Introduction
Gliomas are the most common primary tumors that
arise from glial cells, which still remain incurable despite
treatments including surgical resection, radiotherapy and
chemotherapy. They are considered to be highly invasive, metastatic
and lacking apoptotic cell death. Thus, new treatment strategies
for gliomas are urgently needed.
Nogo-A was first recognized as a myelin-associated
neuronal growth inhibitor after spinal cord injury (1). Molecular cloning of Nogo-A led to the
identification of a neuronal surface glycosylphosphatidylinositol
(GPI)-linked receptor that binds to the 66 amino acid residue
luminal/extracellular domain of Nogo-A (Nogo-66), termed the
Nogo-66 receptor (NgR) (2). Nogo-66
and NgR have been mainly studied for their capabilities to block
axon regeneration and neurite outgrowth. However, their functions
in the normal central nervous system (CNS), especially in the
developing CNS, are also receiving great interest.
In addition to their expression in oligodendrocytes
and neurons, Nogo-A and NgR are detected in glioma cells (3,4).
Although Nogo-A has been intensively studied for its inhibitory
effect on axonal regeneration in the adult CNS, the functions of
Nogo-A and NgR in tumors remain largely elusive. Liao et al
have shown that substratum adherence and migration by human U87MG
glioma cells in culture were significantly attenuated by the
extracellular domains of Nogo-66 and myelin-associated glycoprotein
(MAG) (3). U87MG cells contain
large amounts of endogenous NgR and treatment of these cells with
NgR antibodies results in an increase in their ability to adhere
to, or migrate through, Nogo-66- and MAG-coated substrates.
Therefore, Nogo-66 and MAG may modulate glioma growth and migration
by acting through NgR.
Kilic et al suggest that supression of Nogo-A
led to a decrease in neuronal survival, but the association between
downregulation of NgR and tumor apoptosis remain unexplored
(5).
In the present study, by using an RNA interference
method with C6 cells, NgR expression was downregulated and the
effects of NgR knockdown on tumor apoptosis, migration and cell
adhesion in vitro were investigated. The results implied
potentially important roles for Nogo-66 and NgR in the prevention
of tumor invasion and metastasis as well as in the promotion of
tumor apoptosis.
Materials and methods
Cell lines and culture conditions
Rat C6 glioma cells were cultured in Dulbecco’s
modified Eagle’s medium (DMEM) containing 10% fetal bovine serum,
as described previously (6). Cells
were cultured at 37°C in an incubator containing 5% CO2
and 100% humidity. Cells in the mid-log phase were used for
experiments.
Immunofluorescence staining
C6 cells were grown on coverslips in 24-well culture
plates for 24 h. After fixation for 10 min in 4% formalin or
paraformaldehyde, cells were permeabilized with methanol for 2 min
at room temperature. After fixation/permeabilization, the slides
were rinsed with phosphate-buffered saline (PBS), blocked with 1%
bovine serum albumin (BSA) in PBS for 1 h at room temperature or
overnight at 4°C and incubated with primary antibody (1:500;
anti-Nogo-66 receptor, Santa Cruz Biotechnology). The cells were
washed again with PBS and incubated for 1 h at room temperature
with FITC-conjugated secondary antibody (1:200 dilution). Slides
were washed and rinsed as described above and then mounted in
anti-quenching medium (Sigma, St. Louis, MO, USA). The stained and
mounted slides were stored in the dark at 4°C and fluorescence was
visualized with a laser confocal microscope (FV500; Olympus, Tokyo,
Japan).
Small hairpin RNA (shRNA) design and
transfection
To knock down NgR expression, a vector-based short
hairpin RNA (shRNA) expression system was used. The shRNA vector
(pGenesil1.1) was purchased from Wuhan Genesil Biotechnology Co.,
Ltd. The designation of 21 nucleotide target sequences was based on
a computer algorithm and 5′-AATCTCACCATCCTGTGGCTG-3′ was selected
as the target sequence. The negative control was pGenesil1.1
containing a non-effective scrambled shRNA. For transfection, C6
cells were seeded in 6-well plates to 80% confluency and
transfected with 4 μg of plasmid DNA using Lipofectamine 2000
(Invitrogen, Carlsbad, CA, USA). RNA and proteins from scrambled
shRNA- or shRNA-transfected cells were analyzed 72 h after
transfection.
Detection of NgR mRNA by reverse
transcription polymerase chain reaction (RT-PCR)
Total RNA extraction and cDNA amplification were
performed as described previously (7). The following oligonucleotides were
used for RT-PCR analysis: NgR: 5′-AATGAGCCCAAGGTCACAA-3′ (sense),
5′-CCATGCAGAAAGAGATGCGT-3′ (antisense); β-actin:
5′-GAGAGGGAAATCGTGCGTGAC-3′ (sense), 5′-CAT CTGCTGGAAGGTGGACA-3′
(antisense). The PCR conditions were as follows: predenaturation at
94°C for 10 min; denaturation at 94°C for 50 sec, annealing at 59°C
for 50 sec and extension at 72°C for 1 min; and a final incubation
at 72°C for 7 min. The amplified products were resolved on 1%
agarose by performing homeothermic gel electrophoresis at 80 V for
30 min. The bands were excised and eluted from the gel, purified,
precipitated overnight with ethanol and sequenced. The
electrophoresis results were observed under an ultraviolet lamp and
a density scan of the positive bands was performed. Then, the
refractive index (RI) of NgR mRNA was calculated using the formula
RI = NgR mRNA density/β-actin density × 100%.
Western blot analysis
Cells were lysed with RIPA buffer, electrophoresed
on a 10% SDS-PAGE gel at 100 V for 2 h and electroblotted onto a
polyvinylidene fluoride membrane at 275 mA for 2 h. The membrane
was incubated with 5% fat-free milk in PBS for 2 h at room
temperature. Then, the membrane was incubated in rabbit anti-NgR
primary antibody (1:200; Santa Cruz Biotechnology), rabbit
anti-cleaved caspase-3 mAb (1:300; Cell Signaling Technology), or
mouse anti-β-actin primary antibody (1:1,000; Abcam) overnight at
4°C. After the membranes were washed with PBS three times, they
were incubated in horseradish peroxidase-conjugated anti-rabbit IgG
or anti-mouse IgG (1:5,000; Pierce Chemical Co.) for 1 h at room
temperature. The samples were washed three times with PBS and
developed with an enhanced chemiluminescence reagent (Thermo).
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT)
assay
The proliferation rates of sh-NgR cells and control
cells were measured by an MTT assay. Briefly, NgR-targeted cells or
control cells were plated at an initial density of
5×103/well in 96-well plates and incubated for 1, 2, 3,
4 or 5 days in complete culture medium. Twenty microliters of MTT
(5 mg/ml) (Sigma) was added and the cells were incubated for 4 h.
After the entire medium was discarded, 150 μl of dimethyl sulfoxide
was added into each well. The optical density was determined in a
microplate reader at 490 nm with subtraction of the baseline
reading. Each time-point was repeated six times.
Cell-matrix adhesion assay
The adhesion assay was done as described previously
(8). Briefly, a 96-well microtiter
plate was coated with Matrigel (50 μl/well) (Vigorous
Biotechnology, Beijing, China) for 1 h at 37°C and then blocked
with BSA (10 mg/ml). After being treated with Nogo-66 (15 μg/ml)
(Biosynthesis Biotechnology, Beijing, China) or IgG (10 μg/ml) for
2 h at 37°C, C6 cancer cells were then seeded onto these
components. The cells were allowed to adhere to each well for 1 h
at 37°C and were gently washed three times with PBS. The adhesion
of C6 cancer cells to the extracellular components was counted at
five fields per well at random under a microscope. All experiments
were performed in triplicate.
Cell invasion assay
The invasion activity of C6 cancer cells was
measured by the previously reported method with some modifications
(6). Briefly, C6 cells
(1.25×105 per well) were seeded in the upper chamber
separated with an 8-μm membrane filter coated with 15 μg/ml Nogo-66
or IgG (10 μg/ml), which was diluted into extracellular matrix
(ECM, Sigma). Medium in the upper chamber was supplemented with 10%
fetal bovine ferum (FBS). In the lower chamber, the FBS
concentration was 20%. After incubation for 72 h at 37°C, C6 cells
invading the lower chamber were manually counted under a
microscope. Six randomly selected fields were counted for each
assay. Mean values from six fields were calculated as sample
values. For each group, the cell culture was performed in
triplicate.
Scratch wound migration assay
C6 cells were grown to confluency in 35-mm wells
(3×105 cells/well). Wounds were scratched using a 200 μl
pipette tip and cells were washed three times with PBS. Serum-free
DMEM was then added. Triplicate wells were used per condition and
three fields per well were captured at each time-point over a
period of 24 h. Scratch wound assays were performed for cells from
three independent infections. For experiments in which Nogo-66 was
used, confluent monolayers were treated with 15 μg/ml Nogo-66
before wound initiation.
Wounds were visualized using a Nikon Eclipse TS100
microscope (Nikon, Kingston Upon Thames, UK), images were captured
using a Coolpix 4500 camera (Nikon) and cells migrating through a
central strip were counted in each group.
Flow cytometry of cells
Spontaneous apoptosis of C6 cells was examined by an
Annexin V-FITC/propidium iodide (PI) Apoptosis Detection kit
(BestBio, China). After shRNA vector was transfected for 48 h,
cells were washed with ice-cold PBS and resuspended in 400 μl of 1X
binding buffer and the cells were stained with 5 μl of Annexin V
and 5 μl of PI for 15 min at 4°C in the dark. Then, the stained
cells were analyzed by fluorescence-activated cell sorting (BD
Biosciences, USA) using CellQuest Research Software (BD
Biosciences).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
To observe the extent of spontaneous apoptosis,
cells were cultured on coverslips in 6-well culture plates. Cells
were fixed with 4% formalin for 10 min at 4°C and then
permeabilized with 0.1% Triton-X 100 for 5 min, incubated with
reagents from a fluorescein-conjugated TUNEL kit (Roche, Canada)
for 1 h at 37°C and stained with Hoechst 33342 dye (5 μg/ml) for 15
min at room temperature. After washing with PBS, the number of
apoptotic cells was visualized by using a laser scanning confocal
microscope (FV500; Olympus, Tokyo, Japan) from five random
fields.
Statistical analysis
Statistical analysis was performed using Statistical
Package for the Social Sciences (SPSS; version 11.5; Bizinsight,
Beijing, China). P<0.05 was considered statistically
significant. Intergroup data were compared using analysis of
variance (ANOVA). The quantities of NgR mRNA were consistent with
normal distributions and were analyzed using the
Student-Newman-Keul’s (SNK) test.
Results
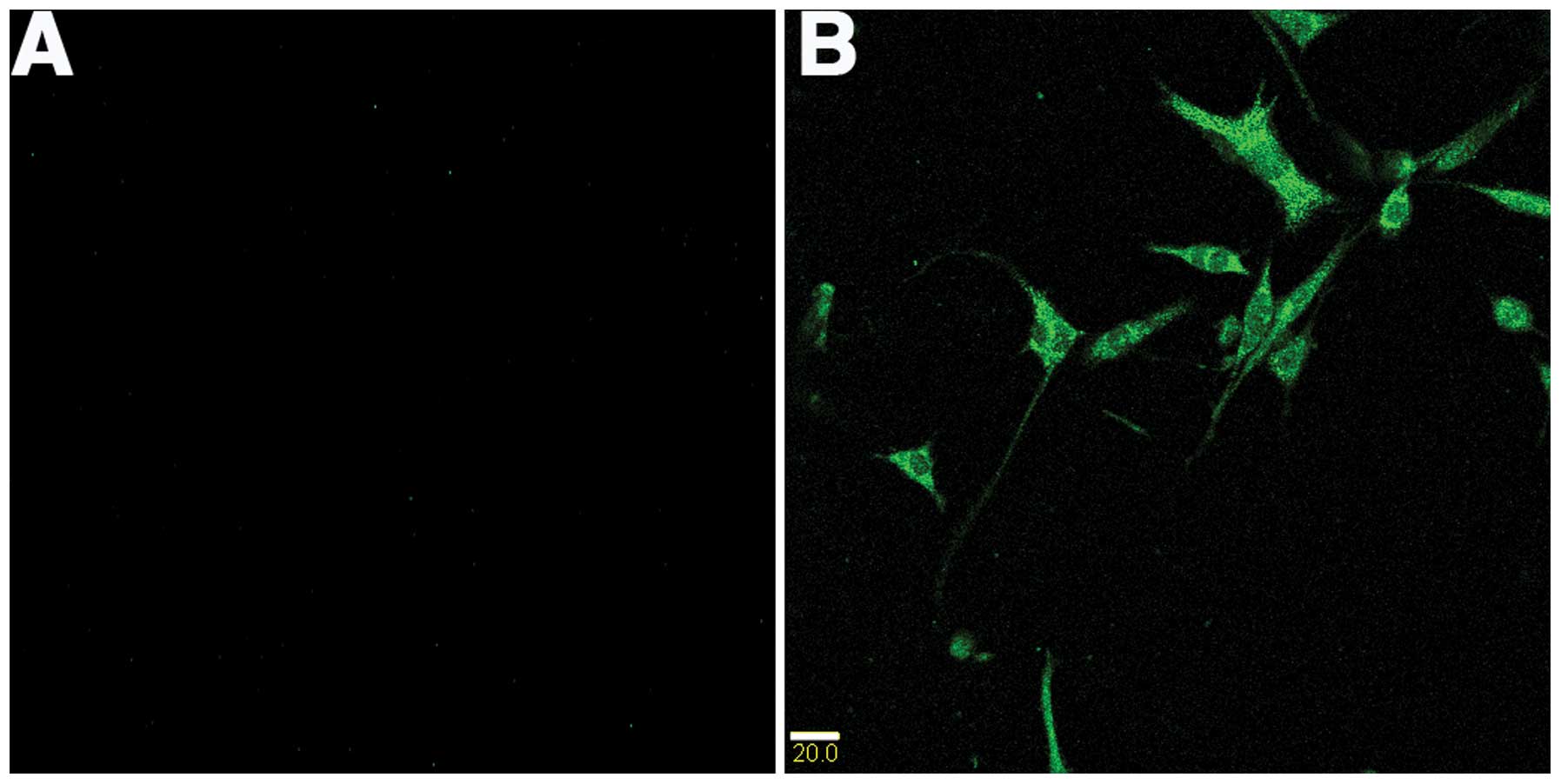
NgR is highly expressed in C6 cells
Immunofluorescence was used to identify the presence
of NgR in C6 cells. The immunofluorescence experiment was performed
with anti-rabbit NgR antibody. As expected, strongly positive
cytoplasmic NgR staining was evident in C6 tumor cell lines
(Fig. 1).
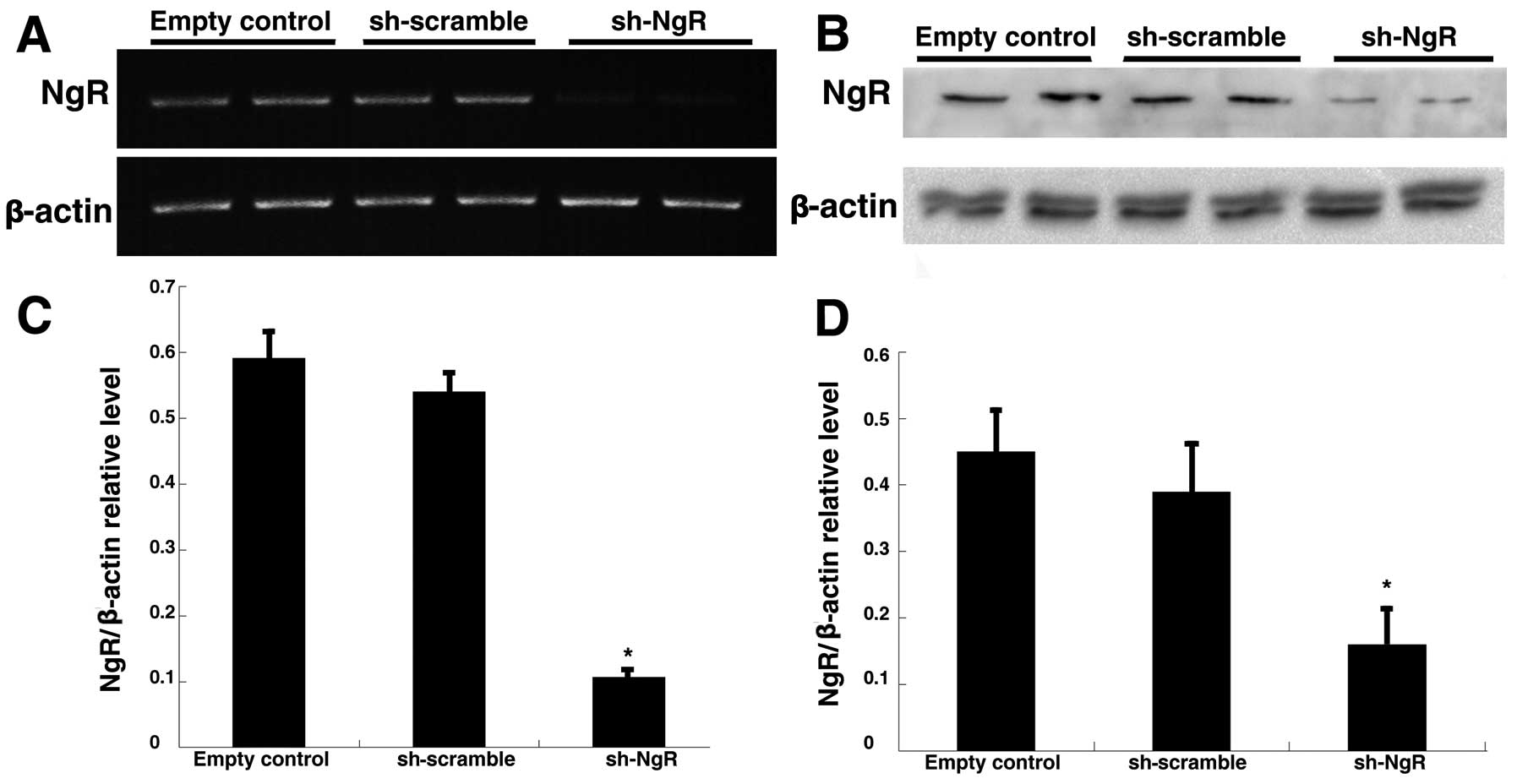
Downregulation of NgR expression by shRNA
in C6 cell lines
RT-PCR analysis was used to detect the changes of
NgR mRNA expression after transfection. A 450-bp band was observed
in the blank control, the scrambled shRNA group and the NgR shRNA
group; and the relative densities of the NgR band compared with the
β-actin band were 0.591±0.038, 0.539±0.028 and 0.105±0.009,
respectively. There was no significant difference in the density of
the NgR mRNA band between the blank control and the scrambled shRNA
groups; whereas compared with the blank control, the density of the
band in the shRNA group was dramatically less (Fig. 2A and C). Similar to the RT-PCR
results, expression of the NgR protein in each group was also
observed by western blotting and the relative densities of the NgR
band compared with the β-actin band were 0.446±0.059, 0.389±0.085
and 0.162±0.048, respectively (Fig. 2B
and D). These data indicate that NgR shRNA can inhibit NgR mRNA
and protein expression after transfection into C6 cells.
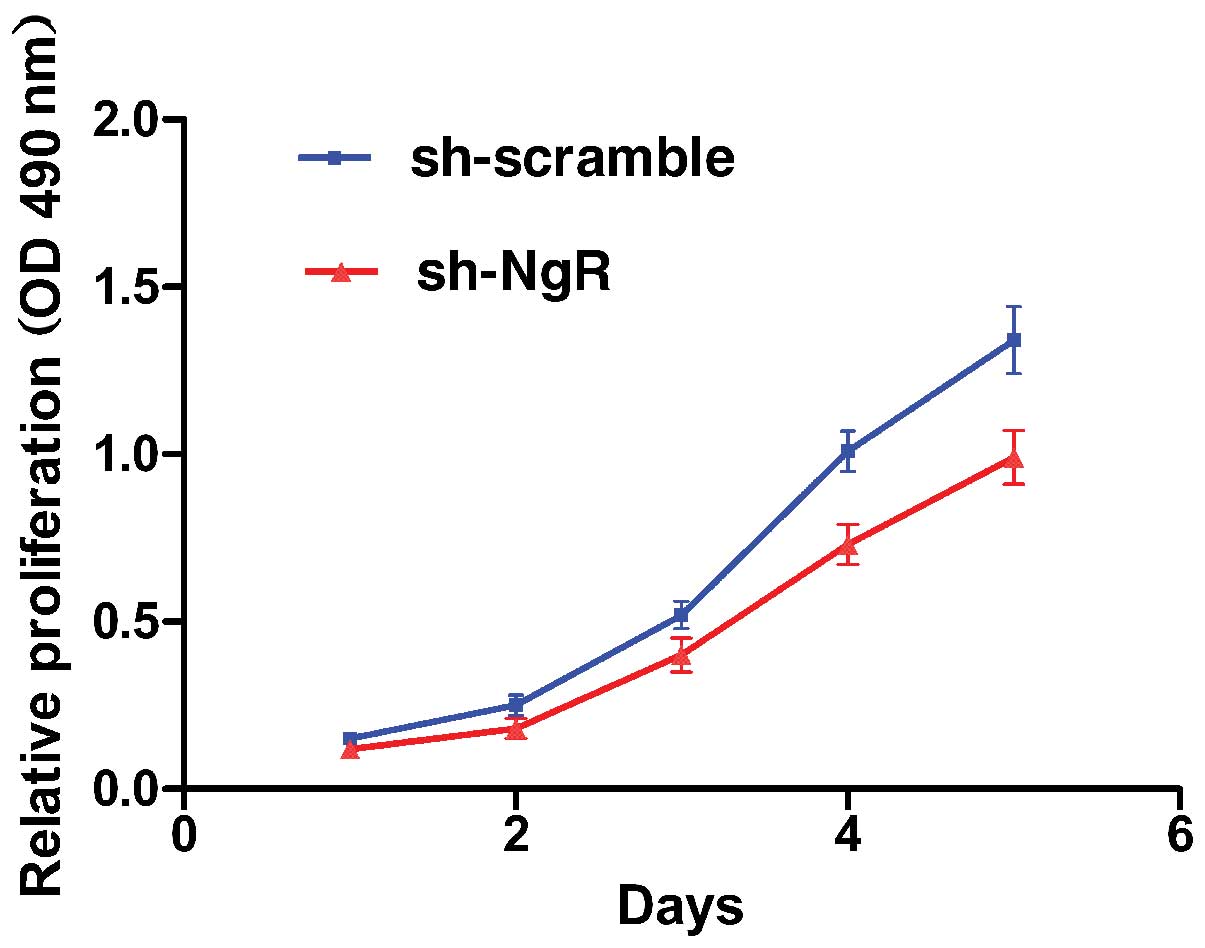
shRNA against NgR suppressed
proliferation of C6 in vitro
Cell proliferation after NgR silencing was assessed
by an MTT assay. As shown in Fig.
3, the sh-NgR cells had a lower proliferation rate than the
sh-scramble cells. Knockdown of NgR led to a decrease in C6 cell
growth at 3 (23.0%), 4 (27.7%) and 5 days (26.1%) (P<0.05),
compared with the scramble control. These results demonstrate that
NgR silencing inhibits the proliferation of C6 cells.
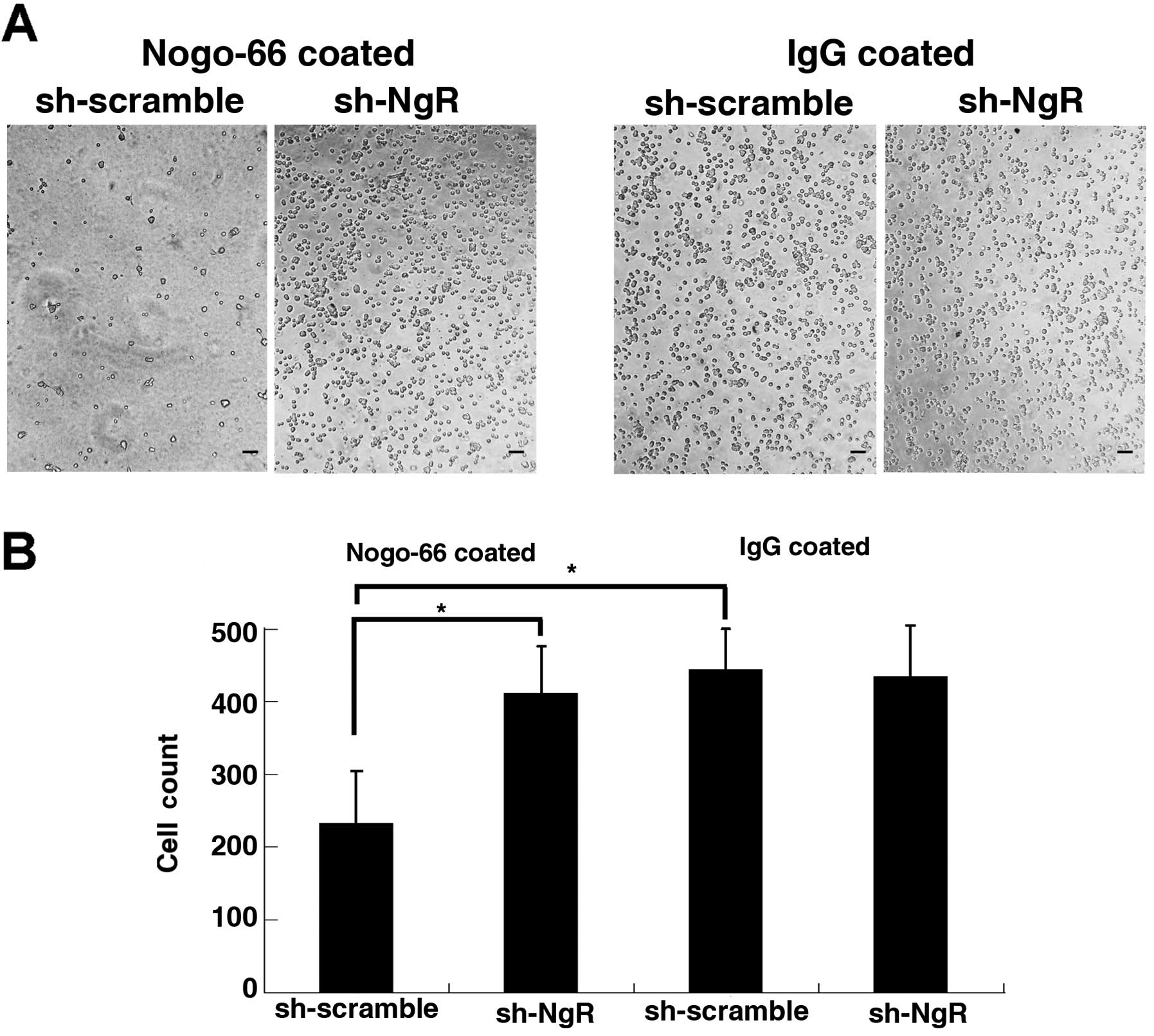
shRNA against NgR enhances cell adhesion
of C6 cells in vitro
In the Nogo-66-coated experiment, 1 h after seeding,
the number of adherent living cells was significantly increased in
the NgR knockdown group compared to the C6 scramble control group
(Fig. 4). As a result, the total
number of cells increased in the NgR knockdown group. In the
IgG-coated experiment, 1 h after seeding, the number of adherent
living cells in the NgR knockdown group was not significantly
different than that in the C6 control group. These results suggest
that NgR knockdown in C6 cells enhances cell adhesion to
Nogo-66-coated Matrigel but does not affect cell adhesion to
IgG-coated Matrigel.
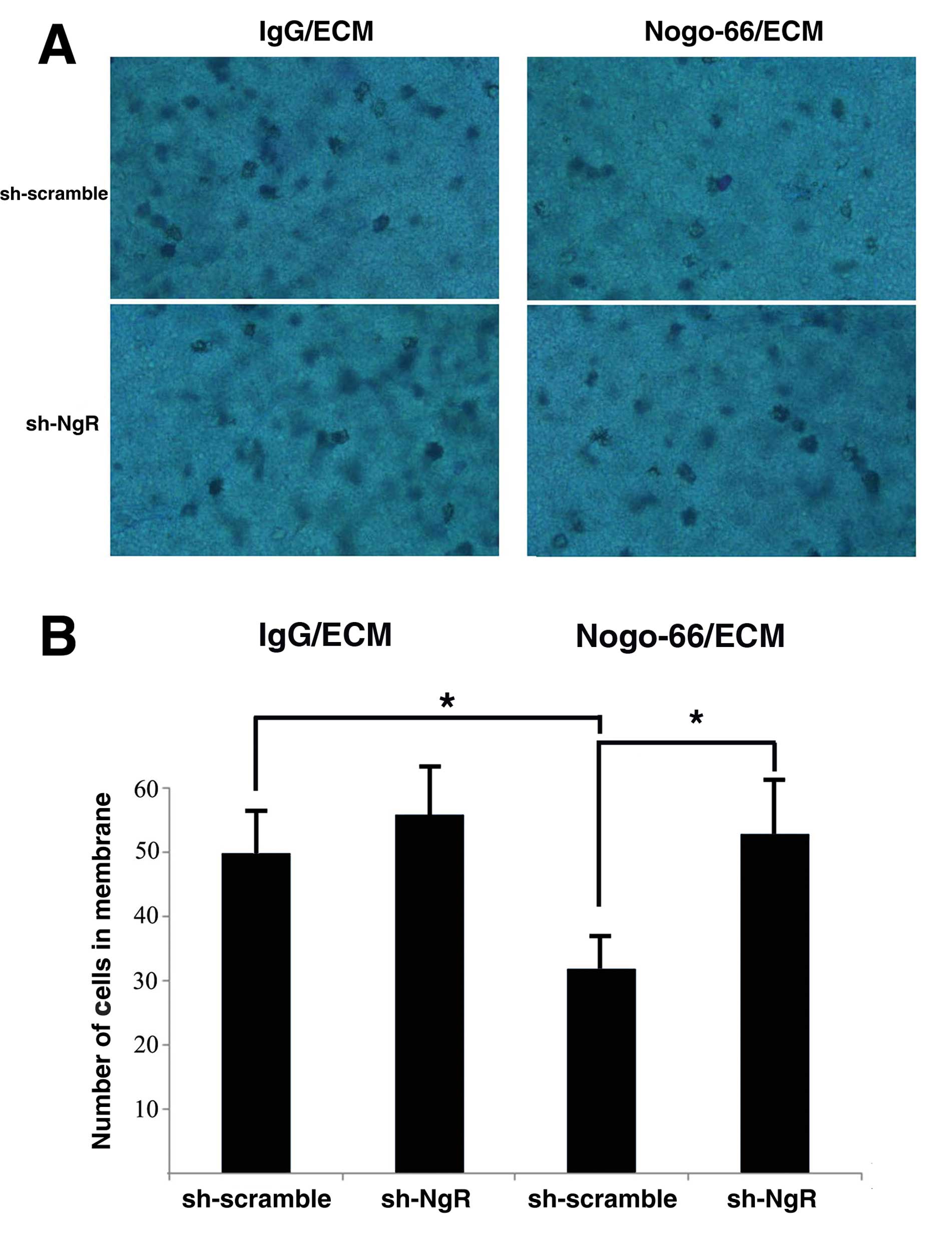
shRNA against NgR enhanced cell invasion
of C6 cells in vitro
We tested whether NgR knockdown affected the
invasion capability of C6 cells by using a Boyden chamber assay.
Cells were seeded in the upper part of a Nogo-66/ECM-coated
invasion chamber containing a low (10%) FCS concentration. After 72
h, cells that migrated in the lower chamber containing a higher
(20%) FCS concentration were stained and counted. In NgR-shRNA C6
cell lines, the number of cells in the membrane was significantly
greater than that of the scramble control (Fig. 5). In the IgG/ECM-coated invasion
chamber, no further decrease in invasion was observed between
NgR-shRNA cells and control cells. These results indicate that
invasion through the Nogo-66-coated membrane was significantly
enhanced by NgR knockdown in C6 cells but did not affect cell
adhesion to the IgG/ECM-coated membrane.
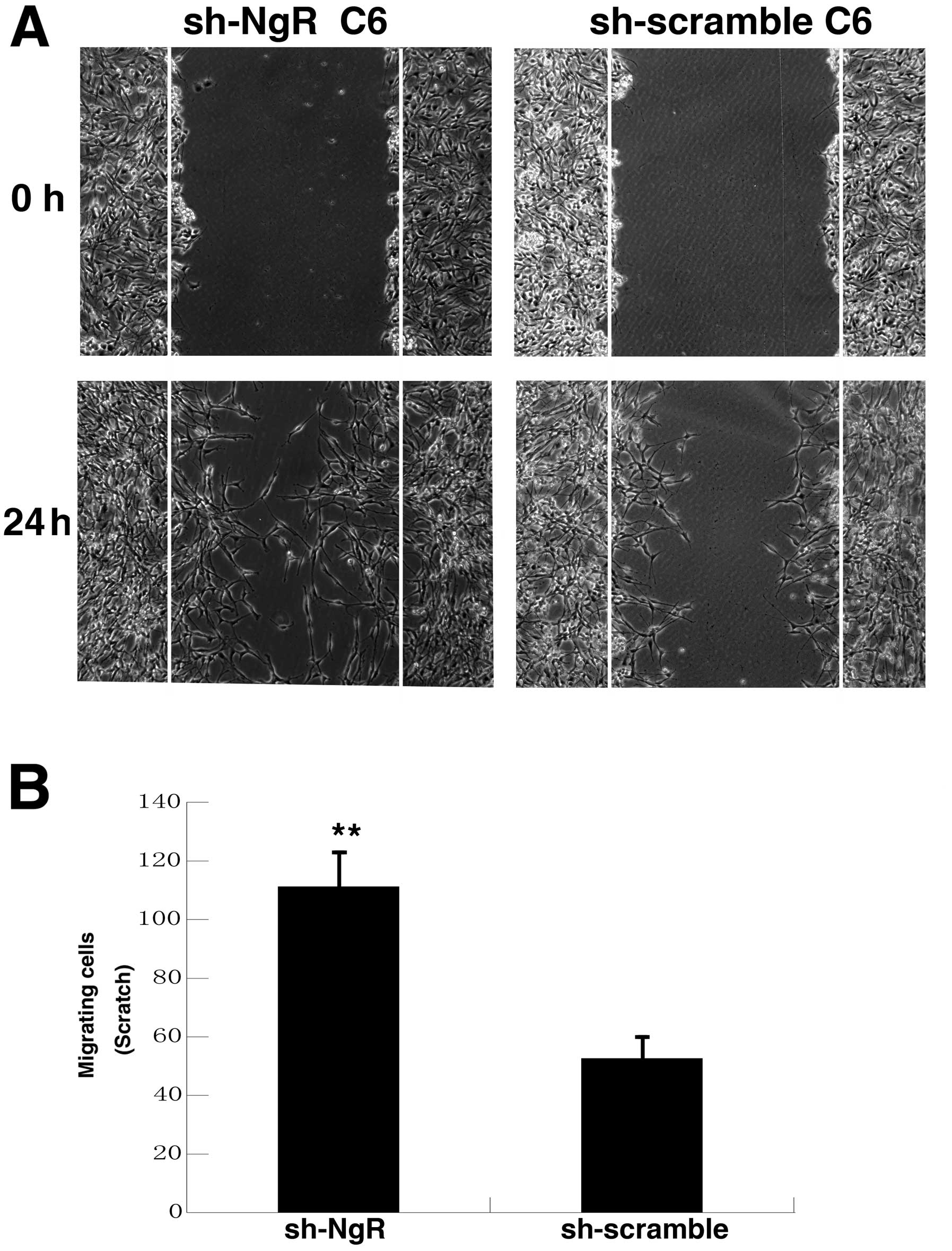
To further test whether NgR knockdown affected the
invasion capability of C6 cells, a scratch wound migration assay
was used. A wound was scratched in a confluent monolayer of
NgR-shRNA cells and scramble control cells coated with Nogo-66
(Fig. 6). The edge of the wound was
monitored over 24 h and images of the same fields were taken 0 and
24 h later. The width of the wound in NgR-shRNA C6 cells was
significantly narrower than that in the control group. This result
indicates that the invasion ability of C6 cells to Nogo-66-coated
coverslips was significantly enhanced by NgR knockdown.
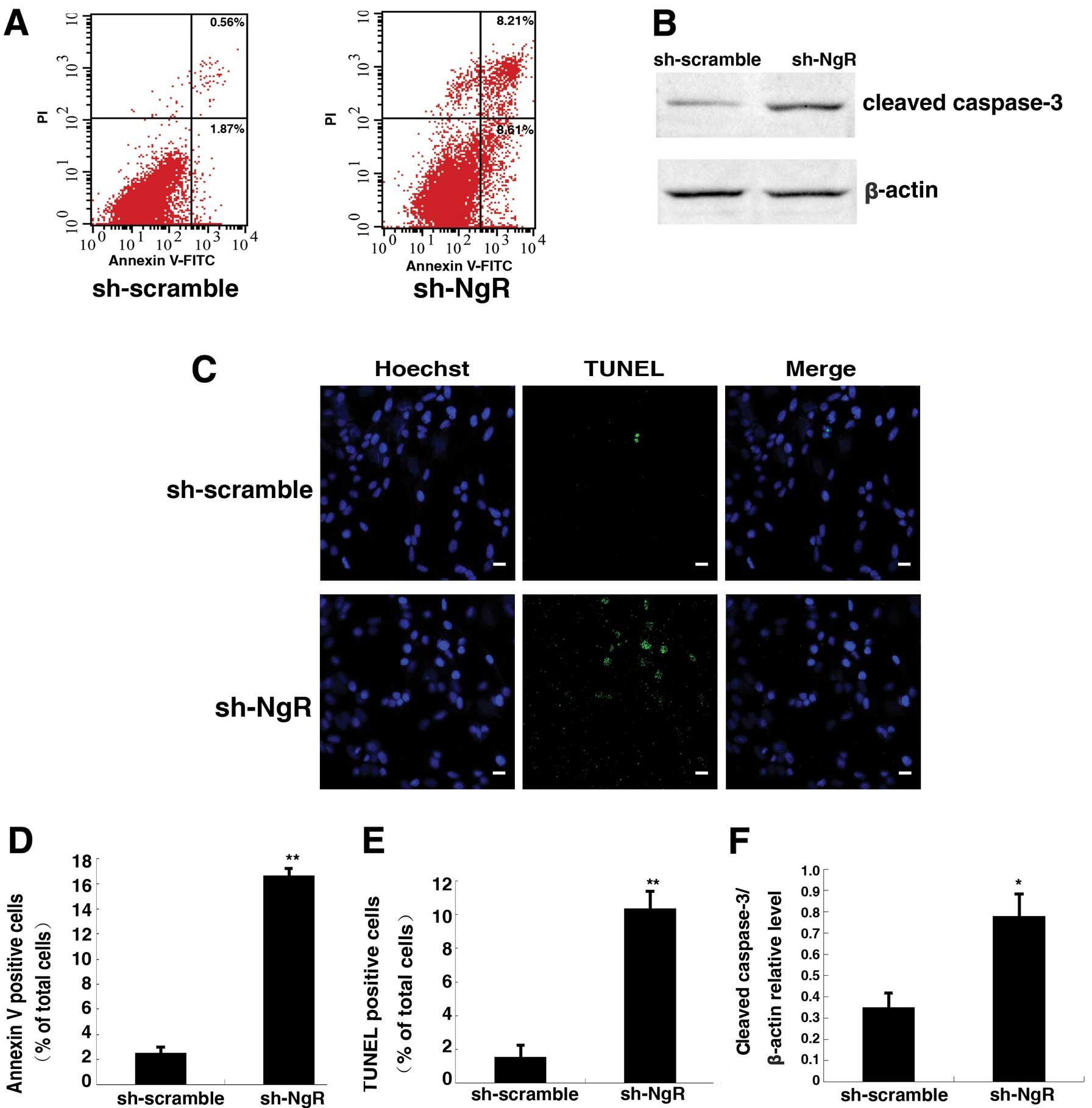
shRNA against NgR increased apoptosis of
C6 cells in vitro
C6 cells stained with the double stain Annexin V/PI
were analyzed by flow cytometry (Fig.
7A and D). The apoptotic rate of C6 cells was 3.21±1.29% in the
scramble control group. After NgR knockdown for 48 h, the rate of
apoptosis increased to 16.63±0.58%. Thus, the rate of apoptosis in
NgR knockdown cells showed a notable increase compared to controls.
NgR knockdown in C6 cells significantly increased the expression of
caspase-3 (active form) when compared with the scramble control
group (Fig. 7B and F). The
increased apoptosis rate in NgR knockdown-C6 cells was also evident
from the TUNEL-positive staining results (Fig. 6C and E). The rate of TUNEL-positive
cells in sh-NgR-C6 cells was 10.21±1.04%, whereas that in the
control group was 1.43±0.69%. These results suggest that knockdown
of NgR increased the apoptotic rate of C6 cells.
Discussion
Glioma is the most common and aggressive form of the
brain tumors. Despite advances in surgical and clinical
neuro-oncology, malignant glioma prognosis remains poor due to its
diffusive and invasive nature (9).
A key contributor to the pathology of brain tumors is their ability
to diffusely infiltrate the parenchyma. Surgical excision of a
malignant brain neoplasm is not sufficient to eliminate neoplastic
cells that have infiltrated normal adjacent brain. Therefore, it is
of primary importance to determine the mechanisms involved in brain
tumor cell adhesion and invasion (10).
The invasion of glioma cells depends upon multiple
factors, including ECM molecules, growth factors and the activity
of intracellular pathways regulating cell motility (11). The brain ECM, composed of
cytotactin/tenascin-C, laminin, thrombospondin, vitronectin and
fibronectin, is synthesized and secreted by astrocytes during
development and is involved in adhesion and migration of neurons
and glia during development (12,13).
In vitro studies using glioma cell lines indicate that brain
tumor cells can use some of these ECM molecules as favorable
substrates for migration (14,15).
We previously investigated the expression of NgR
protein in human astrocytoma (4).
The results showed that the expression of NgR protein markedly
decreased with increasing pathological grades. Double
immunostaining results showed that Nogo-A and NgR were colocalized
on the surface of tumor cells in astrocytoma tissues. Our results
confirmed that the distribution of Nogo-A and NgR at the interface
of the tumor cells was negatively related to tumor malignancy and
supported the hypothesis that Nogo-A restricts migration of tumor
cells. Therefore, NgR may have inhibitory effects on tumor activity
and may also be an attractive therapeutic target for human
astrocytic tumors.
In the present study, we studied the possible roles
of Nogo-66 and NgR in tumor cell migration in vitro.
Immunofluorescence studies showed the presence of NgR in C6 cells.
Our results revealed that NgR-deficient cells had an increased
ability of adhesion and invasion in the presence of Nogo-66,
whereas there were no changes of adhesion and invasion without
Nogo-66. The data suggest that Nogo-A acts as a negative regulator
or ‘brake’ for C6 tumor cells. In addition, this effect may be
mediated by extracellular Nogo-66 via its receptor NgR. A similar
result was obtained by Amberger et al, who found that
low-grade glioma cell lines as well as primary cultures were
strongly sensitive to the inhibitory proteins present in CNS myelin
(16). In contrast, high-grade
glioma cell lines were able to spread and migrate on CNS
myelin-coated culture dishes, demonstrating that within the
gliomas, the ability to overcome the inhibitory effects of CNS
myelin is correlated with the grade of malignancy of the original
tumor. These results suggest that myelin was involved in the
process of invasive migration of malignant gliomas along CNS white
matter fiber tracts.
The nerve tract in the tumor area usually cannot be
eroded and discontinued even though the tract is severely
compressed by a large tumor entity. The reason why the tract is
seldom eroded is not known. The results that myelin molecules in
the nerve tract had the ability to inhibit tumor invasion can
explain this phenomenon.
Various studies have shown that the poor prognosis
of tumors is not only due to over invasion and metastasis but also
due to the loss of natural apoptosis (17). Apoptosis is also a crucial mechanism
for the control of cell proliferation and increased apoptosis
results in glioma cells that are sensitive to chemotherapy and
radiation therapy (18). Our data
suggest that the downregulation of NgR can decrease the
proliferation of C6 cells. Cell apoptosis measured by flow
cytometry and TUNEL assays showed that NgR-deficient cells
increased the apoptosis of C6 cells. Apoptotic changes in
morphology such as the increased ratio of chromatin accumulation,
nuclear condensation and DNA fragmentation were also observed in
the present study. In corroboration with these results, shNgR
treatment resulted in the elevation of cleaved caspase-3 in C6
cells. Silencing of NgR increased the spontaneous apoptosis of C6
cells 3-fold, compared to control cells. Kilic et al found
that Nogo-A and its receptor blockade led to decreased neuronal
survival (5). Our results were
similar to the report by Kilic et al. However, the detailed
mechanism regarding the effect of NgR knockdown on cell apoptosis
was not investigated and there has been no research describing the
effect of NgR knockdown on spontaneous apoptosis in glioma
cells.
Apoptosis typically involves a series of events that
are associated with Bcl-2 family members regulating mitochondrial
release of cytochrome c and activation of a cascade of
caspases and various endoplasmic reticulum stresses, finally
leading to the fragmentation of chromosomal DNA (19,20).
In addition, apoptosis is directly modulated by different members
of the Ras and Rho GTPase superfamilies. Furthermore, Kilic et
al evaluated injured neuronal cells by a TUNEL assay and showed
that Nogo-A blockade decreased neuronal survival through
deactivation of the small GTPases RhoA, Rac1 and RhoB (5). NgR in glioma cells might act as a
tumor apoptosis inhibitor via RhoA deactivation. In the future, we
will undertake in-depth research to confirm our hypothesis.
Recently, it has been reported that Nogo-66 inhibits
adhesion and migration of microglia via RhoA activation and Cdc42
deactivation, but the signal transduction pathways involved in RhoA
triggered by Nogo-66/NgR require more detailed research (21).
In conclusion, we found that Nogo-66 and NgR have
been well-studied for their inhibitory functions on axon growth in
the adult CNS and play a role in the regulation of tumor C6 cell
migration, invasion and apoptosis in vitro. However, many
mechanistic details remain to be studied. For example, whether the
effects of Nogo-66 and NgR on migration are mediated through a
receptor complex that includes Lingo-1 and p75 remains to be
determined. In particular, whether Rho-A is involved in the
signaling pathway downstream of NgR is not known. The precise
mechanism of NgR knockdown on the effect of apoptosis in tumor
cells also remains to be elucidated.
Acknowledgements
This study was supported by research grants from the
National Nature Science Foundation of China (30471775, 30801180)
and the Hubei Research Development Project Foundation
(2005AA301C15).
References
|
1
|
Chen MS, Huber AB, van der Haar ME, Frank
M, Schnell L, Spillmann AA, Christ F and Schwab ME: Nogo-A is a
myelin-associated neurite outgrowth inhibitor and an antigen for
monoclonal antibody IN-1. Nature. 403:434–439. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fournier AE, GrandPre T and Strittmatter
SM: Identification of a receptor mediating Nogo-66 inhibition of
axonal regeneration. Nature. 409:341–346. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liao H, Duka T, Teng FY, Sun L, Bu WY,
Ahmed S, Tang BL and Xiao ZC: Nogo-66 and myelin-associated
glycoprotein (MAG) inhibit the adhesion and migration of Nogo-66
receptor expressing human glioma cells. J Neurochem. 90:1156–1162.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiong N, Shen J, Li S, Li J and Zhao H:
Expression of Nogo-66 receptor in human astrocytoma is correlated
with tumor malignancy. Mol Biol Rep. 39:2625–2632. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kilic E, ElAli A, Kilic U, Guo Z, Ugur M,
Uslu U, Bassetti CL, Schwab ME and Hermann DM: Role of Nogo-A in
neuronal survival in the reperfused ischemic brain. J Cereb Blood
Flow Metab. 30:969–984. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Albini A, Iwamoto Y, Kleinman HK, Martin
GR, Aaronson SA, Kozlowski JM and McEwan RN: A rapid in vitro assay
for quantitating the invasive potential of tumor cells. Cancer Res.
47:3239–3245. 1987.PubMed/NCBI
|
|
7
|
Kawajiri H, Yashiro M, Shinto O, Nakamura
K, Tendo M, Takemura S, Node M, Hamashima Y, Kajimoto T, Sawada T,
et al: A novel transforming growth factor beta receptor kinase
inhibitor, A-77, prevents the peritoneal dissemination of scirrhous
gastric carcinoma. Clin Cancer Res. 14:2850–2860. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nishimura S, Chung YS, Yashiro M, Inoue T
and Sowa M: Role of alpha 2 beta 1- and alpha 3 beta 1-integrin in
the peritoneal implantation of scirrhous gastric carcinoma. Br J
Cancer. 74:1406–1412. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dasari VR, Velpula KK, Kaur K, Fassett D,
Klopfenstein JD, Dinh DH, Gujrati M and Rao JS: Cord blood stem
cell-mediated induction of apoptosis in glioma downregulates
X-linked inhibitor of apoptosis protein (XIAP). PLoS One.
5:e118132010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Friedlander DR, Zagzag D, Shiff B, Cohen
H, Allen JC, Kelly PJ and Grumet M: Migration of brain tumor cells
on extracellular matrix proteins in vitro correlates with tumor
type and grade and involves alphaV and beta1 integrins. Cancer Res.
56:1939–1947. 1996.PubMed/NCBI
|
|
11
|
Yang HW, Menon LG, Black PM, Carroll RS
and Johnson MD: SNAI2/Slug promotes growth and invasion in human
gliomas. BMC Cancer. 10:3012010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eroglu C: The role of astrocyte-secreted
matricellular proteins in central nervous system development and
function. J Cell Commun Signal. 3:167–176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Irintchev A, Rollenhagen A, Troncoso E,
Kiss JZ and Schachner M: Structural and functional aberrations in
the cerebral cortex of tenascin-C deficient mice. Cereb Cortex.
15:950–962. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giese A, Loo MA, Rief MD, Tran N and
Berens ME: Substrates for astrocytoma invasion. Neurosurgery.
37:294–302. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giese A, Rief MD, Loo MA and Berens ME:
Determinants of human astrocytoma migration. Cancer Res.
54:3897–3904. 1994.PubMed/NCBI
|
|
16
|
Amberger VR, Hensel T, Ogata N and Schwab
ME: Spreading and migration of human glioma and rat C6 cells on
central nervous system myelin in vitro is correlated with tumor
malignancy and involves a metalloproteolytic activity. Cancer Res.
58:149–158. 1998.PubMed/NCBI
|
|
17
|
Matsushita S, Nitanda T, Furukawa T,
Sumizawa T, Tani A, Nishimoto K, Akiba S, Miyadera K, Fukushima M,
Yamada Y, et al: The effect of a thymidine phosphorylase inhibitor
on angiogenesis and apoptosis in tumors. Cancer Res. 59:1911–1916.
1999.PubMed/NCBI
|
|
18
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rossi L, De Martino A, Marchese E,
Piccirilli S, Rotilio G and Ciriolo MR: Neurodegeneration in the
animal model of Menkes’ disease involves Bcl-2-linked apoptosis.
Neuroscience. 103:181–188. 2001.
|
|
20
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan J, Zhou X, Guo JJ, Mao L, Wang YJ, Sun
J, Sun LX, Zhang LY, Zhou XF and Liao H: Nogo-66 inhibits adhesion
and migration of microglia via GTPase Rho pathway in vitro. J
Neurochem. 120:721–731. 2012. View Article : Google Scholar
|