Introduction
Ovarian cancer is one of the most common causes of
mortality among all cancers in females and is the leading cause of
mortality from gynecological malignancies. Ovarian epithelial
carcinoma is a common malignant ovarian neoplasm with a poor 5-year
survival rate (<30%). Many factors regulate the rapid growth of
ovarian epithelial carcinoma. The autocrine secretion hypothesis
proposes that as a result of oncogene activation, neoplastic cells
can escape growth-restraining mechanism by independently producing
and responding to their own growth factors (1,2). One
relatively newer but exciting dimension is the role of ghrelin in
malignant cell proliferation (3).
Ghrelin, a 28-amino acid peptide hormone, is
primarily produced and secreted by the gastrointestinal tract
(4). Since its discovery, it has
been implicated in a wide range of physiological activities
(5). Ghrelin is the only currently
identified circulating orexigenic hormone that is able to initiate
food intake. A variety of other and pathophysiological processes
are known to be regulated by ghrelin in addition to ingestive
behavior, including roles in the regulation of growth hormone
release, metabolism, the cardiovascular system and insulin
secretion (5). Ghrelin is the
ligand for the growth hormone secretagogue receptor (GHSR). These
receptors have two known subtypes, GHSR1a and GHSR1b (5,6).
GHSR1a is reserved for ghrelin’s endocrine activities, including
the stimulation of appetite and growth hormone secretion, while the
latter is thought to be devoid of any mechanistic role.
Ghrelin and its receptor are expressed in a number
of cancers and cancer cell lines such as pituitary adenomas,
thyroid follicular cancer, parathyroid adenomas, pancreatic-related
endocrine tumors, oral squamous cell carcinoma, gastric carcinoids,
colon cancer, renal carcinoma, bronchial carcinoid, testicular and
ovarian tumors, adrenocortical tumors, prostate cancer and breast
cancer, and may play a role in processes associated with cancer
progression, including cell proliferation, apoptosis and cell
invasion and migration (7).
However, the reports are controversial. To date, several groups
have reproduced ghrelin’s pro-proliferative effect in neuronal,
adrenal, prostatic, adipose, mammary, chondroblastic and pancreatic
cells (8–11). In contrast to the above mentioned
reports, the anti-proliferative role of ghrelin has been determined
in a few cell types, for example, human CALU-1 lung carcinoma cells
(12).
In the present study, we demonstrated that the
proliferation of human ovarian epithelial carcinoma cells was
inhibited by ghrelin and explored the mediating mechanism and
possible significance.
Materials and methods
Chemicals and reagents
HO-8910, a human ovarian epithelial carcinoma cell
line, was purchased from the American Type Culture Collection
(ATCC, Manassas, VA, USA). RPMI-1640 medium was purchased from
Hyclone Co. (Logan, UT, USA). Recombinant human ghrelin, an
anti-ghrelin neutralizing antibody and the GHSR1a antagonist
D-Lys-3-GH-releasing peptide-6 (D-Lys-3-GHRP-6) were purchased from
Phoenix Pharmaceuticals (Belmont, CA, USA). Goat anti-human GHSR1a,
mouse anti-β-actin antibodies and purified goat IgG were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). All
other antibodies were purchased from Cell Signaling Technology
(Beverly, MA, USA). All other chemicals and drugs were purchased
from Sigma Chemical (St. Louis, MO, USA).
Immunohistochemical analysis
Frozen sections of ovarian samples were incubated
with the goat primary anti-human GHSR1a antibody or purified goat
IgG, horseradish peroxidase-conjugated mouse anti-goat IgG and
3,3-diaminobenzidine successively. Sections were then
counterstained with hematoxylin.
Cell culture
HO-8910 cells were cultured in RPMI-1640 containing
10% fetal bovine serum (FBS) and penicillin/streptomycin (100 U/ml)
in a humidified 37°C incubator. When achieving confluence, the
cells were treated with ghrelin (10−11–10−8
M) for 48 h. For the inhibition experiments, cells were pretreated
with L-leucine or 3-methyladenine (3-MA) for 1 h prior to
stimulation with ghrelin at 10−9 M for 48 h.
RNA extraction and RT-PCR analysis
Total RNAs were isolated using Trizol reagent
according to the manufacturer’s instructions. Total RNA (2 μg) was
reverse-transcribed using reverse transcription system (Promega,
Madison, WI, USA). One microliter of the reaction mixture was
subjected to PCR. The forward and reverse PCR primers were: human
ghrelin, 5′-TGA GCC CTG AAC ACC AGA GAG-3′ and 5′-AAA GCC AGA TGA
GCG CTT CTA-3′ (Genebank sequence ID: NM_016362.3); human GHSR1a,
5′-TCG TGG GTG CCT CGC T-3′ and 5′-CAC CAC TAC AGC CAG CAT TTT C-3′
(Genebank sequence ID: NM_198407.2); human proliferating cell
nuclear antigen (PCNA), 5′-TGT TGG AGG CAC TCA AGG AC-3′ and 5′-TCA
TTG CCG GCG CAT TTT AG-3′ (Genebank sequence ID: NM_002592.2);
human β-actin, 5′-ATC TGG CAC CAC ACC TTC-3′ and 5′-AGC CAG GTC CAG
ACG CA-3′ (Genebank sequence ID: NM_001101.3). All amplification
reactions were performed under the following conditions: 95°C for 5
min, followed by 35 cycles at 95°C for 30 sec, 58°C for 30 sec and
72°C for 60 sec. A 20 μl aliquot of the RT-PCR samples was loaded
onto 1.5% agarose gel. For the quantitative real-time PCR analysis,
the amount of PCR products formed in each cycle was evaluated on
the basis of SYBR-Green I fluorescence. Results were analyzed with
Stratagene Mx3000 software, and mRNA levels were normalized with
respect to the levels of β-actin in each sample. For negative
controls, PCR reactions were performed for the primer pairs in the
absence of the transcript.
Cell proliferation and viability
assays
To determine the effect of ghrelin on HO-8910 cell
proliferation, 30% confluent HO-8910 cells were incubated in
RPMI-1640 media with different concentrations of FBS (0, 5 and 10%)
in the presence or absence of ghrelin (10−9 M) for 48 h.
On completion of the incubation, cultures were typsinized and cell
numbers were determined with an Invitrogen CountessH Automated Cell
Counter (Invitrogen, Carlsbad, CA, USA).
The WST-1 and Cell Counting Kit-8 (CCK-8) assays
were used to determine the effect of ghrelin on HO-8910 cell
viability. Briefly, 1×103 cells/well were incubated in
96-well plates overnight, starved in serum-free medium for 24 h and
treated with indicated reagents. For the WST-1 assay (BioVision
Research Products, Milpitas, CA, USA), cells were incubated with 10
μl of WST-1 reagent for 45 min and the absorbance was measured at
450 nm using the Bio-Rad iMark microplate absorbance reader
(Bio-Rad, USA). For the CCK-8 assay (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan), cells were incubated with 10
μl of CCK-8 solution for 1 h and the absorbance was measured at 450
nm.
Cell cycle analysis
HO-8910 cells, cultured with or without ghrelin
(10−9 M) for 24 h, were trypsinized, fixed and
permeabilized with 70% ethanol. Cells were then labeled with
propidium iodide with RNase A cocktail for 30 min at 37°C, and flow
cytometry was used to determine the cell cycle distribution of the
HO-8910 cells. Data were obtained using the FACSCalibur flow
cytometer (BD Biosciences, USA).
Apoptosis analysis
HO-8910 cells were cultured in a 96-well plate with
or without ghrelin (10−9 M) stimulation. Apoptosis was
assessed by measuring cysteine aspartic acid-specific protease
(caspase) 3/7 activity with the use of the Caspase-Glo®
3/7 assay kit (Promega) according to the manufacturer’s
instructions.
Preparation of cytosolic proteins and
western blot analysis
Following treatment, the cells were packed by
centrifuging the cells for 3 min at 200 × g, and homogenized in
ice-cold fractionation buffer [50 mM Tris-HCl, pH 7.4, 1 mM EDTA,
150 mM NaCl, 1% Triton X-100, 1 mM PMSF, 10 μg/ml leupeptin, 10
μg/ml pepstatin A, 10 μg/ml aprotinin, 1 mM sodium orthovanadate
(Na3VO4), 10 mM sodium pyrophosphate
(Na4P2O7) and 50 mM sodium
fluoride (NaF)]. The cell lysate was incubated on ice for 15 min
and then centrifuged at 20,000 × g for 30 min at 4°C. The cytosolic
fraction was collected and subjected to SDS-PAGE with a 10% running
gel. Protein concentrations were determined by BCA protein assay
kit (Pierce, Rockford, IL, USA). The proteins were transferred to a
polyvinylidene fluoride membrane. The membrane was incubated
successively with 5% bovine serum albumin in Tris-Tween buffered
saline (TTBS) at room temperature for 1 h, with different primary
antibodies at 4°C for 12 h and then with horseradish
peroxidase-labeled secondary antibody for 1 h. After each
incubation, the membrane was washed extensively with TTBS and the
immunoreactive band was detected with ECL detecting reagents
(Pierce).
Statistical analysis
Quantitative data are presented as the means ± SEM
determined from the indicated number of experiments. Statistical
analysis was based on the Student’s t-test for comparison of two
groups or one-way ANOVA for multiple comparisons. P<0.05 was
used to determine statistical significance.
Results
Ghrelin and GHSR1a are expressed in
ovarian epithelial carcinoma in vivo and in vitro
We first examined whether ghrelin and GHSR1a, the
classical ghrelin receptor, are expressed in ovarian epithelial
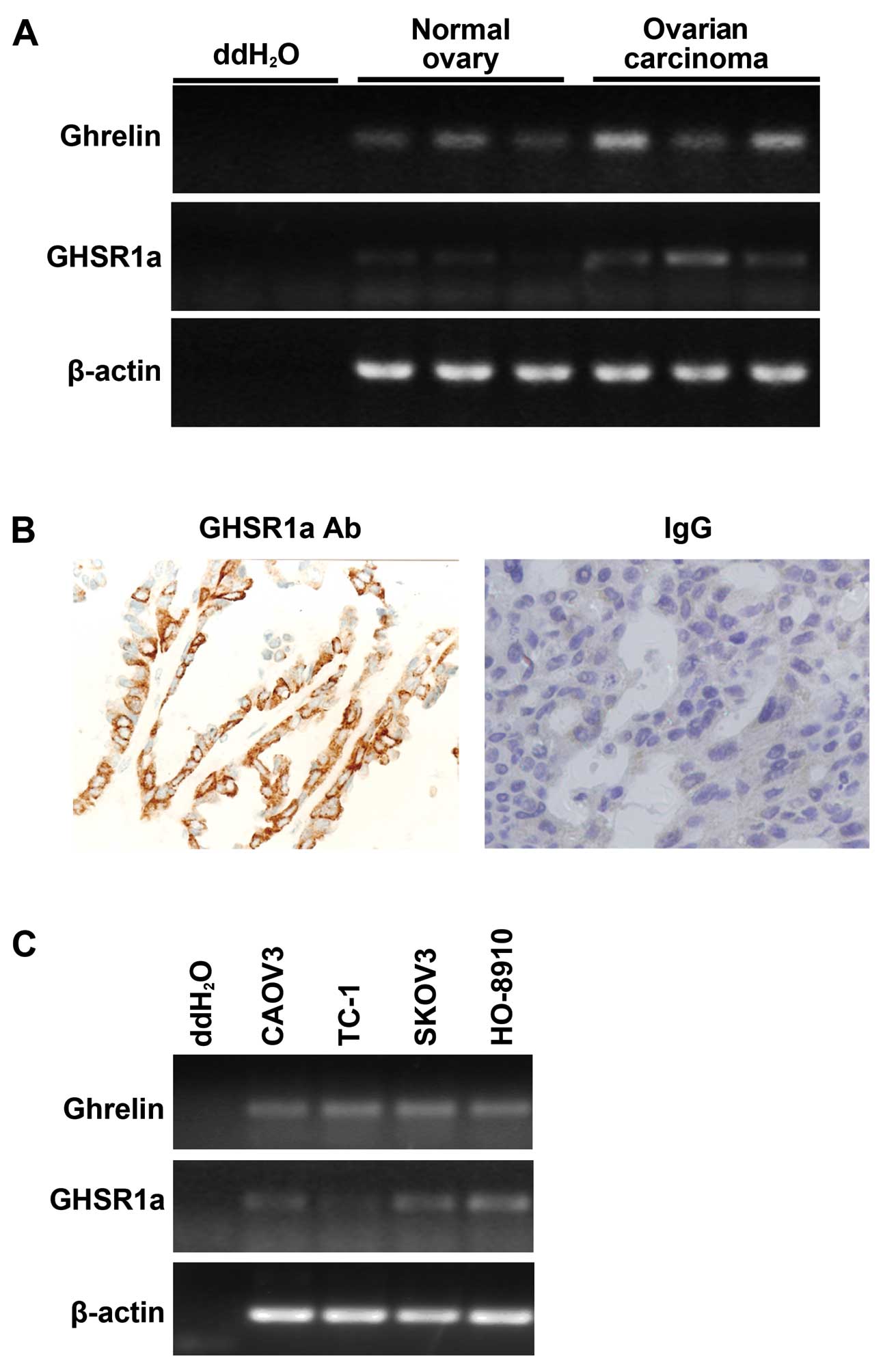
carcinoma tissues in vivo. RT-PCR analysis showed that
ghrelin was expressed in normal ovarian tissues as well as in
ovarian epithelial carcinoma tissues (Fig. 1A), with a higher level of ghrelin in
ovarian epithelial carcinoma. GHSR1a was expressed in ovarian
epithelial carcinoma tissues, but was barely expressed in normal
ovarian tissues (Fig. 1A).
Immunohistochemical staining also showed that GHSR1a was expressed
in ovarian epithelial carcinoma tissues (Fig. 1B). Ghrelin was expressed in all the
human ovarian carcinoma cell lines tested, but the highest
expression of GHSR1a was detected in the HO-8910 cells (Fig. 1C). These results confirmed the
existence of ghrelin and GHSR1a in ovarian epithelial carcinoma and
were consistent with a previous report (13).
Ghrelin inhibits HO-8910 cell
proliferation
We next study the possible role of ghrelin in
ovarian epithelial carcinoma progression. HO-8910, a human ovarian
epithelial carcinoma cell line, was cultured without (control) or
with ghrelin (10−9 M) for 48 h with different
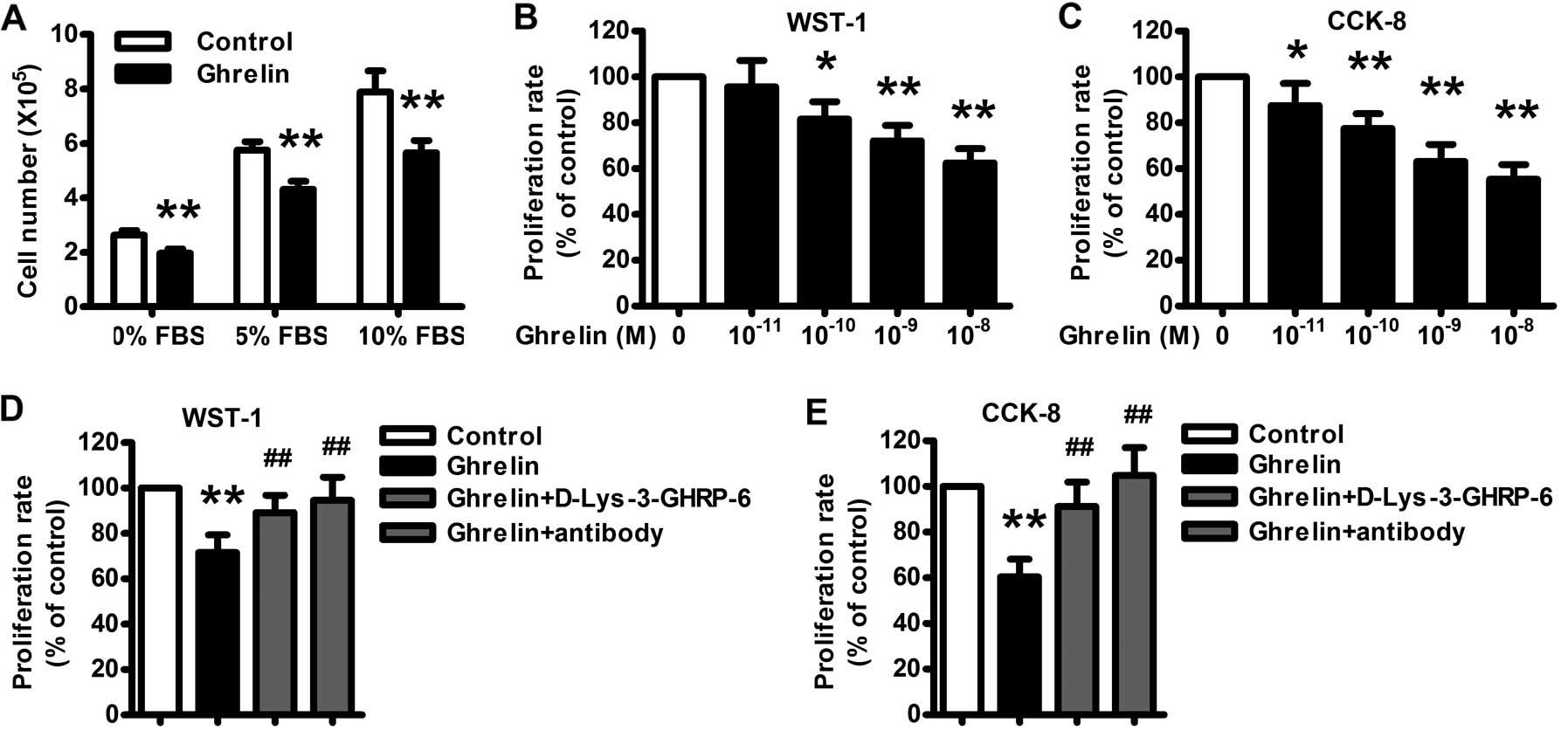
concentrations of FBS. Treatment of HO-8910 cells with ghrelin
resulted in a statistically significant decrease in cell number
compared to the control (Fig. 2A).
Treatment with increasing amounts of serum resulted in a
concentration-dependent increase in cell number. The inhibitory
effect of ghrelin was still significant with increasing
concentrations of serum in the cell culture medium.
The WST-1 and CCK-8 assays also revealed that
ghrelin treatment (10−11–10−8 M) for 48 h
resulted in a concentration-dependent inhibition in HO-8910 cell
proliferation (Fig. 2B and C), with
maximal inhibition of ~62 and 54% of the control noted at
10−8 M of ghrelin. A neutralizing antibody of ghrelin
significantly attenuated the HO-8910 cell proliferation (Fig. 2D and E), similarly as the GHSR1a
antagonist D-Lys-3-GHRP-6, which indicates that ghrelin-mediated
inhibition of HO-8910 cell proliferation depends on the immune
activity of ghrelin and through its receptor.
Alteration of the cell cycle distribution
of HO-8910 cells following ghrelin treatment
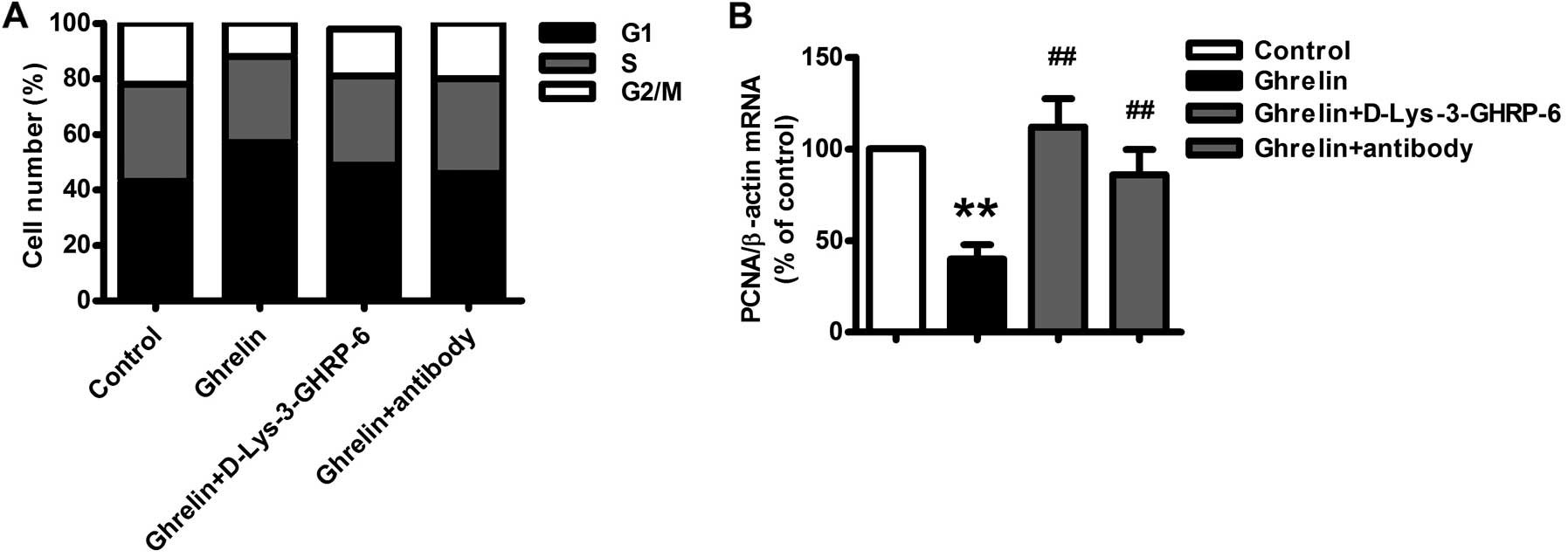
Flow cytometry was used to evaluate the effect of
ghrelin on HO-8910 cell cycle progression. Ghrelin (10−9
M) significantly increased the proportion of cells in the G1 phase
by ~38.1% (P<0.01) (Fig. 3A),
and the proportion of G2/M and S phase cells was decreased by a
comparable degree, which indicated that ghrelin effectively
inhibited the proliferation and growth of HO-8910 cells by G1 phase
arrest. The expression of PCNA, a novel proliferation-related gene
usually highly expressed in the G1/S phase, was also significantly
inhibited by ghrelin (Fig. 3B). A
neutralizing antibody of ghrelin and the GHSR1a antagonist
D-Lys-3-GHRP-6 also significantly attenuated the effect of ghrelin
on the cell cycle (Fig. 3).
Ghrelin promotes apoptosis in HO-8910
cells
The members of the caspase family play key effector
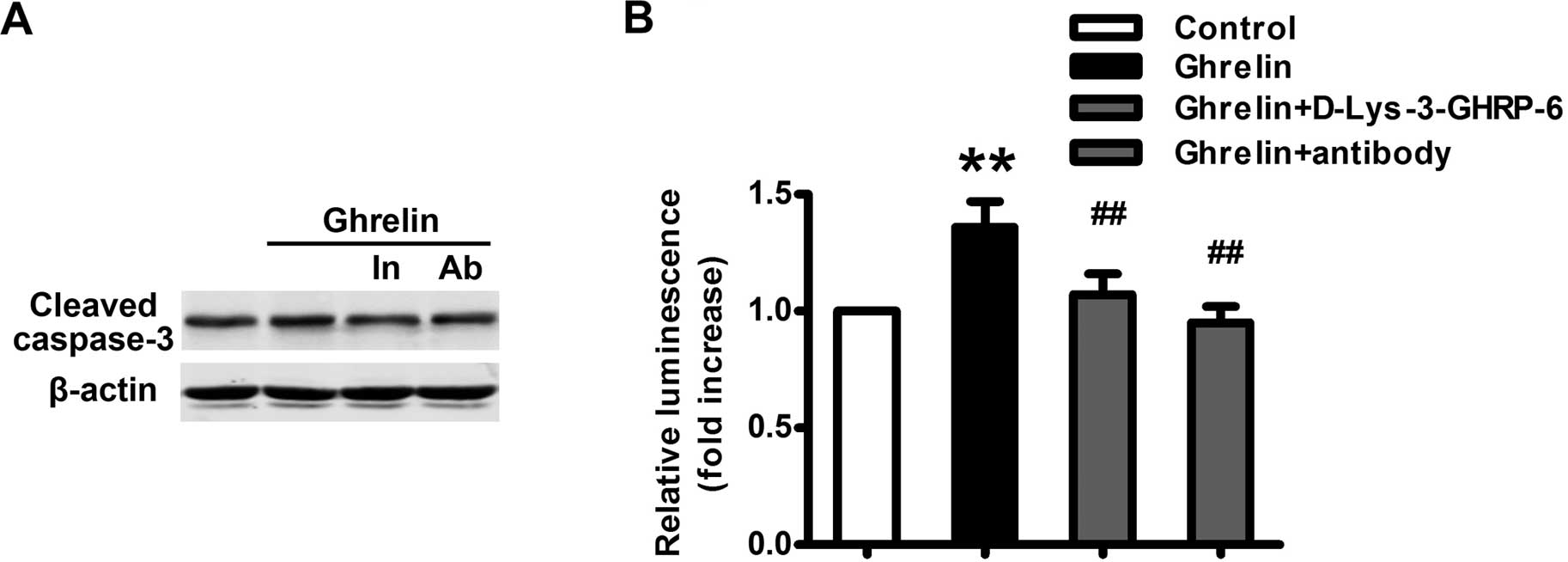
roles in apoptosis in mammalian cells. We next detected the effect
of ghrelin on the apoptosis of HO-8910 cells. As expected, ghrelin
(10−9 M) significantly increased the production of the
apoptosis marker, cleaved caspase-3 (Fig. 4A). The activity of caspase-3 and -7
was also significantly increased following ghrelin treatment
(Fig. 4B). These effects were
dependent on the immune activity of ghrelin and through its
receptor since both the neutralizing antibody of ghrelin and the
GHSR1a antagonist significantly attenuated the effect of ghrelin on
HO-8910 cell apoptosis (Fig.
4).
Mammalian target of rapamycin (mTOR)
signaling pathway is inhibited upon ghrelin stimulation
The mTOR is a central cell-growth regulator that
regulates cell proliferation, and aberrant mTOR activity is linked
to the development of cancer (14).
In the present study, we found that there was a significant
decrease in phosphorylated mTOR (Ser2448) following ghrelin
treatment (10−9 M), as well as the phosphorylation of S6
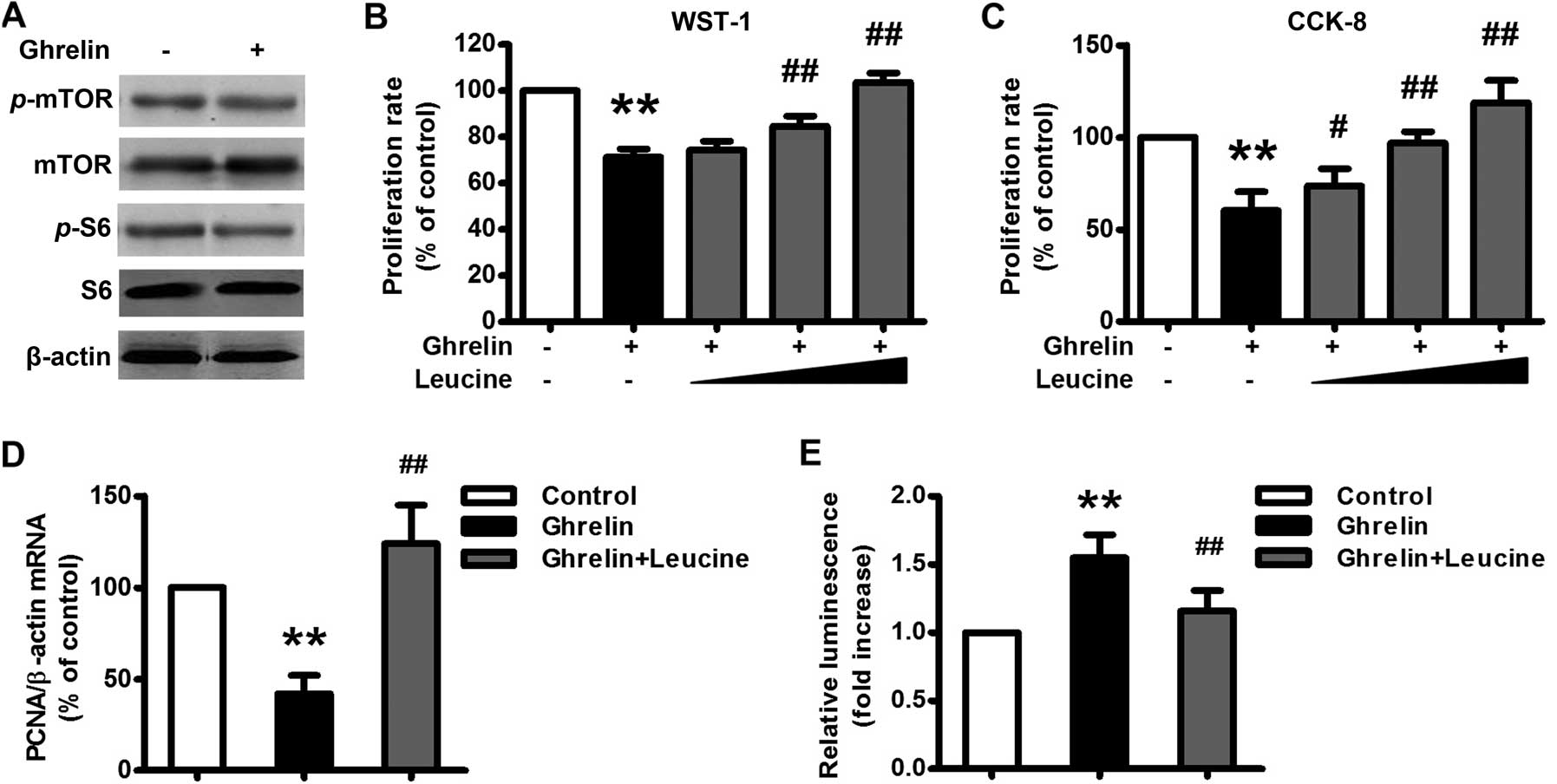
ribosomal protein, a downstream target of mTOR (Fig. 5A). Administration of L-leucine (1,
5, 10 mM), a branched-chain amino acid that has been documented to
activate mTOR signaling (15),
significantly restored ghrelin-inhibited HO-8910 cell proliferation
(Fig. 5B–D). Administration of
L-leucine (5 mM) also significantly attenuated ghrelin-induced
HO-8910 cell apoptosis (Fig. 5E).
Taken together, ghrelin inhibited HO-8910 cell proliferation
through inhibition of the mTOR signaling pathway.
Involvement of autophagy following
ghrelin stimulation
Autophagy is a tightly regulated process, and
defects in autophagy have been closely associated with many human
diseases, including cancer (16,17).
To determine whether autophagy is involved in ghrelin-induced
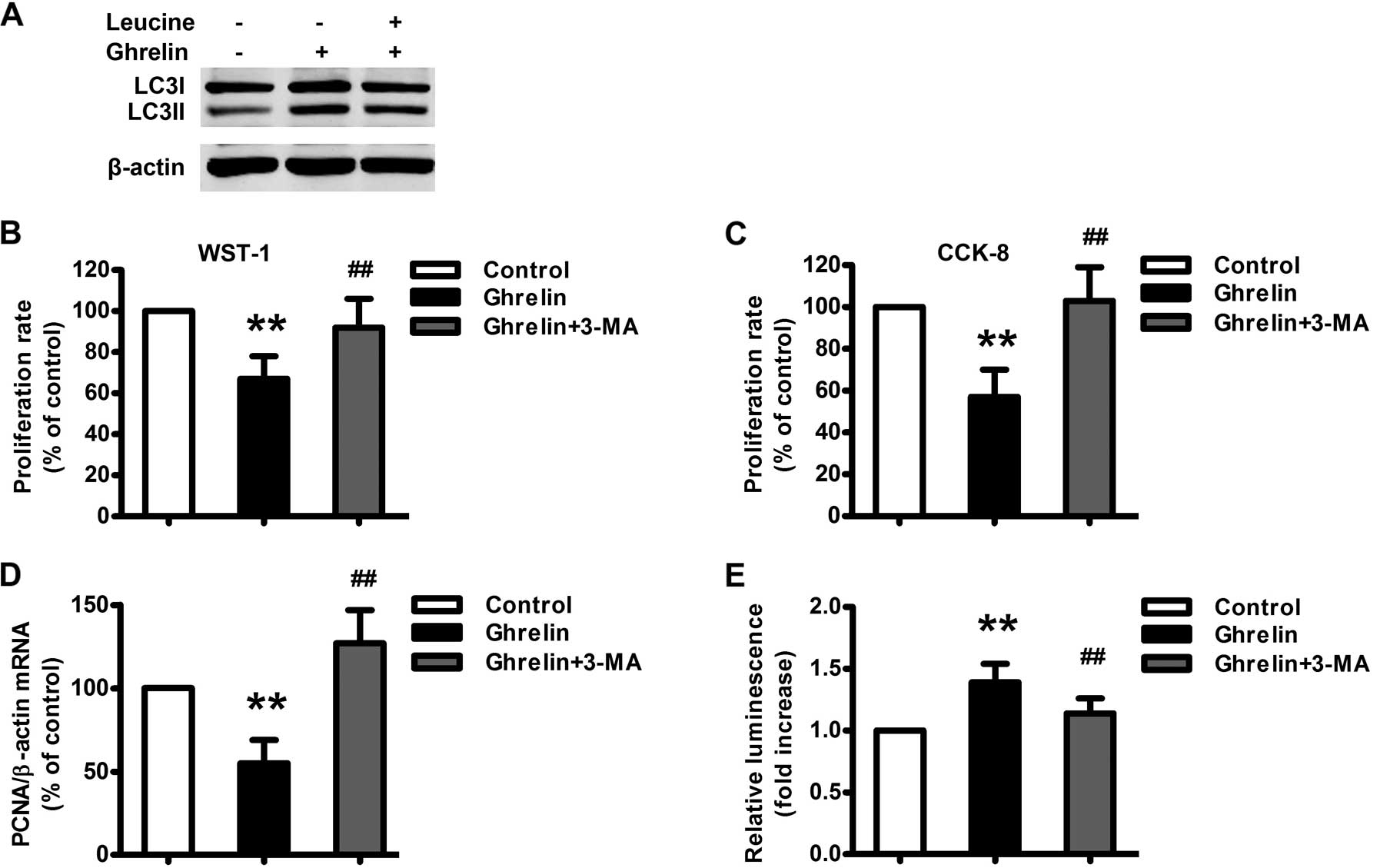
apoptosis, we first examined the effect of ghrelin on autophagosome
formation in HO-8910 cells. Ghrelin (10−9 M)
significantly increased the level of the lipid-conjugated form of
the autophagosome marker light-chain 3-II (LC3II) in HO-8910 cells
(Fig. 6A). To address the potential
role of autophagy in ghrelin-induced apoptosis in HO-8910 cells,
3-methyladenine (3-MA), a pharmacological inhibitor of autophagy,
was used. Pretreatment of 3-MA (5 mM) for 1 h significantly
attenuated ghrelin-induced HO-8910 cell apoptosis (Fig. 6E) and augmented ghrelin-inhibited
HO-8910 cell proliferation (Fig.
6B–D). Furthermore, L-leucine treatment significantly
attenuated ghrelin-induced autophagy (Fig. 6A). Taken together,
autophagy-mediated ghrelin-induced apoptosis, is regulated by the
mTOR signaling pathway.
Discussion
The present study demonstrated that ghrelin
inhibited the proliferation of the human ovarian epithelial
carcinoma cell line HO-8910 through induction of apoptosis and
autophagy via the mTOR signaling pathway and may subsequently
contribute to cancer prevention and therapy. This conclusion is
supported by the following observations. i) Ghrelin and GHSR1a are
expressed in ovarian epithelial carcinoma in vivo and in
vitro. ii) Ghrelin inhibits the proliferation and growth of
HO-8910 cells by G1 phase arrest. iii) Ghrelin enhances HO-8910
cell apoptosis and autophagy. iv) Activation of the mTOR signaling
pathway blocks the effects of ghrelin on autophagy and apoptosis,
thereby reversing the inhibition of HO-8910 cell proliferation. To
the best of our knowledge, this is the first report demonstrating
the involvement of multiple signaling pathways in the
ghrelin-mediated modulation of proliferation in ovarian epithelial
carcinoma.
Ovarian carcinoma is the fourth most common cause of
cancer-related death among women in the United States; although it
is the 12th most common cause of cancer-related death among women
in China, the mortality rate is more than 70% within 5 years.
Although many studies report the effect of ghrelin on the
pathogenesis and progression of malignant tumors, it is seldom
reported in ovarian carcinoma. The possible reason may be the
distribution of ghrelin and GHSR1a expression. Ghrelin has been
found in the stomach, other parts of the gut, adrenal gland,
atrium, breast, buccal mucosa, esophagus, Fallopian tube, fat
tissue, gall bladder, human lymphocytes, ileum, kidney, left colon,
liver, lung, lymph node, muscle, myocardium, ovary, pancreas,
pituitary, placenta, prostate, skin, spleen, testis, thyroid and
vein (13). However, it was
previously reported that the expression of GHSR1a was predominantly
expressed only in the pituitary and at much lower levels in the
thyroid gland, pancreas, spleen, myocardium and adrenal gland, and
not detectable in the ovary (13).
In the present study, we confirmed the expression of
GHSR1a in ovarian epithelial carcinoma tissues, which is consistent
with a previous report (18).
Therefore, ghrelin inhibits HO-8910 cell proliferation through the
interaction with its receptor directly. It has been reported that
in women with ovarian cancer, blood concentrations of active
ghrelin were higher than that in the control group; in contrast,
total ghrelin concentrations in blood were similar in the studied
groups. This alteration resulted in increased ratios of active to
total ghrelin concentration in the peripheral blood of patients
with ovarian cancer (19).
Generally, the structure of ghrelin is unique in that specific
acyl-modification of its third serine occurs. This acylation is
necessary for ghrelin to bind to GHSR1a, and to exert biologic
activity (5). The carboxylic chain
that esterifies the hydroxyl of serine is primarily n-octanoic
acid, but other 6–10 carbon chain residues may also modify the
structure of ghrelin. The enzyme responsible for the acylation of
ghrelin is ghrelin O-acyltransferase (GOAT) (20,21),
which is highly expressed in the stomach, adrenal cortex, spleen,
lung, pituitary, but is barely expressed in the ovary (22). Therefore, although ghrelin is
expressed in ovarian tissues, the effect of active acyl ghrelin on
ovarian carcinoma may still occur through an endocrine rather than
a paracrine/auotcrine pathway. The effect of des-acyl ghrelin, the
main component of plasma total ghrelin but which does not bind to
GHSR1a (23,24), on the pathogenesis and progression
of ovarian epithelial carcinoma is still unknown and warrants
further investigation.
The mTOR, a ubiquitously expressed protein kinase
and important regulator of cell growth and proliferation, is
implicated in cell processes that lead to uncontrolled growth of
cancer cells. L-leucine is a specific activator of mTOR (15), and downstream targets of mTOR
include S6 kinases, S6 and eIF-4E binding protein 1 (25,26).
Exactly how mTOR contributes to cancer is still unclear. It is
believed that mTOR and its downstream signals affect cell
proliferation and tumorigenesis by promoting the translation of
specific mRNAs coding for pro-oncogenic proteins that regulate cell
survival, cell-cycle progression, angiogenesis, energy metabolism
and metastasis (27). Additionally,
the increase in ribosome biogenesis linked to mTOR activation
probably promotes cell proliferation by providing the machinery
required to sustain high levels of cell growth. A number of agents
that target the mTOR pathway have shown potent antitumorigenic
effects (28). The effect of
ghrelin on mTOR activity is controversial; ghrelin was reported to
elicit a marked upregulation or downregulation of the hypothalamic
mTOR signaling pathway. In the present study, we demonstrated the
downregulation of the activities of mTOR and its downstream signal
S6 following ghrelin treatment in HO-8910 cells. This
downregulation mediated ghrelin-inhibited HO-8910 cell
proliferation. Two mTOR complexes are known to exist: mTOR complex
1 (mTORC1) is responsible for nutrient-sensing functions and is
composed of mTOR, G protein-subunit-like protein and raptor; mTORC2
phosphorylates Akt protein kinase B and contains mTOR and rictor
(26). In the present study, we
found that L-leucine significantly attenuated ghrelin-induced
HO-8910 cell apoptosis and therefore increased proliferation,
indicating that mTORC1 mediated the effect of ghrelin. The role of
mTORC2 during the effect of ghrelin remains unclear.
Programmed cell death plays a fundamental role in
animal development and tissue homeostasis (29). Abnormal regulation of this process
is associated with a wide variety of human diseases, including
immunological and developmental disorders and cancer. Apoptosis is
the most important form of programmed cell death. In the present
study, we demonstrated that ghrelin induced the apoptosis of
HO-8910 cells and subsequently inhibited tumor growth. However,
although ghrelin inhibited the proliferation of HO-8910 cells, it
has been previously reported that apoptosis may also induce a
compensative proliferation under a certain environment (30). The balance of ghrelin-regulated
proliferation and apoptosis warrants further discussion.
Autophagy is a dynamic and highly regulated process
of self-digestion. It is a highly conserved cellular process
responsible for removal or recycling of long-lived proteins and
organelles, and provides cells with an alternative source of
nutrients from the reuse of cellular proteins and organelles
(31,32). Induced autophagy can contribute to
or enhance the apoptotic response (33), although limited autophagy in
response to nutrient starvation can prevent the activation of
apoptotic pathways (34). Defects
in autophagy have been closely associated with many human diseases,
including cancer, myopathy and neurodegeneration. Autophagy has
also been implicated in the clearance of pathogens and antigen
presentation. In the present study, we found that ghrelin-induced
autophagy enhanced apoptosis and inhibited proliferation, which
indicates that autophagy is beneficial for controlling tumor growth
following ghrelin stimulation. The inhibitory function of mTORC1 in
autophagy is well established (35,36).
Our finding that activation of mTOR by L-leucine inhibited
ghrelin-induced autophagy is consistent with these reports.
In summary, the present study demonstrated that
ghrelin inhibited the proliferation of human ovarian epithelial
carcinoma HO-8910 cells through the induction of apoptosis and
autophagy via the mTOR signaling pathway. The present study
provides a novel regulatory signaling pathway of ghrelin-regulated
ovarian epithelial carcinoma growth and may contribute to ovarian
cancer prevention and therapy.
Acknowledgements
The present study was supported by the Liaoning
Natural Science Foundation (no. 2009225035) and the Shenyang
Science and Technology Foundation (no. F11-262-9-14) to Y.Z.
References
|
1
|
Billottet C, Janji B, Thiery JP and
Jouanneau J: Rapid tumor development and potent vascularization are
independent events in carcinoma producing FGF-1 or FGF-2. Oncogene.
21:8128–8139. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Di Blasio AM, Cremonesi L, Viganó P, et
al: Basic fibroblast growth factor and its receptor messenger
ribonucleic acids are expressed in human ovarian epithelial
neoplasms. Am J Obstet Gynecol. 169:1517–1523. 1993.PubMed/NCBI
|
|
3
|
Chopin L, Walpole C, Seim I, et al:
Ghrelin and cancer. Mol Cell Endocrinol. 340:65–69. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kojima M, Hosoda H, Date Y, Nakazato M,
Matsuo H and Kangawa K: Ghrelin is a growth-hormone-releasing
acylated peptide from stomach. Nature. 402:656–660. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kojima M and Kangawa K: Ghrelin: structure
and function. Physiol Rev. 85:495–522. 2005. View Article : Google Scholar
|
|
6
|
Smith RG, Leonard R, Bailey AR, et al:
Growth hormone secretagogue receptor family members and ligands.
Endocrine. 14:9–14. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Majchrzak K, Szyszko K, Pawlowski KM,
Motyl T and Król M: A role of ghrelin in cancerogenesis. Pol J Vet
Sci. 15:189–197. 2012.PubMed/NCBI
|
|
8
|
Zhang W, Lin TR, Hu Y, et al: Ghrelin
stimulates neurogenesis in the dorsal motor nucleus of the vagus. J
Physiol. 559:729–737. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SW, Her SJ, Park SJ, et al: Ghrelin
stimulates proliferation and differentiation and inhibits apoptosis
in osteoblastic MC3T3-E1 cells. Bone. 37:359–369. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Vriese C and Delporte C: Autocrine
proliferative effect of ghrelin on leukemic HL-60 and THP-1 cells.
J Endocrinol. 192:199–205. 2007.PubMed/NCBI
|
|
11
|
Waseem T, Javaid-Ur-Rehman, Ahmad F, Azam
M and Qureshi MA: Role of ghrelin axis in colorectal cancer: a
novel association. Peptides. 29:1369–1376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ghè C, Cassoni P, Catapano F, et al: The
antiproliferative effect of synthetic peptidyl GH secretagogues in
human CALU-1 lung carcinoma cells. Endocrinology. 143:484–491.
2002.PubMed/NCBI
|
|
13
|
Gnanapavan S, Kola B, Bustin SA, et al:
The tissue distribution of the mRNA of ghrelin and subtypes of its
receptor, GHS-R, in humans. J Clin Endocrinol Metab. 87:29882002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gomez-Pinillos A and Ferrari AC: mTOR
signaling pathway and mTOR inhibitors in cancer therapy. Hematol
Oncol Clin North Am. 26:483–505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lynch CJ: Role of leucine in the
regulation of mTOR by amino acids: revelations from
structure-activity studies. J Nutr. 131:861S–865S. 2001.PubMed/NCBI
|
|
16
|
Rosenfeldt MT and Ryan KM: The multiple
roles of autophagy in cancer. Carcinogenesis. 32:955–963. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mah LY and Ryan KM: Autophagy and cancer.
Cold Spring Harb Perspect Biol. 4:a0088212012.PubMed/NCBI
|
|
18
|
Gaytan F, Morales C, Barreiro ML, et al:
Expression of growth hormone secretagogue receptor type 1a, the
functional ghrelin receptor, in human ovarian surface epithelium,
mullerian duct derivatives, and ovarian tumors. J Clin Endocrinol
Metab. 90:1798–1804. 2005. View Article : Google Scholar
|
|
19
|
Markowska A, Ziółkowska A,
Jaszczyńska-Nowinka K, Madry R and Malendowicz LK: Elevated blood
plasma concentrations of active ghrelin and obestatin in benign
ovarian neoplasms and ovarian cancers. Eur J Gynaecol Oncol.
30:518–522. 2009.PubMed/NCBI
|
|
20
|
Gutierrez JA, Solenberg PJ, Perkins DR, et
al: Ghrelin octanoylation mediated by an orphan lipid transferase.
Proc Natl Acad Sci USA. 105:6320–6325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang J, Brown MS, Liang G, Grishin NV and
Goldstein JL: Identification of the acyltransferase that
octanoylates ghrelin, an appetite-stimulating peptide hormone.
Cell. 132:387–396. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lim CT, Kola B, Grossman A and Korbonits
M: The expression of ghrelin O-acyltransferase (GOAT) in human
tissues. Endocr J. 58:707–710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang W, Chai B, Li JY, Wang H and
Mulholland MW: Effect of des-acyl ghrelin on adiposity and glucose
metabolism. Endocrinology. 149:4710–4716. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hosoda H, Kojima M, Matsuo H and Kangawa
K: Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin
peptide in gastrointestinal tissue. Biochem Biophys Res Commun.
279:909–913. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Inoki K, Corradetti MN and Guan KL:
Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet.
37:19–24. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Q and Guan KL: Expanding mTOR
signaling. Cell Res. 17:666–681. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
LoRusso PM: Mammalian target of rapamycin
as a rational therapeutic target for breast cancer treatment.
Oncology. 84:43–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fuchs Y and Steller H: Programmed cell
death in animal development and disease. Cell. 147:742–758. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bergmann A and Steller H: Apoptosis, stem
cells, and tissue regeneration. Sci Signal. 3:re82010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Meijer AJ: Amino acids as regulators and
components of nonproteinogenic pathways. J Nutr. 133:2057S–2062S.
2003.PubMed/NCBI
|
|
32
|
Levine B and Klionsky DJ: Development by
self-digestion: molecular mechanisms and biological functions of
autophagy. Dev Cell. 6:463–477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Crighton D, Wilkinson S, O’Prey J, et al:
DRAM, a p53-induced modulator of autophagy, is critical for
apoptosis. Cell. 126:121–134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang H, Bosch-Marce M, Shimoda LA, et al:
Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic
response to hypoxia. J Biol Chem. 283:10892–10903. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jung CH, Ro SH, Cao J, Otto NM and Kim DH:
mTOR regulation of autophagy. FEBS Lett. 584:1287–1295. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang YY, Juhász G, Goraksha-Hicks P, et
al: Nutrient-dependent regulation of autophagy through the target
of rapamycin pathway. Biochem Soc Trans. 37:232–236. 2009.
View Article : Google Scholar : PubMed/NCBI
|