Introduction
Circulating proteins have been studied as prognostic
markers in melanoma (1). The
prognostic value of serum lactate dehydrogenase (LDH) level in
stage IV melanoma is such that it has been included in the American
Joint Committee on Cancer (AJCC) staging system (2). Others have been investigated (1), particularly S100 and melanoma
inhibitory antigen (MIA) but they were reported to be of no
clinical utility in early-stage melanoma patients (AJCC stage
I–III) (3,4).
Osteopontin, encoded by gene SPP1, is a
multifunctional extracellular matrix glycoprotein produced by cells
of many lineages, shown to be important in cancer cell adhesion,
cell motility and survival (5).
Osteopontin induces phosphatidylinositol 3-kinase (PI3K) activation
(6,7) and acts on transcription factor nuclear
factor κB (NF-κB) (8,9), potentially allowing it to regulate
cell proliferation, differentiation and apoptosis.
SPP1 gene expression was reported to be
associated with melanoma progression in whole-genome gene
expression profiling (10), and was
later confirmed in immunohistochemical studies (11–13).
Our group confirmed increased expression of SPP1 in primary
tumours to be of independent prognostic value for melanoma
(14) using an agnostic approach;
utilising the Illumina cDNA-mediated annealing, selection,
extension and ligation (DASL) platform which measures expression of
502 cancer-related genes. Other groups reported supportive evidence
using different gene expression assays (15,16) or
immunohistochemistry (11,17,18).
Increased blood levels of osteopontin have been
described as being associated with progression in many types of
cancers (5), including cancer of
the breast (19), head and neck
(20) and liver (21). An increased osteopontin level was
reported to be a predictor of outcome in non-small cell lung cancer
(NSCLC) (22) and to be reduced
after tumour resection of NSCLC (23). No association was found between
osteopontin levels and disease course in mesothelioma (24). A small number of studies have shown
that osteopontin levels were increased in uveal melanoma (25,26)
and were highly correlated with the presence of liver metastasis
(27).
Elevated osteopontin plasma concentrations have very
recently been reported in two studies concerning metastatic
melanoma (13,28). We report here for the first time a
pilot study examining the potential prognostic utility of plasma
osteopontin in early-stage disease patients (AJCC I to III)
analysing the effect on risk of death from melanoma or from any
cause and taking into account factors already known to be of
prognostic value.
Materials and methods
Patients and samples
One hundred and eighty-five patients were identified
from participants bled at recruitment to the Leeds Melanoma Cohort
and for whom stored plasma samples were available (29). Participants were recruited to the
study within 3–6 months after diagnosis, when possible. Samples
were selected as follows: i) 76 samples from participants who were
believed to be disease-free at venepuncture (53 treated stage I/II,
23 treated stage III), and who have not relapsed in the subsequent
period of a median of 7.5 years (range, 1.1–11.2); ii) 82 from
participants who were believed to be disease-free at sampling but
subsequently relapsed (57 treated stage I/II, 25 treated stage
III); and iii) 27 who had metastatic disease at sampling (17
untreated stage III, 10 untreated stage IV). A patient was defined
as disease-free if they had had their primary melanoma excised or
their lymph nodes removed and there was no known clinical evidence
of further disease. A minimum period of 6 weeks between surgery and
venepuncture was used based on a study in NSCLC patients which
showed that osteopontin plasma levels were elevated in the period
of 6 weeks after surgery possibly due to the involvement of
osteopontin in wound healing (23).
Thirty healthy controls were also included in the study to compare
osteopontin levels with those in the normal population. No
difference in age and gender was observed between controls and
cases. The study was approved by the national ethics committee,
MREC, and informed consent was obtained from all participants for
studies on survival from melanoma.
All samples were collected into EDTA and separated
by centrifugation at 1,500 × g for 15 min, prior to storage in
aliquots at −80°C. Some samples stored from participants in the
Leeds Melanoma Cohort had been mailed to the laboratory resulting
in variation in the time from venepuncture to processing with a
median of 1 day (range, 0–4 days). There are no published data for
the stability of osteopontin plasma levels in stored samples.
Therefore, to investigate the potential impact of delays in
processing we first investigated the stability of osteopontin
levels over time. In order to do this, additional plasma samples
were obtained from 5 melanoma patients and 4 healthy volunteers
with informed consent. Two 4-ml tubes of blood were collected from
each person; one sample was processed immediately after
venepuncture and plasma was stored at −80°C, and the other was
processed similarly but after being left at room temperature for 4
days. In these samples osteopontin levels were measured to
determine whether there was change due to variation in processing
time.
Enzyme-linked immunosorbent assay (ELISA)
of plasma osteopontin
An ELISA assay kit (Quantikine; R&D Systems) was
used to measure osteopontin levels according to the protocol. Prior
to use the assay was validated examining intra- and inter-assay
precision, parallelism and recovery, using recombinant osteopontin
protein purchased from Abcam and interference as previously
described (30,31). EDTA plasma samples were used for the
analysis as proteolytic cleavage of osteopontin by thrombin during
the clotting process occurs in serum samples. In each assay a low-
and a high-quality control sample with known concentration was
analysed.
Statistical analyses
All samples were assayed in duplicate [acceptable
coefficients of variation (CVs) being <10%], and the osteopontin
concentrations (ng/ml) presented here are the mean of the two
replicates. The potential effects of differences in sample
processing time on osteopontin plasma concentrations were assessed
using the results from the matched samples processed at different
time-points (same day vs. 4 days after venepuncture) and analysed
using the Wilcoxon matched-pairs signed-ranks test.
First, osteopontin differences between healthy
controls and cases grouped according to AJCC stage at venepuncture
were compared using the Kruskal-Wallis test and multiple linear
regression. Second, we looked at osteopontin as a prognostic
indicator for patients with no evident disease so that patients
with metastatic disease at sampling were excluded from subsequent
analysis.
Factors previously shown to be associated with
survival in the Leeds Melanoma Cohort were assessed for association
with osteopontin level: Breslow thickness, body mass index (BMI),
AJCC stage, tumour site and mitotic rate, age at diagnosis, gender,
tumour ulceration (ulcerated, not ulcerated), sentinel node biopsy
(SNB) status, and vitamin D serum levels (nmol/l, adjusted for
season) (29). Mann-Whitney U
tests, Spearman correlations and Kruskal-Wallis tests were used
where appropriate. Multiple linear regression was used to identify
possible independent predictors of osteopontin level.
Odds ratios (OR) and 95% confidence intervals (CI)
were estimated from logistic regression models for the effect of
osteopontin levels on risk of death from melanoma and death from
any causes. Due to the skewed frequency distribution of
osteopontin, the log-transformed osteopontin level (to the base 2)
was entered into the models so that the estimated OR would be
interpreted as the OR associated with a doubling of osteopontin
level at recruitment. Osteopontin level was also considered as a
categorical variable by grouping into approximate tertiles (≤49.35,
>49.35 to ≤64.34, >64.34). Both unadjusted and adjusted ORs
were calculated for osteopontin; the adjustment variables were:
age, gender, BMI, site of the primary, season-adjusted vitamin D
level and stage at sampling. Here, the vitamin D variable was
grouped into six categories, to show the effect based on 20 nmol/l
increments (≤20, >20 to ≤40, >40 to ≤60, >60 to ≤80,
>80 to ≤100 and >100). Secondary analyses incorporated
time-to-event data and Kaplan-Meier curves were plotted. Hazard
ratios (HR) and 95% CI were estimated from Cox proportional hazards
models for the effect of osteopontin level on melanoma-specific
survival (MSS) and overall survival (OS). An arbitrary significance
level of P<0.05 was used. STATA version 10 (32) was used for statistical analyses.
Results
Initial validation aspects
Satisfactory validation of the ELISA assay was
achieved with intra- and inter-assay precision of <10%,
acceptable parallelism and recovery, and no hook effect or
interference from bilirubin, haemolysis, triglycerides or
rheumatoid factor (31). There was
no statistically significant difference in osteopontin levels
between samples processed immediately and four days later (Wilcoxon
matched-pairs signed rank test; P=0.07, data not shown) with the
majority of samples differing by <3% between conditions,
enabling all stored samples to be used in the study.
Cross-sectional analysis of plasma
osteopontin in all cases and healthy controls
The normal range of osteopontin levels, as it was
measured in 30 healthy controls, was 28.6–118.8 ng/ml with a median
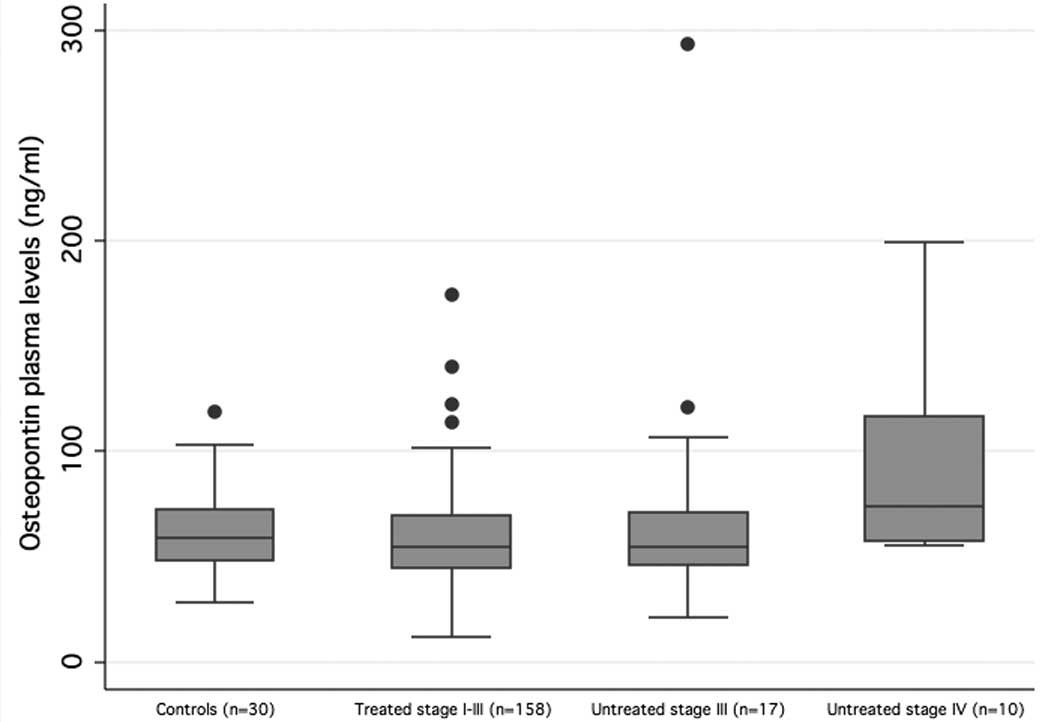
level of 59.2 ng/ml. Of the 185 melanoma patients, 158 had treated
stage I–III, 17 had untreated stage III and 10 had untreated stage
IV disease (median SPP1 level 54.7, 54.6 and 74.0 ng/ml,
respectively) (Fig. 1). A
statistically significant difference in osteopontin levels was
observed between the 4 groups (Kruskal-Wallis test
χ2=8.69; P=0.03; Fig.
1). To explore this further, we performed multiple linear
regression which showed that untreated stage IV patients had
significantly higher osteopontin levels than the controls and the
treated stage I–III patients in age-adjusted models (P=0.004 for
untreated stage IV vs. controls; P<0.001 for untreated stage IV
vs. treated stage I–III patients). No significant differences were
seen between controls and the treated stage I–III or the untreated
stage III patient groups.
In healthy controls, 95% of samples had osteopontin
levels <103.14 ng/ml. This cut-off was, therefore, taken as the
upper end of normal. Patients (2.5%) with treated stage I–III
(4/158), 17.6% of patients with untreated stage III (3/17) and 30%
of patients with untreated stage IV disease (3/10) had levels
higher than this cut-off (Fisher’s exact=0.001, data not shown).
The cut-off that had been previously reported is 76 ng/ml (95th
centile) (33), which is the 80th
centile in our control group.
Osteopontin in patients free of disease
at sampling
Age was positively correlated with osteopontin level
in the disease-free patient group (Spearman’s rho=0.2, P=0.02;
Table I) and overall (Spearman’s
rho=0.21, P=0.004). AJCC stage at sampling was borderline
associated with osteopontin levels (P=0.06; Table I) with a higher median level noted
in patients with treated stage III melanomas compared to those with
treated stage I–II disease (64.3 and 54.1 ng/ml, respectively).
Neither age nor stage at sampling were independent predictors of
osteopontin level in a multiple linear regression model. There was
no difference in osteopontin levels between SNB-positive (n=38) and
SNB-negative (n=41) participants (Table
I).
 | Table IRelationship between osteopontin
levels and other characteristics of the participants who were
disease-free at sampling (univariable analysis). |
Table I
Relationship between osteopontin
levels and other characteristics of the participants who were
disease-free at sampling (univariable analysis).
| Variables | N | Median osteopontin
(range) | Test statistic;
P-value |
|---|
| Osteopontin
(ng/ml) | 158 | 54.7
(27.9–140.0) | |
| Age (years) | 158 | | Spearman’s rho=0.2;
0.02 |
| Gender | 158 | | |
| Male | 85 | 54.6
(27.9–122.4) | Mann-Whitney,
z=−0.4; 0.67 |
| Female | 73 | 54.8
(12.0–174.4) | |
| BMI | 155 | | |
| <18.5 | 1 | 49.5 | |
| ≥18.5 to
<25 | 62 | 56.1
(28.4–174.4) | Kruskal-Wallis
χ2=0.9; 0.82 |
| ≥25 to <30 | 57 | 53.3
(27.9–122.4) | |
| ≥30 | 35 | 54.4
(12.0–113.9) | |
| Breslow thickness
(mm) | 156 | | |
| ≤1 | 11 | 47.7
(30.8–102.2) | |
| >1 to ≤2 | 49 | 54.6
(31.2–174.4) | Kruskal-Wallis
χ2=2.5; 0.47 |
| >2 to ≤4 | 57 | 54.5
(12.0–98.4) | |
| >4 | 39 | 55.2
(27.9–113.9) | |
| Tumour site | 158 | | |
| Trunk | 71 | 53.9
(29.2–122.4) | |
| Head/neck | 16 | 53.0
(29.3–102.0) | Kruskal-Wallis,
χ2=5.7; 0.13 |
| Limbs | 55 | 55.2
(12.0–174.4) | |
| Acral/rare | 16 | 68.0
(27.9–98.9) | |
| Mitotic rate
(mm−2) | 128 | | |
| <1 | 20 | 55.6
(31.2–93.0) | |
| 1–6 | 69 | 52.4
(12.0–140.0) | Kruskal-Wallis,
χ2=3.2; 0.20 |
| >6 | 39 | 56.6
(35.2–98.8) | |
| Ulcerated
tumours | 158 | | |
| Not ulcerated | 98 | 54.6
(28.4–122.4) | Mann-Whitney,
z=−1.0; 0.32 |
| Ulcerated | 60 | 56.6
(12.0–174.4) | |
| Vitamin D
(nmol/l) | 150 | | Spearman’s
rho=−0.1; 0.30 |
| Stage at
sampling | 156 | | |
| Treated I/II | 110 | 54.1
(27.9–122.4) | Mann-Whitney,
z=−1.9; 0.06 |
| Resected III | 48 | 64.3
(28.4–93.2) | |
| SNB status | 79 | | |
| Positive | 38 | 55.2
(28.4–93.2) | Mann-Whitney,
z=−0.1; 0.96 |
| Negative | 41 | 57.6
(30.8–122.4) | |
Logistic regression showed a trend for increased
risk of death from any cause with increasing osteopontin level but
this was not statistically significant [adjusted OR was 1.24 (95%
CI, 0.52–2.96) for the middle vs. lowest tertile, and 1.39 (95% CI,
0.56–3.42) for the highest vs. lowest tertile, adjusted for age,
gender, BMI, site of primary, season-adjusted vitamin D level and
stage at sampling (Table II)].
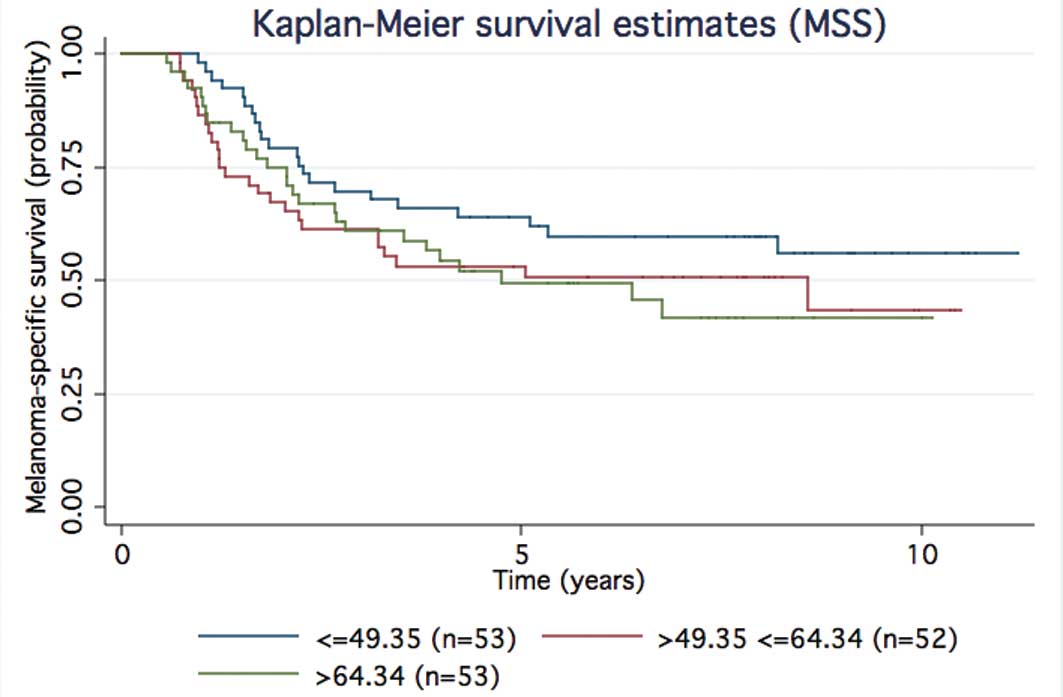
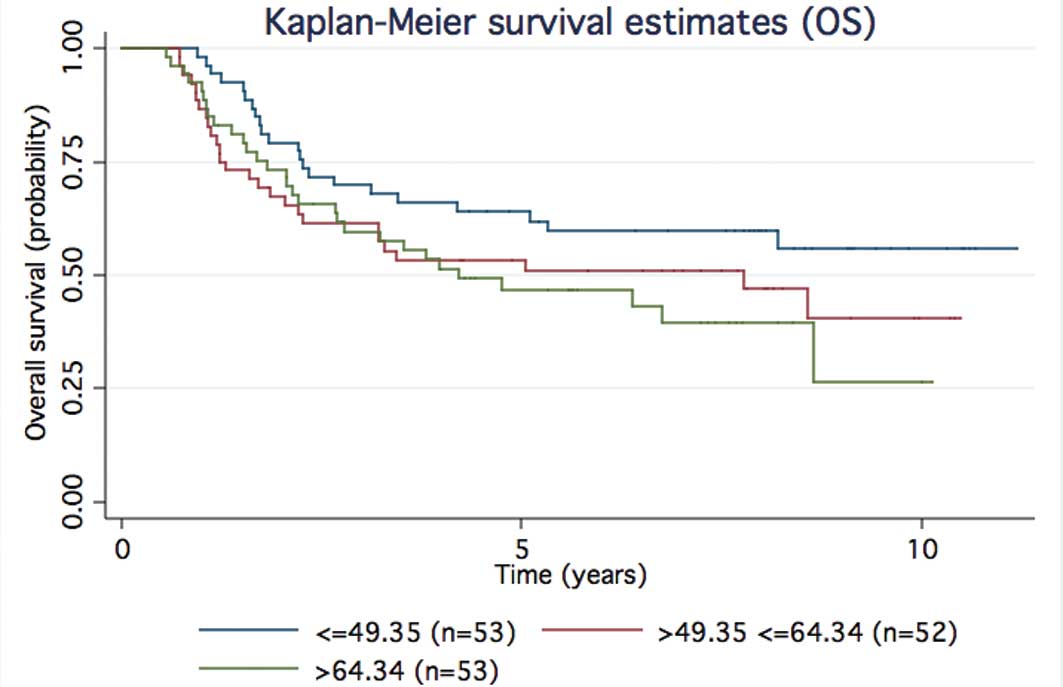
Time-to-event analyses showed support for that trend (Figs. 2 and 3 for MSS and OS, respectively). When the
95th centile cut-off was used 4/158 of the treated stage I–III
patients had higher levels; 3/4 relapsed early (<1.6 years) and
1/4 after 6 years; 154/158 of the treated stage I–III patients had
osteopontin levels below the cut-off and 79/154 were subsequent
relapsers, 60 of which relapsed at <1.6 years.
 | Table IIAssociation of osteopontin plasma
levels with risk of death in participants who were disease-free at
sampling. |
Table II
Association of osteopontin plasma
levels with risk of death in participants who were disease-free at
sampling.
| Alive, dead from
melanoma | OR (95% CI) | P-value | Alive, dead from
any cause | OR (95% CI) | P-value |
|---|
| Continuous
osteopontin |
| Unadjusted
model | 83, 75 | 1.11a (0.60–2.07) | 0.74 | 79, 79 | 1.33a (0.71–2.49) | 0.37 |
| Adjusted
model | 82, 65 | 0.85a (0.42–1.70) | 0.64 | 78, 69 | 1.05a (0.52–2.12) | 0.88 |
| Categorical
osteopontin (tertiles) |
| Unadjusted
model |
| ≤49.35 | 83, 75 | 1 | | 79, 79 | 1 | |
| >49.35 to
≤64.34 | | 1.41
(0.65–3.04) | 0.38 | | 1.52
(0.70–3.29) | 0.29 |
| >64.34 | | 1.46
(0.68–3.15) | 0.33 | | 1.83
(0.85–3.97) | 0.12 |
| Adjusted
model |
| ≤49.35 | 82, 65 | 1 | | 78, 69 | 1 | |
| >49.35 to
≤64.34 | | 1.14
(0.48–2.69) | 0.77 | | 1.24
(0.52–2.96) | 0.63 |
| >64.34 | | 1.02
(0.41–2.52) | 0.96 | | 1.39
(0.56–3.42) | 0.48 |
Discussion
We report a pilot study of osteopontin levels as a
prognostic biomarker. The strength of the present study is that we
looked for the first time at the prognostic value of osteopontin
levels in recently diagnosed patients. The weaknesses are that this
study is underpowered; only a single test sample was available, and
no comparisons were made between osteopontin levels and other blood
markers known to have some prognostic significance, such as
LDH.
Currently, Breslow thickness, tumour ulceration,
mitotic rate, lymph node metastasis, site of distant metastasis and
serum LDH levels are the prognostic markers which are included in
the most recent version of the AJCC staging system. Age, tumour
site and gender are also powerful prognostic factors (34). Even if all these factors are
considered, the variance in survival within stage is still large;
thin tumours for example might progress to advanced disease
(35). Therefore, there is an
urgent need for identification of new prognostic biomarkers. There
is also a need for a screening test to detect early recurrence now
that more effective drug treatments are emerging (36,37).
A clinically useful biomarker should be measured
easily, reliably and at low cost, by a sensitive and specific assay
(38). A number of serological
prognostic biomarkers have been studied in melanoma, but their
clinical utility is still unproven. LDH serum level is most widely
used (2) but is a poor marker of
early recurrence and is commonly somewhat elevated in otherwise
healthy individuals.
Serum levels of S100, MIA and amyloid A have been
identified as potential prognostic biomarkers in advanced disease
(1). However, they failed to
predict outcome in early-stage disease-free disease (39) having a low sensitivity in a subgroup
of patients (3).
Osteopontin plasma levels have recently been
reported to be markedly increased in metastatic melanoma in two
studies (13,28) and the present study provided some
supportive evidence. It has been suggested that osteopontin levels
might best be used in a panel of plasma markers (28). An increase in sensitivity for the
detection of metastatic disease was observed when S100 plasma
levels were combined with osteopontin (13).
A significant difference in osteopontin levels was
observed between healthy controls and patients with melanoma
grouped according to AJCC stage, but most of this difference was
explained by high levels in patients with stage IV disease. Using
the 95th centile measure in healthy controls as a cut-off, however,
showed evidence of a trend to increased rates of results above that
level with disease progression.
When only the disease-free patients were analysed in
logistic regression models adjusted for known prognostic factors we
saw a trend for increased risk of death with increasing osteopontin
level. This provides support for the view that increased
osteopontin levels might predict occult disease. Medical services
in Leeds comply with the UK melanoma guidelines, which state that
there is no need for routine screening (imaging) in stage I–III
patients. Thus, the possibility that there may have been occult
disease in our disease-free patients should be considered.
Serial measurements of osteopontin plasma levels
after diagnosis may prove to be more informative than a single
measurement. A study in uveal melanoma (40) showed a significant increase in
osteopontin level from 12–18 months to 6–12 months prior to
clinical confirmation of metastasis. When we used a measure of
osteopontin level higher than 95th centile, relapse occurred in
significant numbers of melanoma patients with results within the
normal range. It seems unlikely, therefore, that a single
measurement of osteopontin will have sufficient sensitivity and
specificity for use in clinical practice. It may, however, be the
case that biologically different tumours are associated with
increased levels of tumour markers and that a panel of markers will
be necessary to be repeated over time.
Acknowledgements
Recruitment to the Leeds Melanoma Cohort was
facilitated by the UK National Cancer Research Network. We are
thankful to the research staff who collected or managed data: May
Chan, Joanne Gascoyne, Clarissa Nolan, Susan Leake, Birute
Karpavicius, Tricia Mack, Paul King, Sue Haynes, Elaine Fitzgibbon,
Kate Gamble, Saila Waseem, Sandra Tovey, Christy Walker and Paul
Affleck. The present study was funded by Cancer Research UK
(Programme Awards C588/A10589 and C588/A10721, Project Grants
C8216/A6129 and C8216/A8168).
Abbreviations:
|
SPP1
|
secreted phosphoprotein 1
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
DASL
|
cDNA-mediated annealing, selection,
extension and ligation
|
|
SNB
|
sentinel node biopsy
|
|
OR
|
odds ratio
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
|
BMI
|
body mass index
|
|
LDH
|
lactate dehydrogenase
|
|
AJCC
|
American Joint Committee on Cancer
|
|
MIA
|
melanoma inhibitory activity
|
|
NSCLC
|
non-small cell lung cancer
|
|
CV
|
coefficient of variation
|
|
OS
|
overall survival
|
|
MSS
|
melanoma-specific survival
|
References
|
1
|
Gogas H, Eggermont AM, Hauschild A, et al:
Biomarkers in melanoma. Ann Oncol. 20(Suppl 6): vi8–v13. 2009.
View Article : Google Scholar
|
|
2
|
Balch CM, Gershenwald JE, Soong SJ, et al:
Final version of 2009 AJCC melanoma staging and classification. J
Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hofmann MA, Gussmann F, Fritsche A, et al:
Diagnostic value of melanoma inhibitory activity serum marker in
the follow-up of patients with stage I or II cutaneous melanoma.
Melanoma Res. 19:17–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paschen A, Sucker A, Hill B, et al:
Differential clinical significance of individual NKG2D ligands in
melanoma: soluble ULBP2 as an indicator of poor prognosis superior
to S100B. Clin Cancer Res. 15:5208–5215. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rittling SR and Chambers AF: Role of
osteopontin in tumour progression. Br J Cancer. 90:1877–1881. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Das R, Philip S, Mahabeleshwar GH, Bulbule
A and Kundu GC: Osteopontin: it’s role in regulation of cell
motility and nuclear factor kappa B-mediated urokinase type
plasminogen activator expression. IUBMB Life. 57:441–447. 2005.
|
|
7
|
Packer L, Pavey S, Parker A, et al:
Osteopontin is a downstream effector of the PI3-kinase pathway in
melanomas that is inversely correlated with functional PTEN.
Carcinogenesis. 27:1778–1786. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rangaswami H, Bulbule A and Kundu GC:
Osteopontin: role in cell signaling and cancer progression. Trends
Cell Biol. 16:79–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bellahcene A, Castronovo V, Ogbureke KU,
Fisher LW and Fedarko NS: Small integrin-binding ligand N-linked
glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat
Rev Cancer. 8:212–226. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smith AP, Hoek K and Becker D:
Whole-genome expression profiling of the melanoma progression
pathway reveals marked molecular differences between nevi/melanoma
in situ and advanced-stage melanomas. Cancer Biol Ther.
4:1018–1029. 2005. View Article : Google Scholar
|
|
11
|
Rangel J, Nosrati M, Torabian S, et al:
Osteopontin as a molecular prognostic marker for melanoma. Cancer.
112:144–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou Y, Dai DL, Martinka M, et al:
Osteopontin expression correlates with melanoma invasion. J Invest
Dermatol. 124:1044–1052. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maier T, Laubender RP, Sturm RA, et al:
Osteopontin expression in plasma of melanoma patients and in
melanocytic tumours. J Eur Acad Dermatol Venereol. 26:1084–1091.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Conway C, Mitra A, Jewell R, et al: Gene
expression profiling of paraffin-embedded primary melanoma using
the DASL assay identifies increased osteopontin expression as
predictive of reduced relapse-free survival. Clin Cancer Res.
15:6939–6946. 2009. View Article : Google Scholar
|
|
15
|
Jaeger J, Koczan D, Thiesen HJ, et al:
Gene expression signatures for tumor progression, tumor subtype,
and tumor thickness in laser-microdissected melanoma tissues. Clin
Cancer Res. 13:806–815. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Soikkeli J, Podlasz P, Yin M, et al:
Metastatic outgrowth encompasses COL-I, FN1, and POSTN
up-regulation and assembly to fibrillar networks regulating cell
adhesion, migration, and growth. Am J Pathol. 177:387–403. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kashani-Sabet M, Venna S, Nosrati M, et
al: A multimarker prognostic assay for primary cutaneous melanoma.
Clin Cancer Res. 15:6987–6992. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alonso SR, Tracey L, Ortiz P, et al: A
high-throughput study in melanoma identifies epithelial-mesenchymal
transition as a major determinant of metastasis. Cancer Res.
67:3450–3460. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rodrigues LR, Teixeira JA, Schmitt FL,
Paulsson M and Lindmark-Mansson H: The role of osteopontin in tumor
progression and metastasis in breast cancer. Cancer Epidemiol
Biomarkers Prev. 16:1087–1097. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Li L, Wang JT, Kan X and Lu JG:
Elevated content of osteopontin in plasma and tumor tissues of
patients with laryngeal and hypopharyngeal carcinoma associated
with metastasis and prognosis. Med Oncol. 29:1429–1434. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun J, Xu HM, Zhou HJ, et al: The
prognostic significance of preoperative plasma levels of
osteopontin in patients with TNM stage-I of hepatocellular
carcinoma. J Cancer Res Clin Oncol. 136:1–7. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Isa S, Kawaguchi T, Teramukai S, et al:
Serum osteopontin levels are highly prognostic for survival in
advanced non-small cell lung cancer: results from JMTO LC 0004. J
Thorac Oncol. 4:1104–1110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blasberg JD, Pass HI, Goparaju CM, Flores
RM, Lee S and Donington JS: Reduction of elevated plasma
osteopontin levels with resection of non-small-cell lung cancer. J
Clin Oncol. 28:936–941. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wheatley-Price P, Yang B, Patsios D, et
al: Soluble mesothelin-related peptide and osteopontin as markers
of response in malignant mesothelioma. J Clin Oncol. 28:3316–3322.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reiniger IW, Wolf A, Welge-Lussen U,
Mueller AJ, Kampik A and Schaller UC: Osteopontin as a serologic
marker for metastatic uveal melanoma: results of a pilot study. Am
J Ophthalmol. 143:705–707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haritoglou I, Wolf A, Maier T, Haritoglou
C, Hein R and Schaller UC: Osteopontin and ‘melanoma inhibitory
activity’: comparison of two serological tumor markers in
metastatic uveal melanoma patients. Ophthalmologica. 223:239–243.
2009.
|
|
27
|
Kadkol SS, Lin AY, Barak V, et al:
Osteopontin expression and serum levels in metastatic uveal
melanoma: a pilot study. Invest Ophthalmol Vis Sci. 47:802–806.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kluger HM, Hoyt K, Bacchiocchi A, et al:
Plasma markers for identifying patients with metastatic melanoma.
Clin Cancer Res. 17:2417–2425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Newton-Bishop JA, Beswick S,
Randerson-Moor J, et al: Serum 25-hydroxyvitamin D3 levels are
associated with Breslow thickness at presentation and survival from
melanoma. J Clin Oncol. 27:5439–5444. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wind TC, Messenger MP, Thompson D, Selby
PJ and Banks RE: Measuring carbonic anhydrase IX as a hypoxia
biomarker: differences in concentrations in serum and plasma using
a commercial enzyme-linked immunosorbent assay due to influences of
metal ions. Ann Clin Biochem. 48:112–120. 2011. View Article : Google Scholar
|
|
31
|
Sim SH, Messenger MP, Gregory WM, et al:
Prognostic utility of pre-operative circulating osteopontin,
carbonic anhydrase IX and CRP in renal cell carcinoma. Br J Cancer.
107:1131–1137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
StataCorp: Stata Statistical Software:
Release 10. College Station, TX: StataCorp LP; 2007
|
|
33
|
Sennels HP, Jacobsen S, Jensen T, et al:
Biological variation and reference intervals for circulating
osteopontin, osteoprotegerin, total soluble receptor activator of
nuclear factor kappa B ligand and high-sensitivity C-reactive
protein. Scand J Clin Lab Invest. 67:821–835. 2007. View Article : Google Scholar
|
|
34
|
Homsi J, Kashani-Sabet M, Messina JL and
Daud A: Cutaneous melanoma: prognostic factors. Cancer Control.
12:223–229. 2005.
|
|
35
|
Slingluff CL Jr, Vollmer RT, Reintgen DS
and Seigler HF: Lethal ‘thin’ malignant melanoma. Identifying
patients at risk. Ann Surg. 208:150–161. 1988.
|
|
36
|
Flaherty KT, Puzanov I, Kim KB, et al:
Inhibition of mutated, activated BRAF in metastatic melanoma. N
Engl J Med. 363:809–819. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Robert C, Thomas L, Bondarenko I, et al:
Ipilimumab plus dacarbazine for previously untreated metastatic
melanoma. N Engl J Med. 364:2517–2526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kulasingam V and Diamandis EP: Strategies
for discovering novel cancer biomarkers through utilization of
emerging technologies. Nat Clin Pract Oncol. 5:588–599. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Utikal J, Schadendorf D and Ugurel S:
Serologic and immunohistochemical prognostic biomarkers of
cutaneous malignancies. Arch Dermatol Res. 298:469–477. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Barak V, Kaiserman I, Frenkel S, Hendler
K, Kalickman I and Pe’er J: The dynamics of serum tumor markers in
predicting metastatic uveal melanoma (part 1). Anticancer Res.
31:345–349. 2011.PubMed/NCBI
|