Introduction
Ovarian cancer is associated with the highest
mortality rate among women than all other gynecological cancers in
the world (1). Surgery to remove as
much of the tumor as possible following platinum-based chemotherapy
are the standard methods used to cure the disease (1). Although these intense surgical and
chemotherapeutic treatments ease the state of illness, ovarian
cancer more often than not recurs with the neoplasm usually
distributed throughout the peritoneum. Again, platinum-based
chemotherapy is often used to treat recurrent ovarian cancers, yet,
many the ovarian cancer cells are resistant to platinum-based
agents. Multiple theories have attempted to explain why cancer
cells are resistant to platinum-based chemotherapy. In the case of
ovarian cancer, dysfunction of sex steroids such as progesterone
(P4) may play an important role. Importantly, serum P4 levels are
elevated in patients with ovarian cancer when compared with
age-matched controls (2), and P4
levels are also higher in the peritoneal fluid of ovarian cancer
patients (3). Epidemiologically,
postmenopausal smokers with significantly elevated levels of P4
show a negative prognostic effect for ovarian cancer-specific
survival, while the negative effect of smoking was found to
diminish with increasing time since the point a former smoker stops
smoking (4–6). Although the binding of P4 to the
progesterone receptor (PGR) leads to a protective effect in
vitro, the anti-apoptotic effect of P4 may be mediated by other
less known receptors such as progesterone receptor membrane
component 1 or 2 (PGRMC1/2). Research has demonstrated that P4
shows a significant anti-apoptotic effect through PGRMC1/2 in
vitro and in vivo(7).
These biphasic abilities of P4 to either induce or inhibit
apoptosis could also explain why clinical trials have failed to
show a beneficial effect of P4 on ovarian cancer progression.
Regarding the ambiguous and crucial role of P4 in ovarian cancer,
particularly in chemotherapy resistance, additional detailed
mechanisms involved including PGRMC1/2 and signal pathways were
thoroughly investigated in the present study.
Materials and methods
Cells and reagents
The ovarian cancer cell line HO-8910 (a human
ovarian cancer cell line established from a patient with
poorly-differentiated serous carcinoma) was obtained from the
American Type Culture Collection (ATCC, Rockville, MD, USA).
HO-8910 cells were sustained in Dulbecco’s modified Eagle’s medium
(DMEM) (Invitrogen) with 10% (v/v) fetal bovine serum (FBS; Gibco),
100 IU/ml penicillin and 100 ng/ml streptomycin (PAA Laboratories
GmbH, Pasching, Austria) in a 37°C, 5% CO2 humidified
atmosphere. Cisplatin (CDDP) was a kind gift from Wei Zhu
(Department of Oncology, The First Affiliated Hospital of Nanjing
Medical University, China). Progesterone (P4) and the specific
inhibitor of PI3K, LY294002, were purchased from Sigma (St. Louis,
MO, USA).
MTT cell viability assay
Cell viability of HO-8910 cells was examined by MTT
assays according to the standard methods. Briefly, HO-8910 cells
were seeded onto a 96-well plate (2,000 cells/well). After 12 h,
medium was replaced with conditional medium containing 10% FBS as
well as DMSO, P4 (1 μM), CDDP (30 nM), or P4 (1 μM) plus CDDP (30
nM), respectively. After incubation for another 48 h, 15 μl MTT (1
μg/μl) was added into the medium. DMSO (200 μl/well) was used to
dissolve the formazan product. Optical density was detected at a
wavelength of 560 nm.
Cell cycle analysis
The cell cycle was analyzed by flow cytometry
(Beckman Coulter, Inc., Miami, FL, USA) according to the standard
methods and as previously described (8).
Wound healing assay
A monolayer of HO-8910 cells at 80% confluence
supplied with DMEM containing 0.5% FBS in 12-well plates was
scratched using a sterilized 10-μl pipette tip, washed and then
subjected to P4 (1 μM), CDDP (30 nM), or P4 (1 μM) plus CDDP (30
nM), respectively. The migration of cells into the wound was imaged
and monitored at 0, 12 and 24 h after treatment. The images were
analyzed by Image-Pro Plus software (Media Cybernetics, Silver
Spring, MD, USA). Results were obtained from three independent
experiments.
Transwell chamber migration assay
Migration of HO-8910 cells through 8-μm pores was
examined using a Transwell cell culture chamber (Corning Costar).
The lower chamber was filled with DMEM containing 10% FBS. Cells
(100 μl) at a density of 2×105/ml were seeded onto the
upper chamber with the culture containing P4 (1 μM), CDDP (30 nM)
or P4 (1 μM) plus CDDP (30 nM), respectively. Chambers were
incubated for 18 h at 37°C. The cells remaining on the top surface
of the membrane were removed with application of a cotton swab
followed by three PBS washes. The cells on the bottom surface of
the membrane were fixed, stained (0.1% crystal violet), and
quantified by counting 5 fields on the membrane under a ×20
objective.
RT-PCR
RT-PCR was used to characterize the expression of
PGR, PGRMC1 and PGRMC2 in HO-8910 cells. Using this protocol, total
RNA was isolated from HO-8910 cells using the RNeasy Plus Mini kit
(Qiagen, Valencia, CA, USA). To ensure no DNA contamination of the
RNA, which could lead to false-positive results, the RNA samples
were treated with DNase I (Invitrogen) before reverse
transcription. Then cDNA was synthesized by incubating 1 μg of RNA
with oligo-dT and Muloney murine leukemia virus reverse
transcriptase (Invitrogen). Primers for the subsequent PCRs were as
follows: PGR forward, 5′-GAT GCT ATA TTT TGC GCC TGA-3′ and
reverse, 5′-CTC CTT TTT GCC TCA AAC CA-3′ (266 bp); PGRMC1 forward,
5′-GCA AGC TTT GGC GAA AAT CA-3′ and reverse, 5′-CCC CTC GCA TGT
CCA ATC AT-3′ (121 bp); PGRMC2 forward, 5′-AGG GGA AGA ACC GTC AGA
AT-3′ and reverse, 5′-AAG CCC CAC CAG ACA TTA CA-3′ (280 bp).
Reaction times were: 1 min at 94°C then 35 cycles of 30 sec at
94°C, 30 sec at 60°C and 60 sec at 72°C, then 10 min at 72°C. The
amplified products were resolved by electrophoresis on an ethidium
bromide-treated 2% agarose gel. Finally, the relative level of gene
expression was expressed as the ratio of the target gene mean value
to the geometric mean value of the reference gene, β-actin.
Antibodies and western blot analysis
Anti-phospho-PTEN (Ser380) rabbit polyclonal
antibodies (pAb), anti-PTEN rabbit pAb, anti-phospho-AKT (Ser473)
mouse monoclonal antibodies (mAb), anti-AKT rabbit pAb,
anti-phospho-GSK-3β (Ser9) rabbit pAb, anti-GSK-3β rabbit pAb were
obtained from Cell Signaling Technology (Danvers, MA, USA).
Anti-GAPDH mouse mAb, anti-α-tubulin mouse mAb, horseradish
peroxidase (HRP)-conjugated goat anti-mouse and anti-rabbit IgG
were products of Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Western blot analysis was performed as previously described
(9,10).
Statistical analysis
All experiments were performed at least in
triplicate. Numerical data were expressed as the means ± SD. Two
group comparisons were analyzed by the two-sided Student’s t-test.
P-values were calculated and a P-value of <0.05 was considered
to indicate a statistically significant result.
Results
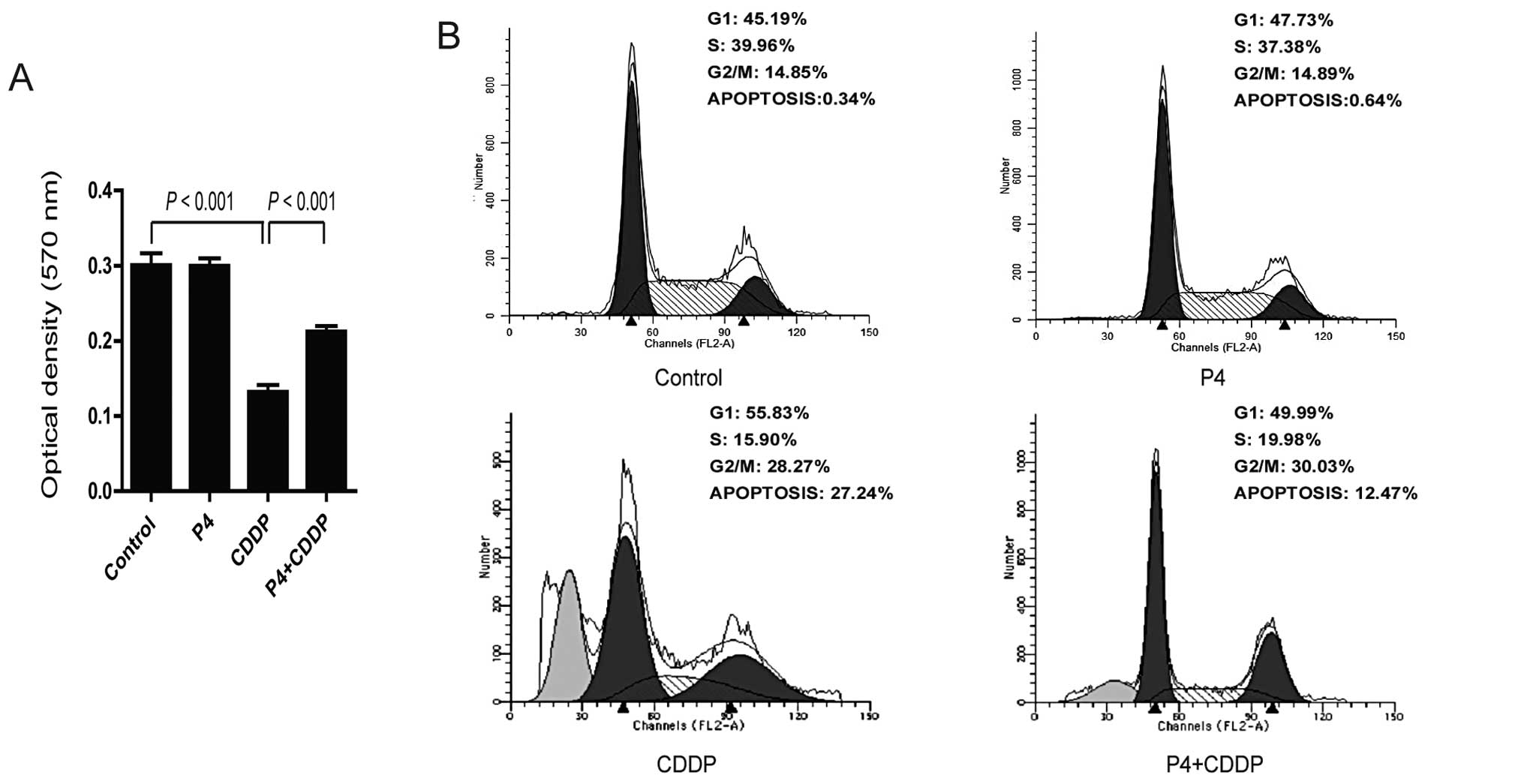
Progesterone attenuates cisplatin-induced
inhibition of proliferation and cell cycle arrest in ovarian cancer
cells
To determine the effect of progesterone on the
cytotoxic effect of cisplatin, HO-8910 cells in DMEM with 5% serum
were exposed to P4 (1 μM), CDDP (30 nM), or P4 (1 μM) plus CDDP (30
nM), respectively. MTT assay was then carried out to examine the
effect of progesterone on cell proliferation. P4 alone did not have
any effect on cell proliferation, and CDDP alone significantly
inhibited the proliferation of HO-8910 cells. Notably, in the
presence of P4, the inhibition of proliferation by CDDP was
significantly attenuated (Fig. 1A).
Consistent with these results, CDDP alone induced cell cycle arrest
with a lower percentage (15.90%) of cells in the S-phase when
compared with these percentages in the cells exposed to P4 (37.38%)
or the control (39.96%) (Fig. 1B).
There was a higher fraction of P4 plus CDDP-treated cells in the
S-phase (19.98%) than the fraction of cells treated with CDDP
alone. Taken together, these data indicate that P4 attenuates the
toxicity effect of CDDP in HO-8910 cells.
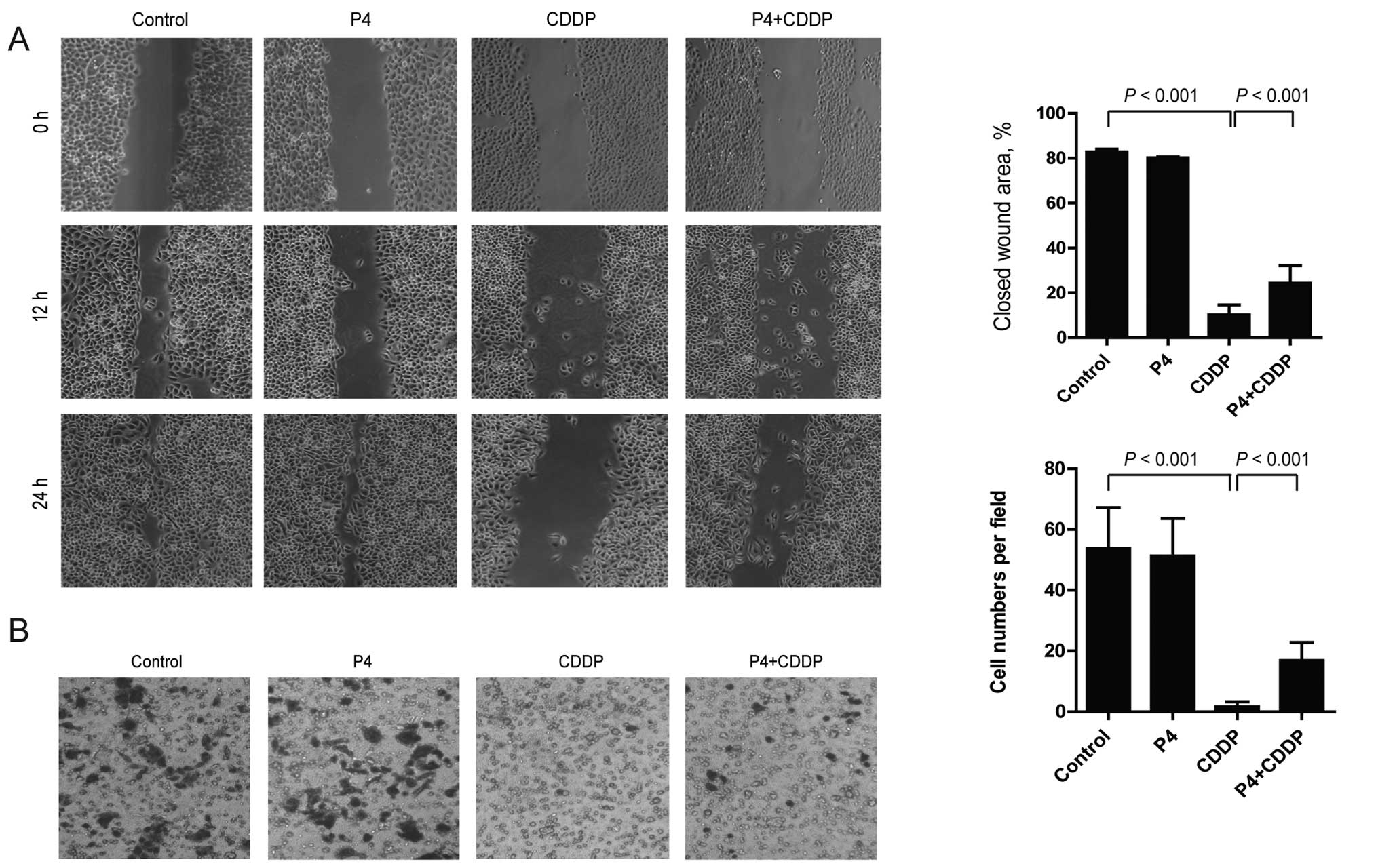
Capability of migration and invasion are
partially restored by progesterone in cisplatin-treated HO-8910
cells
To determine the effect of P4 on migration and
invasion of CDDP-treated HO-8910 cells, a wound healing assay and a
Transwell chamber invasion assay were carried out. In the wound
healing assay, CDDP significantly inhibited the migration of
HO-8910 cells, while P4 attenuated the effect of CDDP and partially
restored the migratory capability of the HO-8910 cells. After 24 h
of treatment, the area of the wound closure of the CDDP-treated
HO-8910 cells was decreased from 82.74±0.66% of the control to
10.25±2.17%, while that of cells treated with P4 plus CDDP was
increased to 24.28±3.94% compared with that of cells treated with
CDDP alone (Fig. 2A). The same
results were observed in the invasion assay. CDDP absolutely
blocked the invasion of the HO-8910 cells from the upper to the
lower chamber, however, P4 restored the action. After treatment of
drugs for 18 h, the number of invasive CDDP-treated HO-8910 cells
on the lower chamber was 2±1 per field, compared to the number of
control or P4-treated cells which totaled 53±13 or 51±11 per field,
respectively. Notably, the number of P4 plus CDDP-treated cells was
increased to 17±6 per field (Fig.
2B).
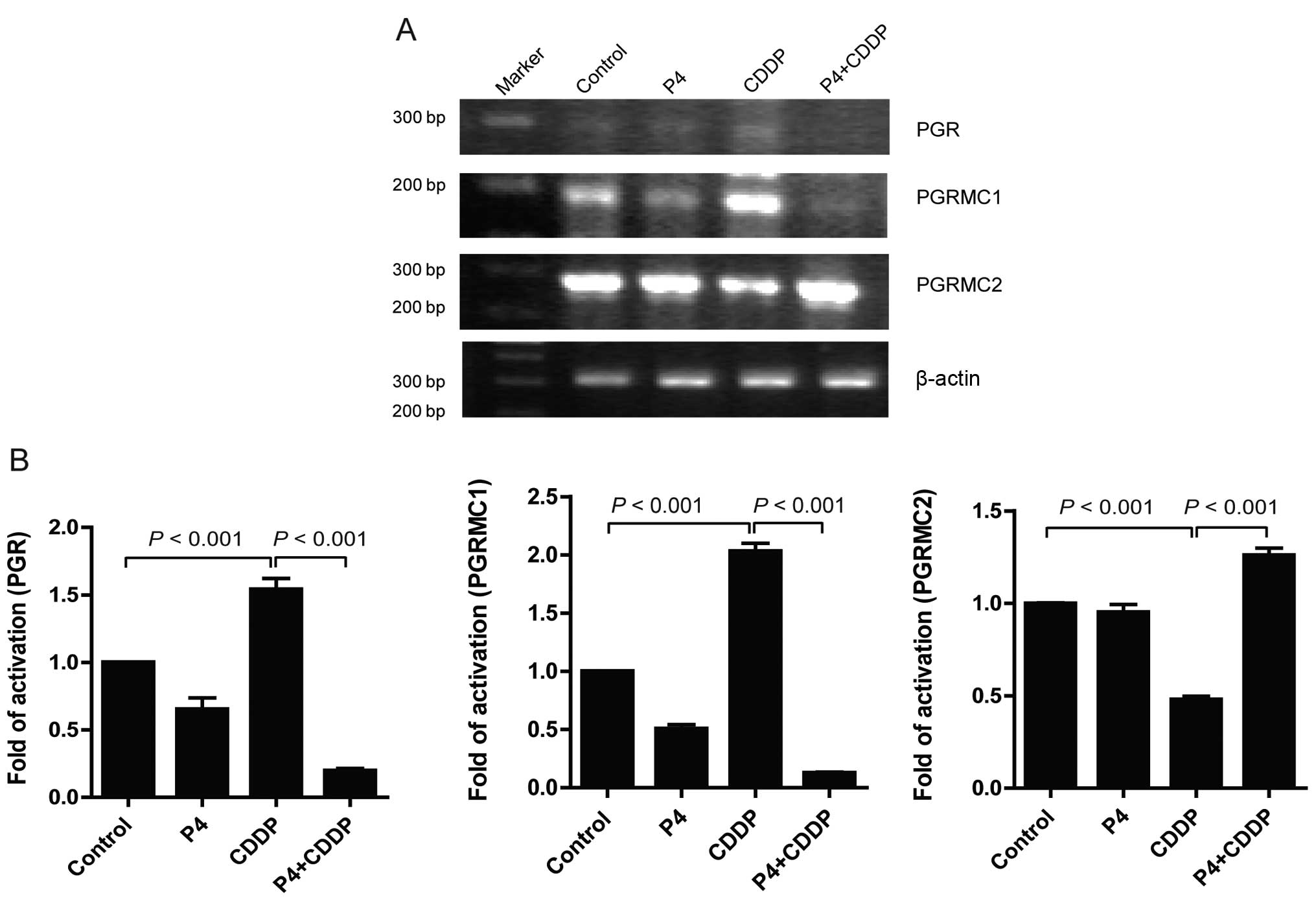
Multiple effect of progesterone on
receptor expression profile in cisplatin-treated HO-8910 cells
Binding to receptors may be the initial step of
progesterone to activate downstream signals and transcriptional
factors. To determine the influence of P4 on the expression profile
of PGR, PGRMC1 and PGRMC2, RT-PCR was carried out to detect the
mRNA levels of these receptors in HO-8910 cells exposed to P4 (1
μM), CDDP (30 nM), or P4 (1 μM) plus CDDP (30 nM), respectively. In
the present study, the data showed a consistent result with that of
Albrecht et al(11).
Treatment with CDDP alone induced an increase in PGRMC1 mRNA by
~102.67% compared to the control, while PGRMC2 mRNA was reduced by
~52.33%. Meanwhile, a significant upregulation of mRNA
transcription of the PGR by CDDP alone was also observed (Fig. 3). Notably, we first observed that P4
significantly inhibited the mRNA transcription of PGRMC1 and PGR in
the CDDP-treated cells, whereas CDDP enhanced mRNA transcription of
PGRMC2 (Fig. 3). It appeared that
the expression profile of PGR/PGRMC1/2 must be tightly correlated
with the anti-apoptotic effect of P4 on CDDP-resistance.
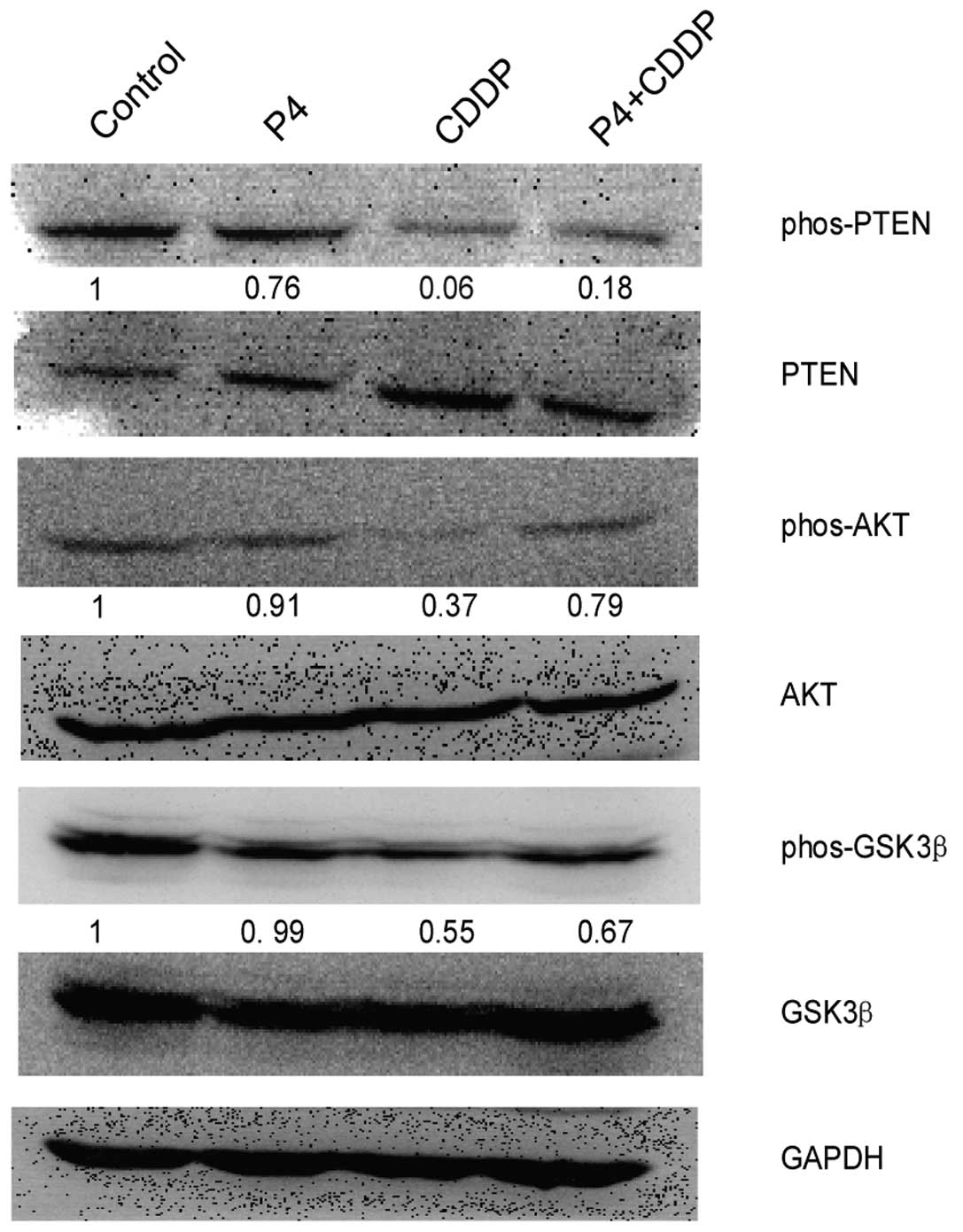
Progesterone upregulates AKT signaling in
cisplatin-treated HO-8910 cells
PI3K/AKT signaling is a classical pathway which has
been shown to be involved in many pathophysiological functions such
as cell proliferation, migration, angiogenesis and tumorigenesis
(12–14). Since we found that P4 rescued
HO-8910 cells from CDDP-induced inhibitory effects, we inferred
that PI3K/AKT signaling may be involved in this action. In the
present study, immunoblot analysis showed that HO-8910 cells
exposed to both P4 and CDDP (0.79-fold of activation normalized to
the control) had a higher level of phos-AKT than the cells exposed
to CDDP alone (0.37-fold of activation). Meanwhile, higher
phos-PTEN and phos-GSK-3β were also detected; the value of
phos-PTEN was 0.18–0.06 and that of phos-GSK-3β was 0.67–0.55,
comparatively (Fig. 4). In the
present study, we also found that P4 alone slightly downregulated
the expression of phos-AKT, but the effect was not significant.
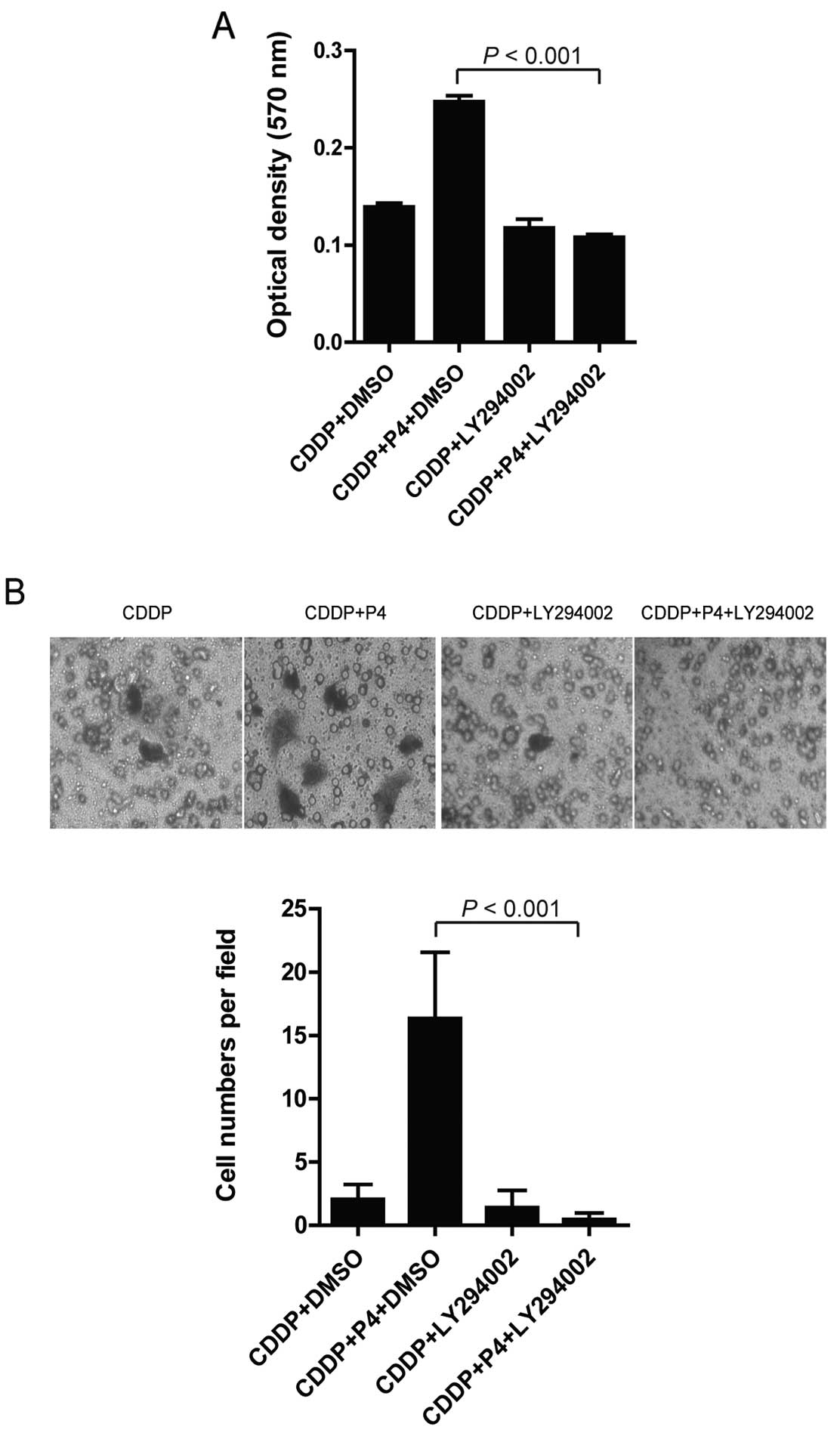
Inhibition of PI3K/AKT signaling blocks
the anti-apoptotic effects of progesterone in HO-8910 cells treated
with cisplatin
To confirm the role of PI3K/AKT activation in the
P4-mediated anti-apoptotic effects, LY294002, an inhibitor of
PI3k/AKT signaling was added to the cell cultures before cell
exposure to P4 (1 μM), CDDP (30 nM), or P4 (1 μM) plus CDDP (30 nM)
as mentioned above. Inhibition of PI3K/AKT signaling by LY294002
significantly abrogated the anti-apoptotic effect of P4 in the
CDDP-treated cells. Our data showed that HO-8910 cells treated with
LY294002 had a high rate of apoptosis following addition of P4 (1
μM) plus CDDP (30 nM) to the culture compared to that of the cells
without LY294002 treatment (Fig.
5A). Consistent with this observation, HO-8910 cells pretreated
with LY294002 and following P4 plus CDDP exposure restored the
capability of migration (Fig. 5B).
These striking findings confirmed a central role of AKT in the
protective effect of P4 in the CDDP-resistant ovarian cancer
HO-8910 cells.
Discussion
Ovarian cancer is the sixth most common cancer among
women worldwide and is considered the leading cause of death from
gynaecological malignancies in western countries (1,15). The
occurrence and development of cancer is a complicated process;
pathogenic organisms, inflammatory factors, hormones followed by
signal alterations are responsible (16–22).
Regarding ovarian cancer as an endocrine neoplasia, dysfunction of
the hormone system should be particularly noted. Until recently,
many researchers have focused on the functions of P4 in ovarian
cancer. Several authors have shown a suppressive effect of P4 on
cancer development (23). However,
the biologic effects of P4 correspond to the concentration of the
drug, as described by Syed et al who reported that P4 at low
concentrations exerts a growth promoting action while P4 at higher
concentrations exerts a growth inhibitory effect (24–27).
In the present study, we used a dose of 1 μM P4 to detect its
effect on CDDP-treated ovarian cancer cells. Our data revealed an
anti-apoptotic effect of P4 with findings that P4 attenuated the
toxic effect of CDDP and protected HO-8910 cells from CDDP-induced
apoptosis in vitro. This finding is consistent with those
reported by Peluso et al(28,29).
Importantly, as a hallmark of cancer malignancy, migration and
invasion of HO-8910 cells were also restored by P4 following CDDP
treatment.
To date, the mechanism by which P4 confer its
anti-proliferative effect has been elucidated. The progesterone
receptor (PGR) which has been proven relevant to the progression of
ovarian cancer patients is responsible for the action (30–32).
PGR is normally highly expressed on ovarian epithelial cells, while
a decline in expression indicates a malignant alteration. Since P4
can mediate its transcriptional activity through a consensus
glucocorticoid response element (GRE), which is not found in the
PGRMC1 promoter but in PGR ones (33), the antiproliferative effect of P4
may be due in part to the antagonism of the binding of P4 to PGR.
The present study first revealed a lower expression of PGR in the
HO-8910 cell line in concordance with other ovarian cancer cell
lines as previously described (34). Notably, CDDP increases the level of
PGR as well as PGRMC1, while conversely decreases the level of
PGRMC2. Most importantly, in CDDP-treated HO-8910 cells, P4
completely reversed the receptor expression profile by decreasing
the level of PGR and PGRMC1, while increasing the level of PGRMC2.
These results suggest the possibility that PGRMC2 instead of PGRMC1
may be crucial to the anti-apoptotic effect of P4 in CDDP-resistant
HO-8910 cells. PGRMC1 and PGRMC2 both contain a cytochrome b5-like
heme/steroid-binding domain and belong to the membrane-associated
progesterone receptor (MAPR) family (35). Currently, PGRMC1 which is highly
expressed in ovarian cancer and other cancer cells and was claimed
to play an important role in chemotherapy resistance, has been
extensively characterized in in vitro studies. Comparably
little is known about PGRMC2. PGRMC2, sharing an amino acid
identity of ~89%, is strongly homologous to PGRMC1, particularly
regarding the intracellular portion containing the cytochrome
b5-like domain (35). The present
data contradict the notion that PGRMC1 plays an important role in
CDDP-resistance of P4 as reported by Peluso et al(29). Instead, our data support those of
Albrecht et al(11) who
reported that overexpression or knockdown of PGRMC1 had no effect
on CDDP-induced apoptosis while overexpression of PGRMC2 induced
significant CDDP resistance. Notably, a combination of P4 plus CDDP
in the present study induced downregulation of PGRMC1 as well as
PGR, while upregulating PGRMC2.
Given the high homology between PGRMC1 and 2, and
the capability of both to interact with cytochrome P450 which has
been shown to play a vital role in tumor progression and metastasis
(36,37), the pathophysiological effect of
PGRMC1/2 on carcinogenesis should be further studied.
In addition to the alteration of the hormone
microenvironment, signaling pathways may also play a crucial role
in the process of carcinogenesis. PI3K/AKT is a classical signal
transduction pathway which has been found to be involved in many
types of cancers, including ovarian. The abnormal activation of
PI3K/AKT may lead to the consequent onset, maintenance and
chemo-resistance of ovarian cancer (38,39).
In the present study, significant activation of the PI3K/AKT
pathway was detected in HO-8910 cells upon treatment with a
combination of P4 and CDDP when compared with CDDP alone-treated
cells. Notably, inhibition of the pathway by a chemical drug
blocked the anti-apoptotic effect of P4 in CDDP-treated cells.
Although, other signaling pathways may also play a role in the
anti-apoptotic action of P4, PI3K/AKT signaling at least in part
plays a role in this case.
Since PGRMC2 binds to cytochrome P450 proteins, 3A4
and 21A2, while the former is associated with the activation of
PI3K/AKT signaling in endothelial cells, the promising crosstalk
between the P4/PGRMC1/2 pathway and PI3K/AKT signaling should be
further investigated.
Taken together, P4 protects ovarian cancer cells
from CDDP-induced apoptosis through activation of PI3K/AKT survival
signaling as well as by modulating the PGR/PGRMC1/2 expression
profile. Thus, the clinical combination of PI3K/AKT signaling
inhibitors or a PGRMC antagonist with platinum-based chemotherapy
may benefit the state and prognosis of ovarian cancer patients.
Acknowledgements
The present study was supported by the Department of
Laboratory Medicine of Jiangsu Provincial Hospital of Traditional
Chinese Medicine.
References
|
1
|
Salzberg M, Thurlimann B, Bonnefois H, et
al: Current concepts of treatment strategies in advanced or
recurrent ovarian cancer. Oncology. 68:293–298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O’Brien ME, Dowsett M, Fryatt I and
Wiltshaw E: Steroid hormone profile in postmenopausal women with
ovarian cancer. Eur J Cancer. 30A:442–445. 1994.PubMed/NCBI
|
|
3
|
Halperin R, Hadas E, Langer R, Bukovsky I
and Schneider D: Peritoneal fluid gonadotropins and ovarian
hormones in patients with ovarian cancer. Int J Gynecol Cancer.
9:502–507. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Modugno F, Ness RB and Cottreau CM:
Cigarette smoking and the risk of mucinous and nonmucinous
epithelial ovarian cancer. Epidemiology. 13:467–471. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Franks AL, Lee NC, Kendrick JS, Rubin GL
and Layde PM: Cigarette smoking and the risk of epithelial ovarian
cancer. Am J Epidemiol. 126:112–117. 1987.PubMed/NCBI
|
|
6
|
Green A, Purdie D, Bain C, Siskind V and
Webb PM: Cigarette smoking and risk of epithelial ovarian cancer
(Australia). Cancer Causes Control. 12:713–719. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu L, Wang J, Zhao L, et al: Progesterone
increases rat neural progenitor cell cycle gene expression and
proliferation via extracellularly regulated kinase and progesterone
receptor membrane components 1 and 2. Endocrinology. 150:3186–3196.
2009. View Article : Google Scholar
|
|
8
|
Chen X, Cheng L, Jia X, et al: Human
immunodeficiency virus type 1 Tat accelerates Kaposi
sarcoma-associated herpesvirus Kaposin A-mediated tumorigenesis of
transformed fibroblasts in vitro as well as in nude and
immunocompetent mice. Neoplasia. 11:1272–1284. 2009.
|
|
9
|
Tang Q, Qin D, Lv Z, et al: Herpes simplex
virus type 2 triggers reactivation of Kaposi’s sarcoma-associated
herpesvirus from latency and collaborates with HIV-1 Tat. PLoS One.
7:e316522012.PubMed/NCBI
|
|
10
|
Qin D, Feng N, Fan W, et al: Activation of
PI3K/AKT and ERK MAPK signal pathways is required for the induction
of lytic cycle replication of Kaposi’s sarcoma-associated
herpesvirus by herpes simplex virus type 1. BMC Microbiol.
11:2402011.PubMed/NCBI
|
|
11
|
Albrecht C, Huck V, Wehling M and Wendler
A: In vitro inhibition of SKOV-3 cell migration as a distinctive
feature of progesterone receptor membrane component type 2 versus
type 1. Steroids. 77:1543–1550. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rodon J, Dienstmann R, Serra V and
Tabernero J: Development of PI3K inhibitors: lessons learned from
early clinical trials. Nat Rev Clin Oncol. 10:143–153. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu J, Chen C and Zhao KN:
Phosphatidylinositol 3-kinass signalings as a therapeutic target
for cervical cancer. Curr Cancer Drug Targets. 13:143–156. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Polak R and Buitenhuis M: The PI3K/PKB
signaling module as key regulator of hematopoiesis: implications
for therapeutic strategies in leukemia. Blood. 119:911–923. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun CC, Ramirez PT and Bodurka DC: Quality
of life for patients with epithelial ovarian cancer. Nat Clin Pract
Oncol. 4:18–29. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tangjitgamol S, Manusirivithaya S,
Khunnarong J, Jesadapatarakul S and Tanwanich S: Expressions of
estrogen and progesterone receptors in epithelial ovarian cancer: a
clinicopathologic study. Int J Gynecol Cancer. 19:620–627. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shanmughapriya S, Senthilkumar G,
Vinodhini K, Das BC, Vasanthi N and Natarajaseenivasan K: Viral and
bacterial aetiologies of epithelial ovarian cancer. Eur J Clin
Microbiol Infect Dis. 31:2311–2317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rosa MI, Silva GD, de Azedo Simoes PW, et
al: The prevalence of human papillomavirus in ovarian cancer: a
systematic review. Int J Gynecol Cancer. 23:437–441. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gonzalez ER: Estrogen, progesterone
receptors in ovarian cancer. JAMA. 245:16261981. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yin G, Chen R, Alvero AB, et al: TWISTing
stemness, inflammation and proliferation of epithelial ovarian
cancer cells through MIR199A2/214. Oncogene. 29:3545–3553. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maccio A, Madeddu C, Massa D, et al:
Hemoglobin levels correlate with interleukin-6 levels in patients
with advanced untreated epithelial ovarian cancer: role of
inflammation in cancer-related anemia. Blood. 106:362–367. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee KB, Byun HJ, Park SH, Park CY, Lee SH
and Rho SB: CYR61 controls p53 and NF-κB expression through
PI3K/Akt/mTOR pathways in carboplatin-induced ovarian cancer cells.
Cancer Lett. 315:86–95. 2012.PubMed/NCBI
|
|
23
|
Murdoch WJ, Van Kirk EA, Isaak DD and Shen
Y: Progesterone facilitates cisplatin toxicity in epithelial
ovarian cancer cells and xenografts. Gynecol Oncol. 110:251–255.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Seeger H, Wallwiener D and Mueck AO: Is
there a protective role of progestogens on the proliferation of
human ovarian cancer cells in the presence of growth factors? Eur J
Gynaecol Oncol. 27:139–141. 2006.PubMed/NCBI
|
|
25
|
Ho SM: Estrogen, progesterone and
epithelial ovarian cancer. Reprod Biol Endocrinol. 1:732003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fauvet R, Dufournet Etienne C, Poncelet C,
Bringuier AF, Feldmann G and Darai E: Effects of progesterone and
anti-progestin (mifepristone) treatment on proliferation and
apoptosis of the human ovarian cancer cell line, OVCAR-3. Oncol
Rep. 15:743–748. 2006.PubMed/NCBI
|
|
27
|
Syed V, Ulinski G, Mok SC, Yiu GK and Ho
SM: Expression of gonadotropin receptor and growth responses to key
reproductive hormones in normal and malignant human ovarian surface
epithelial cells. Cancer Res. 61:6768–6776. 2001.PubMed/NCBI
|
|
28
|
Peluso JJ, Gawkowska A, Liu X, Shioda T
and Pru JK: Progesterone receptor membrane component-1 regulates
the development and cisplatin sensitivity of human ovarian tumors
in athymic nude mice. Endocrinology. 150:4846–4854. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peluso JJ: Progesterone signaling mediated
through progesterone receptor membrane component-1 in ovarian cells
with special emphasis on ovarian cancer. Steroids. 76:903–909.
2011.
|
|
30
|
Lenhard M, Tereza L, Heublein S, et al:
Steroid hormone receptor expression in ovarian cancer: progesterone
receptor B as prognostic marker for patient survival. BMC Cancer.
12:5532012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leite DB, Junqueira MG, de Carvalho CV, et
al: Progesterone receptor (PROGINS) polymorphism and the risk of
ovarian cancer. Steroids. 73:676–680. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gabra H, Watson JE, Taylor KJ, et al:
Definition and refinement of a region of loss of heterozygosity at
11q23.3–q24.3 in epithelial ovarian cancer associated with poor
prognosis. Cancer Res. 56:950–954. 1996.
|
|
33
|
Petz LN, Nardulli AM, Kim J, Horwitz KB,
Freedman LP and Shapiro DJ: DNA bending is induced by binding of
the glucocorticoid receptor DNA binding domain and progesterone
receptors to their response element. J Steroid Biochem Mol Biol.
60:31–41. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Noguchi T, Kitawaki J, Tamura T, et al:
Relationship between aromatase activity and steroid receptor levels
in ovarian tumors from postmenopausal women. J Steroid Biochem Mol
Biol. 44:657–660. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mifsud W and Bateman A: Membrane-bound
progesterone receptors contain a cytochrome b5-like ligand-binding
domain. Genome Biol. 3:Research00682002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Szczesna-Skorupa E and Kemper B:
Progesterone receptor membrane component 1 inhibits the activity of
drug-metabolizing cytochromes P450 and binds to cytochrome P450
reductase. Mol Pharmacol. 79:340–350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oda S, Nakajima M, Toyoda Y, Fukami T and
Yokoi T: Progesterone receptor membrane component 1 modulates human
cytochrome p450 activities in an isoform-dependent manner. Drug
Metab Dispos. 39:2057–2065. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Leake K, Singhal J, Nagaprashantha LD,
Awasthi S and Singhal SS: RLIP76 regulates PI3K/Akt signaling and
chemo-radiotherapy resistance in pancreatic cancer. PLoS One.
7:e345822012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim TR, Cho EW, Paik SG and Kim IG:
Hypoxia-induced SM22α in A549 cells activates the IGF1R/PI3K/Akt
pathway, conferring cellular resistance against chemo- and
radiation therapy. FEBS Lett. 586:303–309. 2012.
|