Introduction
Gastric cancer is one of the most lethal cancers in
Asia and causes approximately 800,000 deaths worldwide each year,
making it the second leading cause of cancer-related mortality in
the world (1–3). Gastric cancer is thought to arise from
a sequence of multistep processes involving a variety of genetic
events (4,5). Despite advances in multimodality
treatments including targeted therapies, the clinical outcome of
gastric cancer still remains poor due to de novo or
acquisition of chemoresistance during therapy (6–8).
Moreover, the overall 5-year survival rate is less than 35% due to
the high rate of relapse after gastrectomy (9). Thus, understanding the molecular
characteristics of gastric cancer and the development of
chemoresistance is urgently needed in order to improve the clinical
outcome of gastric cancer patients and to develop more effective
treatment strategies.
The Hippo signaling pathway, also known as the
Salvador-Warts-Hippo pathway, was originally discovered in
Drosophila melanogaster(10–12).
The pathway core components in the fly, including Hippo, Sav, Wts,
Yki and Mats, are highly conserved in mammals as Mst1/2, WW45,
LATS1/2, YAP and Mob1, respectively (11,13,14).
Recently, several studies have clearly shown the role of the Hippo
pathway in controlling organ size and other biological processes
including cell fate determination, mitosis and pluripotency
(11,15,16).
YAP is a key effector protein in the Hippo pathway and is
negatively regulated by the Mst1/2-LATS1/2-Mob1 complex through
direct phosphorylation (11). The
phosphorylation of YAP (pYAP) inhibits cell proliferation;
therefore, impairment of the Hippo signaling pathway has been
implicated in many human cancers including gastric cancer (17–22).
Natural products from vegetables are well
acknowledged for their possible roles as chemopreventive agents
(23). 3,3′-Diindolylmethane (DIM)
is a principal product converted from the bioactive phytochemical
indole-3-carbinol (I3C) that is found in abundance in cruciferous
vegetables (24). I3C is chemically
unstable in aqueous environments and is rapidly converted to DIM,
which is a major condensation product in the stomach (25–27).
DIM is the predominant bioactive compound in plasma (27), and recent studies have shown that
DIM has anticancer effects in both in vivo and in
vitro models of various types of cancers (28–37).
However, the biological function of DIM, and its possible use as an
antitumor agent for human gastric cancer, is unknown. Several
studies have revealed the importance of the novel Hippo
tumor-suppressor pathway in regulating cell proliferation,
apoptosis and tumorigenesis in gastric cancer. Therefore, the
purpose of the present study was to investigate the biological
function of DIM in human gastric cancer cells and to elucidate
whether DIM regulates the Hippo signaling pathway. Our results
demonstrated that DIM activates the Hippo signaling pathway in
sensitizing gastric cancer cells in vitro and inhibits
gastric cancer tumors in vivo.
Materials and methods
Cell culture and reagents
The human gastric cancer cell lines SNU-1 and
SNU-484 were purchased from the Korean Cell Line Bank (Seoul
National University, Korea). Cancer cells were maintained in
RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS),
100 mg/ml streptomycin, and 100 IU/ml penicillin (all from
Gibco-BRL, Grand Island, NY, USA) as a monolayer in 100-mm dishes
(BD Biosciences, Rockville, MD, USA) under standard conditions at
37°C in a 5% CO2 humidified atmosphere. All experiments
were performed with the cells at 60–80% confluence. DIM was
purchased from LKT Laboratories (St. Paul, MN, USA). The following
antibodies were obtained from the following commercial sources:
cyclin D1, CDK4, CDK6, p27, cleaved-caspase-9, pro-caspase-3,
cleaved-poly(ADP-ribose) polymerase (PARP), Mst1, Mst2, LATS1,
pLATS1, Mob1, pMob1, Sav1, YAP, pYAP, Akt, pAkt (Cell Signaling
Technology, Inc., Beverly, MA, USA); CDK2 and Ras association
domain family 1 (RASSF1) antibody (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA).
Cell growth inhibition by MTT assay
The effect of DIM on cell proliferation was
determined by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay as described previously (38). Briefly, cells were plated in 96-well
plates (SPL, Seoul, Korea) at 1×104 cells/well. After 24
h of incubation under standard conditions, the cells were treated
with the indicated DIM concentration for 72 h. Cell viability was
assessed by a scanning multi-well spectrophotometer (SpectraMAX
340; Molecular Devices Co., Sunnyvale, CA, USA).
Soft agar colony formation assay
The bottom layer of soft agar (1%) was prepared in a
6-well plate, and the top layer (0.7%) was prepared with
5×104 SNU-1 or SNU-484 cells/well in a single-cell
suspension. The cells were divided into 2 groups: i) the control
group (blank group) and ii) the experimental group (DIM, 100 μM).
The cells were cultured in an incubator at 37°C with 5%
CO2 for 2 weeks and observed for colony formation by
microscopy. Colonies of >30 cells were counted, and the
experiments were repeated in triplicate.
Cell cycle analysis
To determine the effect of DIM on the cell cycle,
cells were incubated in 100-mm dishes and were treated for 24 h
with various DIM concentrations. Subsequently, cells were washed
with PBS and the nuclei were stained with propidium iodide (PI)
(Sigma Chemical, St. Louis, MO, USA) as described previously
(28,38). The percentage of cells in the
different cell cycle phases was measured with a FACstar flow
cytometer (Becton-Dickinson, San Jose, CA, USA) and analyzed using
Becton-Dickinson software (Lysis II and CellFit).
Western blot analysis
Briefly, the human gastric cancer cell lines SNU-1
and SNU-484 were plated and allowed to attach for 24 h. DIM was
added to the cell cultures at the indicated concentrations for 72
h. Cells with or without DIM were harvested and suspended in lysis
buffer (Intron Biotechnology, Korea). Extracts were incubated on
ice for 10 min and centrifuged at 13,200 rpm for 20 min at 4°C.
After centrifugation, the supernatant was collected and the protein
concentration was determined using a BSA protein assay kit (Pierce
Biotechnology, Inc., Rockford, IL, USA). Whole lysate was resolved
on a SDS-PAGE gel and transferred to PVDF membranes (Bio-Rad,
Hercules, CA, USA). Membranes were probed with the specific primary
antibodies and then with peroxidase-conjugated secondary
antibodies. The bands were visualized with the enhanced
chemiluminescence kit (Amersham, Arlington Heights, IL, USA). The
following antibodies were used: antibody against cyclin D1, CDK2,
CDK4, CDK6, p53, cleaved-PARP, cleaved-caspase-9, pro-caspase-3,
Mst1, Mst2, pLATS1, LATS1, Mob1, pMob1, Sav1, pYAP, YAP, pAkt, Akt,
RASSF1, β-catenin, pβ-catenin, β-actin and GAPDH.
Immunoprecipitation
Cells were scraped in PBS and suspended in lysis
buffer (20 mM Tris-HCl pH 8, 137 mM NaCl, 10% glycerol, 1% Nonidet
P-40 and 2 mM EDTA). Extracts were incubated on ice for 1 h and
pelleted by centrifugation at 12,000 rpm for 10 min. Protein
quantification was determined using a BSA protein assay kit. Whole
lysates (1.5 mg) were incubated with Protein G-Sepharose (Sigma)
for 1 h at 4°C for pre-clearing and centrifuged 10,000 rpm for 5
min at 4°C. The supernatant was incubated with anti-RASSF1 at 4°C
overnight. Antibody-antigen complexes were collected on Protein
G-Sepharose. Immunoprecipitates were resolved by SDS-PAGE,
transferred to PVDF membranes, and probed with the specific primary
antibodies followed by peroxidase-conjugated secondary antibodies.
The bands were visualized with the enhanced chemiluminescence kit.
The following antibodies were used: antibody against Mst1, Mst2,
LATS1, Mob1, Sav1 and GAPDH.
In vivo studies
Based on our in vitro results, we designed
studies to determine the effects of DIM on xenografted human
gastric tumors in nude mice. Animal experiments adhered to NIH
guidelines (USA) and were performed under approval of the
Institutional Animal Care and Use Committee of Chonbuk National
University. Four-week-old female SPF/VAF immunodeficient mice were
purchased from Orient Bio (Korea). The mice were allowed to
acclimate to local conditions for 1 week prior to injection with
cancer cells. Thirteen mice were injected subcutaneously (s.c.)
into the right flank with 0.1 ml Matrigel containing
3.5×106 human gastric cancer cells (SNU-484). The mice
were randomized into 2 groups 1 week after tumor implantation: i)
the untreated control group (n=5, DMSO in 50 μl PBS daily) and ii)
the DIM-treated group (n=5, 10 mg/kg in 50 μl PBS once daily).
Gastric primary tumors were excised, and the final tumor volume was
measured once every 3 days using a caliper and calculated as
(width)2 × length/2. The experiment was terminated on
day 39. Half of the tumor tissue was prepared for western blotting
and the other half was snap frozen in liquid nitrogen and stored at
−80°C.
Statistical analysis
In vitro experiments were repeated >3
times. The statistical significant difference between experimental
groups and the control was determined by one-way ANOVA and then
later compared among groups with an unpaired Student’s t-test.
Results are expressed as the means ± SE. A p-value <0.05 was
considered to indicate a statistically significant result.
Results
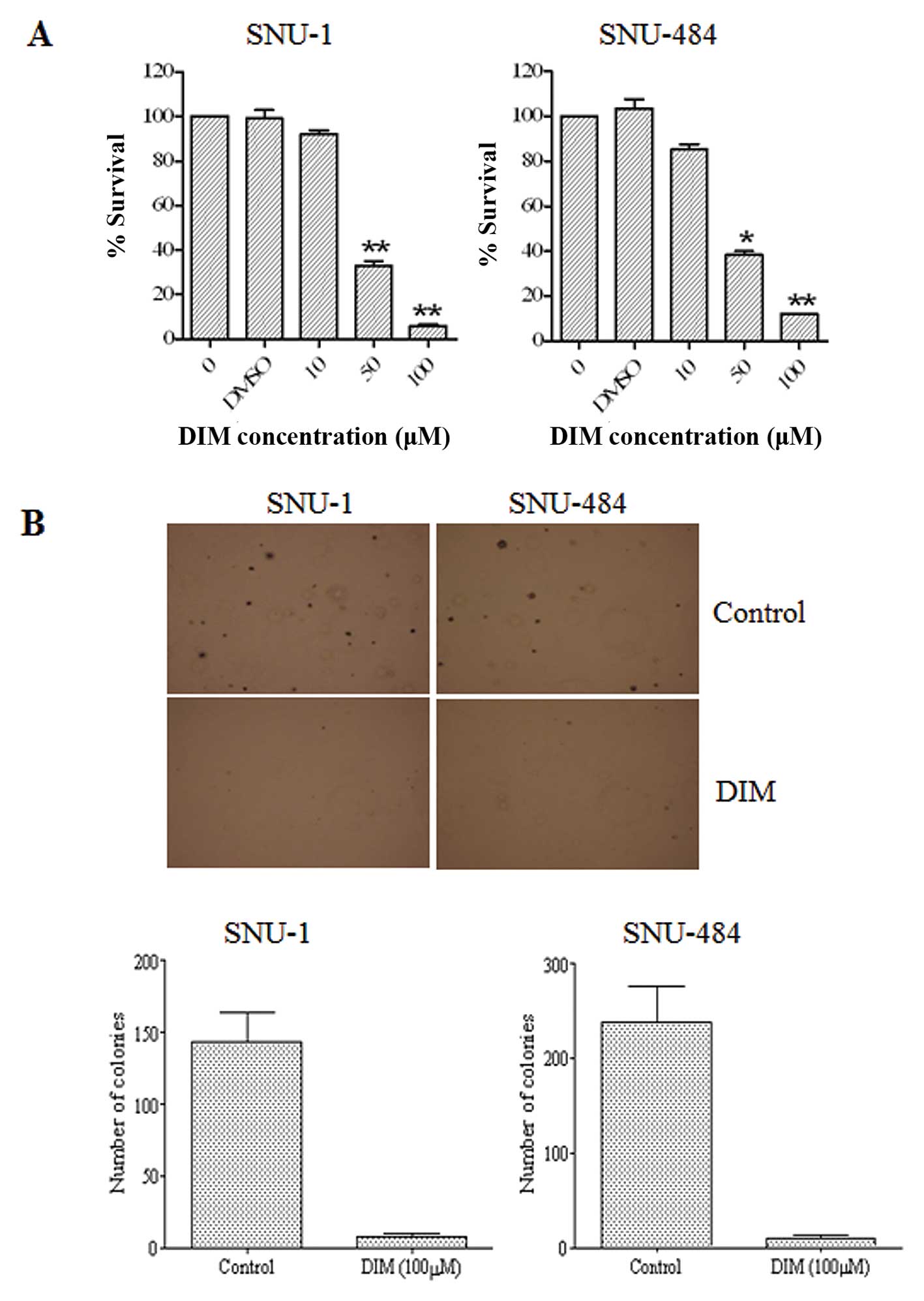
DIM inhibits the proliferation of gastric
cancer cells
The effects of DIM on human gastric adenocarcinoma
cell growth were examined using SNU-1 and SNU-484 cells by
treatment with various DIM concentrations for 72 h. MTT assay
analyses revealed that DIM significantly suppressed the
proliferation of the cell lines in a dose-dependent manner, showing
>50% cell growth inhibition at 50 μM in the two cell lines
(Fig. 1A). The effects of DIM on
SNU-1 and SNU-484 gastric cancer cell colony formation were
evaluated using a soft agar cloning assay. SNU-1 and SNU-484
gastric cancer cells were cultured in medium with 100 μM DIM for 2
weeks, and colony formation was observed by microscopy. As shown in
Fig. 1B, DIM significantly
inhibited colony formation of SNU-1 and SNU-484 cells when compared
to the control. These results suggest that DIM significantly
inhibits the growth of gastric cancer cells.
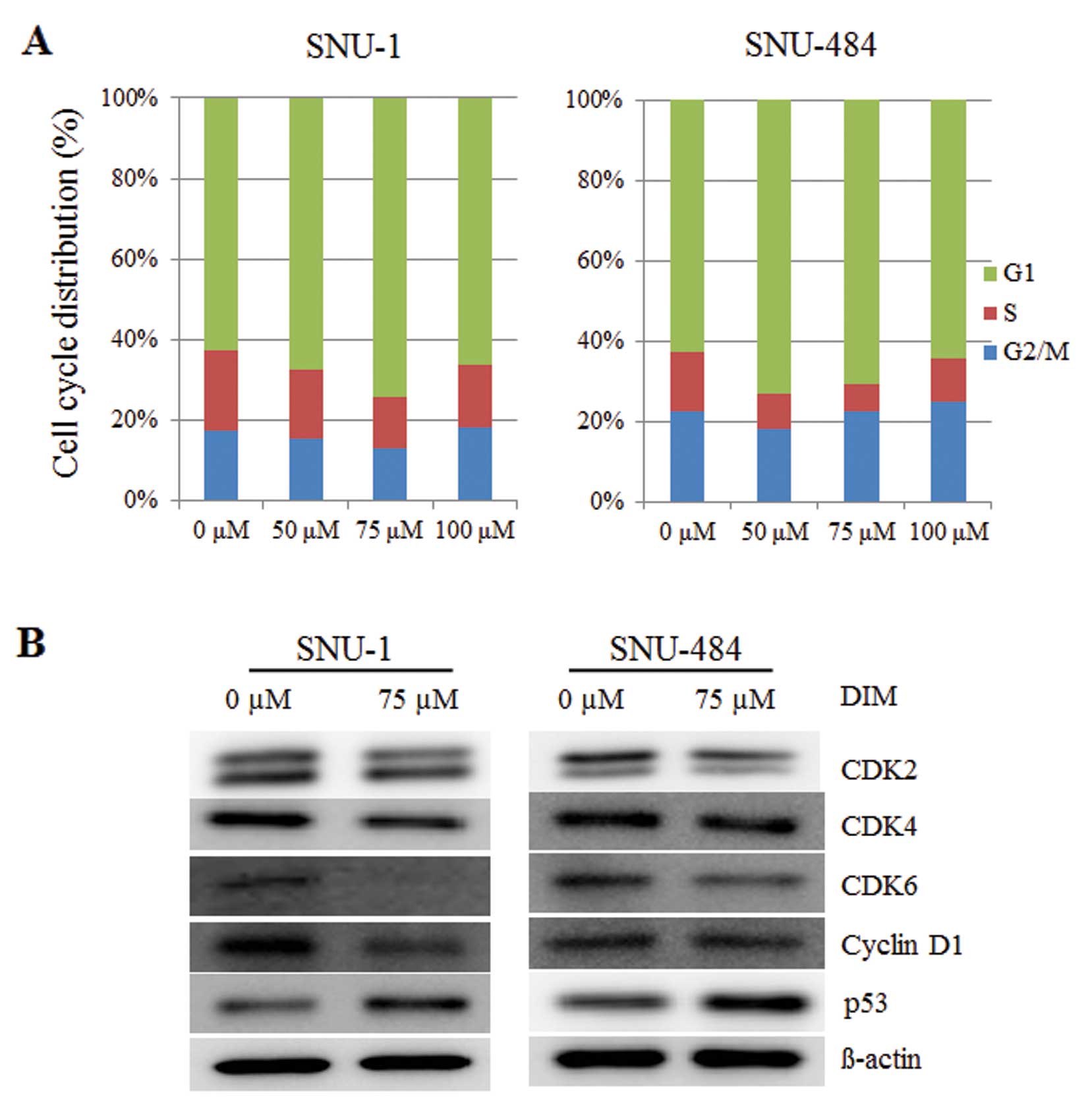
DIM induces cell cycle arrest in gastric
cancer cells
Fluorescence-activated cell sorting (FACS) analysis
was performed to characterize whether DIM regulates cell cycle
progression in human gastric cancer cells. As shown in Fig. 2A, DIM treatment resulted in a
significant increase in the proportion of the cell population in
the G1 phase of the cell cycle at 24 h in SNU-1 and SNU-484 human
gastric cancer cells. These results suggest that DIM induced G1
phase arrest in human gastric cancer cells in a dose-dependent
manner. The effects of DIM on CDK2, CDK4, CDK6 and cyclin D1
protein levels were examined to identify the regulation of G1 cell
cycle regulatory proteins in human gastric cancer cells. A 75-μM
DIM treatment in SNU-1 and SNU-484 cells downregulated the
production of CDK2, CDK4, CDK6, cyclin D1 protein levels at 24 h
(Fig. 2B). Since DNA damage
checkpoints play a critical role in maintaining the integrity of
the genome by arresting cell cycle progression in response to
damaged or incompletely replicated DNA, and G1 checkpoint arrest in
mammalian cells is mediated by the action of p53 protein, we also
investigated the effects of DIM on the induction of the p53 protein
level. A 75-μM DIM treatment in SNU-1 and SNU-484 cells resulted in
the upregulation of the p53 protein level leading to cell cycle
arrest in the G1 phase (Fig. 2B).
These results indicate that DIM induced G1 phase arrest in human
gastric cancer cells through changes in the levels of cell cycle
regulatory proteins.
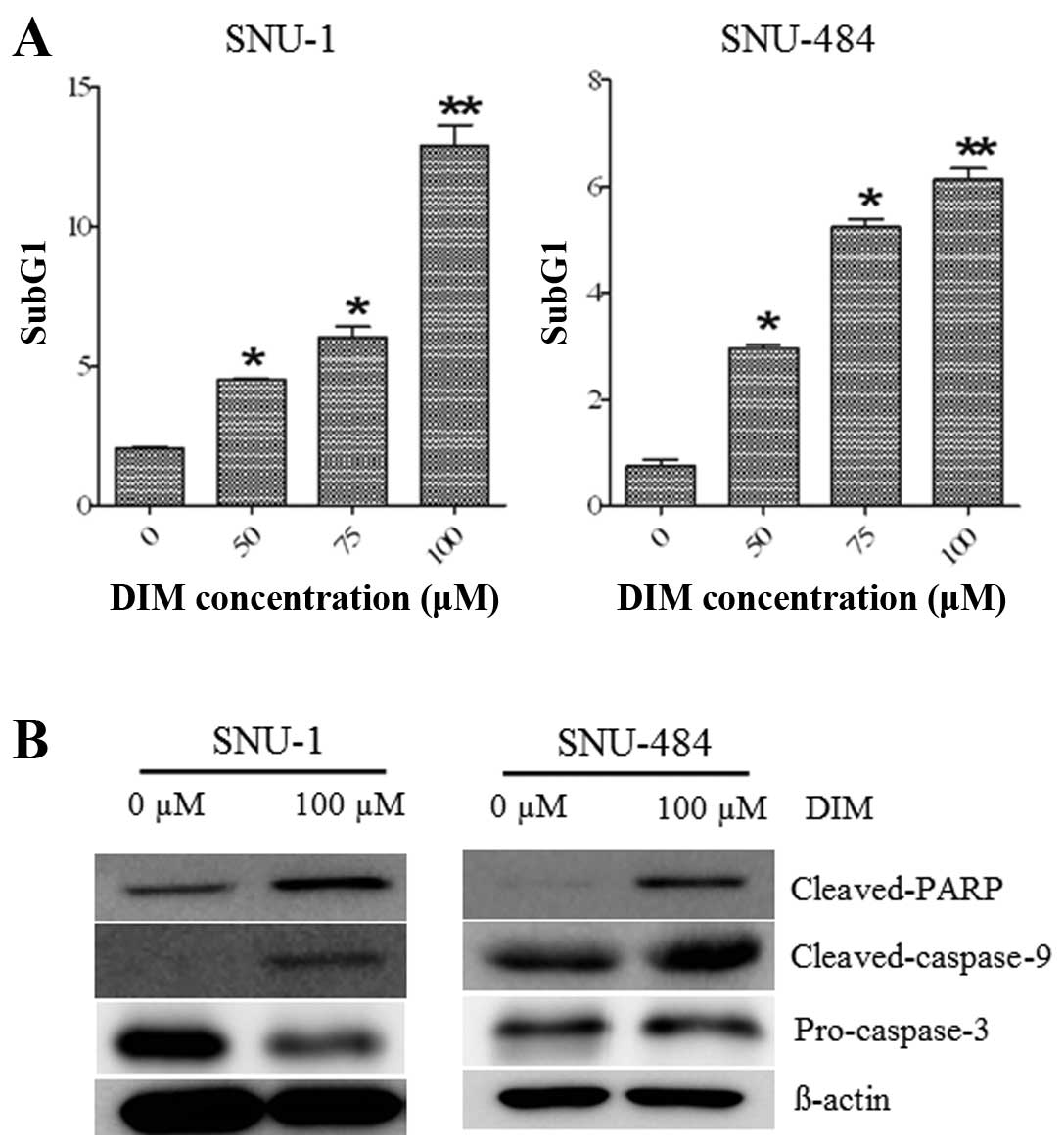
DIM induces apoptosis in gastric cancer
cells
As shown in Fig. 3A,
DIM treatment in SNU-1 and SNU-484 gastric cancer cells showed an
increased accumulation of cells in the sub-G1 phase in a
dose-dependent manner. To test whether DIM induces apoptotic cell
death in gastric cancer cells, we further investigated the
cleaved-caspase-9, cleaved-PARP, and pro-caspase-3 protein levels
using western blot analysis. As shown in Fig. 3B, treatment with 100 μM DIM for 72 h
inhibited pro-caspase-3 protein levels and increased levels of the
cleaved form of PARP and cleaved-caspase-9, hallmarks of apoptosis,
in the SNU-1 and SNU-484 gastric cancer cells. These results
indicate that DIM induced apoptotic cell death in human gastric
cancer cells. Overall, these results support the notion that the
observed decline in cell viability by DIM was in part due to cell
cycle arrest and induction of apoptosis.
DIM activates the Hippo signaling pathway
resulting in YAP inactivation
The Hippo signaling pathway is a tumor-suppressor
signaling system that inhibits cell proliferation and promotes cell
apoptosis. To investigate whether DIM mediates gastric cancer cell
death via the Hippo signaling pathway, we examined the protein
levels of Mst1/2, LATS1, pLASTS1, Mob1, pMob1, and Sav1 after DIM
treatment. As shown in Fig. 4,
western blot analysis revealed that treatment with 100 μM DIM for
72 h significantly increased the production of pLATS1, pMOB1, and
Sav1 in SNU-1 and SNU-484 cells. We also analyzed the production of
YAP and pYAP after treatment with DIM. We found that the production
of pYAP was significantly increased and YAP protein levels were
decreased following treatment with 100 μM DIM for 72 h in gastric
cancer cells. These results suggest that DIM may trigger the Hippo
signaling pathway cascade that leads to phosphorylation,
cytoplasmic retention and functional inactivation of YAP.
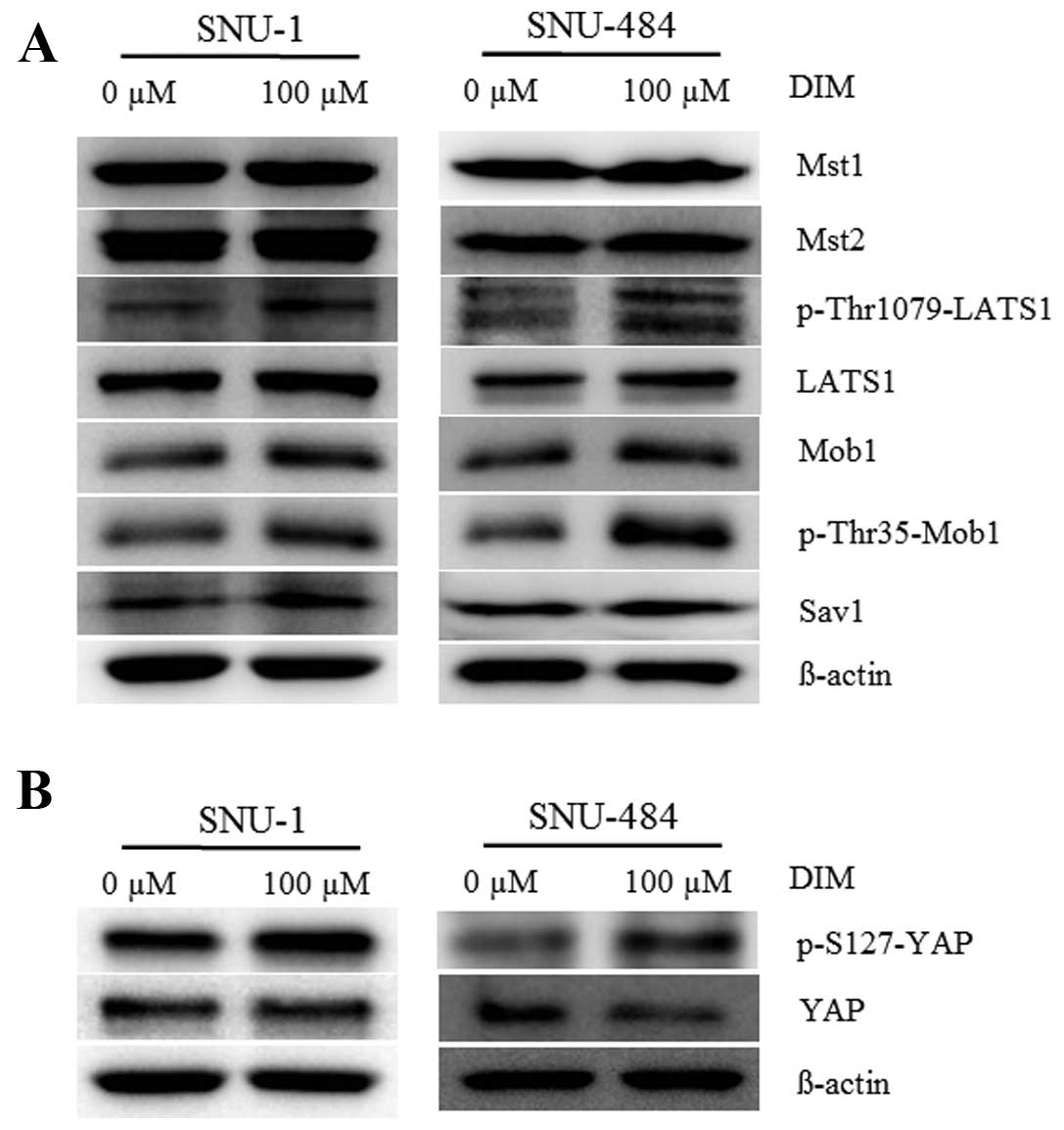 | Figure 4Effects of DIM on the Hippo signaling
pathway in gastric cancer cell lines (SNU-1 and SNU-484). pYAP,
YAP, Mst1/2, pLATS1, LATS1, Mob1, pMob1, and Sav1 levels were
analyzed by western blotting in SNU-1 and SNU-484 cell lines after
treatment with and without DIM. Cells were harvested at 72 h, and
immunoblotted with the indicated antibodies. β-actin was used as an
internal control. DIM, 3,3′-diindolylmethane. |
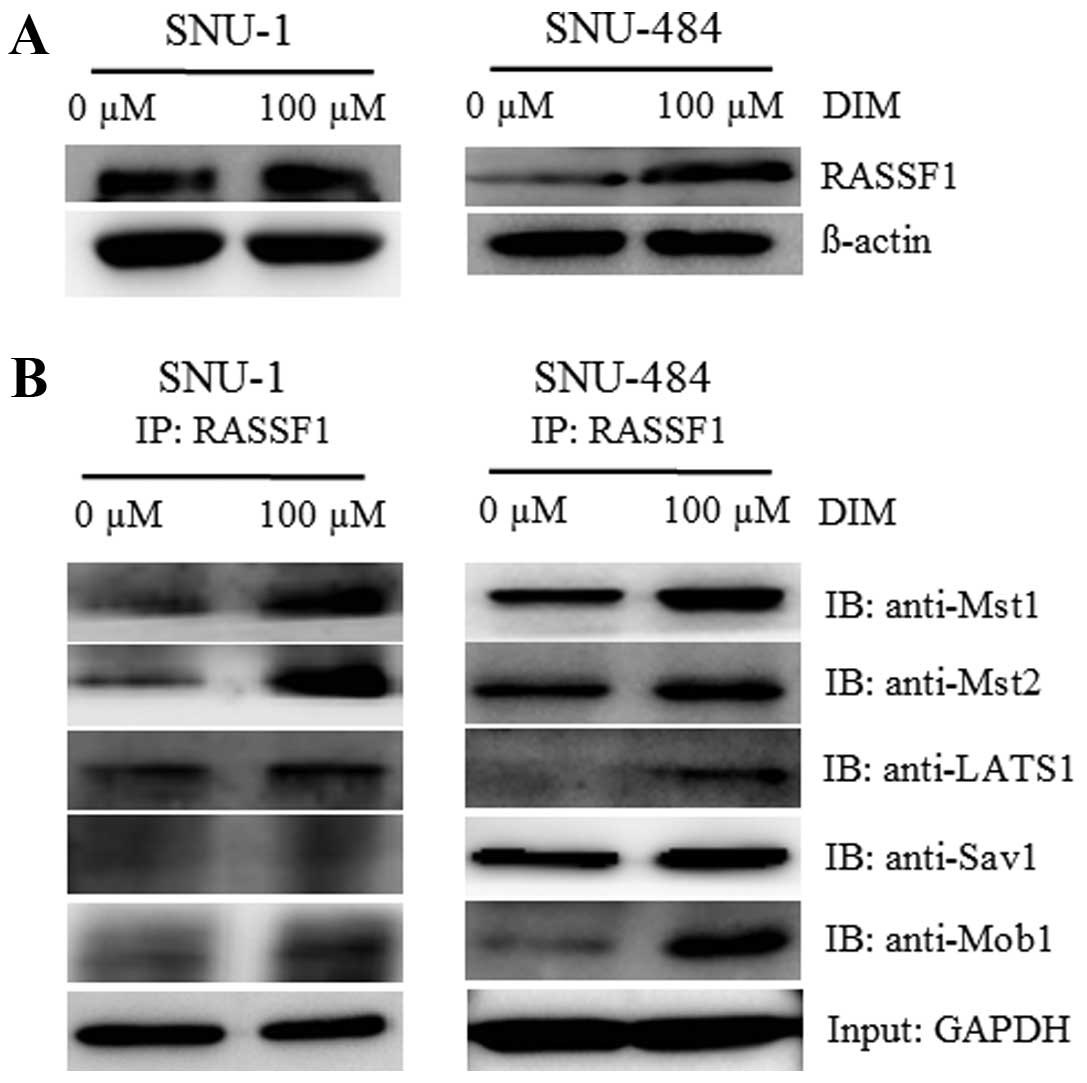
DIM stimulates Mst1/2-RASSF1 interaction
in gastric cancer cells
To address the question of how DIM activates the
Hippo signaling pathway, we investigated the possible role of
RASSF1 that has been identified as an upstream regulator of the
Hippo signaling pathway (39,40).
RASSF1 has been reported to interact with the mammalian kinases,
Mst1, and Mst2 and activate them by promoting their
autophosphorylation and subsequent phosphorylation of the
downstream LATS1 kinase (41). In
addition, several studies have revealed that Hippo signaling is
tightly regulated by protein-protein interactions (14). Therefore, we tested whether RASSF1
associates with Hippo signaling pathway molecules due to DIM
treatment. As shown in Fig. 5A,
endogenous RASSF1 protein production was significantly increased
following treatment with 100 μM DIM in the SNU-1 and SNU-484 cells.
In addition, a dramatic increased interaction between the Mst1/2-
LATS1-Sav1-Mob1 complex and RASSF1 was observed in the presence of
the same DIM dose as analyzed by immunoprecipitation followed by
immunoblotting (Fig. 5B). These
results indicate that DIM may activate the Hippo signaling pathway
through RASSF1 activation, which increases the
coimmunoprecipitation of RASSF1 with Hippo signaling molecules.
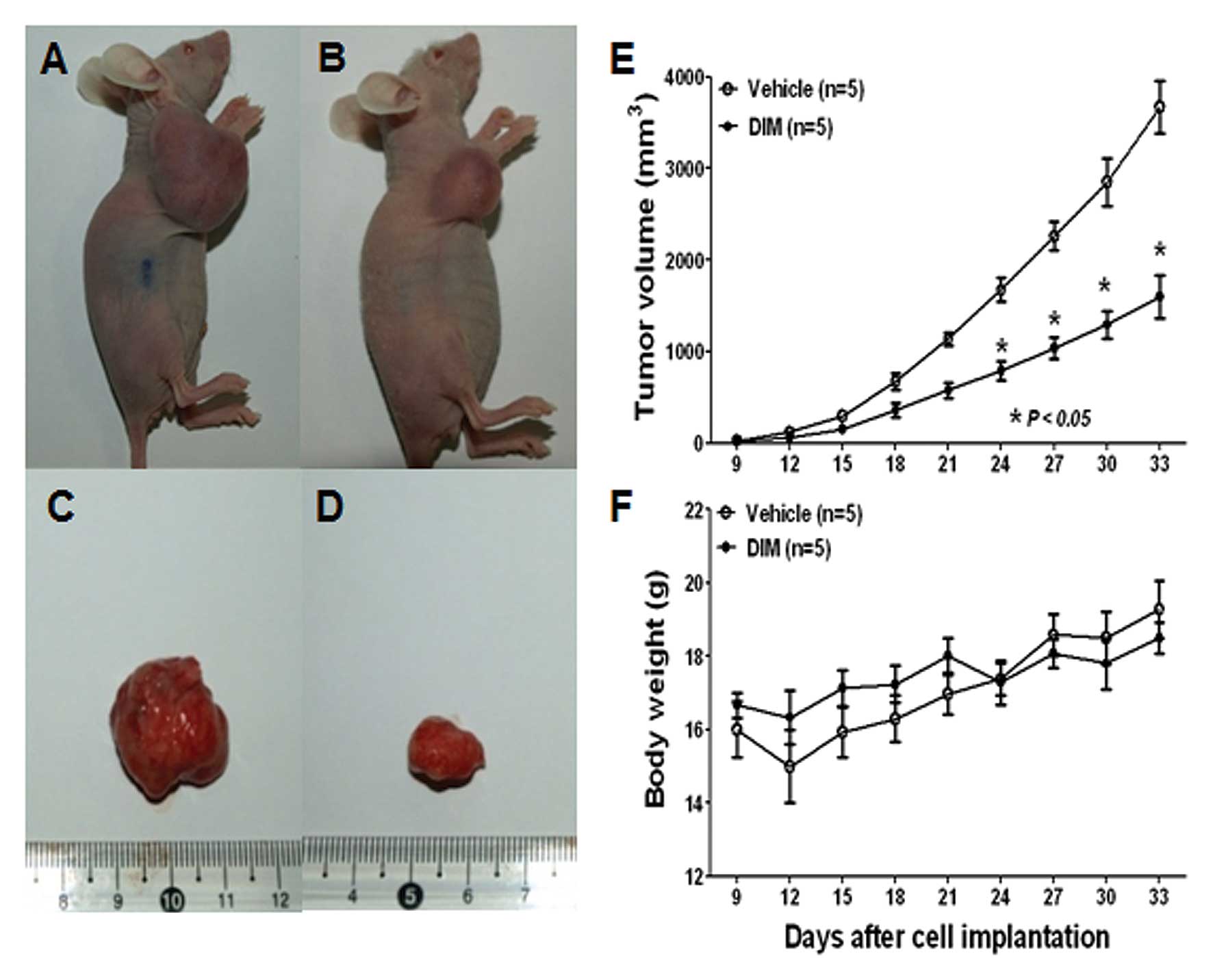
DIM inhibits the growth of SNU-484 tumor
xenografts in immunodeficient mice
Since we observed an inhibition of gastric cancer
cell viability by DIM in vitro, we evaluated whether these
observations could be translated into an animal model system in
vivo. To address this issue, SPF/VAF immunodeficient mice were
inoculated in the right flank with SNU-484 human gastric cancer
cells. Mice were randomized into two groups and were treated daily
s.c. with either vehicle or DIM (10 mg/kg) for 30 days. Tumor
volume and the weight of mice were recorded once every 3 days using
calipers (Fig. 6). As shown in
Fig. 6A–E, DIM treatment resulted
in a marked inhibition of SNU-484 xenograft tumor growth. Notably,
the body weight of mice from both groups did not significantly
differ from the vehicle control following 30 days of drug exposure
(Fig. 6F), suggesting that DIM has
no severe toxicity to the mice. Taken together, these findings
demonstrate that DIM administration significantly inhibited SNU-484
xenograft growth in vivo mediated by the inactivation of
YAP.
Discussion
The present study demonstrated for the first time
that human gastric cancer cells exposed to DIM resulted in an
increase in apoptosis, suggesting that DIM could be used for the
treatment of gastric cancer. Despite the fact that many studies
have revealed that DIM has antitumor effects in a variety of cancer
cells including prostate, breast, pancreas, and esophageal cancer
cells through the NF-κB, Akt, MAPK, p53, AR, and ER pathways
(5,11,28,31,34,42),
no information is available regarding the functional role of the
Hippo signal transduction pathway in mediating DIM-induced
lethality in gastric cancer cells. We found that DIM was effective
in sensitizing gastric cancer cells through activation of the Hippo
signaling pathway. In addition, our results provide convincing
evidence in support of the antitumor effects of DIM on xenograft
gastric tumors in vivo. Therefore, activation of the Hippo
pathway may mediate key contributions in gastric cancer cell death
induced by DIM treatment.
The evolutionarily conserved Hippo signaling pathway
plays an important role in both organ size control and
tumorigenesis, and thus deregulation of this pathway has been
implicated in human cancers (43).
Since the interruption of any factor in this pathway can bring
about tumorigenesis, it appears as though YAP is the major Hippo
pathway downstream effector that functions as an oncogene. In the
present study, DIM dramatically increased the protein levels of
pLATS1, Mob1, pMob1, and Sav1 in gastric cancer cells, while Mst1/2
levels were not altered. Intriguingly, the production of pYAP being
significantly induced by DIM supports the notion that DIM activates
the Hippo signaling pathway cascade leading to the inactivation of
YAP function. Our observations are in agreement with previous
reports demonstrating that Hippo core components can negatively
regulate YAP through direct phosphorylation, and the activated
LATS-Mob1 complex phosphorylates YAP leading to enhanced
interactions with 14-3-3 proteins and cytoplasmic sequestration
(11). Thus, the pYAP induced by
DIM may limit YAP activity by preventing nuclear accumulation and
decreasing the expression of positive regulators for cell growth
and activators of apoptosis. In fact, we found that DIM suppressed
the proliferation of various gastric cancer cell lines including
SNU-1 and SNU-484 mediated by an antiproliferative effect by
inducing G1 cell cycle arrest. The G1 cell cycle arrest was
accompanied by the reduced production of CDK2, CDK4, CDK6 and
cyclin D1 protein levels and the increased production of p53
protein levels. DIM also induced poly(ADP-ribose) polymerase
cleavage, caspase-9 cleavage and diminished pro-caspase-3 protein
levels. These apoptotic effects of DIM on gastric cancer cells are
in agreement with previous reports regarding other cancer cell
lines (7,28,29,37).
Therefore, the induction of apoptosis in gastric cancer cells by
DIM treatment is associated with the activation of the LATS-Mob1
complex and pYAP.
The Ras association domain family 1 (RASSF1) protein
has been demonstrated to be involved in several growth regulatory
and pro-apoptotic pathways. The RASSF1 gene is localized on
chromosome 3p21.3 and is found to be frequently silenced by
promoter hypermethylation in a variety of cancers (39,40,44,45).
Targeted disruption of the RASSF1 gene in mice resulted in
spontaneous and carcinogen-induced tumorigenesis (46), indicating that RASSF1 serves as an
important tumor-suppressor. Recent research has shown that Hippo
signal transduction pathway activity is regulated by RASSF1, an
emerging pathway implicated in the control of cell growth,
apoptosis, and tumor-suppression (20,41,47).
In addition, several studies have shown that RASSF1 interacts with
the mammalian Mst1/2, and promotes phosphorylation of Mst1/2 and
their downstream gene products (48,49),
suggesting that RASSF1-Mst1/2 may have intriguing tumor-suppressing
characteristics. In fact, a recent study revealed that RASSF1
prevented dephosphorylation of Mst1/2 by binding to Mst1/2,
supporting the maintenance of pMst1/2 phosphorylation (41). In the present study, we found that
endogenous RASSF1 protein levels were significantly increased by
DIM treatment in gastric cancer cells. The binding of Mst1/2, LAST1
and Mob1 increased when interacting with RASSF1, suggesting that
DIM stimulates RASSF1 with the Mst1/2-LAST-Mob complex to promote
an active state of the Mst kinases, favoring the induction of pYAP
that inactivates cell proliferation. Therefore, these results
indicate that RASSF1 interacts with Mst1/2 by DIM treatment and the
cytotoxic effects of DIM on gastric cancer cells are mediated
through the Hippo signaling pathway.
Our in vitro results were recapitulated in
vivo using a gastric tumor xenograft animal model. We found
that DIM significantly inhibited the growth of gastric tumors.
Particularly, an increase in pYAP in the DIM-treated tumors was
observed. These in vivo results were consistent with our
molecular studies in vitro, which obviously provide strong
support that Hippo signaling and the YAP pathway play a significant
role in gastric cancer cell proliferation and may therefore be
potential targets for the treatment of gastric cancer. Therefore,
our study suggests that DIM acts as a promising agent against
gastric tumors in vivo as it not only reduced tumor growth
but also suppressed YAP activation that strongly correlated with
the stimulation of tumor growth.
In conclusion, our study provided strong evidence
that DIM induces human gastric cancer cell death that is due in
part to activation of the Hippo signal transduction pathway and the
inactivation of YAP. Our in vitro findings together with our
in vivo results provide important implications of DIM as a
chemopreventive or therapeutic agent for improving the outcome of
gastric cancer patients.
Acknowledgements
This study was supported by the Bumsuk Academic
Research Fund in 2011.
References
|
1
|
Kong D, Banerjee S, Huang W, et al:
Mammalian target of rapamycin repression by 3,3′-diindolylmethane
inhibits invasion and angiogenesis in platelet-derived growth
factor-D-overexpressing PC3 cells. Cancer Res. 68:1927–1934.
2008.
|
|
2
|
Shin HR, Carlos MC and Varghese C: Cancer
control in the Asia Pacific region: current status and concerns.
Jpn J Clin Oncol. 42:867–881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
4
|
Tan IB, Ng I, Tai WM and Tan P:
Understanding the genetic basis of gastric cancer: recent advances.
Expert Rev Gastroenterol Hepatol. 6:335–341. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoffmann W: Stem cells, self-renewal and
cancer of the gastric epithelium. Curr Med Chem. 19:5975–5983.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho JY, Lim JY, Cheong JH, et al: Gene
expression signature-based prognostic risk score in gastric cancer.
Clin Cancer Res. 17:1850–1857. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hundahl SA, Phillips JL and Menck HR: The
National Cancer Data Base Report on poor survival of U.S. gastric
carcinoma patients treated with gastrectomy: Fifth Edition American
Joint Committee on Cancer staging, proximal disease, and the
‘different disease’ hypothesis. Cancer. 88:921–932. 2000.PubMed/NCBI
|
|
8
|
Kovoor PA and Hwang J: Treatment of
resectable gastric cancer: current standards of care. Exp Rev
Anticancer Ther. 9:135–142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rausei S, Dionigi G, Rovera F, et al: A
decade in gastric cancer curative surgery: evidence of progress
(1999–2009). World J Gastrointest Surg. 4:45–54. 2012.PubMed/NCBI
|
|
10
|
Harvey KF, Pfleger CM and Hariharan IK:
The Drosophila Mst ortholog, hippo, restricts growth
and cell proliferation and promotes apoptosis. Cell. 114:457–467.
2003.
|
|
11
|
Harvey K and Tapon N: The
Salvador-Warts-Hippo pathway - an emerging tumour-suppressor
network. Nat Rev Cancer. 7:182–191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grusche FA, Richardson HE and Harvey KF:
Upstream regulation of the hippo size control pathway. Curr Biol.
20:R574–R582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harvey KF and Hariharan IK: The hippo
pathway. Cold Spring Harb Perspect Biol. 4:a0112882012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeng Q and Hong W: The emerging role of
the hippo pathway in cell contact inhibition, organ size control,
and cancer development in mammals. Cancer Cell. 13:188–192. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Edgar BA: From cell structure to
transcription: Hippo forges a new path. Cell. 124:267–273. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao B, Li L, Lei Q and Guan KL: The
Hippo-YAP pathway in organ size control and tumorigenesis: an
updated version. Genes Dev. 24:862–874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu ZP, Zhu JS, Zhang Q and Wang XY: A
breakdown of the Hippo pathway in gastric cancer.
Hepatogastroenterology. 58:1611–1617. 2011.PubMed/NCBI
|
|
18
|
Zhou Z, Zhu JS, Xu ZP and Zhang Q:
Lentiviral vector-mediated siRNA knockdown of the YAP gene inhibits
growth and induces apoptosis in the SGC7901 gastriccancer cell
line. Mol Med Rep. 4:1075–1082. 2011.PubMed/NCBI
|
|
19
|
Min B, Kim MK, Zhang JW, et al:
Identification of RUNX3 as a component of the MST/Hpo signaling
pathway. J Cell Physiol. 227:839–849. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshihama Y, Izumisawa Y, Akimoto K, et
al: High expression of KIBRA in low atypical protein kinase
C-expressing gastric cancer correlates with lymphatic invasion and
poor prognosis. Cancer Sci. 104:259–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song M, Cheong JH and Kim H, Noh SH and
Kim H: Nuclear expression of Yes-associated protein 1 correlates
with poor prognosis in intestinal type gastric cancer. Anticancer
Res. 32:3827–3834. 2012.PubMed/NCBI
|
|
22
|
Lam-Himlin DM, Daniels JA, Gayyed MF, et
al: The hippo pathway in human upper gastrointestinal dysplasia and
carcinoma: a novel oncogenic pathway. Int J Gastrointest Cancer.
37:103–109. 2006.PubMed/NCBI
|
|
23
|
Seymour JD, Calle EE, Flagg EW, Coates RJ,
Ford ES and Thun MJ; American Cancer Society. Diet Quality Index as
a predictor of short-term mortality in the American Cancer Society
Cancer Prevention Study II Nutrition Cohort. Am J Epidemiol.
157:980–988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao N, Cheng S, Budhraja A, et al:
3,3′-Diindolylmethane exhibits antileukemic activity in vitro and
in vivo through a Akt-dependent process. PloS One.
7:e317832012.
|
|
25
|
Grose KR and Bjeldanes LF: Oligomerization
of indole-3-carbinol in aqueous acid. Chem Res Toxicol. 5:188–193.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Keck AS and Finley JW: Cruciferous
vegetables: cancer protective mechanisms of glucosinolate
hydrolysis products and selenium. Integr Cancer Ther. 3:5–12. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Banerjee S, Wang Z, Kong D and Sarkar FH:
3,3′-Diindolyl-methane enhances chemosensitivity of multiple
chemotherapeutic agents in pancreatic cancer. Cancer Res.
69:5592–5600. 2009.
|
|
28
|
Kim SJ, Lee JS and Kim SM:
3,3′-Diindolylmethane suppresses growth of human esophageal
squamous cancer cells by G1 cell cycle arrest. Oncol Rep.
27:1669–1673. 2012.
|
|
29
|
Ali S, Banerjee S, Ahmad A, El-Rayes BF,
Philip PA and Sarkar FH: Apoptosis-inducing effect of erlotinib is
potentiated by 3,3′-diindolylmethane in vitro and in vivo using an
orthotopic model of pancreatic cancer. Mol Cancer Ther.
7:1708–1719. 2008.PubMed/NCBI
|
|
30
|
Chinnakannu K, Chen D, Li Y, et al: Cell
cycle-dependent effects of 3,3′-diindolylmethane on proliferation
and apoptosis of prostate cancer cells. J Cell Physiol. 219:94–99.
2009.
|
|
31
|
Bhuiyan MM, Li Y, Banerjee S, et al:
Down-regulation of androgen receptor by 3,3′-diindolylmethane
contributes to inhibition of cell proliferation and induction of
apoptosis in both hormone-sensitive LNCaP and insensitive C4-2B
prostate cancer cells. Cancer Res. 66:10064–10072. 2006.
|
|
32
|
Li Y, Chinni SR and Sarkar FH: Selective
growth regulatory and pro-apoptotic effects of DIM is mediated by
AKT and NF-kappaB pathways in prostate cancer cells. Front Biosci.
10:236–243. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nachshon-Kedmi M, Fares FA and Yannai S:
Therapeutic activity of 3,3′-diindolylmethane on prostate cancer in
an in vivo model. Prostate. 61:153–160. 2004.
|
|
34
|
Rahman KW and Sarkar FH: Inhibition of
nuclear translocation of nuclear factor-κB contributes to
3,3′-diindolylmethane-induced apoptosis in breast cancer cells.
Cancer Res. 65:364–371. 2005.
|
|
35
|
Abdelrahim M, Newman K, Vanderlaag K,
Samudio I and Safe S: 3,3′-diindolylmethane (DIM) and its
derivatives induce apoptosis in pancreatic cancer cells through
endoplasmic reticulum stress-dependent upregulation of DR5.
Carcinogenesis. 27:717–728. 2006.
|
|
36
|
Carter TH, Liu K, Ralph W Jr, et al:
Diindolylmethane alters gene expression in human keratinocytes in
vitro. J Nutr. 132:3314–3324. 2002.PubMed/NCBI
|
|
37
|
Azmi AS, Ahmad A, Banerjee S, Rangnekar
VM, Mohammad RM and Sarkar FH: Chemoprevention of pancreatic
cancer: characterization of Par-4 and its modulation by 3,3′
diindolylmethane (DIM). Pharm Res. 25:2117–2124. 2008.PubMed/NCBI
|
|
38
|
Kim SJ, Chung MJ, Kim JS, et al:
Deciphering the role of paclitaxel in the SKGT4 human esophageal
adenocarcinoma cell line. Int J Oncol. 39:1587–1591.
2011.PubMed/NCBI
|
|
39
|
Dammann R, Li C, Yoon JH, Chin PL, Bates S
and Pfeifer GP: Epigenetic inactivation of a RAS association domain
family protein from the lung tumour suppressor locus 3p21.3. Nat
Genet. 25:315–319. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dammann R, Schagdarsurengin U, Seidel C,
et al: The tumor suppressor RASSF1A in human carcinogenesis: an
update. Histol Histopathol. 20:645–663. 2005.PubMed/NCBI
|
|
41
|
Guo C, Zhang X and Pfeifer GP: The tumor
suppressor RASSF1A prevents dephosphorylation of the mammalian
STE20-like kinases MST1 and MST2. J Biol Chem. 286:6253–6261. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen Y, Xu J, Jhala N, et al: Fas-mediated
apoptosis in cholangiocarcinoma cells is enhanced by
3,3′-diindolylmethane through inhibition of AKT signaling and
FLICE-like inhibitory protein. Am J Pathol. 169:1833–1842.
2006.PubMed/NCBI
|
|
43
|
Saucedo LJ and Edgar BA: Filling out the
Hippo pathway. Nat Rev Mol Cell Biol. 8:613–621. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Burbee DG, Forgacs E, Zöchbauer-Müller S,
et al: Epigenetic inactivation of RASSF1A in lung and breast
cancers and malignant phenotype suppression. J Natl Cancer Inst.
93:691–699. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Agathanggelou A, Cooper WN and Latif F:
Role of the Ras-association domain family 1 tumor suppressor gene
in human cancers. Cancer Res. 65:3497–3508. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tommasi S, Dammann R, Zhang Z, et al:
Tumor susceptibility of Rassf1a knockout mice. Cancer Res.
65:92–98. 2005.PubMed/NCBI
|
|
47
|
Vichalkovski A, Gresko E, Cornils H,
Hergovich A, Schmitz D and Hemmings BA: NDR kinase is activated by
RASSF1A/MST1 in response to Fas receptor stimulation and promotes
apoptosis. Curr Biol. 18:1889–1895. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Guo C, Tommasi S, Liu L, Yee JK, Dammann R
and Pfeifer GP: RASSF1A is part of a complex similar to the
Drosophila Hippo/Salvador/Lats tumor-suppressor network.
Curr Biol. 17:700–705. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Matallanas D, Romano D, Yee K, et al:
RASSF1A elicits apoptosis through an MST2 pathway directing
proapoptotic transcription by the p73 tumor suppressor protein. Mol
Cell. 27:962–975. 2007. View Article : Google Scholar : PubMed/NCBI
|