Introduction
Thyroid cancer, particularly papillary thyroid
carcinoma, is one of the most common malignancies in the world and
is, generally, of indolent character. Papillary thyroid carcinoma
sometimes contains poorly differentiated components and has an
aggressive behavior and leads to a poor prognosis (1). However, the molecular mechanism
remains unclear. It was recently reported that the accumulation of
multiple genetic alterations that can activate PI3K/Akt and MAPK
pathways promotes thyroid carcinoma aggressiveness and progression
to poorly differentiated thyroid carcinoma and
undifferentiated/anaplastic thyroid carcinoma (2).
Growth factors and their specific cell surface
receptors are known to play several physiological roles in cell
growth and differentiation and are also involved in the development
and progression of cancer through the PI3K/Akt and/or MAPK pathways
(2,3). In particular, the EGF protein family,
such as EGF and transforming growth factor α (TGF-α), which
encompasses a number of mitogens that appear to share similar amino
acid sequences, can also modulate a number of integrin-dependent
functions, including cell adhesion, migration and cytoskeletal
organization. However, the mechanisms underlying these phenomena
are less clear.
HB-EGF is a heparin-binding member of the EGF family
first identified in the condition medium of the U-937
macrophage-like cell line (4).
HB-EGF is synthesized as a transmembrane precursor that can be
cleaved enzymatically to release a soluble 14–20 kDa (4,5). The
soluble form is a potent paracrine and/or autocrine mitogen for
fibroblasts (6), smooth muscle
cells (SMCs) (4,7,8),
keratinocytes (9,10) and some cancer cells (11). HB-EGF has also been involved in
wound healing (9) and in processes
involving SMC hyperplasia such as atherosclerosis (12), pulmonary hypertension (13) and uterine leiomyomas (14). On the other hand, the transmembrane
form of HB-EGF has some different functions; it works as a
juxtacrine growth and adhesion factor, and uniquely it is also the
receptor of diphtheria toxin (DT) (15,16).
To date, HB-EGF has been reported to be a promising
therapeutic target for ovarian, breast, gastric and endometrial
cancer (17,18). Although overexpression of HB-EGF is
found in several types of cancer (19–21),
the underlying molecular mechanisms remain unclear. Furthermore,
there have been some reports on the contribution of HB-EGF in
cancer metastasis and invasion of ovarian cancer cells and head and
neck cancer cells (17,22). By contrast, a previous study showed
that increased expression of HER4, one of the receptors of HB-EGF,
was observed in thyroid papillary carcinomas compared to
non-neoplastic thyroid tissues like HER1 (23). Of note, HB-EGF has been shown to be
a potent chemotactic factor but not a mitogen for cell expressing
HER4, in contrast to the ability of HB-EGF to stimulate both these
activities in cells expressing HER1 (24).
In the present study, we evaluated the possibility
of HB-EGF in cell growth and invasion of thyroid cancer cells. We
demonstrated that HB-EGF was not only a potent mitogen but also a
chemotactic factor in thyroid cancer cells, as previously described
for fibroblasts, SMC and keratinocytes. In addition, in clinical
immunohistochemical study, we also investigated the expression of
HB-EGF proteins and its receptors, HER1 and HER4, in human thyroid
cancer tissues, suggesting that a novel role of HB-EGF-induced
chemotaxis might be mediated by tyrosine phosphorylation not only
of HER1 but also of HER4 in the thyroid cancer cells.
Materials and methods
Reagents
HB-EGF was prepared from U-937 cell conditioned
medium as previously described (4,7). EGF
was obtained from R&D Systems (Minneapolis, MN, USA).
Tyrphostin AG1478, a specific inhibitor of EGF receptor tyrosine
kinase, was obtained from Calbiochem (La Jolla, CA, USA). RPMI-1640
was purchased from Nissui Pharmaceutical Co., Ltd., (Tokyo, Japan),
fetal bovine serum (FBS) from Dainippon Pharmaceutical Co., Ltd.,
(Osaka, Japan) and trypsin-EDTA solution and
penicillin-streptomycin solution from Gibco-BRL (Gaithersburg, MD,
USA). The other chemicals and reagents are described below.
Cells and culture
Human thyroid carcinoma cell lines, 8305C and SW579,
were obtained from the Japanese Collection of Research Bioresources
(JCRB) (HSRRB; Health Science Research Resources Bank, Osaka,
Japan) and ATCC (Rockville, MD, USA), respectively. 8305C was
derived from an undifferentiated thyroid carcinoma (25) and SW579 from a squamous cell
carcinoma of thyroid (26). Cells
were maintained in continuous culture at 37°C in a 5%
CO2 humidified atmosphere. Cells were grown in RPMI-1640
medium supplemented with 10% heat-inactivated FBS. Penicillin (100
U/ml) and streptomycin (100 μg/ml) were added to the
media.
Tissue samples
Thyroid tissues specimens were obtained from the
patients undergoing thyroid surgery at the Nara Medical University
Hospital (Kashihara, Japan). The present study was approved by the
Ethics Committee of the Nara Medical University School of Medicine.
Written informed consent for this study was obtained from each
patient. The median age of the patients was 59.6±10.6 years (range,
29–81 years). All samples were prepared from the surgical specimens
immediately after their removal from the patients and fixed in 10%
buffered formalin. The histology was examined by one of the authors
(M.N.), and the samples were classified according to the WHO
criteria (27). For
immunohistochemistry, 33 samples from 24 patients were obtained and
included the following cases: 9 normal thyroids, 2 hyperplasias, 2
adenomatous goiters, 4 follicular adenomas, 3 follicular
carcinomas, 11 papillary carcinomas and 2 undifferentiated
carcinomas.
Cell proliferation assays
Cell proliferation was determined by the Cell
Counting Kit-8 (Dojindo, Kumamoto, Japan). 8305C cells were grown
overnight in RPMI-1640 medium with 10% heat-inactivated FBS onto
96-well plates (5,000 cells/well) at 37°C in a 5% humidified
atmosphere. After washing with phosphate-buffered saline (PBS), the
cells were incubated in 100 μl/well of serum-free RPMI-1640
medium with 0.1% bovine serum albumin (Fraction V; Sigma).
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H
tetrazolium, monosodium salt (WST-8) was added to the cells (0.5
mM/well), after 48 h of the treatment with varying concentrations
of HB-EGF. The absorbance of each well was measured at 455 nm with
a reference wavelength at 650 nm with MTP-32 microplate reader
(Corona Electric Co., Ltd., Ibaragi, Japan). A strong correlation
was confirmed between the cell proliferation by this assay and
those as measured by counting the number of the cells (28).
Migration assays
Cell migration was evaluated using a modified Boyden
chamber assay (24,29,30).
Eight-micron Nucleopore polyvinylpyrrolidine-free polycarbonate
filters (Cambridge, MA, USA) were coated with 10.0 μg/ml
fibronectin (Iwaki, Chiba, Japan) in PBS for 30 min at room
temperature and allowed to air dry. The filter was placed over a
48-well chamber (NeuroProbe, Cabin John, MD, USA) containing
varying concentrations of HB-EGF in serum-free RPMI-1640 medium
with 0.1% BSA in the lower chamber. After trypsinization, 10,000
cells in 50 μl of serum-free medium were added to the wells
in the upper chamber. In the checkerboard assay, varying
concentrations of HB-EGF were also added to the upper chamber
wells. The chamber was then placed in a 37°C with 5.0%
CO2 humidified incubator for 3 h. Next, the upper
surface of the filter was scraped to remove non-migratory cells.
The filter was subsequently fixed in 10% buffered formalin for 10
min, washed with PBS and stained with hematoxylin for 10 min. Total
cell number per well of the lower surface were counted visually as
an index of the cell migration. Inhibition assays were conducted in
a similar manner with the addition of tyrphostin AG1478 to the
upper and lower chambers. The cells were incubated in serum-free
medium with tyrphostin AG1478 for 1 h prior to placement in the
chamber.
Wound assay
Cell migration was also assessed by a modified in
vitro wound assay (31). Cells
were plated in complete medium (serum-free RPMI-1640 medium with
0.1% BSA) on 6-well plates. Initial plating was adjusted to yield
subconfluent monolayers at the same cell density after 24 h. The
monolayers were then wounded by scratching a line with a plastic
scriber, and after washing with PBS, were incubated for the
indicated time in the complete medium. The experiment was
terminated by fixing the cells, followed by staining with
hematoxylin. The distance between the advancing cells on both sides
in the controls was compared with that in the presence of HB-EGF
and the migratory activity was quantified by counting the cells
that had migrated into the cell-free space on photographic
enlargements (31–33).
Immunohistochemistry and
immunohistochemical evaluation
Immunohistochemical study for HB-EGF, HER1 and HER4
was performed using the avidin-biotin-complex (ABC) method for 9
normal thyroid tissues, 2 hyperplasias, 2 adenomatous goiters, 4
follicular adenomas, 3 follicular carcinomas, 11 papillary
carcinomas and 2 undifferentiated carcinomas. Anti-HB-EGF antibody,
H-1 antibody, which was generated to synthetic peptides located in
cytoplasmic domains, and anti-HER4 polyclonal antibody were
established by our coworker (12,19),
and used at the concentration of 1:500 and 1:200, respectively.
Anti-HER1 polyclonal antibody was purchased from Upstate
Biotechnology, Inc., (Lake Placid, NY, USA) and applied at 1:100.
Slices (4 μm) of tissue section were deparaffinized and
endogenous peroxidase activity was blocked with 0.3% hydrogen
peroxide and 0.1% sodium azide in distilled water for 15 min. For
immunohistochemistry of HER1, we performed antigen retrieval by
incubating the sections with 0.03 mol/l citrate buffer (pH 6.0) and
heated to 121°C for 20 min in pressure cooker. After three rinses
in PBS pH 7.2 PBS, 10% bovine serum albumin (Wako, Osaka, Japan)
was applied for 10 min to block the non-specific reaction. Sections
were incubated with the primary antibody for 60 min at room
temperature. After rinsing in PBS, they were treated with
biotinylated rabbit anti-sheep IgG (Vector Laboratories,
Burlingame, CA, USA) at the concentration of 1:200 for anti-HER1
antibody or biotinylated anti-rabbit IgG (Nichirei, Tokyo, Japan)
at the concentration of 1:1 for anti-HB-EGF and anti-HER4
antibodies for 15 min. Again after rinsing in PBS, the sections
reacted with the ABC (Dako, Copenhagen, Denmark) at the
concentration of 1:300 for 15 min. The peroxidase reaction was
visualized by incubating the sections with 0.02%
3,3′-diaminobenzidine tetrahydrochloride in 0.05 M Tris buffer (pH
7.6) with 0.01% hydrogen peroxide. The sections were counterstained
with hematoxylin. Sections for negative control were prepared by
using normal mouse serum instead of primary antibody.
We classified the results into four groups by
positive cell rate: (−), 0–5% of the positive cells; (+), 5–50% of
positive; (++), 50–75% of positive; (+++), 75–100% of positive.
Immunofluorescence study
Immunofluorescence study of the transmembrane form
of HB-EGF (proHB-EGF) proteins was performed with indirect
immunofluorescence techniques for 8305C cells. Cells were washed
with PBS and fixed with 4% paraformaldehyde. After washing in PBS,
the cells were incubated with anti-HB-EGF antibody, H-1 antibody,
for 30 min at room temperature. After rinsing in PBS, they were
stained with fluorescein isothiocyanate washed in PBS. Cells were
photographed using a fluorescence microscope (Olympus, Tokyo,
Japan).
RNA extraction
Total cellular RNA from culture cells was extracted
by the acid guanidium-phenol-chloroform technique using the TRIzol
(Gibco-BRL) (34). The total RNA
was resuspended in diethylpyrocarbonate-treated water and stored at
−80°C until use.
RT-PCR
RT-PCR was performed as previously described
(35). Single-strand cDNA was
generated from 10 μg of total cellular RNA in a 25-μl
reaction mixture containing 2.5 μM oligo (dT) 18-primer, 5
mM MgCl2 10 mM Tris-HCl, 50 mM KCl (pH 8.3), 1 mM d-NTP
mixture, 1 U/μl RNase inhibitor and 0.25 U/μl AMV
reverse transcriptase (Takara, Kyoto, Japan) for 1 h at 42°C. The
reaction was terminated by inactivating the transcriptase at 65°C
for 10 min. A 1-μl aliquot of this solution was removed for
subsequent first-round PCR by adding each sample to 100 μl
of a solution containing 2.5 mM MgCl2, 10 mM Tris-HCl,
50 mM KCl (pH 8.3), 200 μM dATP, 200 μM dCTP, 200
μM dGTP, 200 μM dTTP, 100 pmol of each of primer A
(5′-TCCTCCAAGCCACAAGCACT-3′) and B (5′-AGAAGCCCCACGATGACCAG-3′) for
HB-EGF, and 2.5 units of Taq polymerase. The initial step was 94°C
for 2 min for denaturing cDNA, and then PCR was carried out for 30
cycles (30 sec at 94°C, 1 min at 55 and 1 min at 72°C). Negative
controls were performed without adding the template cDNA. PCR
products (10 μl) were stained with ethidium bromide and
analyzed by 1.5% agarose gel electrophoresis. To eliminate degraded
RNA, amplification of β-actin was also performed as previously
described (36).
Diphtheria toxin (DT) sensitivity
DT sensitivity was performed for 8305C cells, since
proHB-EGF is uniquely the receptor for DT (16). Approximately 10,000 cells/well were
plated in a 24-well plate and incubated for 16 h. After washing
each well with cold PBS, 0.5 ml/well of Ham’s F12 medium was added
and the cells were exposed to DT (3.3–1,000 ng/ml) for 5 h.
Subsequently, 10 μl/well of 3H-Leu (100
μCi/ml) was added to each well and the cells were incubated
for 1 h. The cells were harvested with trypsin and the extent of
radiolabel incorporation was measured by beta-counter (Walla,
Turku, Finland).
Results
Cell proliferation analysis
EGF and TGF-α have been demonstrated to be potent
mitogenic factors for thyroid carcinoma cells (23,37–39).
To determine whether the cell growth of thyroid cancer 8305C and
SW579 cells is modulated by HB-EGF, we studied the effects of
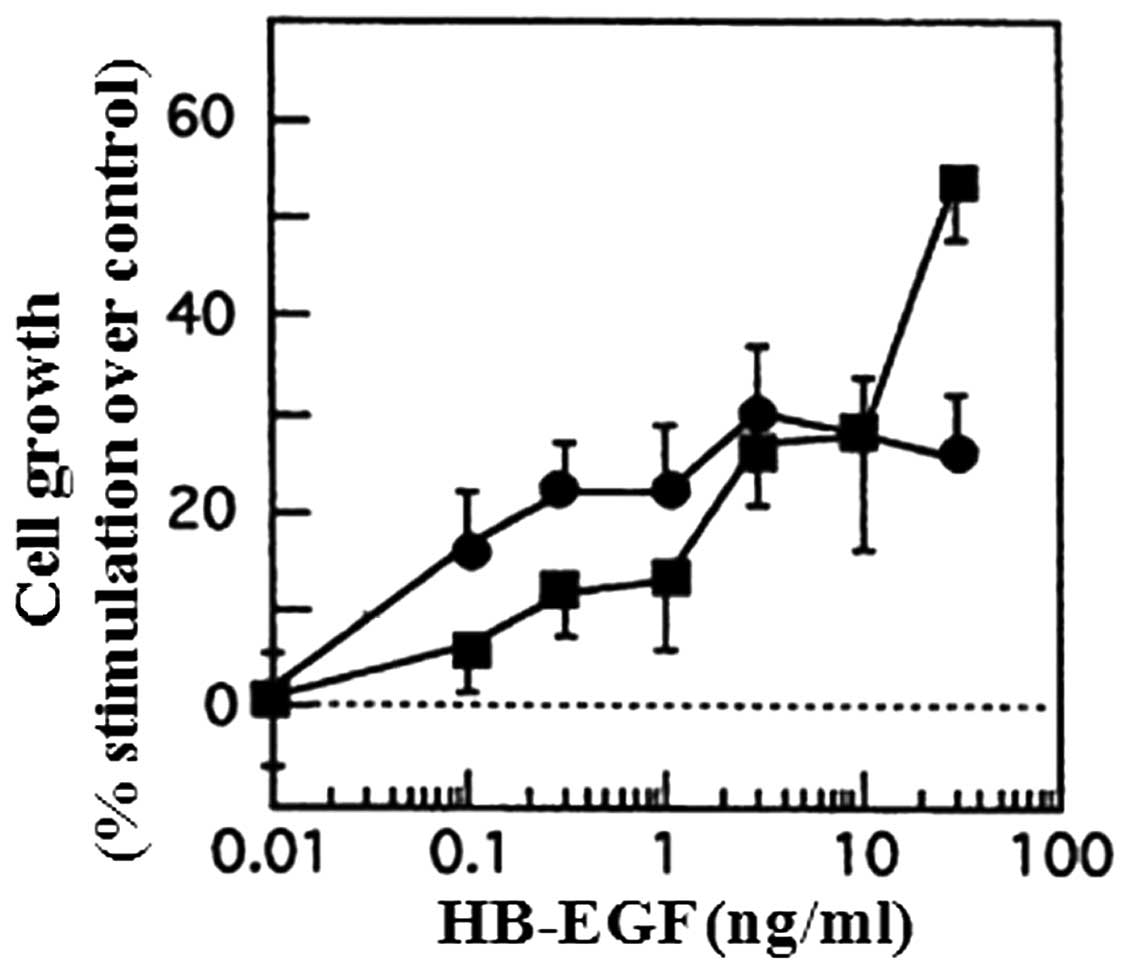
HB-EGF on cell proliferation. HB-EGF enhanced the growth of both
cell lines in a dose-dependent manner (Fig. 1). In 8305C and SW579 cells,
half-maximal growth stimulation occurred at 15 and 1 ng/ml,
respectively.
Cell migration by HB-EGF
It has previously been reported that HB-EGF is a
potent chemotactic factor as well as a powerful mitogenic factor
for SMC (12). To test whether the
cell migration of 8305C and SW579 cells is modulated by HB-EGF, a
wound assay and a modified Boyden chamber assay were performed.
In a wound assay, wounds of 1 mm width were made in
subconfluent monolayers of 8305C cells on 10 μg/ml
fibronectin-coated plates, and the cells were allowed to migrate
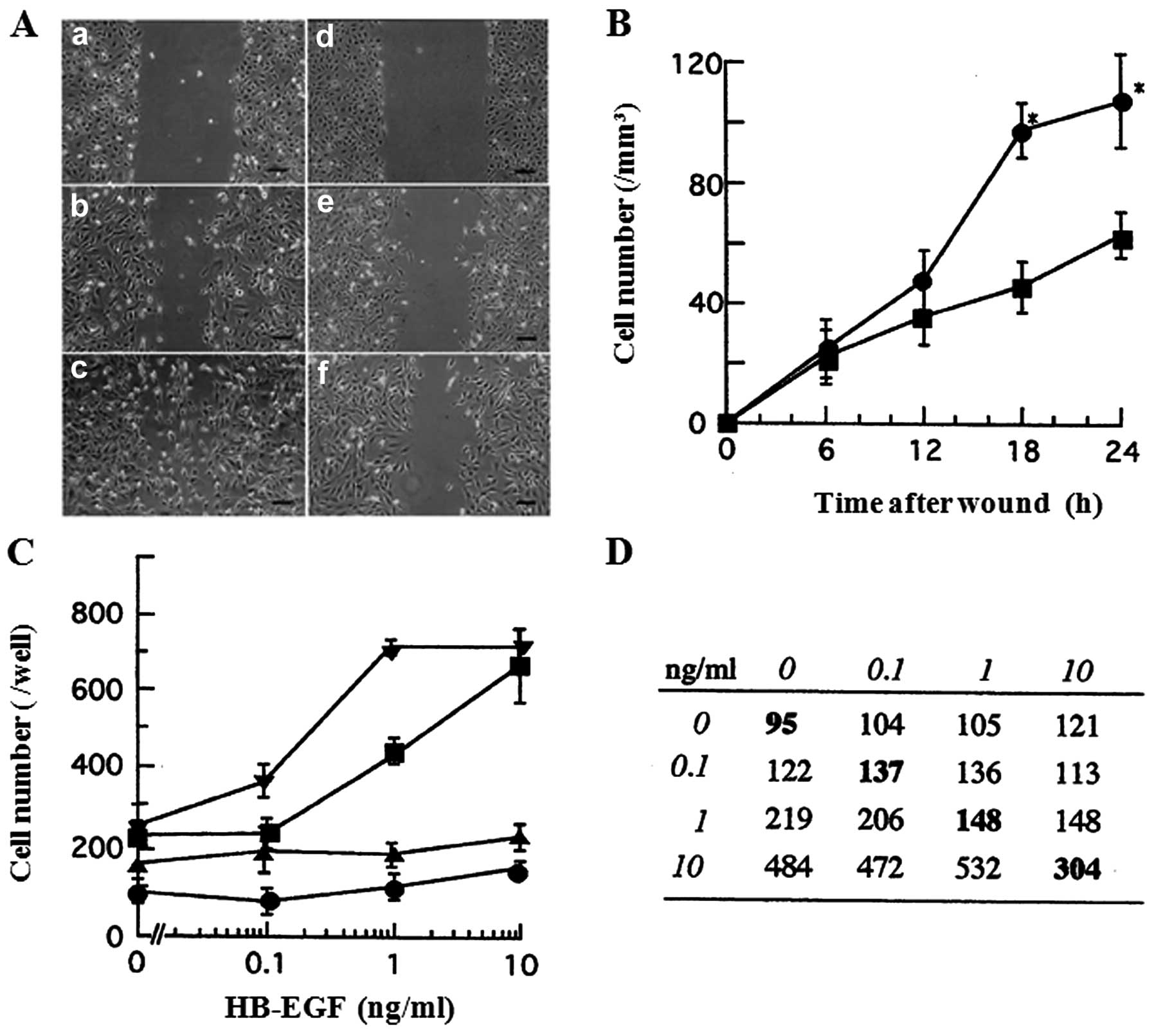
into the cell-free area over a 24-h period (Fig. 2A). Cell migration in this assay was
quantified by counting the cells that had advanced into the wounded
cell-free area from a number of randomly selected 1-mm segments of
the initial edge. As shown in Fig.
2B, at 6 and 12 h after the wounding, no significant
differences were observed in the cell migration between the
presence and the absence of HB-EGF. However, at 18 and 24 h, 10
ng/ml HB-EGF significantly stimulated the cell migration as
compared to HB-EGF-free condition (P<0.05). These results
indicate that HB-EGF enhances the cell migration of 8305C cells in
a time-dependent manner.
In a modified Boyden chamber assay, HB-EGF induced
chemotaxis of 8305C and SW579 cells at the concentrations with
half-maximal stimulation of 1 and 0.3 ng/ml, respectively (Fig. 2C). Furthermore, the checkerboard
assay, in which varying concentrations of HB-EGF were placed in the
upper and lower wells of Boyden chamber apparatus, was performed
for 8305C cells, to verify whether HB-EGF stimulates chemotaxis or
chemokinesis. Cell migration to HB-EGF was predominantly consistent
with chemotactic response as seen in Fig. 2D. Furthermore, when HB-EGF was
present only in the upper wells, the increase in cell migration was
minimal. These results indicate that HB-EGF stimulates directional
chemotaxis rather than significant stimulation of random cell
motility.
Inhibition of cell migration by
tyrphostin AG1478
HB-EGF has been reported to activate HER1 tyrosine
phosphorylation and then to stimulate proliferation and chemotaxis
in cells expressing HER1 (21). To
verify whether HB-EGF stimulates HER1-mediated chemotaxis in cancer
cells, we tested the inhibitory effect of tyrphostin AG1478 in
HB-EGF-induced chemotaxis. In a modified Boyden chamber chemotaxis
assay, the chemotactic effects of HB-EGF for 8305C and SW579 cells
were markedly inhibited by the pretreatment with 100 nM tyrphostin
AG1478 for 60 min prior to this assay (Fig. 2C). Furthermore, the migration with
the pretreatment of tyrphostin AG1478 was more suppressed even
without HB-EGF (Fig. 2C). In a
wound assay, the migration was also inhibited by tyrphostin AG1478
(data not shown). These data suggest that HB-EGF-induced
chemotactic effects could be mediated by HER1.
Expression of proHB-EGF and its receptors
in thyroid carcinoma cells
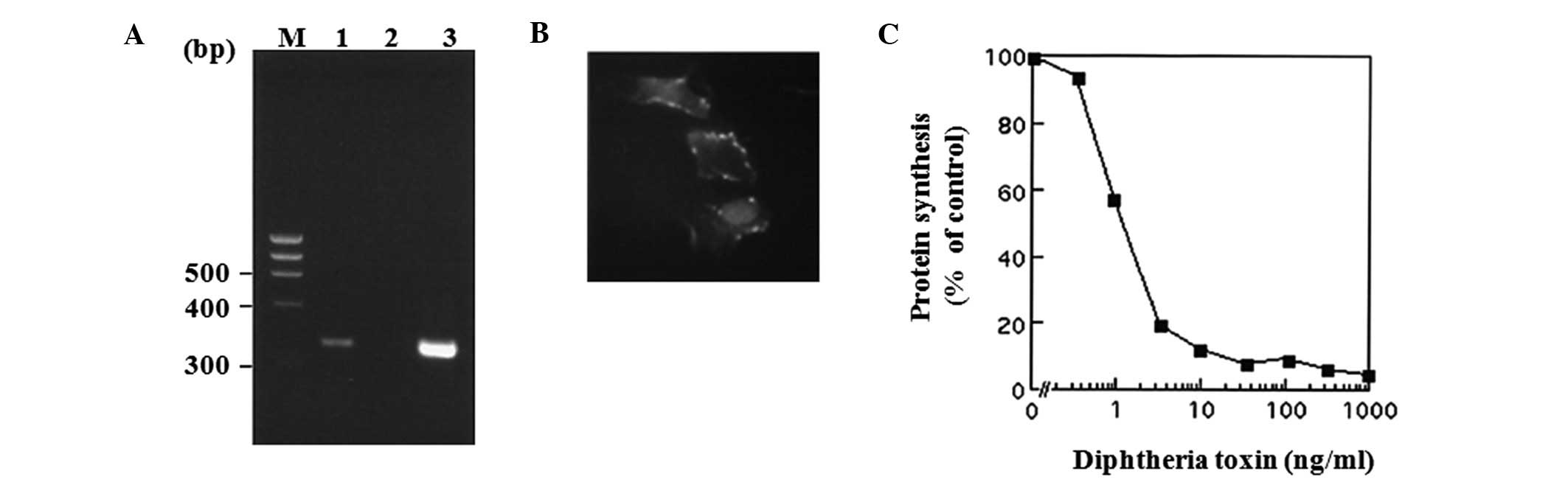
To verify whether HB-EGF mRNA and proHB-EGF protein
are expressed in 8305C cells, RT-PCR and immunofluorescence study
were performed. HB-EGF mRNA expression in 8305C cells was detected
with RT-PCR (Fig. 3A), and the
protein expression was also observed in the cell surface by
immunofluorescence staining with FITC-conjugated anti-rabbit
immunoglobulin (Fig. 3B). Moreover,
the functional presence of proHB-EGF protein was also confirmed by
demonstration of specific sensitivity to DT (Fig. 3C), indicating that proHB-EGF can be
available for DT-binding and toxin internalization as previously
reported in prostate cancer cell line LNCaP cells (40). In addition, HER1 protein and mRNA
expression in 8305C and SW579 cells (data not shown) were detected
with both immunofluorescence study using FACSCalibur
(Becton-Dickinson, San Jose, CA, USA) and RT-PCR, but HER4 was
not.
Immunohistochemistry
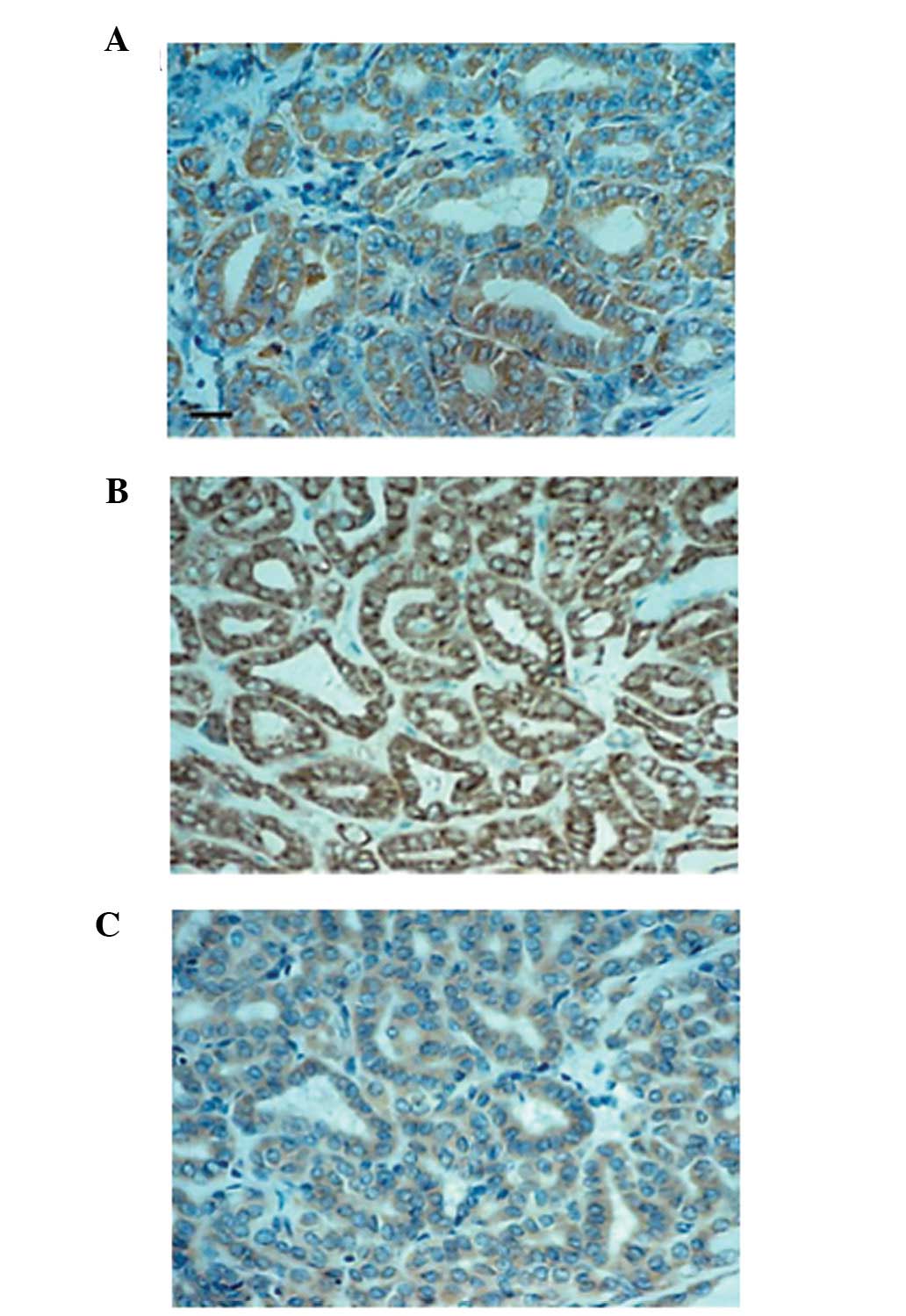
The results of HB-EGF, HER1 and HER4 in thyroid
tissues are presented in Table I.
HB-EGF and HER4 staining in differentiated thyroid carcinoma
tissues, such as papillary carcinomas, were localized in cytoplasm
and/or cell membrane of the cells, whereas HER1 immunoreactivity
was observed predominantly in cell membrane as shown Fig. 4. The intensity of HB-EGF and HER4
immunostaining in carcinomas was stronger and the number of
positive cells was higher than in normal tissues. In some
undifferentiated thyroid carcinoma tissues, however, HB-EGF
staining was negative, although HER4 staining was strongly
positive. On the other hand, HER1 was expressed widely in most
malignant and benign tissues of the thyroid (Table I).
 | Table IImmunohistochemical expression of
HB-EGF, HER1 and HER4 in thyroid tissues. |
Table I
Immunohistochemical expression of
HB-EGF, HER1 and HER4 in thyroid tissues.
| | HB-EGF
reactivity | HER1
reactivity | HER4
reactivity |
|---|
| |
|
|
|
|---|
| Tissue
diagnosis | na | (−) | (+) | (++) | (+++) | (−) | (+) | (++) | (+++) | (−) | (+) | (++) | (+++) |
|---|
| Normal | 9 | 5 | 4 | 0 | 0 | 0 | 1 | 1 | 7 | 6 | 3 | 0 | 0 |
| Adenomatous
goiter | 2 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 0 |
| Hyperplasia | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 0 |
| Follicular
adenoma | 4 | 0 | 1 | 3 | 0 | 0 | 1 | 0 | 3 | 0 | 4 | 0 | 0 |
| Follicular
carcinoma | 3 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 3 | 0 | 1 | 2 | 0 |
| Papillary
carcinoma | 11 | 0 | 3 | 2 | 6 | 0 | 0 | 2 | 9 | 4 | 2 | 2 | 3 |
|
Undifferentiated/anaplastic carcinoma | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 1 |
Discussion
EGF family members, such as EGF and TGF-α, stimulate
not only cell growth but also cell migration in cancer cells.
Thyroid cancer cells also express EGF, TGF-α and its receptor,
HER1, suggesting that they regulate thyroid cancer cell growth and
invasion by autocrine and/or paracrine mechanisms (37–39,41).
While HB-EGF has been shown to be a potent chemotactic factor as
well as a potent mitogen for fibroblasts, SMC and keratinocytes,
the effects of HB-EGF for cancer cells have been reported as
follows (5,42): i) HB-EGF gene expression has been
detected in a variety of tumor-derived cell lines, including
prostate, breast, colon, pancreas, ovarian, head and neck carcinoma
and melanoma (17,21,22);
and ii) enhanced HB-EGF gene expression in tumors such as
pancreatic, liver and gastric tumors, has been detected compared to
normal tissues (19–21). These data suggest that it can work
in an autocrine manner (21).
In the present study, we provide evidence that
HB-EGF might not only be a potent mitogen but also a chemotactic
factor for thyroid cancer cells, 8305C and SW579, in an autocrine
and/or paracrine manner, and that the cell migration could be a
chemotactic pattern as well as a chemokinetic one. These results
favor the effect of HB-EGF on cancer invasion and metastasis in
ovarian cancer and head and neck cancer cells (17,22).
In addition, HB-EGF induced a bell shaped dose response curve in
the Boyden chamber assay. These data indicate that HB-EGF could act
as the soluble form (sHB-EGF) (30). The chemotactic effects of HB-EGF for
8305C and SW579 cells were markedly inhibited by tyrphostin AG1478.
Cell migratory activity with the pretreatment of tyrphostin AG1478
was more suppressed than that in the absence of exogenous HB-EGF
(Fig. 3), suggesting that HB-EGF
could act as an autocrine chemotactic factor for thyroid carcinoma
cells. ProHB-EGF protein expression was detected in 8305C and SW579
cells with immunofluorescence technique and with the demonstration
of specific sensitivity to DT, and proHB-EGF mRNA expression was
also detected in these cell lines. Furthermore, in
immunohistochemical study, the intensity of HB-EGF immunostaining
was stronger and the rate of positive cells was higher in thyroid
carcinomas. Notably, the proHB-EGF immunoreactivity in
undifferentiated thyroid carcinoma tissues was not always detected,
suggesting that less proHB-EGF immunoreactivity in the
undifferentiated carcinomas might be due to the active processing
of proHB-EGF to sHB-EGF on the cell surface. In addition,
undifferentiated thyroid carcinoma-derived cell line 8305C
expressed HB-EGF mRNA and proHB-EGF protein (Fig. 3). These data may indicate the
hypothesis as follows; when proHB-EGF can be enzymatically cleaved
to rapidly release sHB-EGF in undifferentiated thyroid carcinoma
cells, the tumor cells can show cell growth and migration rapidly
through HER1 and/or HER4 by binding with sHB-EGF. Indeed,
undifferentiated thyroid carcinomas are generally associated with
poor prognosis, with most patients dying within a few months. The
mechanism of its carcinogenesis, rapid growth and metastasis
remains completely unclear. By contrast, differentiated thyroid
carcinoma cells strongly express proHB-EGF, which might act as a
tumor survival factor that induces the resistance to apoptosis due
to the upregulation of p21 like hepatoma (43). Differentiated thyroid carcinomas,
such as papillary carcinomas, at the earliest stage, do not always
show rapid growth, while the large tumor, such as tumor size >4
cm, consists of high risk tumor factor (44), suggesting that sHB-EGF might
stimulate the growth and migration of the large papillary
carcinoma, after proHB-EGF could be cleaved by some proteases. It
has been reported that MMP-3 cleaves HB-EGF to active sHB-EGF at a
specific juxtamembrane site (45).
MDC9/meltrin-g/ADAM9, a member of metalloprotease-disintegrin
family, has been reported to be involved in the processing of
proHB-EGF (46). Moreover, MT1-MMP
co-expressed with HB-EGF in ovarian carcinoma cells has been
reported to potentiate the activity of HB-EGF to promote invasive
tumor growth and spreading in vivo(47). It is noteworthy to speculate whether
some proteases including MMP-3, ADAM9 and MT1-MMP are activated in
thyroid carcinoma as well as in hepatoma and ovarian carcinoma
(43,47). This hypothesis in thyroid cancer
favors the evidence that the production of proMMP-2 and its
MT1-MMP-mediated activation in the carcinoma cell nests play an
important role in the lymph node metastasis of human invasive
papillary thyroid carcinomas (48–50).
Furthermore, the ectodomain shedding of HB-EGF by disintegrin and
metalloproteases has been reported to be a key event of receptor
cross talk, as well as a novel intercellular signaling by their
carboxy-terminal fragments to regulate gene expression directly
(51), which could support our data
that HB-EGF regulates thyroid cancer cell growth and invasion.
It has also been reported that HB-EGF can bind to
HER4 as well as HER1 (24). In the
present study, HER1 mRNA and protein expressions in the thyroid
carcinoma cells were detected with flow cytometry and RT-PCR, but
HER4 was not detected. However, in immunohistochemical study, both
strong staining of HER1 and HER4 were observed in thyroid carcinoma
cells including papillary carcinoma, follicular carcinoma and
undifferentiated carcinoma tissues. However, HER4 tended to be more
diffusely expressed in carcinomas than in benign tumor tissues,
while HER1 was expressed very diffusely in most thyroid tumor
tissues. This staining pattern of HER4 tended to be the same as
that of HB-EGF. It has been demonstrated that papillary thyroid
carcinomas express considerably higher levels of HER4 protein than
non-neoplastic thyroid tissue (23), which is almost in agreement with our
data. By contrast, it has been shown that HB-EGF is a chemotactic
factor but not a mitogen for cells expressing HER4 in contrast to
its ability to stimulate both chemotaxis and proliferation in cells
expressing HER1 (24), suggesting a
possible role for HER4 as well as HER1 in mediating
HB-EGF-stimulated chemotaxis in thyroid carcinoma cells. By
contrast, EGF and TGF-α were also found to be a potent chemotactic
factors for these thyroid carcinoma cells like HB-EGF (37–39),
but these growth factors are different from HB-EGF in that EGF or
TGF-α binds to HER1 alone and that HB-EGF bears a heparin binding
site, which binds to cell surface heparin sulfate proteoglycan, but
not EGF or TGF-α. In addition, the HB-EGF-induced chemotaxis for
the thyroid cells expressing HER1, but not HER4, was inhibited by
tyrphostin AG1478 (Fig. 2B and C).
These results suggest that HB-EGF could induce tyrosine
phosphorylation of HER1 in the thyroid carcinoma cell lines
expressing HER1, while in the thyroid carcinoma tissues expressing
both of HER1 and HER4, HB-EGF might induce tyrosine phosphorylation
of HER1 and/or HER4. Therefore, the immunohistochemical data
indicate that HB-EGF-induced chemotaxis might result from tyrosine
phosphorylation not only of HER1 but also of HER4 in the thyroid
carcinoma cell in vivo. Thus, these findings suggest a novel
role of HB-EGF in the invasion and metastasis of thyroid carcinoma
cells.
In summary, HB-EGF is a potent chemotactic factor as
well as a mitogen that mediates HER1 and/or HER4. HER4-mediated
chemotaxis might induce the invasion and metastasis in thyroid
carcinoma cells, particularly in poorly differentiated papillary
thyroid carcinomas or undifferentiated thyroid carcinomas. Although
additional studies should be carried out to establish the
functional interaction between HB-EGF and HER4 as well as HER1 in
thyroid carcinoma cells, these results can serve as a foundation
for the development of novel therapeutic strategies including
molecular targeted therapy for HB-EGF and EGFR signaling in
undifferentiated thyroid carcinomas.
Acknowledgements
The present study was supported in part by Grants-in
Aid for Scientific Research from the Ministry of Education,
Culture, Sports, Science and Technology of Japan.
References
|
1
|
Ito Y, Hirokawa M, Kihara M, et al:
Prognostic value of poorly differentiated carcinoma in Japanese
Society of Thyroid Surgery in a series of papillary thyroid
carcinoma patients: comparison with risk classification system in
Kuma Hospital. Endocr J. 59:817–821. 2012. View Article : Google Scholar
|
|
2
|
Xing M: Genetic alterations in the
phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer.
Thyroid. 20:697–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stoscheck CM and King LE Jr: Role of
epidermal growth factor in carcinogenesis. Cancer Res.
46:1030–1037. 1986.PubMed/NCBI
|
|
4
|
Higashiyama S, Abraham JA, Miller J,
Fiddes JC and Klagsbrun M: A heparin-binding growth factor secreted
by macrophage-like cells that is related to EGF. Science.
251:936–939. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Raab G, Higashiyama S, Hetelekidis S, et
al: Biosynthesis and processing by phorbol ester of the cells
surface-associated precursor form of heparin-binding EGF-like
growth factor. Biochem Biophys Res Commun. 204:592–597. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Besner G, Higashiyama S and Klagsbrun M:
Isolation and characterization of a macrophage-derived
heparin-binding growth factor. Cell Regul. 1:811–819.
1990.PubMed/NCBI
|
|
7
|
Higashiyama S, Lau K, Besner GE, Abraham
JA and Klagsbrun M: Structure of heparin-binding EGF-like growth
factor. Multiple forms, primary structure, and glycosylation of the
mature protein. J Biol Chem. 267:6205–6212. 1992.PubMed/NCBI
|
|
8
|
Higashiyama S, Abraham JA and Klagsbrun M:
Heparin-binding EGF-like growth factor stimulation of smooth muscle
cell migration: dependence on interactions with cell surface
heparan sulfate. J Cell Biol. 122:933–940. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marikovsky M, Breuing K, Liu PY, et al:
Appearance of heparin-binding EGF-like growth factor in wound fluid
as a response to injury. Proc Natl Acad Sci USA. 90:3889–3893.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hashimoto K, Higashiyama S, Asada H, et
al: Heparin-binding epidermal growth factor-like growth factor is
an autocrine growth factor for human keratinocytes. J Biol Chem.
269:20060–20066. 1994.PubMed/NCBI
|
|
11
|
Peoples GE, Blotnick S, Takahashi K,
Freeman MR, Klagsbrun M and Eberlein TJ: T lymphocytes that
infiltrate tumors and atherosclerotic plaques produce
heparin-binding epidermal growth factor-like growth factor and
basic fibroblast growth factor: a potential pathologic role. Proc
Natl Acad Sci USA. 92:6547–6551. 1995. View Article : Google Scholar
|
|
12
|
Miyagawa J, Higashiyama S, Kawata S, et
al: Localization of heparin-binding EGF-like growth factor in the
smooth muscle cells and macrophages of human atherosclerotic
plaques. J Clin Invest. 95:404–411. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Powell PP, Klagsbrun M, Abraham JA and
Jones RC: Eosinophils expressing heparin-binding EGF-like growth
factor mRNA localize around lung microvessels in pulmonary
hypertension. Am J Pathol. 143:784–793. 1993.PubMed/NCBI
|
|
14
|
Mangrulkar RS, Ono M, Ishikawa M,
Takashima S, Klagsbrun M and Nowak RA: Isolation and
characterization of heparin-binding growth factors in human
leiomyomas and normal myometrium. Biol Reprod. 53:636–646. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Higashiyama S, Iwamoto R, Goishi K, et al:
The membrane protein CD9/DRAP 27 potentiates the juxtacrine growth
factor activity of the membrane-anchored heparin-binding EGF-like
growth factor. J Cell Biol. 128:929–938. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iwamoto R, Higashiyama S, Mitamura T,
Taniguchi N, Klagsbrun M and Mekada E: Heparin-binding EGF-like
growth factor, which acts as the diphtheria toxin receptor, forms a
complex with membrane protein DRAP27/CD9, which up-regulates
functional receptors and diphtheria toxin sensitivity. EMBO J.
13:2322–2330. 1994.
|
|
17
|
Yotsumoto F, Yagi H, Suzuki SO, et al:
Validation of HB-EGF and amphiregulin as targets for human cancer
therapy. Biochem Biophys Res Commun. 365:555–561. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miyamoto S, Hirata M, Yamazaki A, et al:
Heparin-binding EGF-like growth factor is a promising target for
ovarian cancer therapy. Cancer Res. 64:5720–5727. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Inui Y, Higashiyama S, Kawata S, et al:
Expression of heparin-binding epidermal growth factor in human
hepatocellular carcinoma. Gastroenterology. 107:1799–1804.
1994.PubMed/NCBI
|
|
20
|
Naef M, Yokoyama M, Friess H, Buchler MW
and Korc M: Co-expression of heparin-binding EGF-like growth factor
and related peptides in human gastric carcinoma. Int J Cancer.
66:315–321. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kobrin MS, Funatomi H, Friess H, Buchler
MW, Stathis P and Korc M: Induction and expression of
heparin-binding EGF-like growth factor in human pancreatic cancer.
Biochem Biophys Res Commun. 202:1705–1709. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohnishi Y, Inoue H, Furukawa M, Kakudo K
and Nozaki M: Heparin-binding epidermal growth factor-like growth
factor is a potent regulator of invasion activity in oral squamous
cell carcinoma. Oncol Rep. 27:954–958. 2012.PubMed/NCBI
|
|
23
|
Haugen DR, Akslen LA, Varhaug JE and
Lillehaug JR: Expression of c-erbB-3 and c-erbB-4 proteins in
papillary thyroid carcinomas. Cancer Res. 56:1184–1188.
1996.PubMed/NCBI
|
|
24
|
Elenius K, Paul S, Allison G, Sun J and
Klagsbrun M: Activation of HER4 by heparin-binding EGF-like growth
factor stimulates chemotaxis but not proliferation. EMBO J.
16:1268–1278. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ito T, Seyama T, Mizuno T, et al: Unique
association of p53 mutations with undifferentiated but not with
differentiated carcinomas of the thyroid gland. Cancer Res.
52:1369–1371. 1992.PubMed/NCBI
|
|
26
|
Fogh J, Wright WC and Loveless JD: Absence
of HeLa cell contamination in 169 cell lines derived from human
tumors. J Natl Cancer Inst. 58:209–214. 1977.PubMed/NCBI
|
|
27
|
Sobin LH and Wittekind C: International
Union against Cancer. TNM Classification of Malignant Tumours. 6th
edition. Wiley-Liss; New York, NY: 2002
|
|
28
|
Ishiyama M, Miyazono Y, Sasamoto K, Ohkura
Y and Ueno K: A highly water-soluble disulfonated tetrazolium salt
as a chromogenic indicator for NADH as well as cell viability.
Talanta. 44:1299–1305. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boyden S: The chemotactic effect of
mixtures of antibody and antigen on polymorphonuclear leucocytes. J
Exp Med. 115:453–466. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoshida A, Anand-Apte B and Zetter BR:
Differential endothelial migration and proliferation to basic
fibroblast growth factor and vascular endothelial growth factor.
Growth Factors. 13:57–64. 1996. View Article : Google Scholar
|
|
31
|
Goodman SL, Vollmers HP and Birchmeier W:
Control of cell locomotion: perturbation with an antibody directed
against specific glycoproteins. Cell. 41:1029–1038. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mannino RJ, Ballmer K, Zeltner D and
Burger MM: An inhibitor of animal cell growth increases
cell-to-cell adhesion. J Cell Biol. 91:855–859. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Giancotti FG and Ruoslahti E: Elevated
levels of the α5β1fibronectin receptor
suppress the transformed phenotype of Chinese hamster ovary cells.
Cell. 60:849–859. 1990.
|
|
34
|
Chomczynski P and Sacchi N: Single-step
method of RNA isolation by acid guanidinium
thiocyanate-phenol-chloroform extraction. Anal Biochem.
162:156–159. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Datta YH, Adams PT, Drobyski WR, Ethier
SP, Terry VH and Roth MS: Sensitive detection of occult breast
cancer by the reverse-transcriptase polymerase chain reaction. J
Clin Oncol. 12:475–482. 1994.
|
|
36
|
Noguchi S, Aihara T, Nakamori S, et al:
The detection of breast carcinoma micrometastases in axillary lymph
nodes by means of reverse transcriptase-polymerase chain reaction.
Cancer. 74:1595–1600. 1994. View Article : Google Scholar
|
|
37
|
Haugen DR, Akslen LA, Varhaug JE and
Lillehaug JR: Demonstration of a TGF-alpha-EGF-receptor autocrine
loop and c-myc protein over-expression in papillary thyroid
carcinomas. Int J Cancer. 55:37–43. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gorgoulis V, Aninos D, Priftis C, et al:
Expression of epidermal growth factor, transforming growth
factor-alpha and epidermal growth factor receptor in thyroid
tumors. In Vivo. 6:291–296. 1992.PubMed/NCBI
|
|
39
|
Aasland R, Akslen LA, Varhaug JE and
Lillehaug JR: Co-expression of the genes encoding transforming
growth factor-alpha and its receptor in papillary carcinomas of the
thyroid. Int J Cancer. 46:382–387. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Freeman MR, Paul S, Kaefer M, et al:
Heparin-binding EGF-like growth factor in the human prostate:
synthesis predominantly by interstitial and vascular smooth muscle
cells and action as a carcinoma cell mitogen. J Cell Biochem.
68:328–338. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Holting T, Siperstein AE, Clark OH and Duh
QY: Epidermal growth factor (EGF)- and transforming growth factor
alpha-stimulated invasion and growth of follicular thyroid cancer
cells can be blocked by antagonism to the EGF receptor and tyrosine
kinase in vitro. Eur J Endocrinol. 132:229–235. 1995. View Article : Google Scholar
|
|
42
|
Ongusaha PP, Kwak JC, Zwible AJ, et al:
HB-EGF is a potent inducer of tumor growth and angiogenesis. Cancer
Res. 64:5283–5290. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Miyoshi E, Higashiyama S, Nakagawa T,
Hayashi N and Taniguchi N: Membrane-anchored heparin-binding
epidermal growth factor-like growth factor acts as a tumor survival
factor in a hepatoma cell line. J Biol Chem. 272:14349–14355. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shah JP, Loree TR, Dharker D, Strong EW,
Begg C and Vlamis V: Prognostic factors in differentiated carcinoma
of the thyroid gland. Am J Surg. 164:658–661. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Suzuki M, Raab G, Moses MA, Fernandez CA
and Klagsbrun M: Matrix metalloproteinase-3 releases active
heparin-binding EGF-like growth factor by cleavage at a specific
juxtamembrane site. J Biol Chem. 272:31730–31737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Izumi Y, Hirata M, Hasuwa H, et al: A
metalloprotease-disintegrin, MDC9/meltrin-gamma/ADAM9 and PKCdelta
are involved in TPA-induced ectodomain shedding of
membrane-anchored heparin-binding EGF-like growth factor. EMBO J.
17:7260–7272. 1998. View Article : Google Scholar
|
|
47
|
Koshikawa N, Mizushima H, Minegishi T, et
al: Proteolytic activation of heparin-binding EGF-like growth
factor by membrane-type matrix metalloproteinase-1 in ovarian
carcinoma cells. Cancer Sci. 102:111–116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nakamura H, Ueno H, Yamashita K, et al:
Enhanced production and activation of progelatinase A mediated by
membrane-type 1 matrix metalloproteinase in human papillary thyroid
carcinomas. Cancer Res. 59:467–473. 1999.PubMed/NCBI
|
|
49
|
Aust G, Hofmann A, Laue S, Rost A, Kohler
T and Scherbaum WA: Human thyroid carcinoma cell lines and normal
thyrocytes: expression and regulation of matrix metalloproteinase-1
and tissue matrix metalloproteinase inhibitor-1 messenger-RNA and
protein. Thyroid. 7:713–724. 1997. View Article : Google Scholar
|
|
50
|
Hofmann A, Laue S, Rost AK, Scherbaum WA
and Aust G: mRNA levels of membrane-type 1 matrix metalloproteinase
(MT1-MMP), MMP-2, and MMP-9 and of their inhibitors TIMP-2 and
TIMP-3 in normal thyrocytes and thyroid carcinoma cell lines.
Thyroid. 8:203–214. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Higashiyama S, Iwabuki H, Morimoto C,
Hieda M, Inoue H and Matsushita N: Membrane-anchored growth
factors, the epidermal growth factor family: beyond receptor
ligands. Cancer Sci. 99:214–220. 2008. View Article : Google Scholar : PubMed/NCBI
|