Introduction
Breast cancer continues to be a major health threat
to women worldwide despite the recent advances in diagnosis and
treatment. It was estimated that 1.38 million new breast cancer
cases were diagnosed in 2008 (23% of all cancers) (1), and more than 400,000 people die from
breast cancer each year in the world (2). Hence, it is essential to develop new
anticancer drugs and novel regimens that are capable of killing
cancer cells, particularly drug-resistant cells.
TRAIL preferentially exerts its obvious suppression
of growth in a variety of cancer cells without influencing normal
tissues and cells (3) as compared
to tumor necrosis factor (TNF) and FasL which lead to severe liver
damage and lethal inflammatory reactions (4,5).
Nevertheless, recent studies suggest that TRAIL resistance exists
in many tumor cell lines (6),
particularly certain highly malignant tumor cells (7,8). Worse
still, some cancer cells initially sensitive to TRAIL may develop
resistance to TRAIL after repeated use (9). Thus, it is indicated that TRAIL alone
may be ineffective for breast cancer therapy.
Fortunately, many studies have shown that
chemotherapeutic drugs can lead to enhanced apoptosis induction in
TRAIL-resistant tumor cells (10,11).
Cisplatin is a widely used conventional chemotherapeutic drug used
for breast cancer treatment. By forming DNA adducts, cisplatin
leads to DNA damage through p53-dependent and p53-independent
mechanisms (12,13). Yet, it is known to cause severe
side-effects, being toxic to normal cells and organs at the
concentrations essential for effective treatment of malignancies
(14,15).
With the development of molecular biology and
research into the pathogenesis of various types of tumors, gene
therapy has become a potential method for the biotherapy of tumors
(16). Gene therapy applies a
variety of delivery vehicles to transfer therapeutic genes to tumor
cells. Adenovirus is now one of the most prospective delivery
vectors for gene therapy due to advantages, such as low
pathogenicity, lack of immunogenicity, infection of non-dividing
cells and steady integration into the tumor cell genome at a
targeted site (17). To date,
adenovirus as an excellent vector has been used in many clinical
trials for treating certain types of malignancies (18,19).
Moreover, adenovirus possesses a broad host range; breast cancer
cells included (20).
In the present research, two breast cancer cell
lines, MDA-MB-468 and HCC-1937, were treated with Ad-sTRAIL and
cisplatin to investigate their antitumor efficacy in vitro
and explore the molecular mechanism. The results showed that
cisplatin sensitized breast cell lines to TRAIL-induced apoptosis
probably by upregulating the level of DR5, downregulating the
expression of cFLIP and BCL2L1 and inducing activation of
caspase-8. Therefore, Ad-sTRAIL in combination with the
conventional chemotherapeutic agent cisplatin has potential for
clinical cancer therapy.
Materials and methods
Cell lines and reageats
Human breast cancer cell lines MDA-MB-468 and
HCC-1937 were purchased from the Type Culture Collection of the
Chinese Academy of Science (Shanghai, China). MDA-MB-468 cells were
maintained in L-15 medium (Gibco), and HCC-1937 cells were
maintained in RPMI-1640 medium (HyClone), both with 10% fetal
bovine serum (FBS) (HyClone) as recommended by the Type Culture
Collection. Cells were incubated under 5% CO2 and at
37°C. The cells were always detached using 0.25% trypsin (Amresco)
and 0.02% ethylenediaminetetraacetic acid (EDTA). Cisplatin (10 mg)
was purchased from Qilu Pharmaceutical Co., Ltd. (Jinan, China).
The recombinant Ad-sTRAIL (the virus titer was 2.18×109
IFU/ml) was constructed by the Central Laboratory, The Affiliated
Hospital of the Medical College, Qingdao University. DAPI dye and
crystal violet dye were obtained from Beyotime Institute of
Biotechnology. RNAiso reagent (TRIzol reagent), the SuperScript™ II
reverse transcriptase kit and PCR-DRR081A (used for fluorogenic
quantitative RT-PCR) were obtained from Takara Biotechnology Co.,
Ltd. Annexin V-PE (R-phycoerythrin) and propidium iodide (PI) for
flow cytometry were purchased from Invitrogen Corp. Primary
antibodies against DR5 and caspase-8 were purchased from Cell
Signaling Technology. Anti-glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) rabbit polyclonal antibody was obtained from Abcam. Unless
mentioned, all other reagents were obtained from Sigma.
MTT assay
The effect of Ad-sTRAIL and cisplatin on MDA-MB-468
and HCC-1937 cell viability was measured using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Cells (4×104) in 100 μl medium were seeded in
each well of a 96-well plate and were left for 24 h to adhere. The
cells were then treated with different concentrations of cisplatin
for 48 h. Thereafter, 20 μl of MTT (5 mg/ml) was added into each
well and incubated under 5% CO2 and at 37°C for another
4 h. After removal of the culture medium, the cells were lysed in
150 μl of dimethylsulfoxide (DMSO). The plate was shaken until the
crystals were dissolved. Afterwards, the absorption at 490 nm
(OD490) was measured using a microplate reader (Bio-Rad
Laboratories, Hercules, CA, USA). In this way, the half maximal
inhibitory concentration (IC50) of cisplatin was
determined. The cells were then treated with cisplatin (5 μg/ml)
and Ad-sTRAIL (multiplicity of infection MOI =10), alone or
combined for 48 h. The inhibitory rates of the different groups
were calculated using the formula: Cell inhibitory rate (%) = (1 −
OD of the experimental group/OD of the control group) × 100%.
Crystal violet staining assay
The breast cancer cells were seeded in each well of
a 24-well plate with a density of 2×105/well at
subconfluent levels. The cells were treated with various
concentrations of cisplatin and were infected with Ad-sTRAIL at
various MOIs, alone or combined. After 7 day of incubation at 37°C
and 5% CO2, the medium was removed and cells were
exposed to 2% crystal violet in 20% methanol for 15 min. The plates
were then washed with deionized water until the dye was washed off
and finally documented as photographs.
DAPI staining assay
The breast cancer cells were cultured in each well
of a 24-well plate with a density of 3×105/well with 1
ml medium. The cells were then treated with cisplatin (5 μg/ml) and
Ad-sTRAIL (MOI=10), alone or combined. After incubation for 48 h,
cells were collected and centrifuged at 3,000 rpm for 5 min at room
temperature, washed twice in phosphate-buffered saline (PBS), fixed
with 4% formaldehyde at room temperature for 30 min and washed with
PBS twice again after fixation. Afterwards, 200 μl of
4,6-diamidino-2-phenylindole (DAPI; 2 μl/ml) was added into each
well for incubation for 30 min at room temperature avoiding light
exposure. After discarding the DAPI dye, cells were washed with PBS
twice to remove the redundant fluorescent dye. The preparation was
completely resuspended in the antifading mounting medium. The
preparation was then transferred to glass slides for observation
under a fluorescence microscope (DM2500; Leica Microsystems,
Wetzlar, Germany) and images were captured and analyzed.
Flow cytometric analysis
Cells were seeded in each well of 6-well plates at a
density of 5×106 and treated with Ad-sTRAIL and/or
cisplatin for 48 h. Both adherent and suspended cells were
collected, washed in PBS and then suspended in Annexin-binding
buffer. Afterwards, cells were stained with Annexin V-PE or PI to
distinguish apoptotic and dead cells. All steps were conducted in
accordance with the manufacturer’s instructions. Finally, the
stained cells were analyzed by flow cytometry.
Real-time fluorogenic quantitative
RT-PCR
Total mRNA was extracted from the cells of the 4
groups using TRIzol reagent and then quantitated using an
ultraviolet spectrophotometer (Beckman Coulter) by measuring
A260 and A280. Then 0.5 mg mRNA of each
sample was reversely transcribed into cDNA using the PrimeScript
reverse transcriptase kit. To assess the levels of mRNA of the
target gene, we used real-time fluorescence quantitative PCR
analysis based on the SYBR-Green method in A480
real-time thermal cycler (Roche). The PCR reactions in duplicate
were subjected to an initial denaturation at 95°C for 10 sec,
followed by 40 cycles of denaturation at 95°C for 5 sec, annealing
and extension at 60°C for 45 sec. The value of the threshold cycle
(Ct) for each reaction was recorded. The levels of target mRNA
transcripts relative to GAPDH were expressed as the ΔCt
(CtTarget − CtGAPDH) and further quantified
using the 2−ΔΔCT method, where ΔΔCt =
(CtTarget − CtGAPDH)experimental
group − (CtTarget −
CtGAPDH)control group(21). RT-PCR was performed with primer
pairs for TRAIL: forward (5′-AGTGAGAGAAAGAGGTCCTCAG-3′) and reverse
(5′-CCAGAGCCTTTTCATTCTTGGA-3′); for cFLIP: forward
(5′-AGAGTGAGGCGATTTGACCTG-3′) and reverse
(5′-GTCCGAAACAAGGTGAGGGTT-3′); for BCL2L1: forward
(5′-GACTGAATCGGAGATGGAGACC-3′) and reverse
(5′-GCAGTTCAAACTCGTCGCCT-3′); for BCL2: forward
(5′-ATGTGTGTGGAGAGCGTCAACC-3′) and reverse
(5′-TGAGCAGAGTCTTCAGAGACAGCC-3′); and for GAPDH as a control:
forward (5′-TCATGGGTGTGAACCATGAGAA-3′) and reverse
(5′-GGCATGGACTGTGGTCATGAG-3′).
Western blot analysis
Cell samples were lysed in ice-cold lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China) with 1% PMSF
(phenylmethylsulfonyl fluoride) for half an hour, then centrifuged
at 10,000 × g for 20 min at 4°C, and the protein concentration of
the resulting supernatant was determined using the BCA
(bicinchoninic acid) protein assay kit (Beyotime Institute of
Biotechnology). Proteins (50 μg) were separated by 12% SDS-PAGE
electrophoresis and subsequently transferred to PVDF membranes.
Membranes were blocked with 5% non-fat dry milk in TBS/Tween-20
(0.05%, v/v) for 2 h at room temperature and incubated overnight at
4°C with the primary antibodies directed against DR5, caspase-8 and
GAPDH. The blots were washed and incubated with the horseradish
peroxidase-conjugated secondary antibody (DakoCytomation) and
developed with a chemiluminescent substrate, ECL Plus. An
autoradiograph was obtained, and protein levels were measured using
a Fluor-S scanner and Quantity One software for analysis (Bio-Rad
Laboratories).
Statistical analysis
All experiments were conducted at least three times,
and experimental data are expressed by the means ± SD. P-values
were determined by the Student’s t-test. P<0.05 was considered
to indicate a statistically significant result. IC50
values were calculated using the GraphPad Prism version 5.00 for
Windows (GraphPad software, San Diego, CA, USA).
Results
Combination of Ad-sTRAIL and cisplatin
promotes the apoptosis of breast cancer cells
The MDA-MB-468 and HCC-1937 cells were treated with
different concentrations of cisplatin for 48 h, and thereafter the
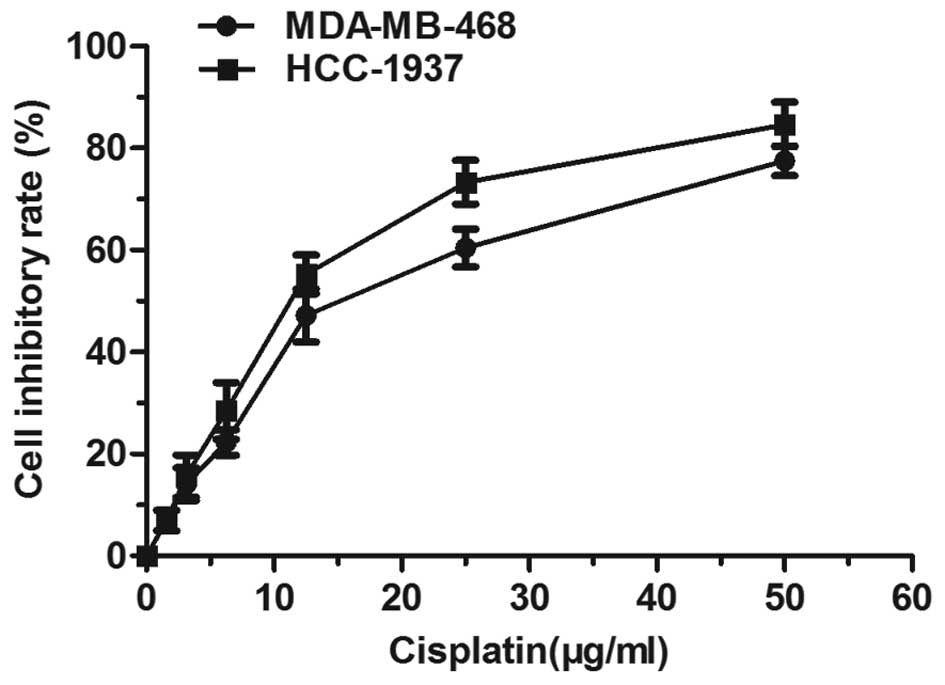
inhibition rate was determined. Fig.
1 shows that cisplatin triggered a dose-dependent inhibition of
cancer cell proliferation. Yet, when the concentration was >30
μg/ml, the inhibition rate increased slowly, entering into a
plateau period. The 50% inhibitory concentration (IC50)
of the MDA-MB-468 cells was 15.32 μg/ml, and that of HCC-1937 at 48
h was 11.91 μg/ml. Examination of the cisplatin effect on
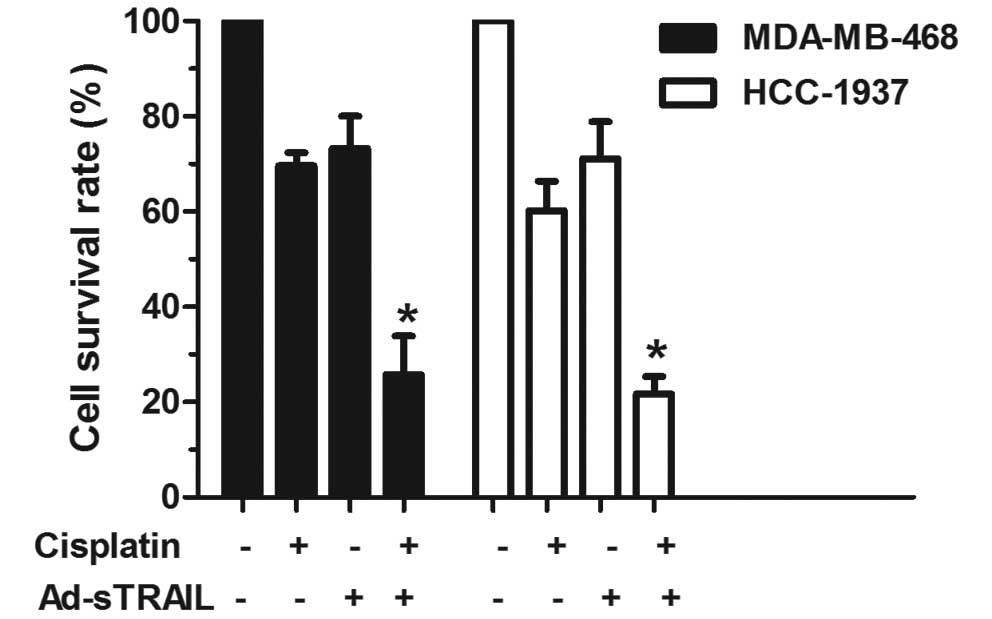
TRAIL-induced apoptosis showed that cisplatin exhibited a
synergistic effect on TRAIL-induced apoptosis in the MDA-MB-468 and
HCC-1937 cell lines (Fig. 2). The
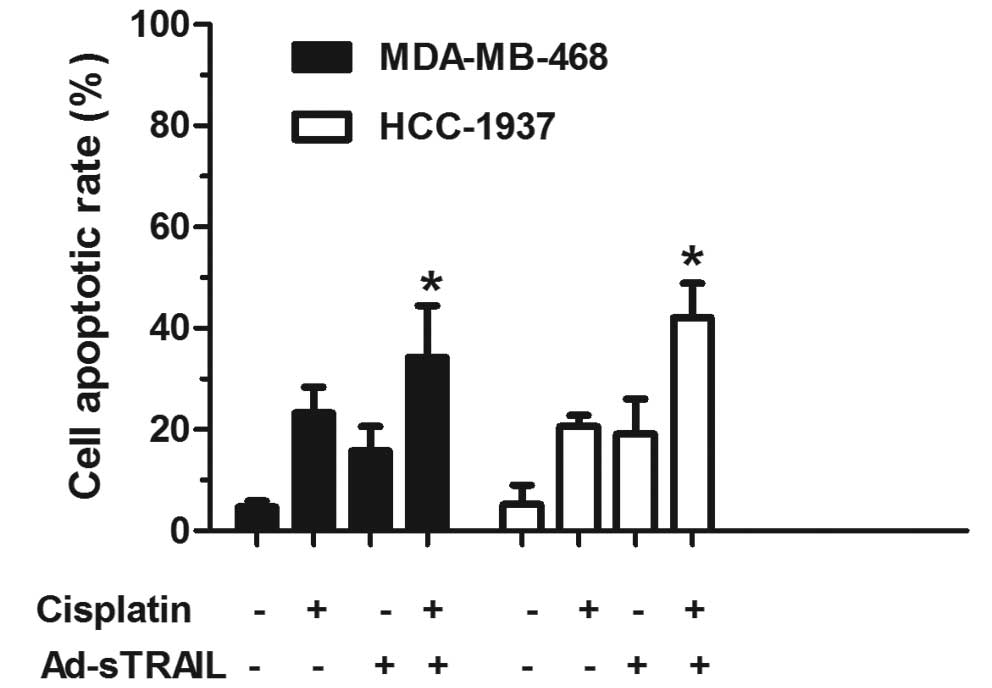
flow cytometric analysis showed that the apoptotic proportion of
MDA-MB-468 and HCC-1937 cells treated with Ad-sTRAIL combined with
cisplatin was significantly higher (34.32 and 42.16%, respectively)
than the apoptotic proportion of cells treated with either agent
alone (Fig. 3). The following
crystal violet assay further confirmed the above observations. The
images indicate that the treatment of cisplatin or Ad-sTRAIL alone
was dose-dependent (Fig. 4).
Moreover, in the condition that the concentration of cisplatin was
6.25 μg/ml plus the MOI of Ad-sTRAIL was 10, the effect of the
combination treatment was more effective than that of cisplatin (25
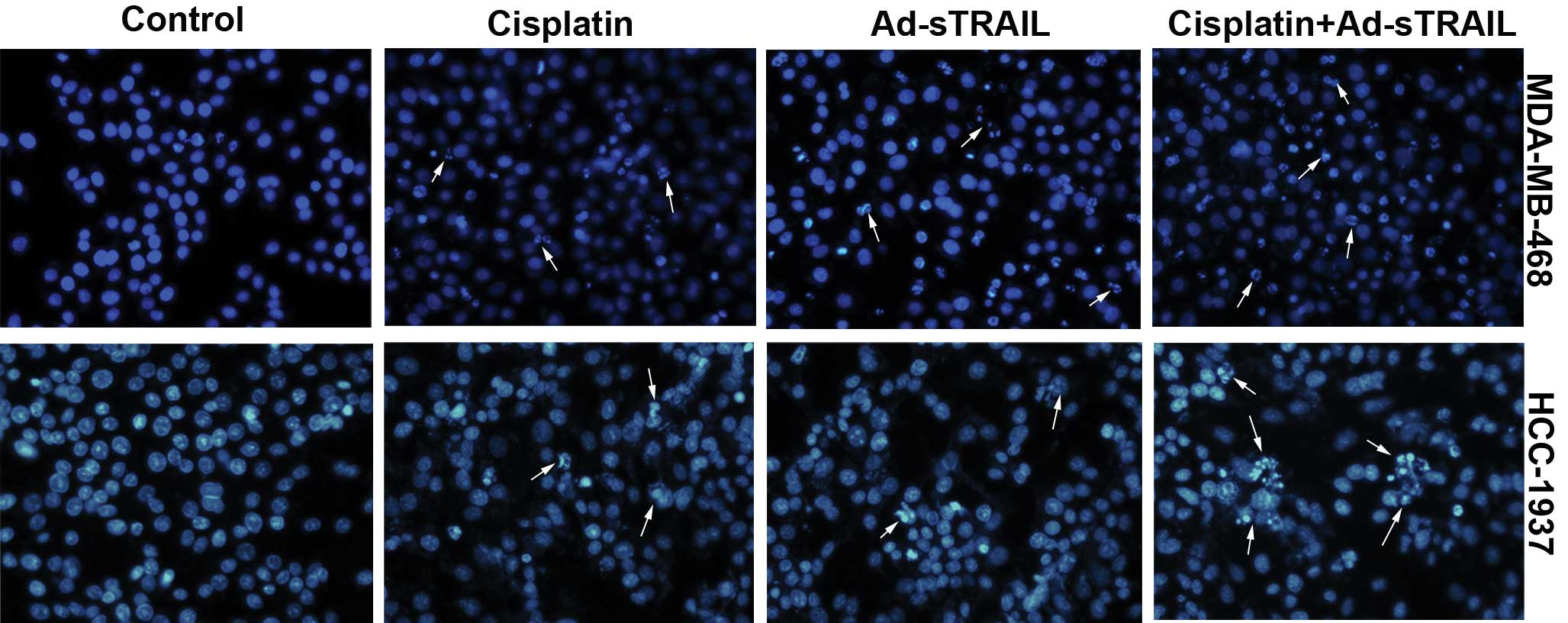
μg/ml) or Ad-sTRAIL (MOI=100) alone. The observations of the DAPI
assay were consistent with the former results. Nuclear changes were
noted which showed that few nuclei of apoptotic cells were noted in
the control groups. In the groups receiving the combined treatment,
large numbers of apoptotic cell nuclei were noted, which were more
than in the groups treated with either agent alone (Fig. 5).
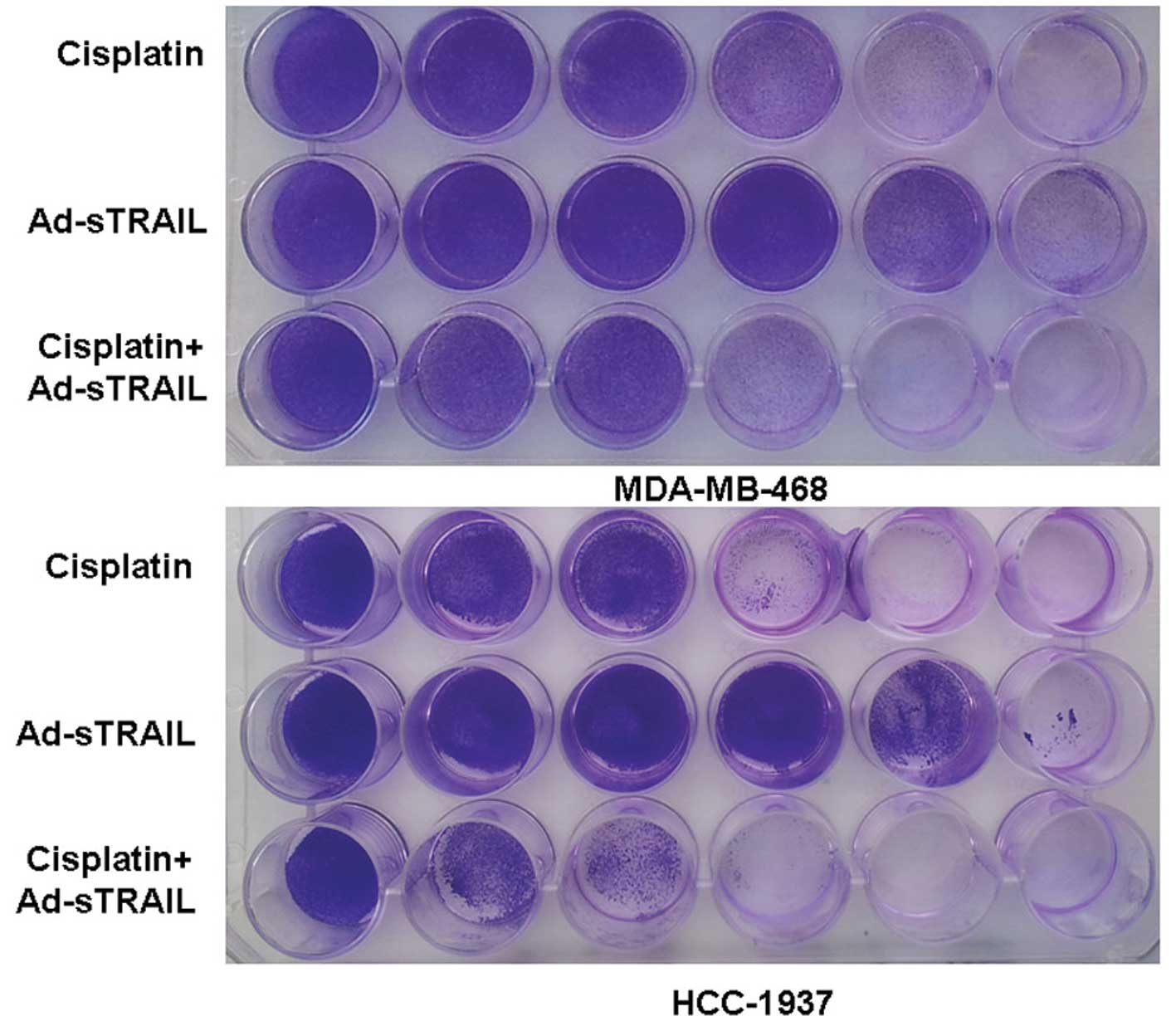 | Figure 4Enhanced suppressive effects of tumor
cell proliferation by treatment with a combination of Ad-sTRAIL and
cisplatin. From left to right, the concentration of cisplatin was
0, 1.57, 3.12, 6.25, 12.5 and 25 μg/ml. In the same order, the MOI
of Ad-sTRAIL was 0, 1, 5, 10, 50 and 100. Similarly, each well of
the third row was treated with the same dosage of cisplatin and
Ad-sTRAIL as the two wells above it. After 7 days of incubation,
cells were stained with 2% crystal violet. The experiment was
repeated three times with essentially the same results. |
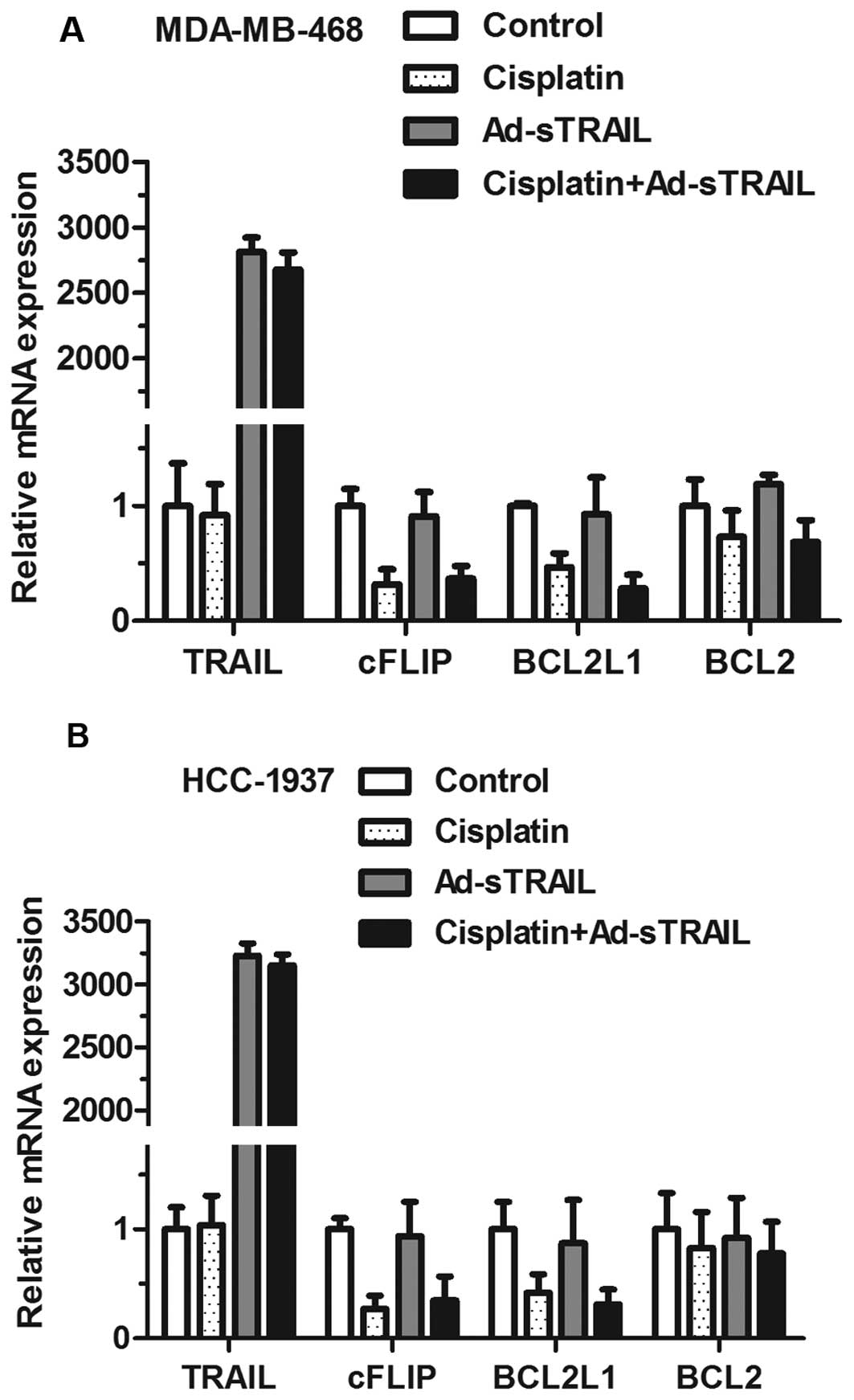
Ad-sTRAIL and cisplatin treatment
downregulates the level of anti-apoptotic molecules cFLIP and
BCL2L1
The effects of Ad-sTRAIL and cisplatin on the
expression of anti-apoptotic molecules cFLIP and BCL2L1 in
MDA-MB-468 and HCC-1937 cell lines were examined. The data in
Fig. 6 indicate that, in the groups
receiving either agent alone, the inhibition of cFLIP mRNA and
BCL2L1 mRNA was more obvious than that in the control group.
Moreover, the maximum effect of inhibition appeared in the group
treated with both agents. In addition, TRAIL mRNA was significantly
enhanced in groups treated with Ad-sTRAIL, while BCL2 mRNA did not
show significant changes whether or not cells were treated with
cisplatin.
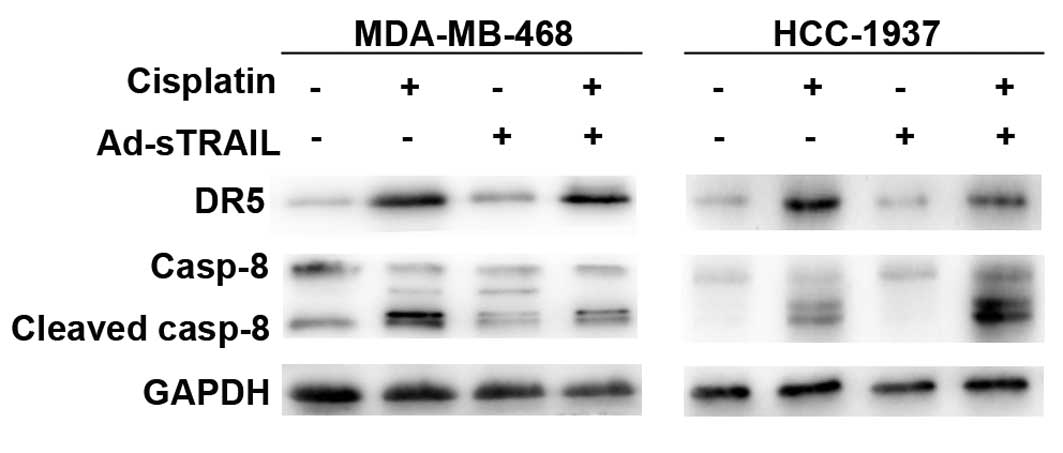
Cisplatin upregulates DR5 and enhances
the activation of caspase-8, thus enhancing the sensitivity to
Ad-sTRAIL of breast cancer cells
We investigated the expression of DR5 in the 4
groups. Fig. 7 indicates that
cisplatin enhanced the expression of DR5 in comparison to the
control group. Expression of DR5 in the groups treated with
Ad-sTRAIL was almost unchanged. Previous studies have confirmed
that caspases play an important part in TRAIL-induced apoptosis
(22). Fig. 7 shows that, in the groups treated
with cisplatin, the activity of caspase-8 was increased compared to
that in the control groups. Furthermore, the groups treated with
Ad-sTRAIL and cisplatin showed a maximum effect for the activity of
caspase-8.
Discussion
Toxic side-effects and resistance of many tumors to
current-treatment strategies, particularly to chemotherapeutic
strategies continue to be a main concern of cancer treatment. Thus,
it is essential to develop more effective therapeutic modalities
which are simultaneously specific for tumor cells with low toxicity
for normal cells. The recombinant adenovirus, a non-pathogenic
virus with a single-stranded DNA genome, has a broad host range and
can be used to transduce breast cancer cells. Thus, it is presently
being used in a number of clinical trials and may become a
prospective component of antitumor therapy. Cisplatin is one of
most efficacious chemotherapeutic drugs for breast cancer; however,
it produces major toxicities to normal cells at the concentrations
effective for cancer treatment. Therefore, the combination of
chemotherapeutic agents and gene therapy is a potential efficacious
strategy for breast cancer treatment (23).
TRAIL resistance is exhibited in many types of tumor
cells; thus, attenuating its monotherapy in the clinic. Shin et
al(24) reported that
TRAIL-resistant human hepatocellular carcinoma becomes sensitive to
TRAIL by co-treatment with cycloheximide and cisplatin,
respectively. Furthermore, Jiang et al(25) also found that AAV-mediated TRAIL
expression in conjunction with cisplatin demonstrated synergistic
therapeutic effects on head and neck cancers. These studies
demonstrated that the combined use of TRAIL and chemotherapeutic
agents is promising. Thus, we applied Ad-sTRAIL and cisplatin
concomitantly to two types of breast cancer cells. In the present
study, we confirmed that MDA-MB-468 and HCC-1937 cells treated with
Ad-sTRAIL and cisplatin exhibited significantly increased apoptosis
than untreated cells. Moreover, the present study also confirmed
that cisplatin promotes the sensitivity of cancer cells to
TRAIL-induced apoptosis by the increased expression of DR5,
suppression of cFLIP and BCL2L1 and activation of caspase-8.
TRAIL induces apoptosis through binding to cell
surface receptors. To date, five receptors of TRAIL have been
identified. Among these, TRAIL-R1 (DR4), TRAIL-R2 (DR5), TRAIL-R3
(DcR1) and TRAIL-R4 (DcR2) are membrane-bound receptors. DR4 and
DR5 contain a cytoplasmic death domain, while DcR1 and DcR2 are
decoy receptors without a cytoplasmic death domain (26). TRAIL is shown to bind with DR4 and
DR5, leading to the formation of Fas-associated protein with death
domain (FADD). FADD and caspase-8 are recruited to form the
death-inducing signaling complex (DISC). Activation of procaspase-8
at the DISC triggers the downstream signaling cascades mainly
through mitochondrial-dependent and mitochondrial-independent
pathways. In the former pathway, activated caspase-8 cleaves Bid
thus forming truncated Bid (tBid) which translocates to the
mitochondria causing release of cytochrome c, the loss of
mitochondrial membrane potential and activation of caspase-9 and
-3. In the latter pathway, activated caspase-8 activates caspase-3
directly. On the whole, activated caspase-3 cleaves target
substrates and finally leads to cell apoptosis in both pathways
(27). Osteoprotegerin, a soluble
receptor for TRAIL may act as a third decoy receptor (28).
Most tumor cells exhibit high expression of DR4 and
DR5, yet with less or no DcR1 and DcR2. Therefore, TRAIL could
promote the apoptosis of tumor cells by combining with DR4 and DR5.
But in most normal tissues and cells, the expression of DcR1 and
DcR2 dominates, and they bind to TRAIL thus protecting normal
tissues from TRAIL-induced apoptosis (29). In the present study, cispatin
treatment upregulated the expression of DR5 and thereafter
sensitized breast cancer cells to TRAIL-induced apoptsis. The
results indicate that the resistance of breast cancer cells to
TRAIL may be partly due to the low expression of DR5.
The BCL2 family consisting of anti-apoptotic and
pro-apoptotic members regulates cell apoptosis by inhibiting or
promoting mitochondrial apoptosis signals. The former includes
BCL2, BCL2L1 and Mcl-1, and the latter includes Bax, Bak, Bid and
Bim (30,31). The anti-apoptotic members of the
BCL2 family carry out their actions by inhibiting the release of
cytochrome c from the mitochondria to the cytoplasm and by
maintaining the integrity of the mitochondrial membrane by
preventing mitochondrial swelling and membrane hyperpolarization
(32,33). In the present study, the expression
of BCL2L1 mRNA was attenuated in cells following treatment of
cisplatin, while BCL2 mRNA did not show significant changes whether
or not cells were treated with cisplatin. Kim et al(34) performed functional genetic screening
to isolate genes interfering with TRAIL-induced apoptosis using a
cDNA retroviral library, and concluded that BCL2L1 but not BCL2
suppressed TRAIL-induced apoptosis in tumor cells. The present
study further indicates that BCL2L1 may play an important role in
the resistance of breast cancer cells to TRAIL.
cFLIP is a newly discovered apoptosis-inhibited
protein which is composed of two isoforms: cFLIPs (a
short form) and cFLIPL (a long form). Both of them
contain two death effector domains (DED) that bind to FADD, thus
preventing caspase-8 binding to FADD and inhibiting cell apoptosis
mediated by TRAIL (35,36). Wang et al(37) reported that knockdown of cFLIP by a
short hairpin RNA (shRNA) rendered resistant pancreatic cells
sensitive to TRAIL-induced apoptosis through the cleavage of
caspase-8 and activation of the mitochondrial pathway. Brooks and
Sayers (38) also demonstrated that
treatment of human renal carcinoma cells with small interfering
oligoribonucleotides (siRNA) for cFLIP caused a reduction in cFLIP
protein and sensitized cells to TRAIL-mediated apoptosis. In the
present study, we showed that the expression of cFLIP was
significantly decreased in cells treated with cispaltin, and cells
exposed to cisplatin plus TRAIL exhibited significantly enhanced
apoptosis in comparison with the untreated cells. Therefore, the
resistance of breast cancer cells to TRAIL was probably in part due
to the overexpression of cFLIP proteins.
Based on the above-mentioned results, it was shown
that TRAIL in combination with cisplatin showed excellent antitumor
efficacy in MDA-MB-468 and HCC-1937 breast cancer cell lines by the
increased expression of DR5, and decreased expression of cFLIP and
BCL2L1. Simultaneously, the combination of cisplatin and Ad-sTRAIL
attenuated the potential toxicity of cisplatin. These findings
prove that the combination of cispaltin and Ad-sTRAIL is a
potential therapeutic mode for breast cancer; and gene therapy may
find its own niche in the comprehensive treatment of malignancies.
Moreover, we demonstrated that the resistance of breast cancer to
TRAIL may be due to the low expression of death receptors and
overexpression of cFLIP; consequently, the mechanisms of the
resistance of various types of tumors to TRAIL remain to be further
clarified.
Acknowledgements
The present study was supported by grants from the
Natural Science Foundation of the Shandong Province of China
(Y2008C48), the Department of Education of the Shandong Province of
China (J11LF05) and the Research Program of the Qingdao South
District Municipal Science and Technology Commission
(2011-5-004-YY).
References
|
1
|
Curado MP: Breast cancer in the world:
incidence and mortality. Salud Publica Mex. 53:372–384.
2011.PubMed/NCBI
|
|
2
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kelley SK and Ashkenazi A: Targeting death
receptors in cancer with Apo2L/TRAIL. Curr Opin Pharmacol.
4:333–339. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ashkenazi A, Pai RC, Fong S, Leung S,
Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert
A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen
H, Shahrokh Z and Schwall RH: Safety and antitumor activity of
recombinant soluble Apo2 ligand. J Clin Invest. 104:155–162. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ryo K, Kamogawa Y, Ikeda I, Yamauchi K,
Yonehara S, Nagata S and Hayashi N: Significance of Fas
antigen-mediated apoptosis in human fulminant hepatic failure. Am J
Gastroenterol. 95:2047–2055. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan S, Qu X, Xu C, Zhu Z, Zhang L, Xu L,
Song N, Teng Y and Liu Y: Down-regulation of Cbl-b by bufalin
results in up-regulation of DR4/DR5 and
sensitization of TRAIL-induced apoptosis in breast cancer cells. J
Cancer Res Clin Oncol. 138:1279–1289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eggert A, Grotzer MA, Zuzak TJ, Wiewrodt
BR, Ho R, Ikegaki N and Brodeur GM: Resistance to tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis
in neuroblastoma cells correlates with a loss of caspase-8
expression. Cancer Res. 61:1314–1319. 2001.
|
|
8
|
Hyer ML, Croxton R, Krajewska M, Krajewski
S, Kress CL, Lu M, Suh N, Sporn MB, Cryns VL, Zapata JM and Reed
JC: Synthetic triterpenoids cooperate with tumor necrosis
factor-related apoptosis-inducing ligand to induce apoptosis of
breast cancer cells. Cancer Res. 65:4799–4808. 2005. View Article : Google Scholar
|
|
9
|
Zhang L and Fang B: Mechanisms of
resistance to TRAIL- inducing apoptosis in cancer. Cancer Gene
Ther. 12:228–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh TR, Shankar S, Chen X, Asim M and
Srivastava RK: Synergistic interactions of chemotherapeutic drugs
and tumor necrosis factor-related apoptosis-inducing ligand/Apo-2
ligand on apoptosis and on regression of breast carcinoma in vivo.
Cancer Res. 63:5390–5400. 2003.
|
|
11
|
Hesry V, Piquet-Pellorce C, Travert M,
Donaghy L, Jégou B, Patard JJ and Guillaudeux T: Sensitvity of
prostate cells to TRAIL-induced apoptosis increases with tumor
progression: DR5 and caspase 8 are key players. Prostate.
66:987–995. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Siddik ZH: Cisplatin: mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagane M, Pan G, Weddle JJ, Dixit VM,
Cavenee WK and Huang HJ: Increased death receptor 5 expression by
chemotherapeutic agents in human gliomas causes synergistic
cytotoxicity with tumor necrosis factor-related apoptosis inducing
ligand in vitro and in vivo. Cancer Res. 60:847–853. 2000.
|
|
14
|
Boulikas T and Vougiouka M: Recent
clinical trials using cisplatin, carboplatin and their combination
chemotherapy drugs. Oncol Rep. 11:559–595. 2004.PubMed/NCBI
|
|
15
|
Burtness B, Goldwasser MA, Flood W, Mattar
B and Forastiere AA; Eastern Cooperative Oncology Group. Phase III
randomized trial of cisplatin plus placebo compared with cisplatin
plus cetuximab in metastatic/recurrent head and neck cancer: an
Eastern Cooperative Oncology Group study. J Clin Oncol.
23:8646–8654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wiezorek J, Holland P and Graves J: Death
receptor agonists as a targeted therapy for cancer. J Clin Cancer
Res. 16:1701–1708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mandel RJ, Manfredsson FP, Foust KD,
Rising A, Reimsnider S, Nash K and Burger C: Recombinant
adeno-associated viral vectors as therapeutic agents to treat
neurological disorders. Mol Ther. 13:463–483. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wagner JA, Reynolds T, Moran ML, Moss RB,
Wine JJ, Flotte TR and Gardner P: Efficient and persistent gene
transfer of AAV-CFTR in maxillary sinus. Lancet. 351:1702–1703.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Huang F, Cai H, Wu Y, He G and Tan
W: The efficacy of combination therapy using adeno-associated
virus-TRAIL targeting to telomerase activity and cisplatin in a
mice model of hepatocellular carcinoma. J Clin Cancer Res.
136:1827–1837. 2010. View Article : Google Scholar
|
|
20
|
Kanazawa T, Urabe M, Mizukami H, Okada T,
Kume A, Nishino H, Monahan J, Kitamura K, Ichimura K and Ozawa K:
γ-rays enhance rAAV-mediated transgene expression and cytocidal
effect of AAV-HSVtk/ganciclovir on cancer cells. Cancer Gene
Ther. 8:99–106. 2001.
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C (T)) method. Methods. 25:402–408. 2001.
|
|
22
|
Lin J, Zhang Z, Zeng S, Zhou S, Liu BF,
Liu Q, Yang J and Luo Q: TRAIL-induced apoptosis proceeding from
caspase-3-dependent and independent pathways in distinct HeLa
cells. Biochem Biophys Res Commun. 346:1136–1141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shankar S and Srivastava RK: Enhancement
of therapeutic potential of TRAIL by cancer chemotherapy and
irradiation: mechanisms and clinical implications. Drug Resist
Updat. 7:139–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shin EC, Seong YR, Kim CH, Kim H, Ahn YS,
Kim K, Kim SJ, Hong SS and Park JH: Human hepatocellular carcinoma
cells resist to TRAIL-induced apoptosis, and the resistance is
abolished by cisplatin. Exp Mol Med. 34:114–122. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang M, Liu Z, Xiang Y, Ma H, Liu S, Liu
Y and Zheng D: Synergistic antitumor effect of AAV-mediated TRAIL
expression combined with cisplatin on head and neck squamous cell
carcinoma. BMC Cancer. 11:54–65. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liping X, Shuping Y and Kaladhar BR:
Enhanced anticancer effect of the combination of cisplatin and
TRAIL in triple-negative breast tumor cells. Mol Cancer Ther.
10:550–557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seol DW, Li J, Seol MH, Park SY, Talanian
RV and Billiar TR: Signaling events triggered by tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL): caspase-8 is
required for TRAIL-induced apoptosis. Cancer Res. 61:1138–1143.
2001.PubMed/NCBI
|
|
28
|
Brunetti G, Colucci S, Rizzi R, Mori G,
Colaianni G, Oranger A, Zallone A, Liso V and Grano M: The role of
OPG/TRA IL complex in multiple meloma: the OPG/TRAIL complex in an
in vitro osteoclastogenesis model derived from human multiple
meloma-bone disease. Ann NY Acad Sci. 1068:334–340. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sheridan JP, Marsters SA, Pitti RM, Gurney
A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood
WI, Goddard AD, Godowski P and Ashkenazi A: Control of
TRAIL-induced apoptosis by a family of signaling and decoy
receptors. Science. 277:818–821. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fulda S, Meyer E and Debatin KM:
Inhibition of TRAIL-induced apoptosis by BCL2 overexpression.
Oncogene. 21:2283–2294. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Van Geelen CM, de Vries EG and de Jong S:
Lessons from TRAIL-resistance mechanisms in colorectal cancer
cells: paving the road to patient-tailored therapy. Drug Resist
Updat. 7:345–358. 2004.PubMed/NCBI
|
|
32
|
Vander Heiden MG, Chandel NS, Williamson
EK, Schumacker PT and Thompson CB: Bcl-Xl regulates the membrane
potential and volume homeostasis of mitochondria. Cell. 91:627–637.
1997.PubMed/NCBI
|
|
33
|
Harris MH and Thompson CB: The role of the
BCL2 family in the regulation of outer mitochondrial membrane
permeability. Cell Death Differ. 7:1182–1191. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim IK and Jung YK, Noh DY, Song YS, Choi
CH, Oh BH, Masuda ES and Jung YK: Functional screening of genes
suppressing TRAIL-induced apoptosis: distinct inhibitory activities
of Bcl-XL and Bcl-2. Br J Cancer. 88:910–917. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee TJ, Lee JT, Park JW and Kwon TK:
Acquired TRAIL resistance in human breast cancer cells are caused
by the sustained cFLIP(L) and XIAP protein levels and ERK
activation. Biochem Biophys Res Commun. 351:1024–1030. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bellail AC, Tse MC, Song JH, Phuphanich S,
Olson JJ, Sun SY and Hao C: DR5-mediated DISC controls caspase-8
cleavage and initiation of apoptosis in human glioblastomas. J Cell
Mol Med. 14:1303–1317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang P, Zhang J, Anita B, Jiang W, Judith
H, Norman MK and Hao C: Inhibition of RIP and c-FLIP enhances
TRAIL-induced apoptosis in pancreatic cancer cells. Cell Signal.
19:2237–2246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brooks AD and Sayers TJ: Reduction of the
antiapoptotic protein cFLIP enhances the susceptibility of human
renal cancer cells to TRAIL apoptosis. Cancer Immunol Immunother.
54:499–505. 2005. View Article : Google Scholar : PubMed/NCBI
|