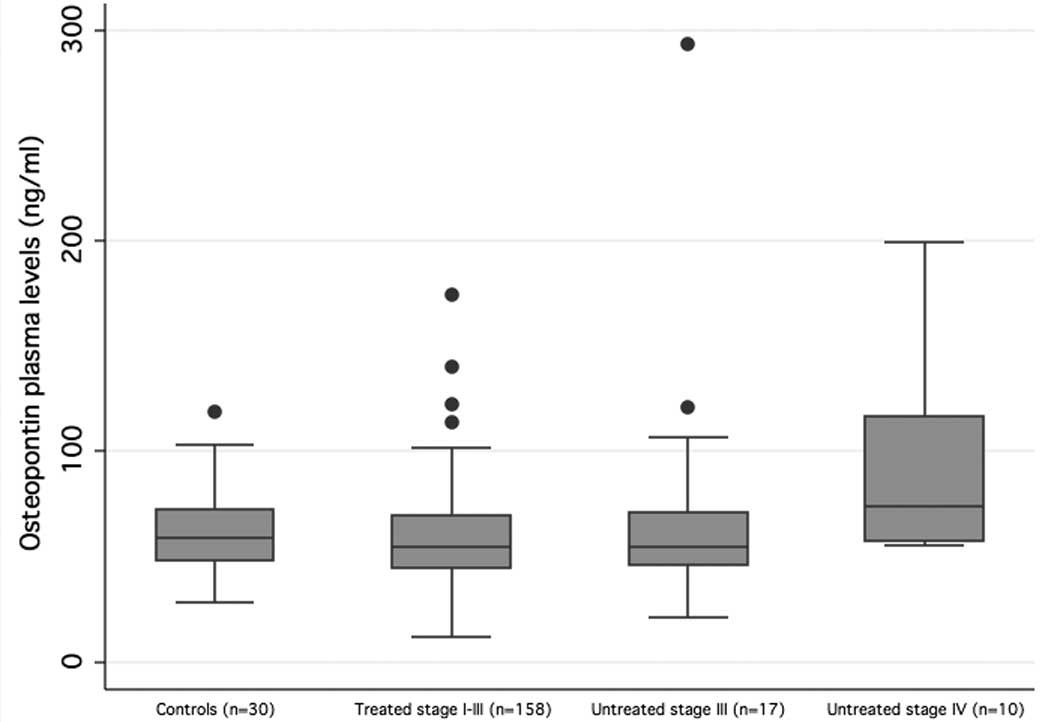
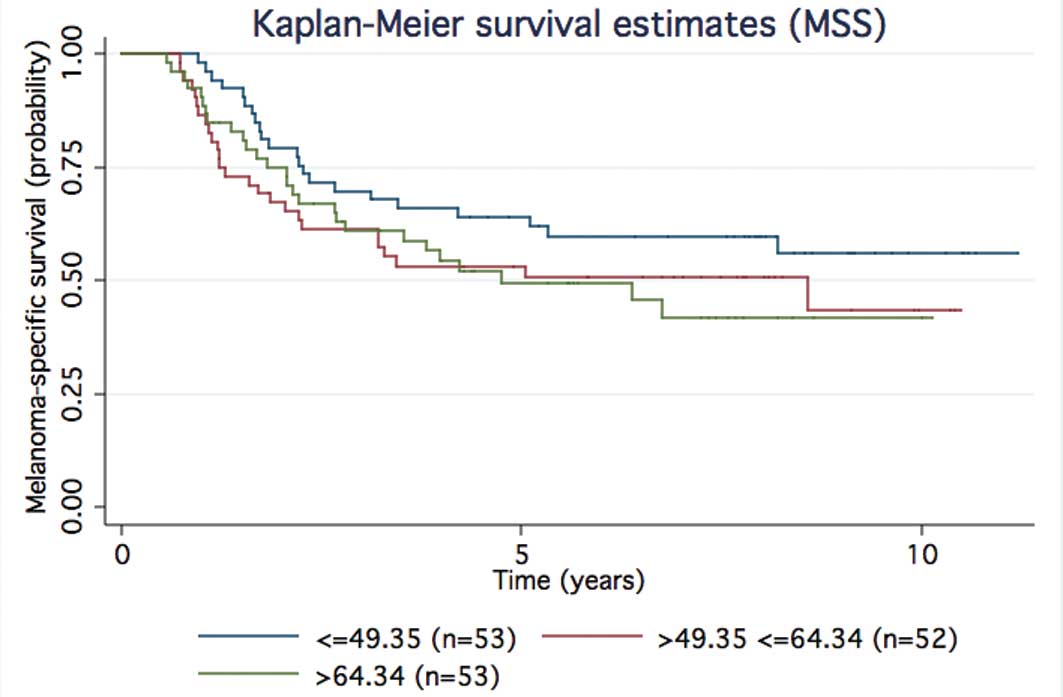
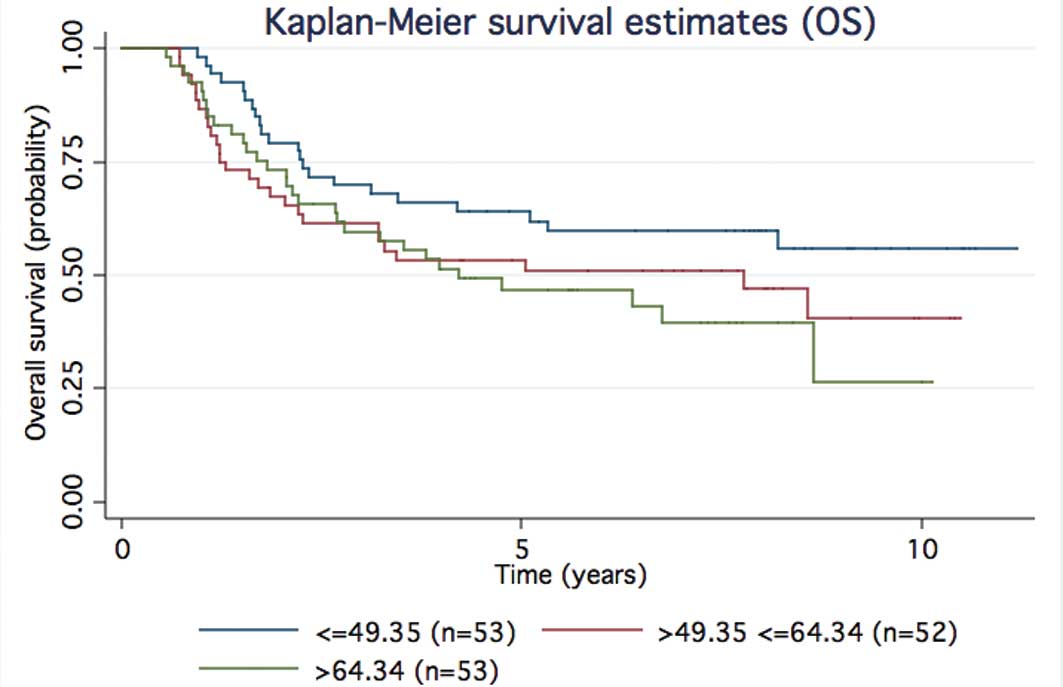
|
1
|
Gogas H, Eggermont AM, Hauschild A, et al:
Biomarkers in melanoma. Ann Oncol. 20(Suppl 6): vi8–v13. 2009.
View Article : Google Scholar
|
|
2
|
Balch CM, Gershenwald JE, Soong SJ, et al:
Final version of 2009 AJCC melanoma staging and classification. J
Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hofmann MA, Gussmann F, Fritsche A, et al:
Diagnostic value of melanoma inhibitory activity serum marker in
the follow-up of patients with stage I or II cutaneous melanoma.
Melanoma Res. 19:17–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paschen A, Sucker A, Hill B, et al:
Differential clinical significance of individual NKG2D ligands in
melanoma: soluble ULBP2 as an indicator of poor prognosis superior
to S100B. Clin Cancer Res. 15:5208–5215. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rittling SR and Chambers AF: Role of
osteopontin in tumour progression. Br J Cancer. 90:1877–1881. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Das R, Philip S, Mahabeleshwar GH, Bulbule
A and Kundu GC: Osteopontin: it’s role in regulation of cell
motility and nuclear factor kappa B-mediated urokinase type
plasminogen activator expression. IUBMB Life. 57:441–447. 2005.
|
|
7
|
Packer L, Pavey S, Parker A, et al:
Osteopontin is a downstream effector of the PI3-kinase pathway in
melanomas that is inversely correlated with functional PTEN.
Carcinogenesis. 27:1778–1786. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rangaswami H, Bulbule A and Kundu GC:
Osteopontin: role in cell signaling and cancer progression. Trends
Cell Biol. 16:79–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bellahcene A, Castronovo V, Ogbureke KU,
Fisher LW and Fedarko NS: Small integrin-binding ligand N-linked
glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat
Rev Cancer. 8:212–226. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smith AP, Hoek K and Becker D:
Whole-genome expression profiling of the melanoma progression
pathway reveals marked molecular differences between nevi/melanoma
in situ and advanced-stage melanomas. Cancer Biol Ther.
4:1018–1029. 2005. View Article : Google Scholar
|
|
11
|
Rangel J, Nosrati M, Torabian S, et al:
Osteopontin as a molecular prognostic marker for melanoma. Cancer.
112:144–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou Y, Dai DL, Martinka M, et al:
Osteopontin expression correlates with melanoma invasion. J Invest
Dermatol. 124:1044–1052. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maier T, Laubender RP, Sturm RA, et al:
Osteopontin expression in plasma of melanoma patients and in
melanocytic tumours. J Eur Acad Dermatol Venereol. 26:1084–1091.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Conway C, Mitra A, Jewell R, et al: Gene
expression profiling of paraffin-embedded primary melanoma using
the DASL assay identifies increased osteopontin expression as
predictive of reduced relapse-free survival. Clin Cancer Res.
15:6939–6946. 2009. View Article : Google Scholar
|
|
15
|
Jaeger J, Koczan D, Thiesen HJ, et al:
Gene expression signatures for tumor progression, tumor subtype,
and tumor thickness in laser-microdissected melanoma tissues. Clin
Cancer Res. 13:806–815. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Soikkeli J, Podlasz P, Yin M, et al:
Metastatic outgrowth encompasses COL-I, FN1, and POSTN
up-regulation and assembly to fibrillar networks regulating cell
adhesion, migration, and growth. Am J Pathol. 177:387–403. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kashani-Sabet M, Venna S, Nosrati M, et
al: A multimarker prognostic assay for primary cutaneous melanoma.
Clin Cancer Res. 15:6987–6992. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alonso SR, Tracey L, Ortiz P, et al: A
high-throughput study in melanoma identifies epithelial-mesenchymal
transition as a major determinant of metastasis. Cancer Res.
67:3450–3460. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rodrigues LR, Teixeira JA, Schmitt FL,
Paulsson M and Lindmark-Mansson H: The role of osteopontin in tumor
progression and metastasis in breast cancer. Cancer Epidemiol
Biomarkers Prev. 16:1087–1097. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Li L, Wang JT, Kan X and Lu JG:
Elevated content of osteopontin in plasma and tumor tissues of
patients with laryngeal and hypopharyngeal carcinoma associated
with metastasis and prognosis. Med Oncol. 29:1429–1434. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun J, Xu HM, Zhou HJ, et al: The
prognostic significance of preoperative plasma levels of
osteopontin in patients with TNM stage-I of hepatocellular
carcinoma. J Cancer Res Clin Oncol. 136:1–7. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Isa S, Kawaguchi T, Teramukai S, et al:
Serum osteopontin levels are highly prognostic for survival in
advanced non-small cell lung cancer: results from JMTO LC 0004. J
Thorac Oncol. 4:1104–1110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blasberg JD, Pass HI, Goparaju CM, Flores
RM, Lee S and Donington JS: Reduction of elevated plasma
osteopontin levels with resection of non-small-cell lung cancer. J
Clin Oncol. 28:936–941. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wheatley-Price P, Yang B, Patsios D, et
al: Soluble mesothelin-related peptide and osteopontin as markers
of response in malignant mesothelioma. J Clin Oncol. 28:3316–3322.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reiniger IW, Wolf A, Welge-Lussen U,
Mueller AJ, Kampik A and Schaller UC: Osteopontin as a serologic
marker for metastatic uveal melanoma: results of a pilot study. Am
J Ophthalmol. 143:705–707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haritoglou I, Wolf A, Maier T, Haritoglou
C, Hein R and Schaller UC: Osteopontin and ‘melanoma inhibitory
activity’: comparison of two serological tumor markers in
metastatic uveal melanoma patients. Ophthalmologica. 223:239–243.
2009.
|
|
27
|
Kadkol SS, Lin AY, Barak V, et al:
Osteopontin expression and serum levels in metastatic uveal
melanoma: a pilot study. Invest Ophthalmol Vis Sci. 47:802–806.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kluger HM, Hoyt K, Bacchiocchi A, et al:
Plasma markers for identifying patients with metastatic melanoma.
Clin Cancer Res. 17:2417–2425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Newton-Bishop JA, Beswick S,
Randerson-Moor J, et al: Serum 25-hydroxyvitamin D3 levels are
associated with Breslow thickness at presentation and survival from
melanoma. J Clin Oncol. 27:5439–5444. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wind TC, Messenger MP, Thompson D, Selby
PJ and Banks RE: Measuring carbonic anhydrase IX as a hypoxia
biomarker: differences in concentrations in serum and plasma using
a commercial enzyme-linked immunosorbent assay due to influences of
metal ions. Ann Clin Biochem. 48:112–120. 2011. View Article : Google Scholar
|
|
31
|
Sim SH, Messenger MP, Gregory WM, et al:
Prognostic utility of pre-operative circulating osteopontin,
carbonic anhydrase IX and CRP in renal cell carcinoma. Br J Cancer.
107:1131–1137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
StataCorp: Stata Statistical Software:
Release 10. College Station, TX: StataCorp LP; 2007
|
|
33
|
Sennels HP, Jacobsen S, Jensen T, et al:
Biological variation and reference intervals for circulating
osteopontin, osteoprotegerin, total soluble receptor activator of
nuclear factor kappa B ligand and high-sensitivity C-reactive
protein. Scand J Clin Lab Invest. 67:821–835. 2007. View Article : Google Scholar
|
|
34
|
Homsi J, Kashani-Sabet M, Messina JL and
Daud A: Cutaneous melanoma: prognostic factors. Cancer Control.
12:223–229. 2005.
|
|
35
|
Slingluff CL Jr, Vollmer RT, Reintgen DS
and Seigler HF: Lethal ‘thin’ malignant melanoma. Identifying
patients at risk. Ann Surg. 208:150–161. 1988.
|
|
36
|
Flaherty KT, Puzanov I, Kim KB, et al:
Inhibition of mutated, activated BRAF in metastatic melanoma. N
Engl J Med. 363:809–819. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Robert C, Thomas L, Bondarenko I, et al:
Ipilimumab plus dacarbazine for previously untreated metastatic
melanoma. N Engl J Med. 364:2517–2526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kulasingam V and Diamandis EP: Strategies
for discovering novel cancer biomarkers through utilization of
emerging technologies. Nat Clin Pract Oncol. 5:588–599. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Utikal J, Schadendorf D and Ugurel S:
Serologic and immunohistochemical prognostic biomarkers of
cutaneous malignancies. Arch Dermatol Res. 298:469–477. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Barak V, Kaiserman I, Frenkel S, Hendler
K, Kalickman I and Pe’er J: The dynamics of serum tumor markers in
predicting metastatic uveal melanoma (part 1). Anticancer Res.
31:345–349. 2011.PubMed/NCBI
|