Introduction
Hepatocellular carcinoma (HCC) is one of the most
common cancers worldwide (1,2). As
for many other tumors, development of HCC is due to a multistep
process with accumulation of genetic and epigenetic alterations in
regulatory genes, leading to activation of oncogenes and
inactivation or loss of tumor suppressor genes (TSGs) (3).
In the last three decades, cancer has been
understood as a summary of altered genetic and epigenetic events.
The epigenetic pathway is, in contrast to genetic events, a
reversible alteration and is characterized by three main
mechanisms: i) DNA hypermethylation leading to inactivation, ii)
DNA hypomethylation causing genomic instability and iii) histone
modifications affecting chromatin conformation (3).
These processes, particularly aberrant DNA
methylation and histone modifications, are closely linked with each
other by a protein complex of transcript activators and repressors
and alter mRNA transcript expression of affected genes (4). Characteristically, DNA methylation
does not change the genetic information, it simply alters the
readability of the DNA and results in inactivation of genes by
subsequent mRNA transcript repression (3). In humans and other mammals, CpG island
methylation is an important physiological mechanism. The
inactivated X-chromosome of females silenced alleles of imprinted
genes or inserted viral genes and repeat elements are inactivated
through promoter methylation (5).
The nucleolar and coiled-body phosphoprotein 1
(NOLC1, also called Nopp140 or NS5ATP13) is a family of proteins
which is characterized by a conserved C-terminal SRP40 domain
(6). NOLC1 is a phosphoprotein
composed of N- and C-terminal domains and a unique central repeated
domain consisting of alternating acidic and basic amino acid
clusters and localized in the nucleolus (7). The highest steady state concentrations
of vertebrate NOLC1 localize within the dense fibrillar component
(DFC) of nucleoli (8,9). NOLC1 was first identified as a nuclear
localization signal-binding protein and is thought to be a
chaperone for shuttling between the nucleolus and the cytoplasm
(9,10). NOLC1 plays an essential role in the
synthesis of rRNA and the biosynthesis of ribosomes. A previous
study revealed that NOLC1 has transcription factor-like activity
(11). By binding to the
transcription factor C/EBP β (also known as AGP/EBP or NF-IL6),
NOPP140 can indirectly activate the transcription of the α-1 acid
glycoprotein gene (11).
Overexpression of the partial or whole NOLC1 cDNA resulted in
mislocalization of nucleolar proteins, improper formation of the
nucleolus, and inhibition of rRNA gene transcription. These
observations suggest that hNopp140 is crucial for normal cell
growth (12).
In our previous study (6), we found that an altered DNA
methylation pattern may play a definitive role in the regulation of
NOLC1 gene expression in human liver cancers. In this report, we
present evidence to support the notion that DNA methylation is a
key mechanism of epigenetic regulation to suppress NOLC1 expression
in HCC cells, and we identified the precise methylation sites in
the NOLC1 gene. This study provides important insight into the
epigenetic regulation in HCC.
Materials and methods
Cell culture and 5-aza-2′-deoxycytidine
treatment
Human normal liver cell lines, L02 and Chang liver,
and human hepatoma cell lines, HepG2 and Huh7, were cultured in
Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10%
fetal bovine serum (FBS) and 1% antibiotics
[penicillin-streptomycin solution (PSN)], under identical
conditions (37°C in humidified 5% CO2/95% air),
respectively. For drug treatment, cell lines were treated with 5
μmol/l 5-aza-2′-deoxycytidine (Aza) (Sigma, St. Louis, MO, USA) for
3 days, changing Aza and medium every 24 h. Control cells were
incubated with culture medium.
There were clear similarities between the results
from the Chang liver and L02 cell. The results from Huh 7 were
similar to those of the HepG2 cells. Therefore, the results from
Chang liver and Huh7 cells are not presented.
Tissues and surgical specimens
HCC paraffin blocks and frozen tissues were obtained
from the archives of the Department of Pathology, Beijing Ditan
Hospital, Capital Medical University, Beijing, China, according to
institutional review board-approved protocol. The use of human
specimens in this research was approved by the ethics committee of
our hospital according to the Declaration of Helsinki. We clearly
confirm that we had all the necessary consent from any patient
involved in the study, including consent to participate in the
study and consent to publish.
RNA extraction and RT-PCR
RNA was extracted from cells or patient tissues
using an RNA isolation reagent (TRIzol; Invitrogen Life
Technologies, Carlsbad, CA, USA). To prevent DNA contamination,
total RNA was treated with RNase-free DNase II (Invitrogen Life
Technologies).
The human glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) gene (forward primer, 5′-TCACCAGGGCTGCTT TTA-3′ and
reverse, 5′-TTCACACCCATGACGAACA-3′) was used as an internal control
in the PCR amplification. A two-step RT-PCR procedure was performed
in all experiments. First, total RNA samples (2 μg/reaction) were
reversely transcribed into cDNAs by RT II reverse transcriptase
(Invitrogen Life Technologies). Then, the cDNAs were used as
templates in PCR with NOLC1 gene-specific primers
(5′-AGCTGGCCTGACGGTATG-3′ and 5′-TTGGTCTGGCTGAGTACCG-3′). The
primers for cyclin D3 were 5′-ATTCCTCTTTGCTTTG CTTTC-3′ and
5′-CAGCAGCAAAGCTGTCAATC-3′. The primers that were used for
amplification of MDM2 were 5′-CGGCACGAGCTAGGATCT and
5′-ACGGCAGCTCCA TGAGTC-3′. The amplification reactions were
performed using AmpliTaq Gold DNA polymerase (Applied Biosystems,
Foster City, CA, USA). The PCR was programmed as follows: 2 min at
95°C and 30–32 cycles of 30 sec at 94°C, 30 sec at 58–62°C, and 30
sec at 72°C, with an extension for 10 min at 72°C. The PCR bands
were visualized under UV light and photographed. Quantitative
real-time PCR was used to measure mRNA levels of urea cycle genes
for the HCC cell lines and NOLC1 mRNA levels for tissues using the
StepOne Plus Real-Time PCR system with SYBR-Green Master Mix
(Applied Biosystems).
Immunohistochemical staining
HCC biopsy specimens were subjected to routine
immunohistochemical staining using a monoclonal antibody directed
against NOLC1 (13), according to a
previously described method (14).
Immunoreactivity, defined as the number of positive tumor cells
over the total tumor cells, was scored independently by two
researchers. The number of NOLC1-positive and -negative HCC cells
was counted under a light microscope at a magnification of ×400.
Only the cells displaying brown nucleoli on the section were
considered NOLC1-positive. For each slide, 7–10 microscopic fields
were randomly chosen. Positive scores were categorized into weak
staining (only one nucleolus was stained), moderate staining (more
than one nucleolus was stained), and strong staining (both the
nucleus and nucleolus of the tumor cells were stained). The average
percentage of NOLC1-positive HCC cells was then calculated for each
group.
Western blot analysis
Lysates from the cultured cells were subjected to
routine western blotting as described previously (15). The antibodies used were monoclonal
anti-mouse antibodies against NOLC1 (13) and β-actin (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), and polyclonal rabbit antibodies
against cyclin D3 and caspase-8 (Abcam, Cambridge, MA, USA). The
results shown are representative of 2 independent experiments.
Bisulfite genomic sequencing
Genomic DNA was purified from cells with the Wizard
Genomic DNA purification kit (Promega Corporation, Madison, WI,
USA). DNA (2 μg) was bisulfite modified with the EZ DNA methylation
direct kit (Zymo Research, Orange, CA, USA). Sequence-specific
primers to amplify the CpG-rich regions of interest were designed
using a computer program (MethPrimer; http://www.urogene.org/methprimer). The primers that
were used for amplification were as follows: NOLC1 upstream CpG
island-1 forward, 5′-TTGGGATGGTATTAGAAAGGGT-3′ and reverse
5′-CTCAAAAACCCAACAAAAACAAT-3′; NOLC1 upstream CpG island-2 forward,
5′-GTTTTTGTTGGGTTTTTGAGT-3′ and reverse 5′-CCTCAATAAAACAAAACTCTA
CCTC-3′. The PCR products were amplified, purified and cloned into
a vector (pGEM-T Easy Vector; Promega Corporation). Clones were
selected through blue-white screening. Finally, the colonies
harboring the insert were sequenced in a 96-well plate using the
M13 reverse and/or forward primers.
Methylation-specific PCR
The bisulfite-treated DNA was amplified using
primers that specifically amplify either the methylated or
unmethylated sequence of the NOLC1 promoter containing four CpG
dinucleotides, respectively. The PCR was performed for 40 cycles,
with annealing temperatures of 58°C for the methylated reaction and
52°C for the unmethylated reaction. The human methylated and
unmethylated DNA was used as a control to verify the specificity of
the primers (Qiagen, Valencia, CA, USA).
Plasmid and transfection
Cells were subcultured and transfected as previously
described (16,17). The cDNA encoding NOLC1, flanked by
BamHI and SalI restriction sites, was cloned into the
mammalian expression vector pCDNA4 (Stratagene Inc., La Jolla, CA,
USA) to generate pCDNA-NOLC1, which expresses an N-terminal
myc-tagged NOLC1 fusion protein. The promoter of NOLC1 was cloned
into the pGL3 plasmid. Subconfluent cells were transiently
transfected with pCDNA-NOLC1 DNA (4 μg/dish) mixed with
Lipofectamine and Plus™ reagent (Invitrogen Life Technologies),
according to the manufacturer’s protocol. Cells were harvested ~48
h after transfection (18).
NOLC1 shRNA transfectants
The shRNA constructs described in Table I were purchased from Open Biosystems
(Huntsville, AL, USA). When the HCC cultured cells had reached
70–80% confluence, the shRNA constructs were transfected into the
HCC cells using the Arrest-In transfection reagent for RNAi (Open
Biosystems) (12).
 | Table IConstruct sequences of the
shRNAs. |
Table I
Construct sequences of the
shRNAs.
| shRNA | Sequence |
|---|
| NOLC1 shRNA |
5′-TGCTGTTGACAGTGAGCGCGACATCTAAGTCTGCAGTTAATAGTGAAGCCACAGATGTATTAACTGCAGACTTAGATGTCTTGCCTACTGCCTCGGA-3′ |
| NS-shRNA |
5′-TGCTGTTGACAGTGAGCGAACCACTAAGCTTCTGTCTTAATAGTGAAGCCACAGATGTATTAAGACAGAAGCTTAGTGGTCTGCCTACTGCCTCGGA-3′ |
Luciferase assay
The cells were transfected with 0.6 μg of firefly
luciferase reporter plasmid and 0.05 μg of control plasmid
containing Renilla luciferase (pRL-TK; Promega Corporation).
A promoterless basic vector (pGL3; Promega Corporation) was used as
a negative control. To confirm the efficiency of transfection
(Lipofectin; Invitrogen Life Technologies), a luciferase expression
vector (pGL3-control; Promega Corporation) was used as a positive
control. After 48 h, the cells were harvested for analysis.
Luciferase enzyme assays and colorimetric β-galactosidase assays
were performed according to the manufacturer’s instructions
(Promega Corporation). Luciferase activity was normalized to
β-galactosidase activity to assess the transfection efficiency.
When indicated, the firefly and Renilla luciferase
activities were measured using the Dual-Luciferase reporter assay
system (Promega Corporation), according to the manufacturer’s
instructions. The Renilla luciferase activity of pRL-TK was
used to normalize the firefly luciferase activity of the reporter
construct. Each transfection experiment was repeated 3 times.
Cell growth and proliferation assay
Cell growth was determined by the colorimetric
tetrazolium derived sodium
3′-[1-(phenylamino-carbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro)benzene-sulfonic
acid hydrate assay (XTT), and DNA synthesis of cells was assessed
by the bromodeoxyuridine (BrdU) incorporation assay (both from
Roche Applied Science, Mannheim, Germany). For the cell growth and
proliferation assays, the cells of each group at 48 h after
treatment were re-seeded onto 96-well plates at a density of
3×103 cells/well. Then XTT and incorporated BrdU were
measured colorimetrically using a microtiter plate reader (Bio-Rad,
Hercules, CA, USA) at a wavelength of 450 nm (19,20).
Determimation of apoptosis
Cells (3×105) were cultured onto 60-mm
SWNHs-coated and non-coated dishes for 48 h. Then apoptotic cells
were identified by using fluorescence-activated cell sorting (FACS)
Annexin V-Fluos (BioLegend) following the protocol of the
manufacturer. Cells having been cultured were washed at 4°C for 30
min in PBS and then stained with Annexin-V staining solution at 4°C
for 3 h. Gels were washed 4 times in PBS at 4°C and fixed at room
temperature with 1% paraformaldehyde (Sigma-Aldrich, St. Louis, MO,
USA) in PBS for 15 min. For counterstaining, 7-AAD (2 μg/ml) was
added to the first washing step. The numbers of total,
Annexin-V-positive, 7-AAD-positive, and double-positive cells were
determined respectively using FACS technique. Apoptosis was
verified by detection of activated caspases (21).
Statistics
Significance was determined using the one-way ANOVA
test for the mean of three different experiments. Significance was
determined using the paired Student’s t-test for the mean of 3
different experiments. Probabilities of ≤0.05 were considered to
indicate statistically significant results.
Results
Low expression of NOLC1 in HCC cell lines
and liver cancer tissues is associated with cyclin D3
To understand the potential mechanisms by which
NOLC1 is regulated in HCC, we detected the NOLC1 expression levels
at the transcription and translation levels in HCC cell lines and
human HCC tissues using immunohistochemical staining and RT-PCR
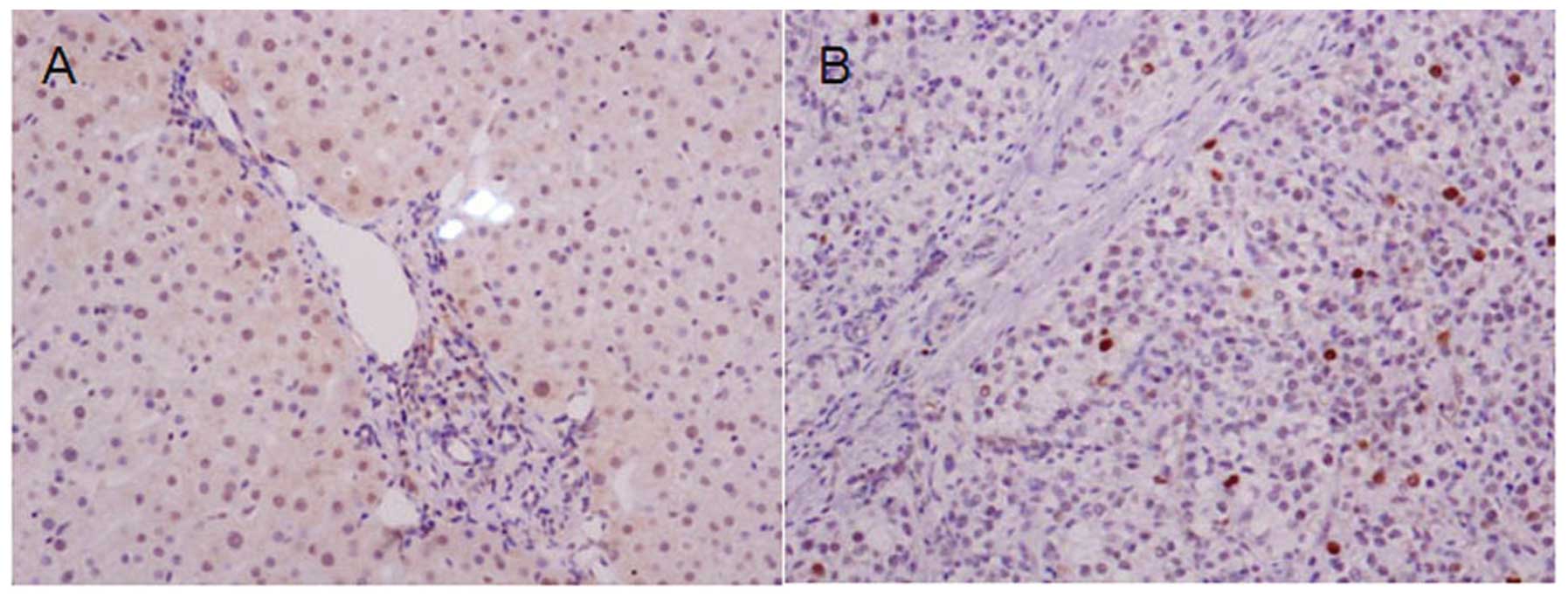
approach. The results showed that NOLC1 was moderately expressed in
the cytoplasm and the nucleus (Fig.
1A). It was expression at a low level in HCC tissues and was
localized in the nucleus (Fig. 1B).
We then examined NOLC1 mRNA expression in 38 pairs of patient
tissues. The NOLC1 expression was decreased in tumor tissues when
compared with that in the matched noncancerous tissues after 32
cycles of PCR amplification (P<0.01) (Fig. 1C). As shown in Fig. 1D, strong expression of NOLC1 protein
was detected in the normal liver cell lines, L02 and Chang liver,
but NOLC1 protein was weakly expressed in hepatoma liver cell lines
HepG2 and Huh7. To correlate the mRNA transcription with protein
expression, Western blot analysis was performed to examine the
NOLC1 protein expression in patient tissues. As shown in Fig. 1E, NOLC1 protein in the tumor samples
was decreased at different degrees when compared with that in the
adjacent noncancerous specimens, particularly in patients 1–7.
Hwang et al(12) found that
NOLC1 plays a role in the regulation of tumorigenesis of
nasopharyngeal carcinoma (NPC) and demonstrated that both NOLC1 and
tumor protein 53 synergistically activate the MDM2 promoter in NPC
cells. The frequent downregulation of miR-138 regulates cyclin D3
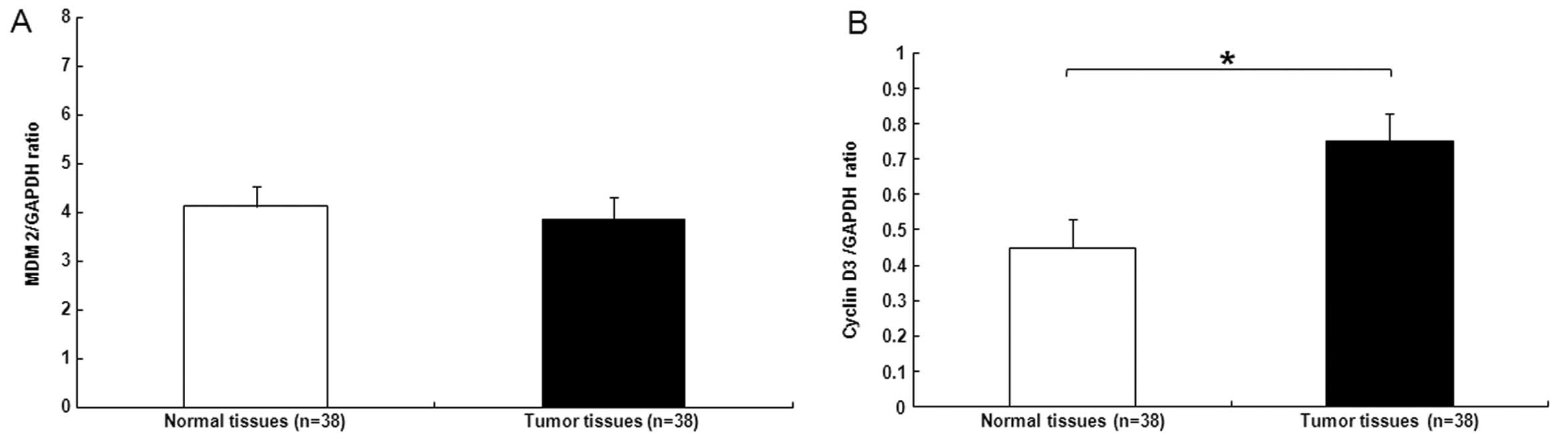
and functions as a tumor-suppressor in HCC (22). We next examined MDM2 and cyclin D3
mRNA expression in patient tissues. MDM2 expression in tumor
tissues was similar to that in the matched noncancerous tissues
(Fig. 2A), while the tumor samples
exhibited an increase in cyclin D3 expression when compared with
that in the adjacent noncancerous specimens (Fig. 2B).
NOLC1 expression is regulated by DNA
methylation in HCC tumor cells
Since many cancer cells exhibit aberrant epigenetic
regulation, it is possible that NOLC1 expression is regulated by
epigenetic modification. To confirm that DNA methylation regulates
NOLC1 expression, detailed methylation analysis of the NOLC1 gene
sequence was performed using genomic DNA extracted from cell lines
and liver tissues. Sequence analysis (GenBank accession no. GI
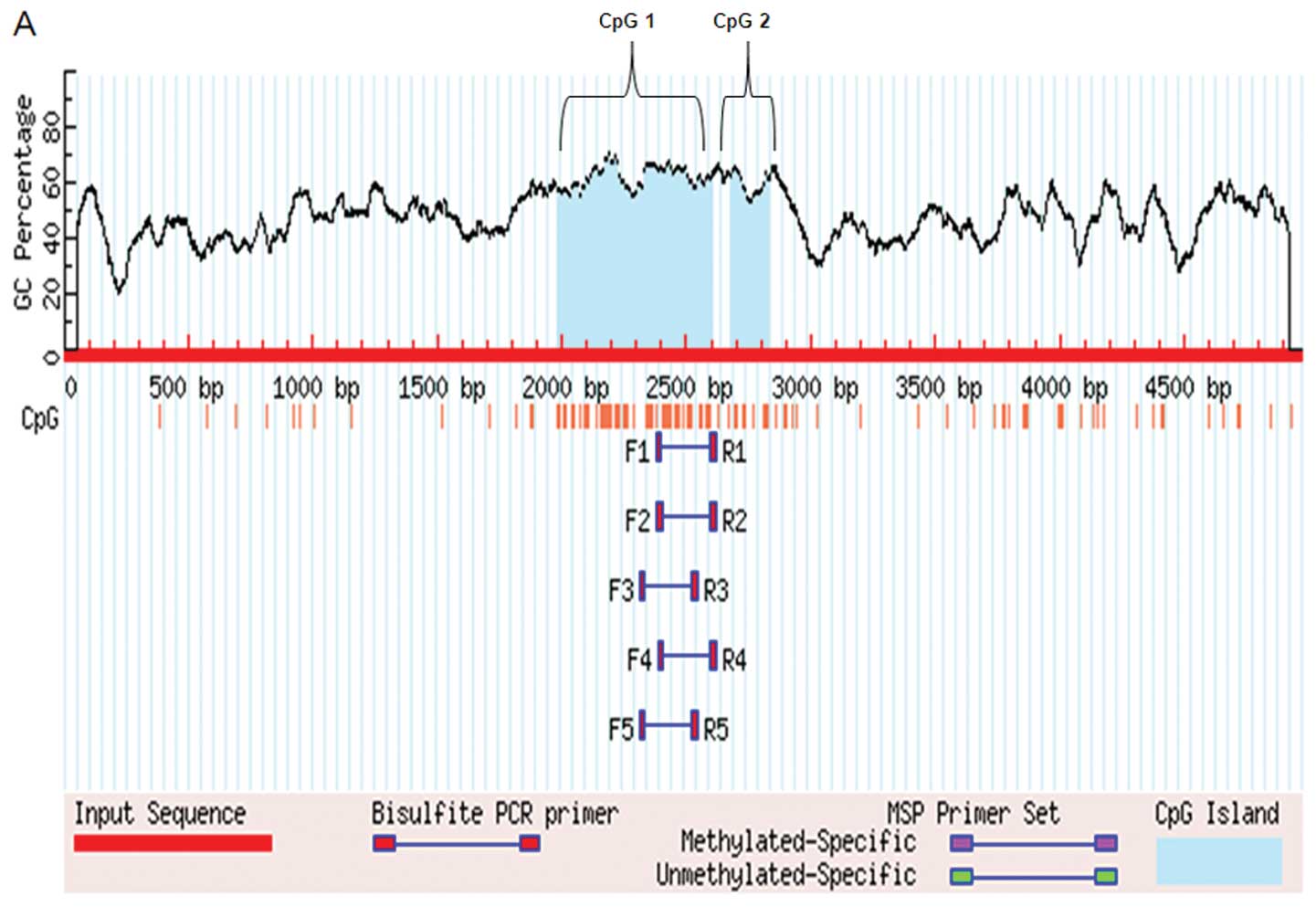
470595142; http://www.ncbi.nlm.nih.gov/mapview) (23) indicated that the NOLC1 promoter has
2 typical CpG islands (CpG1 and CpG2) upstream of it. We predicted
the CpG islands of the NOLC1 promoter: the length of CpG island 1
(CpG1) was 616 bp (−1987 and −2602), and the length of CpG island 2
(CpG2) was 148 bp (−2678 and −2825) (Fig. 3A). To determine whether NOLC1
promoter methylation is associated with the control of NOLC1
expression in liver cell lines and clinical specimens, we performed
methylation-specific PCR and compared the promoter methylation
status in the hepatoma cell lines with that in the normal liver
cell lines, and in tumor tissues with that in paired noncancerous
tissues. Methylation of the 2 CpG dinucleotides in the promoter
region was detectable. As shown in Fig.
3, the results revealed that 4 dinucleotides at the start of
CpG1 were strongly methylated (Fig. 3A
and D), particularly in the hepatoma cell lines and tumor
tissues when compared with that in normal liver cell lines and
adjacent noncancerous tissues (Fig.
3D). The methylation frequencies were 58.07% in CpG1-57, 47.8%
in CpG1-72, 44.9% in CpG1-82 and 37.7% in CpG1-93 (Fig. 3B). But CpG2 was not methylated
(Fig. 3C). The methylation status
in the promoter CpG1 start region appeared to be correlated with
NOLC1 expression levels in the liver cell lines and tissues
specimen.
Effect of the CpG1 island on NOLC1
promoter activity
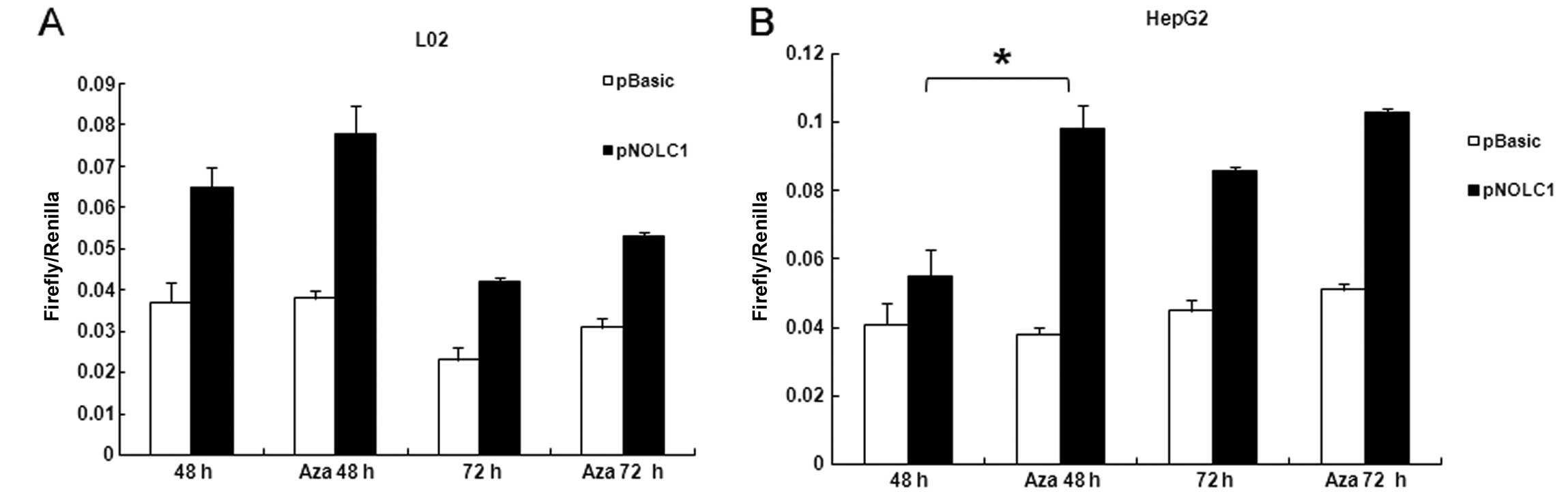
To investigate the possible effect of methylation of
the CpG1 on promoter activity and determine the functional
significance of the CpG1, we generated a reporter gene construct
using a NOLC1 promoter sequence containing CpG1. The reporter
construct (pNOLC1) together with pRL-TK Renilla luciferase
expression vector were transiently transfected in L02 and HepG2
cells, followed by Aza treatment. The promoter activity was
determined by luciferase assay. Firefly and Renilla
luciferase activities were measured at the points indicated.
Renilla luciferase activity was used to normalize firefly
luciferase activity of the reporter constructs. As shown in
Fig. 4, Aza treatment caused a
significant increase in promoter activity in both cell lines,
particular in the HepG2 cells (Fig.
4B). Luciferase activity of pGL3-basic, which has no promoter
element, was not affected by Aza treatment. The CpG1 dinucleotides
in the plasmid were not methylated at transfection (data not
shown). Thus, the data suggest that the 4 CG dinucleotides at the
CpG1 island start site appear to be critical for NOLC1 promoter
activity.
Function of NOLC1 in cell biology
It is uncertain how NOLC1 affects cellular function.
Cells were synchronized at the G1/S boundary by double thymidine
block, and then released into mitosis. After 24 h, BrdU was added
into the medium at the indicated time points to evaluate DNA
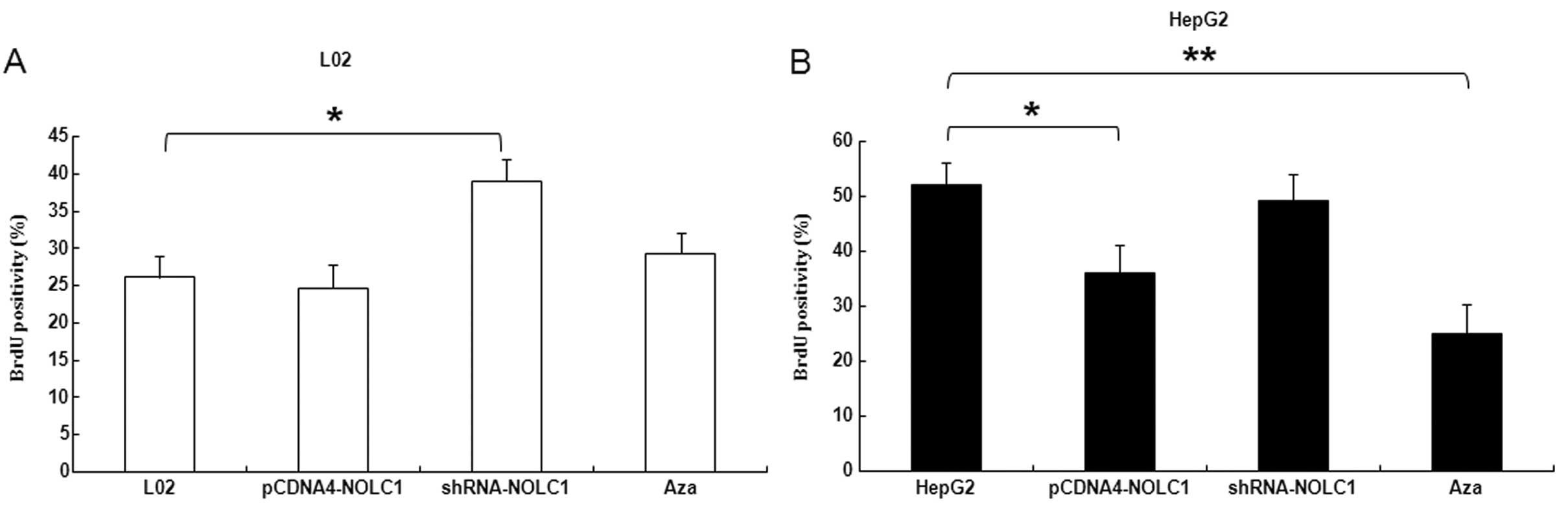
synthesis. As shown in Fig. 5A,
incorporation of BrdU into the control, and accumulation of mitotic
L02 cells was significantly promoted by shRNA interfere NOLC1
(P<0.05). In contrast, overexpression of NOLC1 in HepG2 cells
were significantly delayed at 36 and 48 h, particularly in cells
treated with Aza (P<0.05) (Fig.
5B).
As determined by XTT assays, NOLC1 silencing of L02
cells resulted in a significant increased in cell growth when
compared to that in the control and other cells (P<0.05)
(Fig. 5C). However, Aza treatment
in HepG2 cells significantly inhibited cell growth when compared
with than the control group (P<0.01) (Fig. 5D).
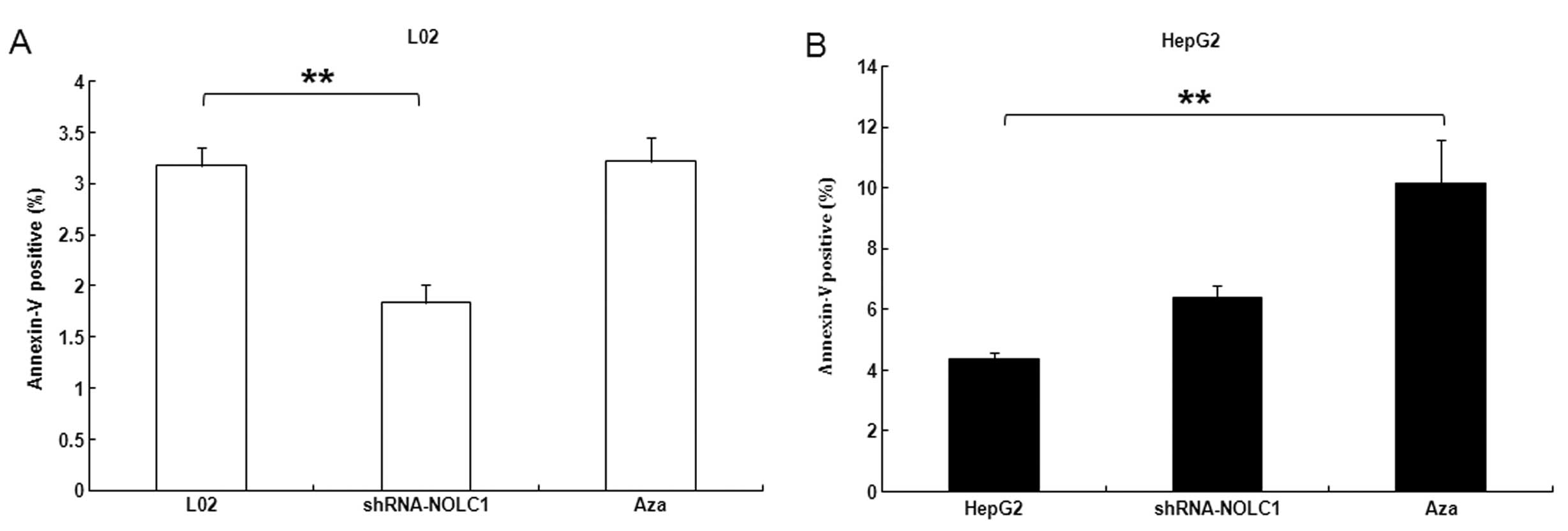
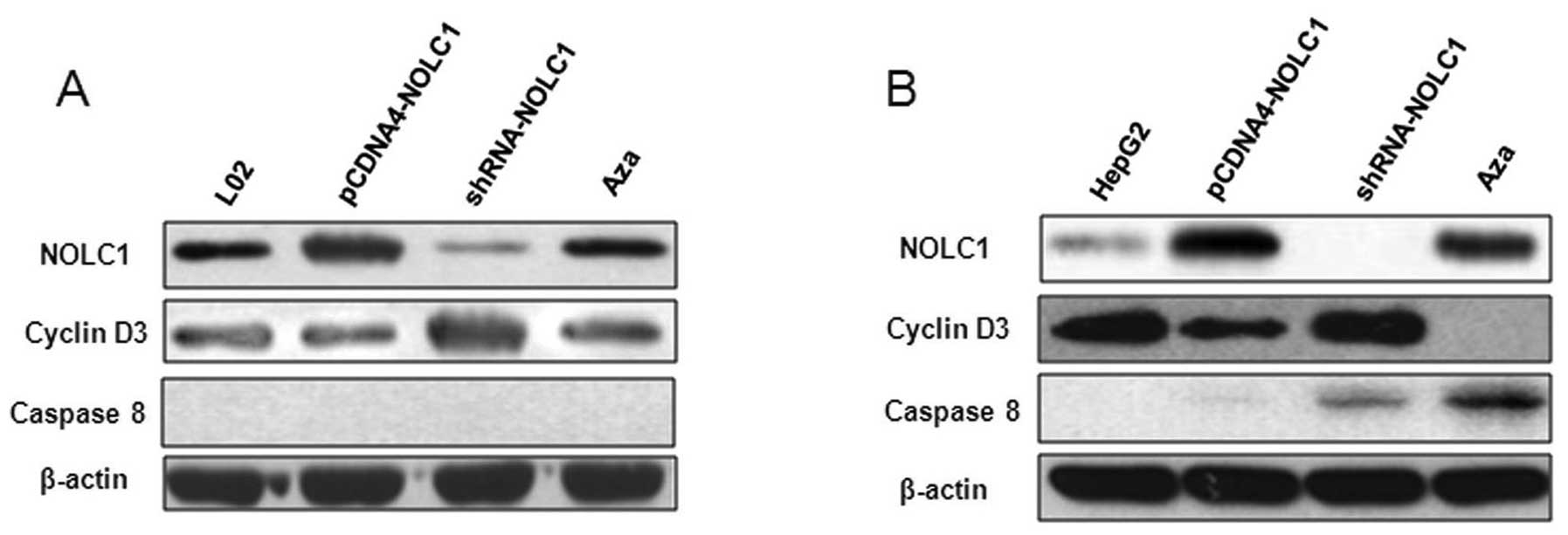
The effect of NOLC1 expression on apoptosis was
determined in L02 and HepG2 cells by flow cytometry. Downregulation
of NOLC1 in L02 cells significantly suppressed apoptosis
(P<0.01) (Fig. 6A). Furthermore,
HepG2 cells treated with Aza exhibited increased apoptosis
(P<0.01) (Fig. 6B), which may be
associated with overexpression of caspase-8 and low levels of
cyclin D3 (Fig. 7B).
Discussion
In high incidence areas, such as Asia and Africa,
HCC is strongly associated with chronic viral hepatitis B and C and
liver cirrhosis; 70–80% of HCC occurs in cirrhotic liver.
Nutritional factors, toxins and metabolic diseases also contribute
to hepatocarcinogenesis (1,2).
Aberrant epigenetic states may predispose to genetic
changes, but genetic changes may also initiate aberrant epigenetic
events. Epigenetic and genetic mechanisms may thus work together to
silence key cellular genes and destabilize the genome, leading to
oncogenic transformation and the observed complexity and
heterogeneity in human cancers, including HCC (24–26).
The development of HCC results from a multistep process beginning
with the accumulation of genetic and epigenetic alterations in
regulatory genes (3). In general,
cancer cells have global hypomethylation, but they have
hypermethylation in some specific genes. DNA hypermethylation in
promoter regions is associated with the silencing of
tumor-suppressor genes because of direct or indirect prevention to
accessing transcription factors in the promoter region (27). Recent studies (3,28,29)
have demonstrated that CpG island hypermethylation, via silencing
of key cancer-related genes, plays a major causal role in cancer,
including HCC (23).
Our study of NOLC1 gene methylation provides a novel
insight into the epigenetic regulation in HCC. NOLC1 plays a role
as an oncogene in NPC tumorigenesis. Although an increasing numbers
of reports have shown that NOLC1 is a multiple functional protein
(30), Hwang et al(12) demonstrated that NOLC1 plays a role
in enhancing NPC tumorigenesis. In our study, NOLC1 expression was
suppressed in hepatoma cell lines and tumor tissues. Moreover,
NOLC1 expression in hepatoma cell lines was restored by treatment
with the demethylating agent Aza and was associated with cyclin D3.
We further demonstrated that the methylation status of the CpG-rich
region (CpG1) in the promoter region was correlated with NOLC1 gene
expression in hepatoma cell lines. Consistently, the NOLC1 promoter
activity was noted in the reporter assay in the hepatoma cell lines
tested. Taken together, these observations indicate that DNA
methylation regulates NOLC1 expression in liver cancer cells.
Most studies investigating the mechanism that
regulates gene expression by CpG methylation focus on CpG islands
in the promoter. In the present study, we found that the NOLC1
promoter region CpG1. Hypermethylation of CpG islands in promoter
sequences is associated with silencing of tumor-suppressor genes
and tumor-related genes by subsequent downregulation of mRNA
transcript expression. Epigenetic silenced genes are involved in
important molecular pathways of carcinogenesis e.g., cell cycle
regulation, apoptosis, DNA repair or cell adhesion (3). As known, the imbalance between cell
proliferation and death is considered to be an early and important
event in the process of carcinogenesis; thus it is desirable to
develop new strategies to induce apoptosis and inhibition of
proliferation in tumor cells. The results of this study
demonstrated that NOLC1 inhibited the proliferation of liver cell
lines, and promoted hepatoma cell apoptosis. When compared with
other types of malignant tumors, in hepatocellular carcinomas,
aberrant methylation of several TSGs and tumor-related genes such
as RASSF1A, hMLH1 or SOCS1 was frequently observed (31). In our study, NOLC1 may play a role
in suppressing HCC tumorigenesis.
Further studies are required to explore the
mechanisms involved in the suppression of NOLC1 gene expression by
DNA methylation. The answer to why NOLC1 plays a role in
suppressing HCC tumorigenesis, but enhancing NPC tumorigenesis
warrants investigation. Gao et al(9) found that NF-κB and CREB positively
regulated the NOLC1 promoter. NOLC1 was found to play a role in the
regulation of tumorigenesis of NPC and both NOLC1 and tumor protein
53 were demonstrated to synergistically activate the MDM2 promoter
in NPC cells (9). We hypothesized
that the key signaling pathway of NOLC1 is different between NPC
and HCC.
From a cell biology point of view, our finding
concerning NOLC1 and its methylation raises an important
conclusion. NOLC1 expression affects the proliferation of liver
cells. To attempt to confirm this hypothesis, we overexpressed the
NOLC1 gene in normal liver cell lines and examined its effect on
the cell phenotype. The normal liver cells with NOLC1 expression
promote proliferation, as our experiment demonstrated (Fig. 5A and C). Restoration of NOLC1
inhibited the proliferation of hepatoma cell lines and was
associated with cyclin D3 (Figs. 5B and
D and 7B). The underlying
mechanisms for these changes remain to be determined. Cyclin D3 as
a target of miR-138 in HCC provides new insights into the
mechanisms underlying tumorigenesis (22). Cyclin D3 is expressed in nearly all
proliferating cells and could promote initiation of the cell cycle
(32–42). Further study of the relationship
between cyclin D3 and the function of NOLC1 or its methylation is
needed to determine the relevant mechanisms.
Nevertheless, methylation of the NOLC1 gene can, at
least, serve as a surrogate marker to reflect the DNA methylation
status in HCC cells; therefore, it can serve as a biomarker for HCC
diagnosis. It is potentially more important to use NOLC1
methylation as an early biomarker for HCC. In addition, the
demethylation agent for NOLC1 can be used as a potential target for
HCC therapy.
In summary, we found that the low expression of
NOLC1 and high levels of aberrant DNA methylation levels of its
promoter in cancer cell lines and tissues are associated with
cyclin D3. The important methylation sites were identified at the
CpG1 start region of the NOLC1 gene. Our findings provide new means
for developing better diagnostic tests and more effective therapies
for HCC.
Acknowledgements
This study was supported by grants from the Healthy
Talent Leadership Programs of Beijing (no. 2009-1-09), the National
Importance Foundation of Infectious Diseases during the Five-Year
Plan Period of China (nos. 2012ZX10002003, 2013ZX10002005 and
2012ZX10004904), and the National Natural Science Foundation of
China (nos. 30600524, 81071990, 81172383 and 81201758).
References
|
1
|
Beasley RP, Hwang LY, Lin CC and Chien CS:
Hepatocellular carcinoma and hepatitis B virus. A prospective study
of 22 707 men in Taiwan. Lancet. 2:1129–1133. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tischoff I and Tannapfe A: DNA methylation
in hepatocellular carcinoma. World J Gastroenterol. 14:1741–1748.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baylin SB and Herman JG: DNA
hypermethylation in tumorigenesis: epigenetics joins genetics.
Trends Genet. 16:168–174. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Riggs AD and Pfeifer GP: X-chromosome
inactivation and cell memory. Trends Genet. 8:169–174. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang Q, Cheng J, Liu Y, Hong Y, Wang JJ
and Zhang SL: Cloning and identification of NS5ATP2 gene and
its spliced variant transactivated by hepatitis C virus
non-structural protein 5A. World J Gastroenterol. 10:1735–1739.
2004.
|
|
7
|
Meier UT: Comparison of the rat nucleolar
protein nopp140 with its yeast homolog SRP40. Differential
phosphorylation in vertebrates and yeast. J Biol Chem.
271:19376–19384. 1996.PubMed/NCBI
|
|
8
|
Meier UT and Blobel G: Nopp140 shuttles on
tracks between nucleolus and cytoplasm. Cell. 70:127–138. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao XS, Wang Q, Li W, Yang B, Song H, Ju
W, Liu SA and Cheng J: Identification of nucleolar and coiled-body
phosphoprotein 1 (NOLC1) minimal promoter regulated by NF-κB and
CREB. BMB Rep. 44:70–75. 2011.PubMed/NCBI
|
|
10
|
Meier UT and Blobel G: A nuclear
localization signal binding protein in the nucleolus. J Cell Biol.
111:2235–2245. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miau LH, Chang CJ, Tsai WH and Lee SC:
Identification and characterization of a nucleolar phosphoprotein.
Nopp140, as a transcription factor. Mol Cell Biol. 17:230–239.
1997.PubMed/NCBI
|
|
12
|
Hwang YC, Lu TY, Huang DY, Kuo YS, Kao CF,
Yeh NH, Wu HC and Lin CT: NOLC1, an enhancer of nasopharyngeal
carcinoma progression, is essential for TP53 to regulate MDM2
expression. Am J Pathol. 175:342–354. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pai CY, Chen HK, Sheu HL and Yeh NH:
Cell-cycle-dependent alterations of a highly phosphorylated
nucleolar protein p130 are associated with nucleologenesis. J Cell
Sci. 108:1911–1920. 1995.PubMed/NCBI
|
|
14
|
Lin CT, Lin CR, Tan GK, Chen W, Dee AN and
Chan WY: The mechanism of Epstein-Barr virus infection in
nasopharyngeal carcinoma cells. Am J Pathol. 150:1745–1756.
1997.PubMed/NCBI
|
|
15
|
Wu HC and Lin CT: Association of
heterotrimeric GTP binding regulatory protein (Go) with mitosis.
Lab Invest. 71:175–181. 1994.PubMed/NCBI
|
|
16
|
Jiang M and Milner J: Bcl-2 constitutively
suppresses p53-dependent apoptosis in colorectal cancer cells.
Genes Dev. 17:832–837. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang M, Rubbi CP and Milner J: Gel-based
application of siRNA to human epithelial cancer cells induces
RNAi-dependent apoptosis. Oligonucleotides. 14:239–248. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim YK, Lee WK, Jin YN, Lee KJ, Jeon HS
and Yu YG: Doxorubicin binds to un-phosphorylated form of hNopp140
and reduces protein kinase CK2-dependent phosphorylation of
hNopp140. J Biochem Mol Biol. 39:774–781. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hamamoto R, Furukawa Y, Morita M, Iimura
Y, Silva FP, Li M, Yagyu R and Nakamura Y: SMYD3 encodes a histone
methyltransferase involved in the proliferation of cancer cells.
Nat Cell Biol. 6:731–740. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ford J, Jiang M and Milner J:
Cancer-specific functions of SIRT1 enable human epithelial cancer
cell growth and survival. Cancer Res. 65:10457–10463. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li H, Bergeron L, Cryns V, Pasternack MS,
Zhu H, Shi L, Greenberg A and Yuan J: Activation of caspase-2 in
apoptosis. J Biol Chem. 272:21010–21017. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang W, Zhao LJ, Tan YX, Ren H and Qi ZT:
MiR-138 induces cell cycle arrest by targeting cyclin D3 in
hepatocellular carcinoma. Carcinogenesis. 33:1113–1120. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu HY, Dong HJ, Robertson K and Liu C:
DNA methylation suppresses expression of the urea cycle enzyme
carbamoyl phosphate synthetase 1 (CPS1) in human hepatocellular
carcinoma. Am J Pathol. 178:652–661. 2011. View Article : Google Scholar
|
|
24
|
Herath NI, Leggett BA and MacDonald GA:
Review of genetic and epigenetic alterations in
hepatocarcinogenesis. J Gastroenterol Hepatol. 21:15–21. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li HP, Leu YW and Chang YS: Epigenetic
changes in virus-associated human cancers. Cell Res. 15:262–271.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Herceg Z: Epigenetics and cancer: towards
an evaluation of the impact of environmental and dietary factors.
Mutagenesis. 22:91–103. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Adrien LR, Schlecht NF, Kawachi N, Smith
RV, Brandwein-Gensler M, Massimi A, Chen S, Prystowsky MB, Childs G
and Belbin TJ: Classification of DNA methylation patterns in tumor
cell genomes using a CpG island microarray. Cytogenet Genome Res.
114:16–23. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lund AH and van Lohuizen M: Epigenetics
and cancer. Genes Dev. 18:2315–2335. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sparmann A and van Lohuizen M: Polycomb
silencers control cell fate, development and cancer. Nat Rev
Cancer. 6:846–856. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lo SJ, Lee CC and Lai HJ: The nucleolus:
reviewing oldies to have new understandings. Cell Res. 16:530–538.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Esteller M, Corn PG, Baylin SB and Herman
JG: A gene hypermethylation profile of human cancer. Cancer Res.
61:3225–3229. 2001.PubMed/NCBI
|
|
32
|
Lin J, Jinno S and Okayama H: Cdk6-cyclin
D3 complex evades inhibition by inhibitor proteins and uniquely
controls cell’s proliferation competence. Oncogene. 20:2000–2009.
2001.PubMed/NCBI
|
|
33
|
Rao CN, Liu YY, Peavey CL and Woodley DT:
Novel extracellular matrix-associated serine proteinase inhibitors
from human skin fibroblasts. Arch Biochem Biophys. 317:311–314.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sprecher CA, Kisiel W, Mathewes S and
Foster DC: Molecular cloning, expression, and partial
characterization of a second human tissue-factor-pathway inhibitor.
Proc Natl Acad Sci USA. 91:3353–3357. 1994. View Article : Google Scholar
|
|
35
|
Rao CN, Cook B, Liu Y, Chilukuri K, Stack
MS, Foster DC, Kisiel W and Woodley DT: HT-1080 fibrosarcoma cell
matrix degradation and invasion are inhibited by the
matrix-associated serine protease inhibitor TFPI-2/33 kDa MSPI. Int
J Cancer. 76:749–756. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rao CN, Mohanam S, Puppala A and Rao JS:
Regulation of ProMMP-1 and ProMMP-3 activation by tissue factor
pathway inhibitor-2/matrix-associated serine protease inhibitor.
Biochem Biophys Res Commun. 255:94–98. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wong CM, Ng YL, Lee JM, Wong CC, Cheung
OF, Chan CY, Tung EK, Ching YP and Ng IO: Tissue factor pathway
inhibitor-2 as a frequently silenced tumor suppressor gene in
hepatocellular carcinoma. Hepatology. 45:1129–1138. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim H, Jen J, Vogelstein B and Hamilton
SR: Clinical and pathological characteristics of sporadic
colorectal carcinomas with DNA replication errors in microsatellite
sequences. Am J Pathol. 145:148–156. 1994.PubMed/NCBI
|
|
39
|
Oliveira C, Seruca R, Seixas M and
Sobrinho-Simões M: The clinicopathological features of gastric
carcinomas with microsatellite instability may be mediated by
mutations of different ‘target genes’: a study of the TGFβ
RII, IGFII R, and BAX genes. Am J Pathol.
153:1211–1219. 1998.PubMed/NCBI
|
|
40
|
Park JH, Cho SB, Lee WS, Park CH, Joo YE,
Kim HS, Choi SK, Rew JS, Lee JH and Kim SJ: Methylation pattern of
DNA repair genes and microsatellite instability in hepatocellular
carcinoma. Korean J Gastroenterol. 48:327–336. 2006.(In
Korean).
|
|
41
|
Matsukura S, Soejima H, Nakagawachi T,
Yakushiji H, Ogawa A, Fukuhara M, Miyazaki K, Nakabeppu Y,
Sekiguchi M and Mukai T: CpG methylation of MGMT and hMLH1 promoter
in hepatocellular carcinoma associated with hepatitis viral
infection. Br J Cancer. 88:521–529. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang L, Bani-Hani A, Montoya DP, Roche PC,
Thibodeau SN, Burgart LJ and Roberts LR: hMLH1 and hMSH2 expression
in human hepatocellular carcinoma. Int J Oncol. 19:567–570.
2001.PubMed/NCBI
|