Introduction
Epigenetic alterations have long been implicated in
both the development and progression of human cancer (1). Epigenetic changes such as DNA
methylation, histone modification and nucleosome remodeling refer
to stable alterations in gene expression with no underlying
modifications in the genetic sequence itself (2). In contrast to genetic mutations,
epigenetic alterations are intrinsically reversible (3) and hence, much research has been
focused on understanding the alterations with the aim to develop
effective therapies. DNA methylation is the foremost type of
epigenetic modification and is well characterized in human breast
cancer (4). Breast cancer is one of
the leading causes of death in the US and an increasing number of
cases per year are reported. Thus, there is an urgent need to
design effective therapies to overcome this disease (5).
Ets family members are characterized by an
evolutionarily conserved DNA binding domain called the Ets domain
and control key cellular processes, including proliferation,
differentiation and apoptosis (6–10).
hPDEF (prostate-derived Ets factor/prostate-specific Ets or PSE),
is one Ets family member in particular that has been intensely
investigated for its role in cancer development and progression.
Initial reports indicate that PDEF acts as an oncogene (11–13).
However, more recent studies suggest that PDEF possesses a
tumor-suppressing function (12,14,15).
Despite these findings, it is still not known how PDEF suppresses
tumor progression. Most of the research conducted to date has used
in vitro studies or correlative immunohistochemical analytic
methods to evaluate the role of PDEF in cancer progression
(11,12).
We undertook the present study, considering the
importance of epigenetic alterations in understanding breast cancer
and the lack of studies involving epigenetic modifications of PDEF
in breast cancer. Using the breast cancer cell line MDA-MB-468, we
provide evidence that PDEF undergoes epigenetic modifications such
as DNA methylation and is positively correlated with the expression
of its target gene p21. These findings provide further
understanding of the mechanisms of breast cancer progression and
facilitate the evaluation of options for designing effective
therapies to treat breast cancer.
Materials and methods
Chemicals
Methylation inhibitor 5-AZA-2′-deoxycytidine (A3656;
Sigma) and various HDAC inhibitors trichostatin A (T-1952; Sigma),
nicotinamide (N0636; Sigma), valproic acid (P4543; Sigma), sodium
butyrate (B5887; Sigma) and SAHA- (auberoyl bis-hydroxamic acid)
(GR323–0100; Biomol) were used.
Cell culture
The human MCF-7, MDA-MB-468 and MDA-MB-231 breast
cancer cell lines were maintained in Dulbecco’s modified Eagle’s
medium (DMEM) (Invitrogen) and the CWR22rv1 and PC3 prostate cancer
cell lines were maintained in RPMI-1640 medium (Invitrogen)
supplemented with 5% fetal bovine serum (HyClone Laboratories) and
1% penicillin/streptomycin at 37°C with 5% CO2.
Isolation of protein following treatment
with the methylation and HDAC inhibitors
Following treatment with either the methylation
inhibitor (5′AZA) or the HDAC inhibitors (TSA, SAHA,
NAD+, VPA and NaB) monolayers of cell were washed twice
with ice cold PBS, and then cell lysates were prepared using RIPA
buffer with protease inhibitor mixture (Thermo Fisher Scientific).
Cellular debris was cleared by centrifugation, and the protein
concentration was determined using the BCA protein assay kit
(Pierce).
Western blot analysis
Equal amounts of protein lysates were separated by
10% SDS-PAGE, transferred to a PVDF membrane (GE Healthcare) and
subsequently blotted with the anti-PDEF antibody (15), actin (A2066; Sigma) and acetyl H4
(H9164; Sigma). HRP-labeled goat anti-rabbit polyclonal antibody
was used as a secondary antibody, and proteins were visualized with
an enhanced chemiluminescence substrate kit (Pierce). Actin and H4
were used as the loading control as required.
Isolation of RNA and RT-PCR
Total RNA was isolated from the cell lines after
treatment with 5′AZA (1 μM) or TSA (0.5 μM) for 24 h using TRIzol
reagent (Invitrogen) according to the manufacturer’s instructions.
A two-step RT-PCR was used to analyze the mRNA expression of PDEF
(15). cDNA was created using
oligo(dT) primer and the Moloney murine leukemia virus reverse
transcriptase (MMLV-RT) enzyme according to the manufacturer’s
instructions (Invitrogen). Standard PCR techniques were then
conducted with gene-specific primers.
3-(4,5-Dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT)
in vitro proliferation assay
As previously described (16), MDA-MB-468 cells were seeded at 1,000
cells/well in a 96-well dish and allowed to grow at 37°C with 5%
CO2. The following day cells were treated with the
methylation inhibitor 5-AZA-2′-deoxycytidine at one single dose of
1 μM. Growth was assayed for 5 days; every day 10 μl/well of MTT
reagent (5 mg/ml) was added and incubation was carried out at 37°C
with 5% CO2 for 3 h. The medium was aspirated, and 100
μl of DMSO was added and mixed until a purple color formed. The
cell samples were measured using a plate reader at 570 nm. All
experiments were conducted in triplicates.
Statistical analysis
All the experiments were carried out for minimum of
three times or as mentioned. Statistical analysis was based on a
minimum of three replicates using Microsoft Office Excel. The
results were considered significant when the P-value was
<0.05.
Results
Expression levels of PDEF in the
different breast and prostate cancer cell lines
In order to investigate the epigenetic alterations
of PDEF, we analyzed the expression of PDEF protein in various
breast (MCF7, MDA-MB-231 and MDA-MB-468) and prostate (CWR22Rv1 and
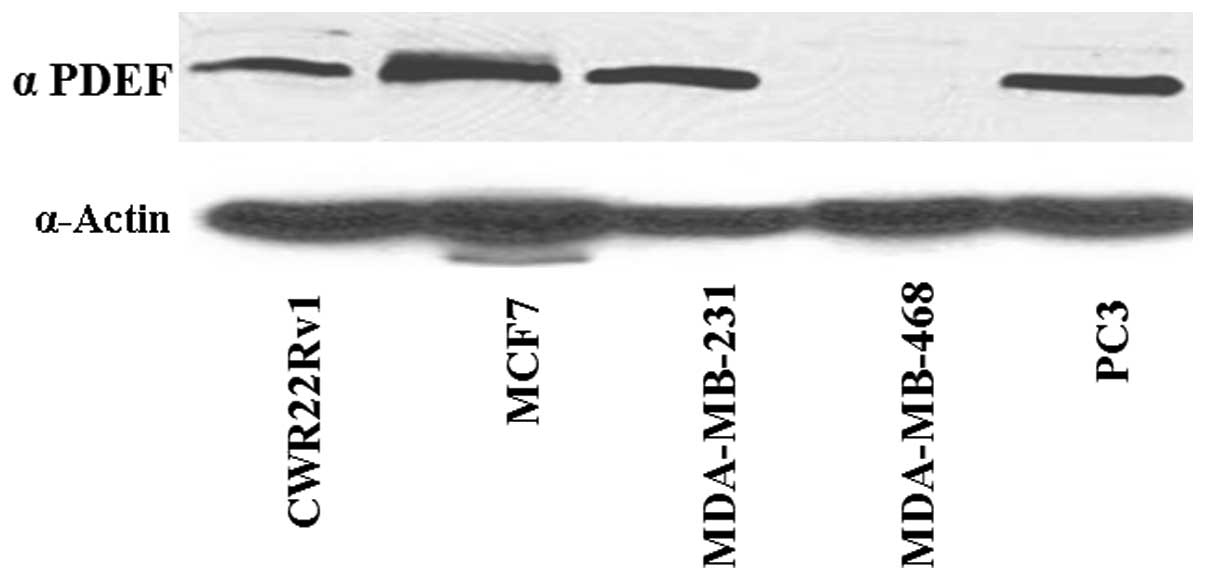
PC3) cancer cell lines. All of the cell lines tested expressed PDEF
protein except for the breast cancer cell line MDA-MB-468 (Fig. 1). Observation of undetectable PDEF
protein expression in MDA-MB-468 human breast cancer cells
encouraged us to use these cells as the cellular model for further
epigenetic studies.
Inhibition of DNA methylation enhances
PDEF expression in MDA-MB-468 cells
To further investigate the epigenetic modifications
of PDEF, we treated the MDA-MB-468 cells with a DNA methylation
inhibitor 5′AZA. 5′AZA has been widely used in epigenetic research
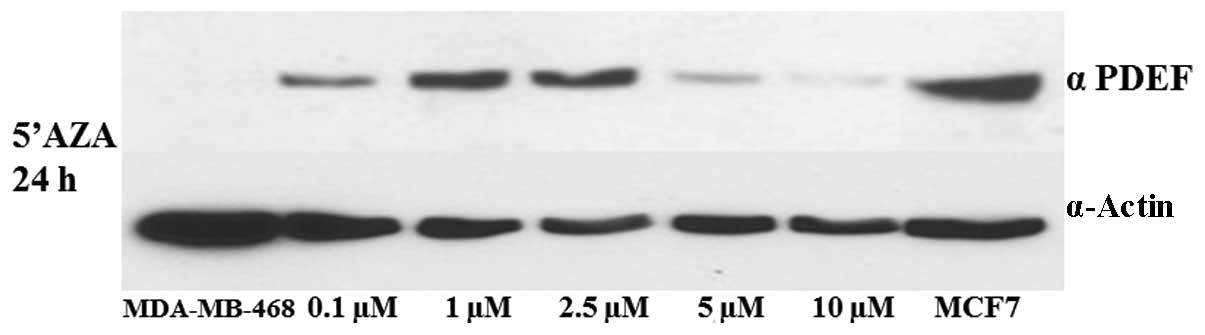
as an inhibitor that blocks DNA methylation to occur (17). Dose-dependent studies for 24 h
revealed that expression of PDEF was enhanced at a dose of 0.1 μM
of 5′AZA up to a dose of 2.5 μM (Fig.
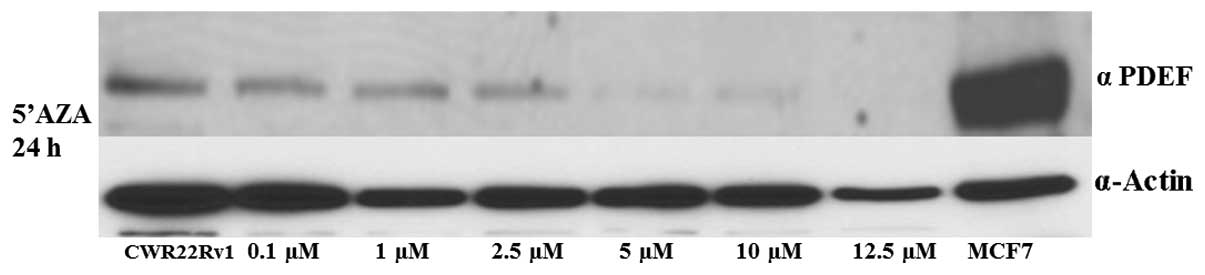
2). We also studied the effect of 5′AZA treatment at the same
doses in CWR22Rv1, a prostate cancer cell line, which exhibits weak
PDEF protein expression (Fig. 1,
lane 1) in comparison to the other cell lines used in the study.
CWR22Rv1 cells did not show any significant change following
treatment with the methylation inhibitor 5′AZA at the same 24-h
time-point (Fig. 3). HDAC
inhibitors such as TSA, SAHA, sodium butyrate, valproic acid and
nicotinamide are able to cause epigenetic changes and regulate gene
expression (18–20). However, we did not observe any
significant effect of these HDAC inhibitors on PDEF gene expression
in the MDA-MB-468 and CWR22Rv1 cells at different times and dosages
(data not shown). These observations clearly indicate that PDEF
undergoes DNA methylation as an epigenetic alteration in breast
cancer cells.
5′AZA treatment significantly inhibits
the proliferation rate of MDA-MB-468 cells in vitro
To further investigate the functional effects of
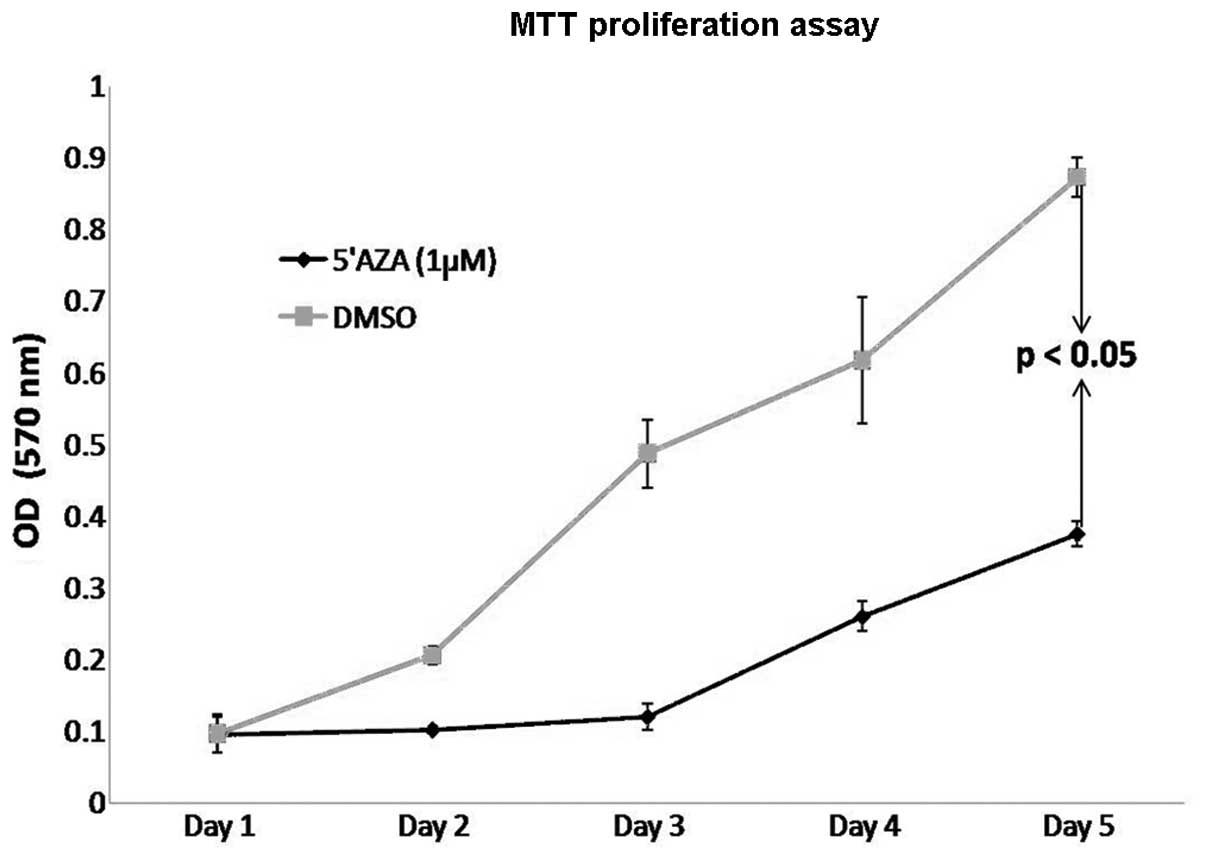
5′AZA treatment on cell proliferation in vitro, MDA-MB-468
breast cancer cells were treated with 5′AZA. The cells were treated
with 5′AZA at a concentration of 1 μM, and the proliferation rate
was analyzed by MTT assay for a 5-day time period. As shown in
Fig. 4, 5′AZA treatment led to a
significant decrease in the proliferation rate of MDA-MB-468 cells
when compared to the controls (DMSO-treated cells). These data
indicate that PDEF expression may interfere with the epigenetic
modifications such as DNA methylation of mammary tumor cells to
decrease or slow the growth or proliferation rate of these
cells.
Analysis of the protein level of the cell
cycle inhibitor p21 and its correlation with PDEF expression
PDEF controls cell proliferation and mammary tumor
progression by transcriptional regulation of the cell cycle
inhibitor p21/CIP1 (15). To
determine the correlation of PDEF and its target gene p21,
expression of p21 was determined in MDA-MB-468 cells following
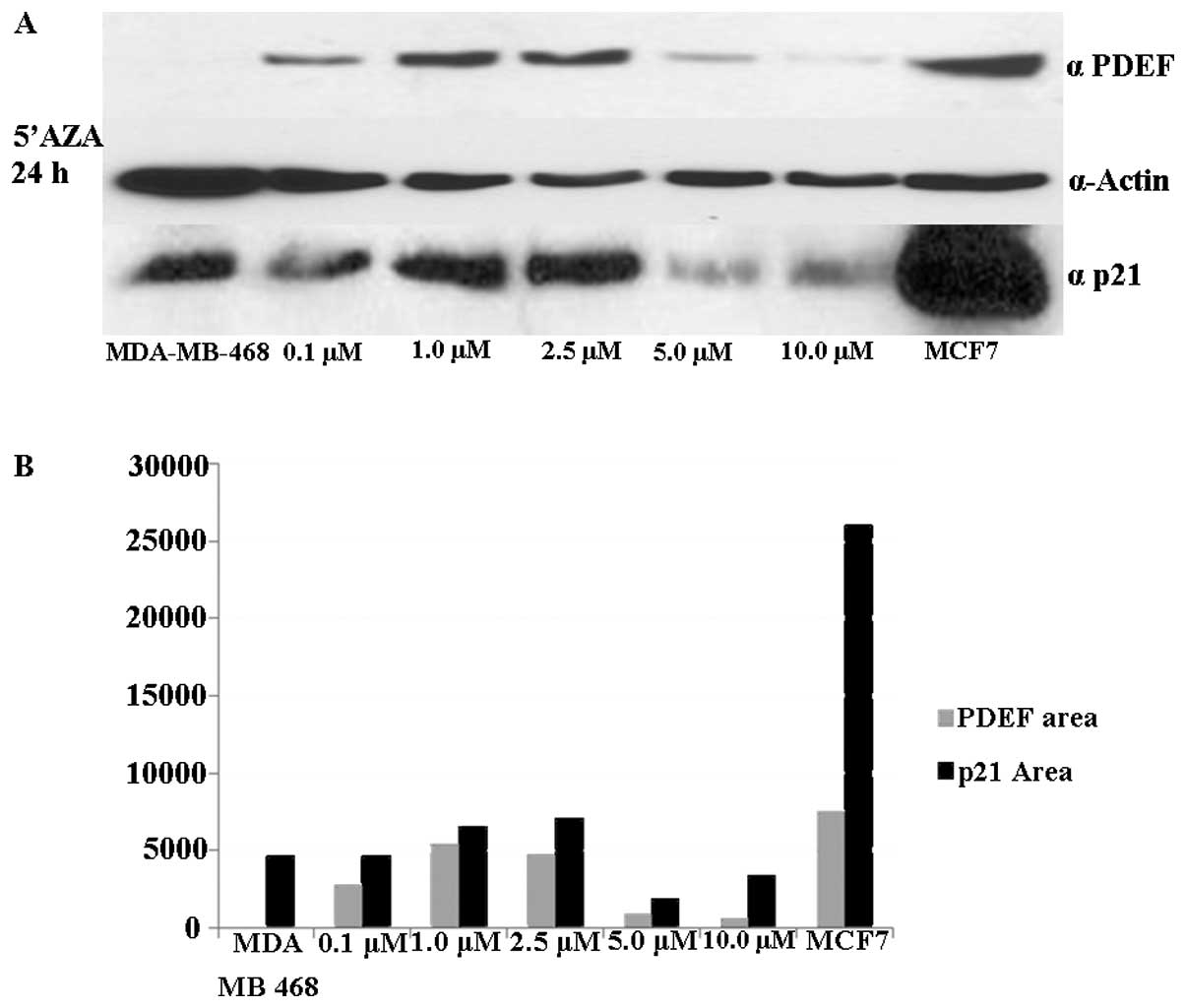
5′AZA treatment. As shown in Fig.
5A, an increased expression of p21 was observed following the
same expression pattern as that of PDEF. The p21 protein level was
increased following 5′AZA treatment at a range of doses from 0.1 to
2.5 mM (Fig. 5B).
Discussion
PDEF, a member of the Ets family, has been studied
intensively since its initial discovery in 2000 (13). The role of PDEF in normal growth and
development as well as in disease states such as cancer is well
established (6–8,10). We
previously demonstrated a tumor-suppressive role of PDEF in mammary
tumor progression and demonstrated the transcriptional regulation
of cell cycle inhibitor p21/CIP1 by PDEF (15). In another study, using iTRAQ
labeling and proteomic analysis methods the importance of PDEF was
established in prostate cancer cells. We demonstrated an
association between stathmin, a microtubule-associated protein and
PDEF in prostate cancer progression (16).
Epigenetic silencing of a gene can be reversible and
therefore, can reactivate gene expression. In the present study, we
provide evidence for the epigenetic modifications of PDEF in an
in vitro experimental system. After examining various breast
and prostate cancer cell lines, we found that only MDA-MB-468
cancer cell line showed no detectable PDEF protein expression
(Fig. 1). Thus, we considered the
MDA-MB-468 cancer cell line suitable for studying the epigenetic
modifications of PDEF gene expression. The MDA-MB-468 cell line was
treated with the well-known DNA methylation inhibitor 5′AZA. 5′AZA
has been used in many studies and has also been used in cancer
therapy (21,22). We observed a dose-dependent
enhancement in the PDEF protein expression in MDA-MB-468 cells
following 5′AZA treatment (Fig. 2).
However, with higher concentrations of 5′AZA treatment the PDEF
protein expression was reduced. One of the reasons for this
reduction could be ubiquitin degradation or 5′AZA toxicity to the
cells at higher doses. Notably, DNA demethylation of PDEF was found
to be more specific to the MDA-MB-468 breast cancer cell line, as
DNA demethylation in the prostate cancer cell line CWR22Rv1 did not
show any significant effects (Fig.
3). We further analyzed other epigenetic modifications such as
histone acetylation or deacetylation in both the MDA-MB-468 human
breast cancer cell line and CWR22Rv1 human prostate cancer cells
and did not see any significant changes (data not shown). PDEF in
the present study only displayed DNA methylation alteration
specific to MDA-MB-468 breast cancer cells. This clearly suggests
that in a mammary tumor in vitro experimental design, PDEF
exhibited DNA methylation modification.
PDEF has been shown to be associated with many
different target genes, such as p21/CIP1, maspin, survivin, p62 and
PSA (23–28), to exert its effects based on the
co-receptors present and the internal cell environment. In the
present study, with human breast cancer cells, PDEF acted as an
inhibitor of cell proliferation (Fig.
4). DNA methylation inhibitors have already been used in
clinical trials as single-molecule agents and in combinatorial
therapies (29–31). The fact that PDEF undergoes DNA
methylation reveals the importance to study the underlying
mechanism of this epigenetic alteration and the role of PDEF and
its correlation with p21 as a target gene.
In conclusion, this is the first report to
demonstrate that PDEF undergoes DNA methylation in breast cancer
cells. The present study provides insight into the mechanisms
involved in the DNA methylation of PDEF. Our report unravels the
possibility of further research into the epigenetic DNA methylation
of PDEF and the target genes involved in this process in the aim of
designing effective and specific PDEF cancer therapeutics.
Acknowledgements
The authors would like to thank Dr Asish K. Ghosh
and Dr George P. Tuszynski for their helpful discussions and
comments. The present study was supported by a Northwestern
Prostate Cancer SPORE pilot project fund to M.Z.
References
|
1
|
Lustberg MB and Ramaswamy B: Epigenetic
therapy in breast cancer. Curr Breast Cancer Rep. 3:34–43. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang X, Yan L and Davidson NE: DNA
methylation in breast cancer. Endocr Relat Cancer. 8:115–127. 2001.
View Article : Google Scholar
|
|
3
|
Connolly R and Stearns V: Epigenetics as a
therapeutic target in breast cancer. J Mammary Gland Biol
Neoplasia. 17:191–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Locke WJ and Clark SJ: Epigenome
remodelling in breast cancer: insights from an early in vitro model
of carcinogenesis. Breast Cancer Res. 14:2152012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel R, DeSantis C, Virgo K, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oikawa T: ETS transcription factors:
possible targets for cancer therapy. Cancer Sci. 95:626–633. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galang CK, Muller WJ, Foos G, Oshima RG
and Hauser CA: Changes in the expression of many Ets family
transcription factors and of potential target genes in normal
mammary tissue and tumors. J Biol Chem. 279:11281–11292. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsu T, Trojanowska M and Watson DK: Ets
proteins in biological control and cancer. J Cell Biochem.
91:896–903. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li R, Pei H and Watson DK: Regulation of
Ets function by protein - protein interactions. Oncogene.
19:6514–6523. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seth A and Watson DK: ETS transcription
factors and their emerging roles in human cancer. Eur J Cancer.
41:2462–2478. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ghadersohi A and Sood AK: Prostate
epithelium-derived Ets transcription factor mRNA is overexpressed
in human breast tumors and is a candidate breast tumor marker and a
breast tumor antigen. Clin Cancer Res. 7:2731–2738. 2001.PubMed/NCBI
|
|
12
|
Sood AK, Saxena R, Groth J, et al:
Expression characteristics of prostate-derived Ets factor support a
role in breast and prostate cancer progression. Hum Pathol.
38:1628–1638. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamada N, Tamai Y, Miyamoto H and Nozaki
M: Cloning and expression of the mouse Pse gene encoding a
novel Ets family member. Gene. 241:267–274. 2000.
|
|
14
|
Ghadersohi A, Odunsi K, Lele S, et al:
Prostate derived Ets transcription factor shows better
tumor-association than other cancer-associated molecules. Oncol
Rep. 11:453–458. 2004.
|
|
15
|
Schaefer JS, Sabherwal Y, Shi HY, et al:
Transcriptional regulation of p21/CIP1 cell cycle inhibitor by PDEF
controls cell proliferation and mammary tumor progression. J Biol
Chem. 285:11258–11269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sabherwal Y, Mahajan N, Helseth DL,
Gassmann M, Shi H and Zhang M: PDEF downregulates stathmin
expression in prostate cancer. Int J Oncol. 40:1889–1899.
2012.PubMed/NCBI
|
|
17
|
Ghoshal K and Bai S: DNA
methyltransferases as targets for cancer therapy. Drugs Today.
43:395–422. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Acharya MR, Sparreboom A, Venitz J and
Figg WD: Rational development of histone deacetylase inhibitors as
anticancer agents: a review. Mol Pharmacol. 68:917–932. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsuzaki Y, Sowa Y, Hirose T, Yokota T
and Sakai T: Histone deacetylase inhibitors: promising agents for
‘gene-regulating chemoprevention’ and ‘molecular-targeting
prevention’ of cancer. Environ Health Prev Med. 8:157–160.
2003.
|
|
20
|
Ropero S and Esteller M: The role of
histone deacetylases (HDACs) in human cancer. Mol Oncol. 1:19–25.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Braiteh F, Soriano AO, Garcia-Manero G, et
al: Phase I study of epigenetic modulation with 5-azacytidine and
valproic acid in patients with advanced cancers. Clin Cancer Res.
14:6296–6301. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karahoca M and Momparler RL:
Pharmacokinetic and pharmacodynamic analysis of
5-aza-2′-deoxycytidine (decitabine) in the design of its
dose-schedule for cancer therapy. Clin Epigenetics. 5:32013.
|
|
23
|
Feldman RJ, Sementchenko VI, Gayed M,
Fraig MM and Watson DK: Pdef expression in human breast cancer is
correlated with invasive potential and altered gene expression.
Cancer Res. 63:4626–4631. 2003.
|
|
24
|
Ghadersohi A, Pan D, Fayazi Z, Hicks DG,
Winston JS and Li F: Prostate-derived Ets transcription factor
(PDEF) downregulates survivin expression and inhibits breast cancer
cell growth in vitro and xenograft tumor formation in vivo. Breast
Cancer Res Treat. 102:19–30. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oettgen P, Finger E, Sun Z, et al: PDEF, a
novel prostate epithelium-specific Ets transcription factor,
interacts with the androgen receptor and activates
prostate-specific antigen gene expression. J Biol Chem.
275:1216–1225. 2000. View Article : Google Scholar
|
|
26
|
Thompson HG, Harris JW, Wold BJ, Lin F and
Brody JP: p62 overexpression in breast tumors and regulation by
prostate-derived Ets factor in breast cancer cells. Oncogene.
22:2322–2333. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang M, Maass N, Magit D and Sager R:
Transactivation through Ets and Ap1 transcription sites determines
the expression of the tumor-suppressing gene maspin. Cell Growth
Differ. 8:179–186. 1997.PubMed/NCBI
|
|
28
|
Zhang M, Magit D and Sager R: Expression
of maspin in prostate cells is regulated by a positive Ets element
and a negative hormonal responsive element site recognized by
androgen receptor. Proc Natl Acad Sci USA. 94:5673–5678. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gros C, Fahy J, Halby L, et al: DNA
methylation inhibitors in cancer: recent and future approaches.
Biochimie. 94:2280–2296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marks PW: Decitabine for acute myeloid
leukemia. Expert Rev Anticancer Ther. 12:299–305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nebbioso A, Carafa V, Benedetti R and
Altucci L: Trials with ‘epigenetic’ drugs: an update. Mol Oncol.
6:657–682. 2012.
|