast Cancer. Apr 5–2012.(Epub ahead of
print).
|
|
3
|
Magee JA, Piskounova E and Morrison SJ:
Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer
Cell. 21:283–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu C, Cao X, Zhang Y, Xu H, Zhang R, Wu
Y, Lu P and Jin F: Co-expression of Oct-4 and Nestin in human
breast cancers. Mol Biol Rep. 39:5875–5881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Louderbough JM and Schroeder JA:
Understanding the dual nature of CD44 in breast cancer progression.
Mol Cancer Res. 9:1573–1586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ren L, Hong SH, Chen QR, Briggs J,
Cassavaugh J, Srinivasan S, Lizardo MM, Mendoza A, Xia AY, Avadhani
N, et al: Dysregulation of ezrin phosphorylation prevents
metastasis and alters cellular metabolism in osteosarcoma. Cancer
Res. 72:1001–1012. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bulut G, Hong SH, Chen K, Beauchamp EM,
Rahim S, Kosturko GW, Glasgow E, Dakshanamurthy S, Lee HS, Daar I,
et al: Small molecule inhibitors of ezrin inhibit the invasive
phenotype of osteosarcoma cells. Oncogene. 31:269–281. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boldrini E, Peres SV, Morini S and de
Camargo B: Immuno-expression of Ezrin and CD44 in patients with
osteosarcoma. J Pediatr Hematol Oncol. 32:e213–e217. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dontu G, Abdallah WM, Foley JM, Jackson
KW, Clarke MF, Kawamura MJ and Wicha MS: In vitro propagation and
transcriptional profiling of human mammary stem/progenitor cells.
Genes Dev. 17:1253–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu W, Rui H, Wang J, Lin S, He Y, Chen M,
Li Q, Ye Z, Zhang S, Chan SC, et al: Axin is a scaffold protein in
TGF-β signaling that promotes degradation of Smad7 by Arkadia. EMBO
J. 25:1646–1658. 2006.
|
|
12
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(in German).
|
|
13
|
Liu S, Clouthier SG and Wicha MS: Role of
microRNAs in the regulation of breast cancer stem cells. J Mammary
Gland Biol Neoplasia. 17:15–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Countercurrents Series and Narod SA. A
model for breast cancer risk based on stem-cell theory. Curr Oncol.
19:9–11. 2012.PubMed/NCBI
|
|
15
|
Liu CG, Lu Y, Wang BB, Zhang YJ, Zhang RS,
Lu Y, Chen B, Xu H, Jin F and Lu P: Clinical implications of stem
cell gene Oct-4 expression in breast cancer. Ann Surg.
253:1165–1171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gould KL, Bretscher A, Esch FS and Hunter
T: cDNA cloning and sequencing of the protein-tyrosine kinase
substrate, ezrin, reveals homology to band 4.1. EMBO J.
8:4133–4142. 1989.PubMed/NCBI
|
|
17
|
Hart SP, Rossi AG, Haslett C and
Dransfield I: Characterization of the effects of cross-linking of
macrophage CD44 associated with increased phagocytosis of apoptotic
PMN. PLoS One. 7:e331422012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang YY, Chen WL, Huang ZQ, Yang ZH, Zhang
B, Wang JG, Li HG and Li JS: Expression of the
membrane-cytoskeletal linker Ezrin in salivary gland adenoid cystic
carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
112:96–104. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hertweck MK, Erdfelder F and Kreuzer KA:
CD44 in hematological neoplasias. Ann Hematol. 90:493–508. 2011.
View Article : Google Scholar : PubMed/NCBI
|