Introduction
Malignant glioma is the most common primary brain
tumor, accounting for approximately 80% of malignant tumors in the
central nervous system. It has a very poor prognosis, mainly due to
its resistance to radiotherapy, chemotherapy and adjuvant therapies
(1–3). Generally, patients diagnosed with
glioblastoma, the most malignant form of glioma, live approximately
only 1 year after diagnosis (4).
Despite the marked developments in the therapy of other types of
cancer, the median survival rate of malignant glioma has not
improved in the past years (5).
Therefore, a more effective therapeutic strategy is urgently
required.
Receptor for activated C-kinase 1 (RACK1) is a
member of the intracellular receptors for activated protein kinase
C (PKC) (6). RACK1 is widely
expressed in the central nervous system, and is involved in
multiple crucial cellular process including cell growth,
proliferation, apoptosis and migration (7–9). In
recent years, accumulating evidence has shown that RACK1 plays an
important role in various types of cancer (10–12).
Therefore, we hypothesized that RACK1 may act as an important
regulator in the development and progression of malignant
glioma.
In this study, we first determined the mRNA and
protein expression levels of RACK1 in glioma tissues of different
grades as well as in normal brain tissues. Then, the mRNA and
protein expression of RACK1 was determined in two types of human
glioma cell lines U87 and CHG-5. Using RACK1-specific siRNA, the
effects as well as the molecular mechanism of RACK1 downregulation
involved in proliferation, apoptosis and invasion of U87 and CHG-5
cells were further investigated in vitro and in
vivo.
Materials and methods
Materials and reagents
The protocol used in the present study was approved
by the Ethics Committee of Central South University. Written
informed consent was obtained from each participant. Forty-five
glioma tissues (10 cases of WHO I, 13 cases of WHO II, 12 cases of
WHO III, and 10 cases of WHO IV) were obtained from patients who
underwent surgery from October 2011 to October 2012 at the
Department of Neurosurgery, Xiangya Hospital of Central South
University (Hunan, China), and 10 normal brain tissues from
patients with cerebral trauma were used as controls. The human
glioma U87 (WHO IV) and CHG-5 (WHO II) cell lines were obtained
from the Cell Bank of Central South University. Dulbecco’s modified
Eagle’s medium (DMEM), opti-MEM, fetal bovine serum (FBS) and
Lipofectamine 2000 transfection agent were purchased from
Invitrogen Life Technologies (USA). MTT was obtained from Sigma
(USA). The bicinchoninic acid (BCA) protein assay kit was purchased
from Pierce Biotechnology, Inc. (USA). PVDF membrane was obtained
from Pall (USA). All siRNAs and antibodies were obtained from Santa
Cruz Biotechnology, Inc. (USA). The Annexin V-FITC Apoptosis
Detection kit was purchased from Biovision (USA). Matrigel was
purchased form BD Biosciences (USA).
Cell culture
U87 and CHG-5 cell lines were cultured in DMEM
containing 10% FBS, 100 U/ml penicillin and 100 U/ml streptomycin.
Cells were incubated at 37°C in a humidified incubator of 95% air
and 5% CO2.
Real-time RT-PCR
The RACK1 mRNA expression in tissues or cell lines
was detected by real-time RT-PCR (ABI 7500). Specific primers for
different genes in this study were synthesized by BGI Company
(Guangzhou, China). Specific primers for RACK1 were: sense,
5′-GAGCAAATGACCCTTCGT-3′ and antisense, 5′-GTAGTGCCCGTTGTGAGA-3′.
Specific primers for Bax were: sense, 5′-CCCGAGAGGTCTTTTTCC GAG-3′
and antisense, 5′-CCAGCCCATGATGGTTCTGAT-3′. Specific primers for
Bcl-2 were: sense, 5′-GGTGGGGTCAT GTGTGTGG-3′ and antisense,
5′-CGGTTCAGGTACTCAGTCATCC-3′. Human glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) primers were used as a control (sense,
5′-GGCAGCCCAGAACATCATCC-3′ and antisense,
5′-GCCAGCCCAAGCATCAAAG-3′). All amplifications were performed in 3
parallel samples.
Western blotting
Cells were lysed by cold RIPA lysis, and the protein
concentrations were determined using BCA protein assay kit. Then,
proteins of 20 μg/lane were loaded on 12% SDS-PAGE to separate, and
then electrophoretically transferred to PVDF membranes. Proteins on
the membranes were then probed using primary antibodies according
to the supplier’s protocol. Following incubation with secondary
antibodies, results were visualized with peroxidase and an enhanced
chemiluminescence system (Pierce Biotechnology, Inc.) and
quantified by Quantity One software (Bio-Rad, USA).
siRNA interference
Human glioma U87 and CHG-5 cells were seeded at a
density of 105 cells/well in 6-well plates and cultured
in DMEM containing 10% FBS. After incubating at 37°C, 5%
CO2 for 24 h, U87 and CHG-5 cells were transfected with
siRNA and Lipofectamine 2000 according to the supplier’s
instruction. Briefly, 100 nmol siRNA and 5 μl Lipofectamine 2000
were diluted in opti-MEM to a final volume of 800 μl. After mixing
for 20 min at room temperature, the siRNA/Lipofectamine 2000
mixture was added. Cells were incubated at 37°C 5% CO2
for 6 h. Following incubation, the mixture was replaced with DMEM
containing 10% FBS for 24 h.
Proliferation assay
Cell proliferation was determined by MTT assay.
After 24 h post-transfection, the transfection medium in each well
was replaced by DMEM medium containing 10% FBS used before, and was
cultured for 12, 24, 36, 48 and 60 h. Then, the medium was replaced
by 100 μl of fresh serum-free medium and cultured with 0.5 g/l MTT.
Following incubation at 37°C for 4 h, the MTT medium was removed by
aspiration and 50 μl of DMSO was added to each well. Following
incubation at 37°C for a further 10 min, the A540 of each sample
was measured using a plate reader.
Apoptosis analysis
Flow cytometry was used to determine the cell
apoptosis with the Annexin V-FITC Apoptosis Detection kit. After 24
h post-transfection, cells were harvested and washed with cold PBS
twice. Subsequently, 106 cells were resuspended in 200
μl binding buffer supplemented with 10 μl Annexin V-FITC and 5 μl
PI-PE, and incubated in the dark for 30 min. Then, 300 μl binding
buffer was added followed by flow cytometry assay.
Transwell matrix penetration assay
Cells (105) of different groups in 200 μl
serum-free DMEM were plated on the upper chamber plated on the top
side of polycarbonate Transwell filter coated with Matrigel and
incubated at 37°C for 24 h. Subsequently, cells inside the upper
chamber were removed. Cells on the lower membrane surface were
fixed in 1% paraformaldehyde, stained with 0.1% crystal violet and
counted.
Tumorigenesis in nude mice
All animal experiments were approved by the Animal
Care and Use Committee of Central South University. RACK1-specific
shRNA lentivirus, or non-specific shRNA lentivirus, were purchased
from Santa Cruz Biotechnology, Inc. Ten million U87 cells, which
were without any treatment or infected with RACK1-specific shRNA
lentivirus, or non-specific shRNA lentivirus, were injected
subcutaneously into 8-week old nude mice (n=5), which were
maintained under pathogen-limited conditions. The xenograft tumors
were measured at 10, 15, 20, 25 and 30 days. We calculated the
tumor volume according to V (mm) = D2 (mm2) × L (mm)/2,
where D is the smallest perpendicular tumor diameters while L is
the largest perpendicular tumor diameters. The animals were
sacrificed at 30 days after implantation. All tumors were
photographed and weighed.
Statistical analysis
Data are presented as means ± standard deviation
(SD). SPSS 17.0 software was used to perform statistical analyses
using a two-tailed Student’s t-test or one-way ANOVA. P<0.05 was
considered to indicate statistically significant differences. All
experiments were repeated at least 3 times.
Results
Upregulation of RACK1 in human glioma
tissues and glioma cell lines
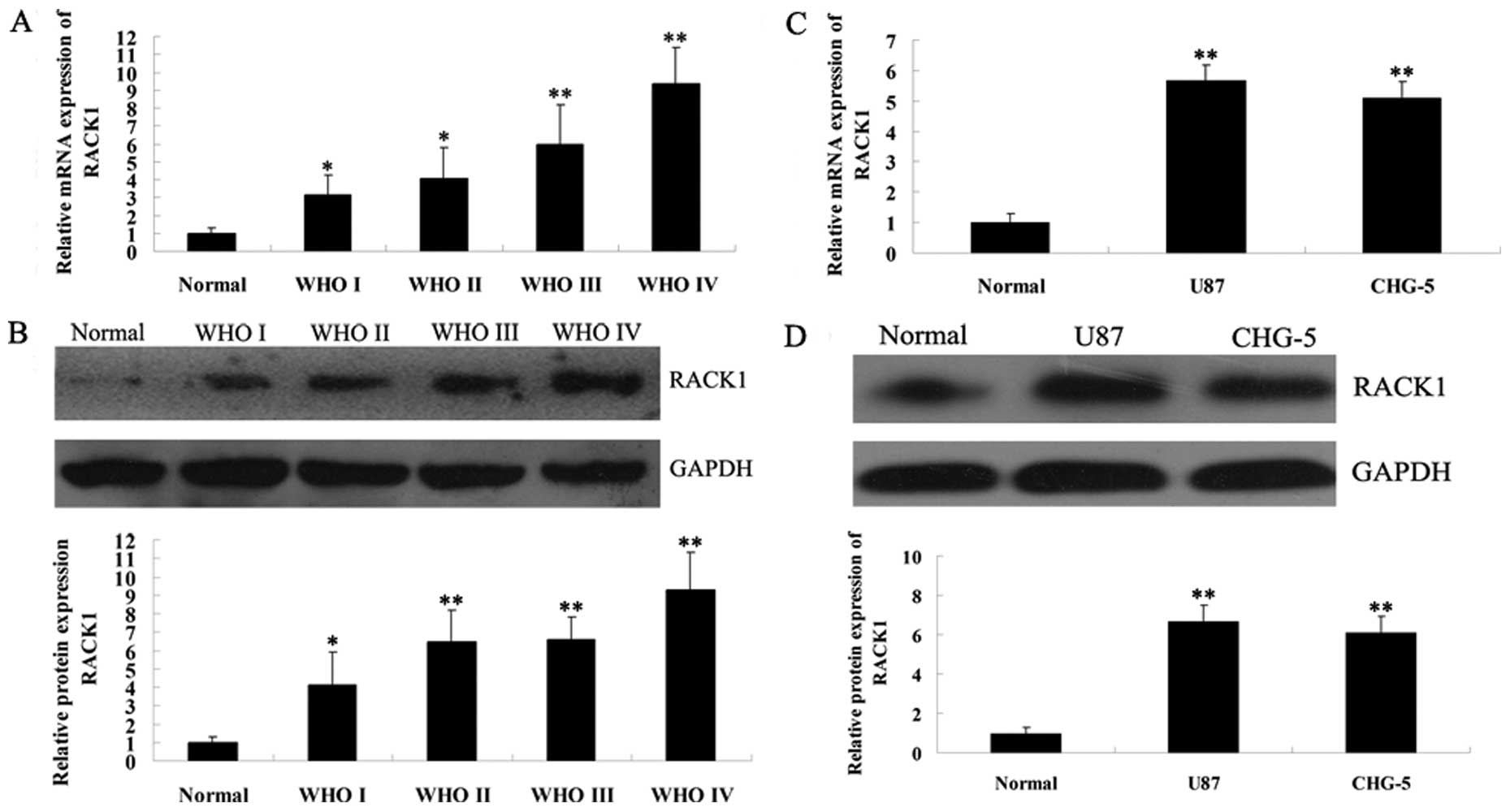
To preliminarily investigate the relationship
between RACK1 expression and glioma, we first examined the mRNA and
protein expression levels of RACK1 in normal brain tissues, gliomas
of different grades and 2 glioma cell lines. As shown in Fig. 1A and B, real-time PCR and western
blotting results showed that the expression of RACK1 showed an
increasing tendency with the malignancy of glioma. We further
determined the expression of RACK1 in 2 human glioma cell lines,
U87 (grade IV) and CHG-5 (grade II). As shown in Fig. 1C and D, real-time PCR and western
blotting results demonstrated that RACK1 expression was also
upregulated in U87 and CHG-5 cells compared to normal brain tissues
(P<0.01). However, there was no difference of RACK1 expression
between these 2 glioma cell lines (P>0.05). Accordingly, these
data above indicate that the increased expression of RACK1 may be
associated with the malignancy of glioma.
siRNA-induced RACK1 downregulation in U87
and CHG-5 cells
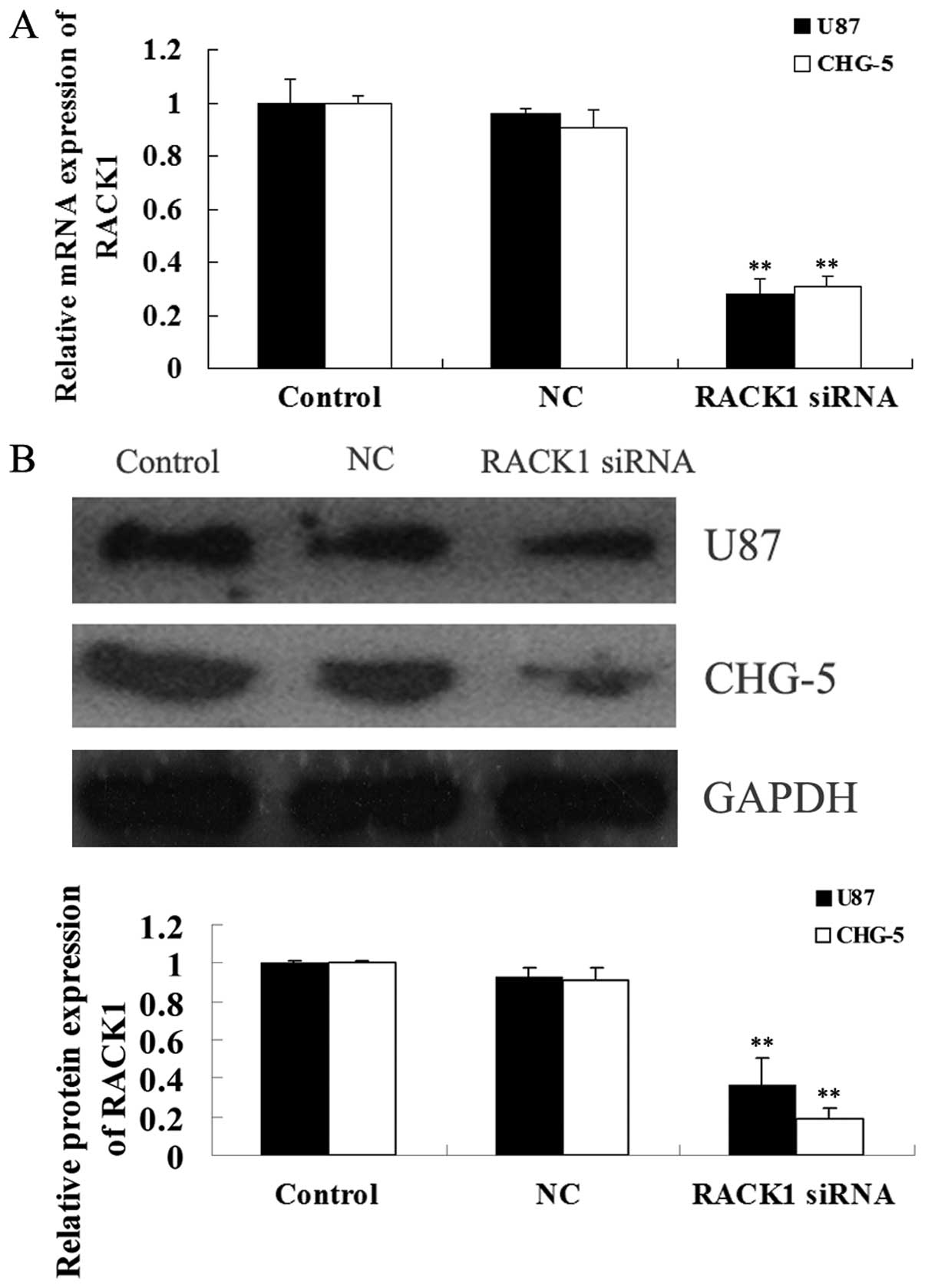
Due to the increased expression of RACK1 in glioma
tissues and cell lines, we used RACK1-specific siRNA to
downregulate the expression of RACK1 in U87 and CHG-5 cells to
further investigate the role of RACK1 in glioma. After
transfection, we first determined the mRNA and protein expression
of RACK1 in U87 and CHG-5 cells using real-time RT-PCR and western
blotting, respectively. As shown in Fig. 2, after transfection with
RACK1-specific siRNA, the mRNA and protein levels of RACK1 in U87
and CHG-5 cells were effectively downregulated (P<0.01), when
compared with those in the untreated (control) and the non-specific
siRNA group (NC).
siRNA-induced RACK1 downregulation
suppresses proliferation of glioma U87 and CHG-5 cells
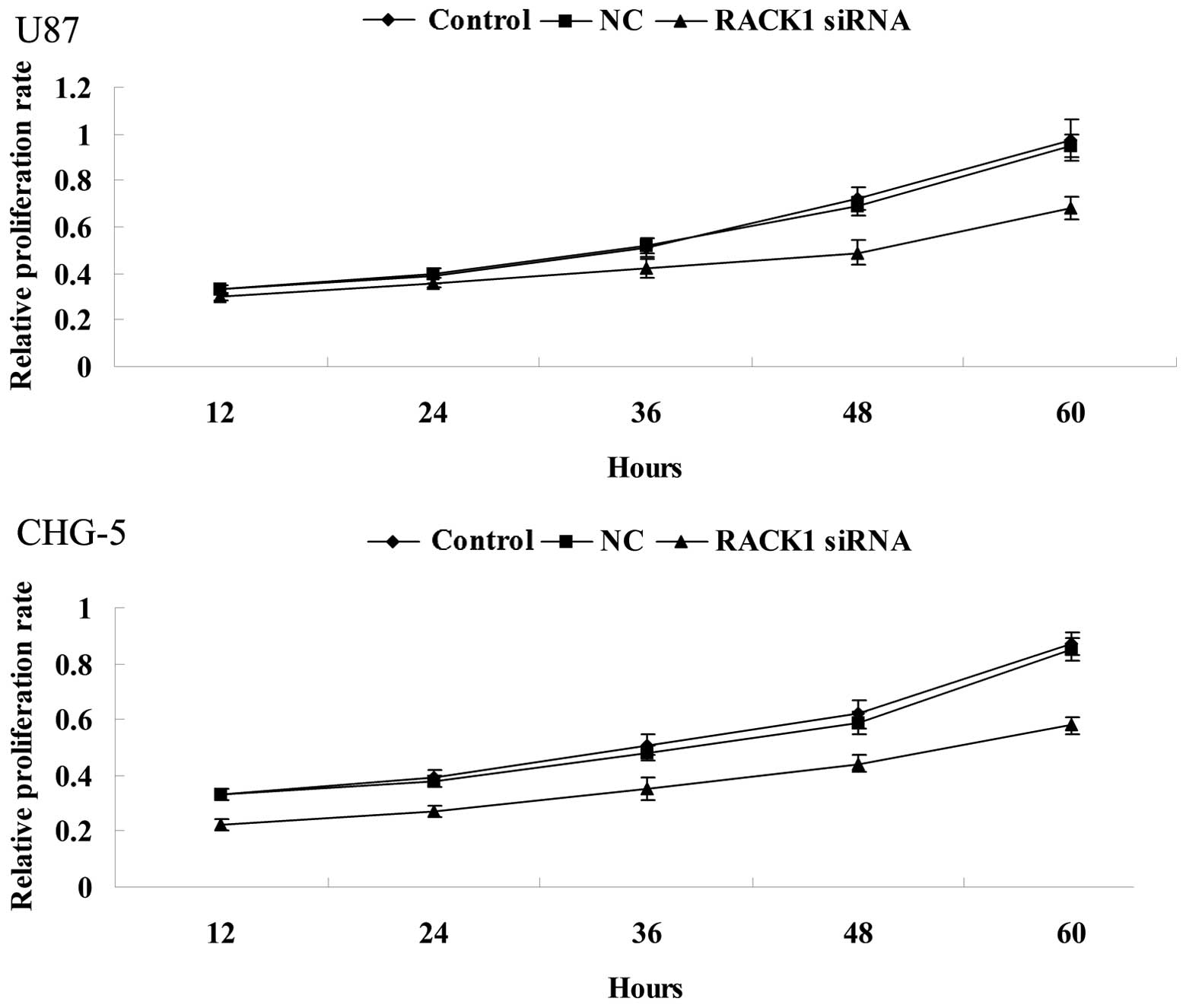
To further investigate the role of RACK1 in glioma
cells in vitro, MTT assay was performed to determine the
effect of siRNA-induced RACK1 downregulation on proliferation of
U87 and CHG-5 cells. As shown in Fig.
3, MTT assay demonstrated that in RACK1-downregulated U87 and
CHG-5 cells, the cell proliferation rate was lower when compared
with controls (P<0.01). These results suggest that RACK1
promotes proliferation of human glioma cells.
siRNA-induced RACK1 downregulation
enhances apoptosis of glioma U87 and CHG-5 cells
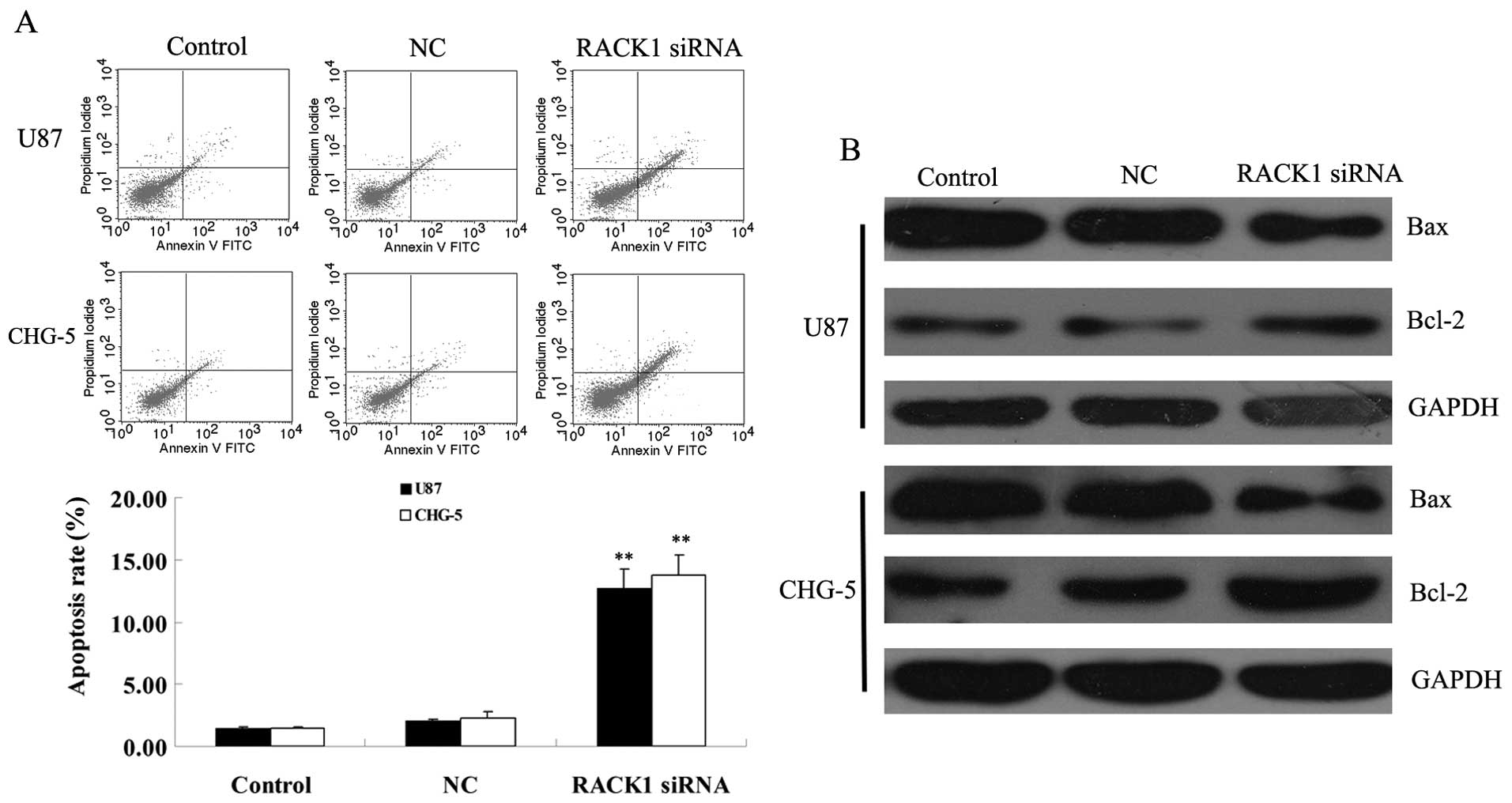
We further investigated the effect of RACK1
downregulation on apoptosis of glioma cells. As shown in Fig. 4, in RACK1-downregulated U87 and
CHG-5 cells, the cell apoptosis rate was much higher when compared
with controls (P<0.01), suggesting that RACK1 may play an
inhibitory role in the regulation of apoptosis of human glioma
cells. We then examined the expression of apoptotic-related genes
in each group. Real-time PCR assay showed that the mRNA expression
of pro-apoptotic gene Bax was upregulated, while the mRNA
expression of anti-apoptotic gene Bcl-2 was reduced, in
RACK1-downregulated glioma U87 and CHG-5 cells, when compared with
those in controls. These results indicated that RACK1 may suppress
apoptosis of glioma cells in vitro through directly or
indirectly regulating the expression of apoptotis-related genes,
Bax and Bcl-2.
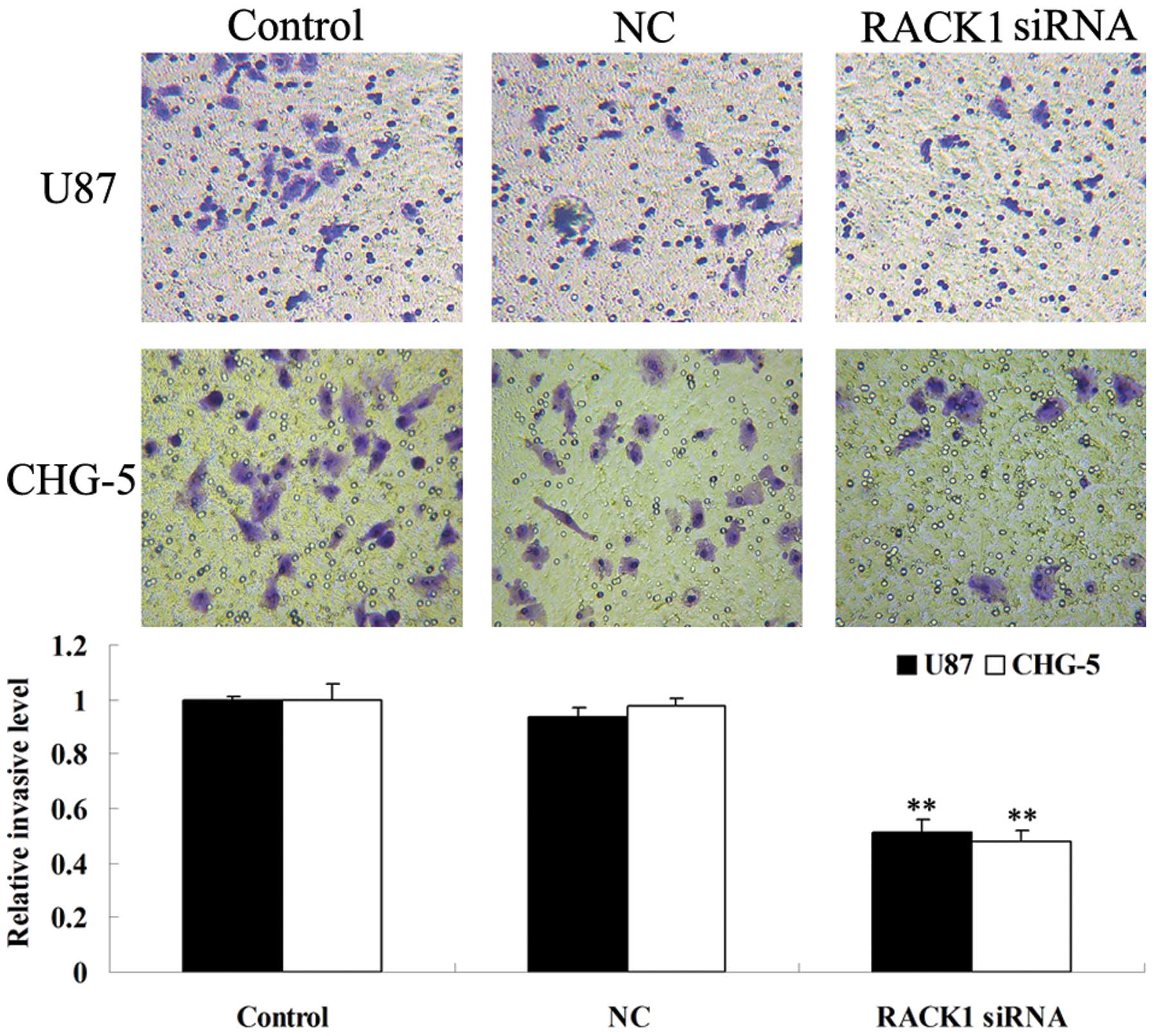
siRNA-induced RACK1 downregulation
inhibits invasion of glioma U87 and CHG-5 cells
The alternations of cell invasion ability of human
glioma U87 and CHG-5 cells were examined after transfection with
RACK1-specific siRNA. As shown in Fig.
5, RACK1-downregulated cells showed decreased invasion ability
when compared with controls (P<0.05). These data indicate that
RACK1 promotes invasion ability of human glioma cells in
vitro.
siRNA-induced RACK1 downregulation
suppresses survival, apoptosis, migration, and proliferation
relative signaling pathways in U87 and CHG-5 cells
Bax is a pro-apoptotic gene, while Bcl-2 has an
anti-apoptotic function. It has been well established that the
ratio of Bax/Bcl-2 protein plays a crucial role in regulating cell
apoptosis. Hence, we applied real-time RT-PCR to determine the mRNA
expression of Bax and Bcl-2 in U87 and CHG-5 cells of each group.
As shown in Fig. 6A, the relative
mRNA level of Bax was significantly upregulated in U87 and CHG-5
cells after transfection with RACK1-specific siRNA, when compared
with that in the control group (P<0.01). However, the relative
mRNA level of Bcl-2 was decreased in U87 and CHG-5 cells after
transfection with RACK1-specific siRNA, compared with that in the
control group (P<0.01). These data partly explain the above
findings that forced downregulation of RACK1 promotes the apoptosis
of U87 and CHG-5 cells.
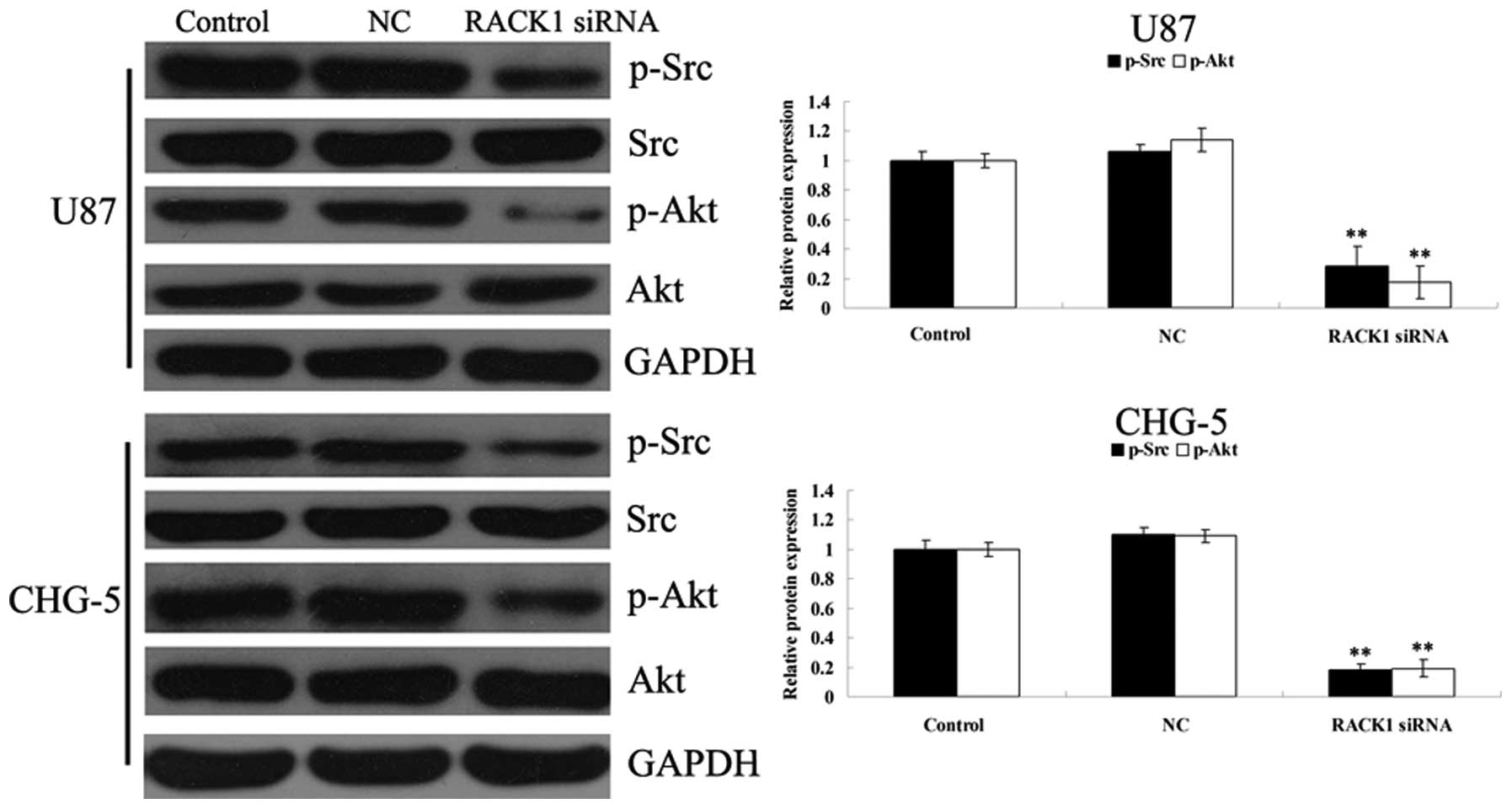
Src/Akt signaling pathway plays a crucial role in
the regulation of survival, proliferation and migration of multiple
types of cells. It has been reported that RACK1 induces colon cell
apoptosis, partly by suppressing Src activity in Akt pathway.
However, whether a similar molecular mechanism exists in glioma
cells remains unknown. Thus, we determined the activity of Src/Akt
signaling pathway in RACK1-downregulated U87 and CHG-5 cells. As
shown in Fig. 6B, the
phosphorylation levels of Src and Akt were much lower in U87 and
CHG-5 cells after transfection with RACK1-specific siRNA, when
compared with those in controls (P<0.05). These results indicate
that the effects of RACK1 on the survival, proliferation, invasion
of glioma cells may be involved in the regulation of the Src/Akt
signal pathway.
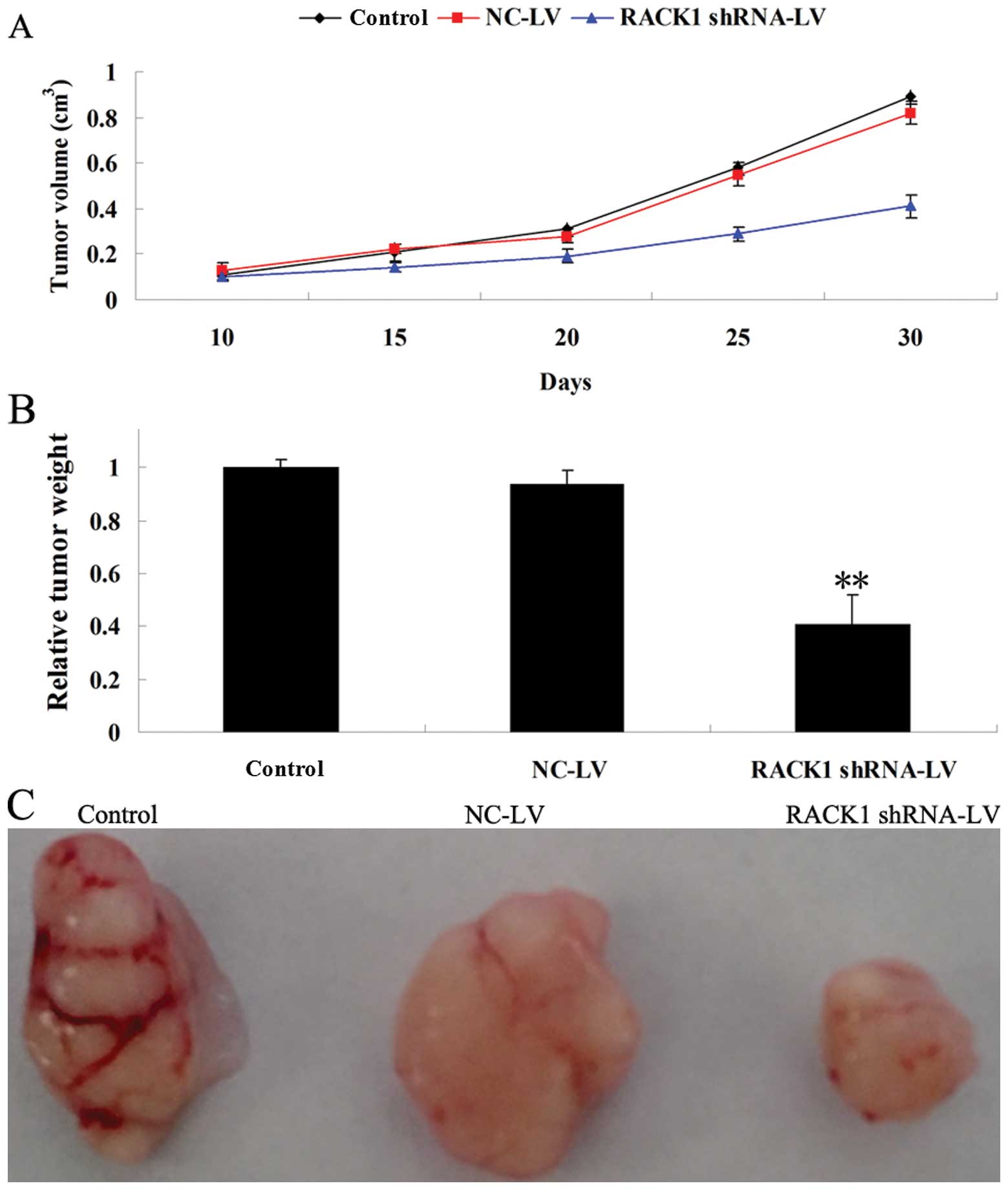
Forced downregulation of RACK1 suppresses
tumor xenograft growth in nude mice
To further investigate the role of RACK1 in
vivo, a tumor xenograft animal model was conducted using U87
cells, in which RACK1 was successfully knocked down by lentivirus
infection. After all animals were subcutaneously implanted with the
infected tumor cells, the size of tumors in the RACK1-specific
shRNA group was significantly smaller than that in the control and
NC group (Fig. 7A). As shown in
Fig. 7B, the average tumor weight
in the RACK1-specific shRNA group was markedly lower than that in
the control and NC group. Moreover, the tumor size in the
RACK1-specific shRNA group was much smaller than that in the
control and NC group (Fig. 7C).
These results are consistent with those of the cell proliferation
assay, indicating that forced downregulation of RACK1 significantly
inhibits tumor growth, and that RACK1 plays a positive regulatory
role in glioma growth in vivo.
Discussion
As a receptor for activated PKC, RACK1 has effects
on multiple cellular processes, such as cell proliferation,
apoptosis and migration (7–9). As a result, its dysregulation may
contribute to tumorigenesis. In fact, RACK1 has been demonstrated
to be involved in various types of malignant tumors including
hepatocellular carcinoma, lung cancer, colon cancer, prostate
cancer, cervical carcinoma, oral squamous cell carcinoma and
neuroblastoma (13–19). However, whether RACK1 plays a role
in malignant glioma as well as the mechanism involved remains
largely unknown.
In the present study, we first examined the
expression of RACK1 in glioma tissues of different grades as well
as 2 glioma cell lines, U87 and CHG-5. We found that the RACK1
expression was significantly higher in glioma tissues and cell
lines when compared to normal brain tissues. It is worth noting
that the expression of RACK1 was gradually upregulated with the
malignancy of glioma, indicating that RACK1 is associated with the
development and progression of gliomas, and hence may become a
promising therapeutic target for this cancer.
Based on these clinical findings, we speculated that
forced downregulation of RACK1 may suppress the development of
gliomas. To test this hypothesis, RACK1-specific siRNA was applied
to successfully downregulate the RACK1 expression in human glioma
U87 and CHG-5 cells. As expected, RACK1 downregulation
significantly inhibited the proliferation and invasion in U87 and
CHG-5 cells. The Bcl-2 family plays an essential role in the
regulation of cell survival and apoptosis, including both
pro-apoptotic members such as Bax, as well as anti-apoptotic
members such as Bcl-2. The balance between Bax and Bcl-2 determines
the susceptibility of cells to the apoptotic signal (20). We found that siRNA-induced RACK1
downregulation notably promoted cell apoptosis of U87 and CHG-5
cells, partly through suppressing Bax expression and upregulating
Bcl-2 expression, and hence breaking this balance for maintaining
cell survival.
Based on these findings in vitro, we further
applied tumor xenograft animal models to test whether RACK1
downregulation has an inhibitory effect on tumor growth in
vivo, and found that stable downregulation of RACK1 in human
glioma U87 cells markedly suppressed the tumor growth in
vivo. These findings suggest that RACK1 may play a crucial role
in tumorigenesis and progression of glioma in vitro and
in vivo.
Src is a protein tyrosine kinase and participates in
the regulation of multiple cellular processes including cell
survival, proliferation and migration (21,22).
Accumulating evidence has revealed that Src could be recruited by
RACK1, and acts as an oncogene in some types of cancer including
glioma (23–26). Akt, also known as protein kinase B
(PKB), acts in downstream of Src and plays an important role in the
regulation of cell survival, proliferation and migration (27,28).
However, whether Src/Akt signaling activity is involved in
RACK1-mediated glioma development has yet to be investigated. The
present study demonstrated that forced RACK1 downregulation
suppressed the activity of Src/Akt signaling pathway in U87 and
CHG-5 cells. These findings suggest that siRNA-induced RACK1
downregulation inhibits glioma development partly via suppressing
Src/Akt signaling activity. Our results are consistent with the
findings of Mamidipudi and Cartwright (29) that RACK1 promotes mitochondrial cell
death and blocked Akt-mediated cell survival, partly via
suppressing Src activity, in colon cancer cells, indicating that
this regulatory mechanism may exist in multiple types of cancer
cells.
In conclusion, the present study indicated that the
upregulation of RACK1 is a common event in glioma, and that RACK1
plays a critical role for glioma development and progression in
vitro and in vivo. Moreover, the underlying mechanism
involves RACK1-mediated SRC/Akt signaling activity. Thus, the
present study suggests that RACK1 may be a novel promising
therapeutic target for glioma treatment.
References
|
1
|
Stewart LA: Chemotherapy in adult
high-grade glioma: a systematic review and meta-analysis of
individual patient data from 12 randomised trials. Lancet.
359:1011–1018. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu VF, Yang J, Lebrun DG and Li M:
Understanding the role of cytokines in Glioblastoma Multiforme
pathogenesis. Cancer Lett. 316:139–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sathornsumetee S, Reardon DA, Desjardins
A, Quinn JA, Vredenburgh JJ and Rich JN: Molecularly targeted
therapy for malignant glioma. Cancer. 110:13–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Linz U: Commentary on effects of
radiotherapy with concomitant and adjuvant temozolomide versus
radiotherapy alone on survival in glioblastoma in a randomised
phase III study: 5-year analysis of the EORTC-NCIC trial (Lancet
Oncol 2009;10:459–466). Cancer. 116:1844–1846. 2010.PubMed/NCBI
|
|
5
|
Pulkkanen KJ and Yla-Herttuala S: Gene
therapy for malignant glioma: current clinical status. Mol Ther.
12:585–598. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dorn GW 2nd and Mochly-Rosen D:
Intracellular transport mechanisms of signal transducers. Annu Rev
Physiol. 64:407–429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adams DR, Ron D and Kiely PA: RACK1, a
multifaceted scaffolding protein: structure and function. Cell
Commun Signal. 9:222011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wan L, Xie Y, Su L, Liu Y, Wang Y and Wang
Z: RACK1 affects morphine reward via BDNF. Brain Res. 1416:26–34.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Battaini F, Pascale A, Paoletti R and
Govoni S: The role of anchoring protein RACK1 in PKC activation in
the ageing rat brain. Trends Neurosci. 20:410–415. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marqués N, Sesé M, Cánovas V, et al:
Regulation of protein translation and c-Jun expression by prostate
tumor overexpressed 1. Oncogene. Mar 04–2013.(Epub ahead of print).
View Article : Google Scholar
|
|
11
|
Guo Y, Wang W, Wang J, et al: Receptor for
activated C kinase 1 promotes hepatocellular carcinoma growth by
enhancing mitogen-activated protein kinase kinase 7 activity.
Hepatology. 57:140–151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao XX, Xu JD, Xu JW, et al: RACK1
promotes breast carcinoma migration/metastasis via activation of
the RhoA/Rho kinase pathway. Breast Cancer Res Treat. 126:555–563.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trerotola M, Li J, Alberti S and Languino
LR: Trop-2 inhibits prostate cancer cell adhesion to fibronectin
through the β1 integrin-RACK1 axis. J Cell Physiol. 227:3670–3677.
2012.PubMed/NCBI
|
|
14
|
Wu J, Meng J, Du Y, et al: RACK1 promotes
the proliferation, migration and invasion capacity of mouse
hepatocellular carcinoma cell line in vitro probably by PI3K/Rac1
signaling pathway. Biomed Pharmacother. 67:313–319. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruan Y, Sun L, Hao Y, et al: Ribosomal
RACK1 promotes chemoresistance and growth in human hepatocellular
carcinoma. J Clin Invest. 122:2554–2566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li G, Ji XD, Gao H, et al: EphB3
suppresses non-small-cell lung cancer metastasis via a
PP2A/RACK1/Akt signalling complex. Nat Commun. 3:6672012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu F, Zhang C, Wu WJ and Wu YM: RACK1
downregulation suppresses migration and proliferation of
neuroblastoma cell lines. Oncol Rep. 27:1646–1652. 2012.PubMed/NCBI
|
|
18
|
Li J, Guo Y, Feng X, et al: Receptor for
activated C kinase 1 (RACK1): a regulator for migration and
invasion in oral squamous cell carcinoma cells. J Cancer Res Clin
Oncol. 138:563–571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang F, Osawa T, Tsuchida R, Yuasa Y and
Shibuya M: Downregulation of receptor for activated C-kinase 1
(RACK1) suppresses tumor growth by inhibiting tumor cell
proliferation and tumor-associated angiogenesis. Cancer Sci.
102:2007–2013. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Delft MF, Wei AH, Mason KD, et al: The
BH3 mimetic ABT-737 targets selective Bcl-2 proteins and
efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized.
Cancer Cell. 10:389–399. 2006.PubMed/NCBI
|
|
21
|
Gelman IH: Src-family tyrosine kinases as
therapeutic targets in advanced cancer. Front Biosci. 3:801–807.
2011.PubMed/NCBI
|
|
22
|
Aleshin A and Finn RS: SRC: a century of
science brought to the clinic. Neoplasia. 12:599–607.
2010.PubMed/NCBI
|
|
23
|
Doan AT and Huttenlocher A: RACK1
regulates Src activity and modulates paxillin dynamics during cell
migration. Exp Cell Res. 313:2667–2679. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huveldt D, Lewis-Tuffin LJ, Carlson BL, et
al: Targeting Src family kinases inhibits bevacizumab-induced
glioma cell invasion. PLoS One. 8:e565052013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stedt H, Alasaarela L, Samaranayake H, et
al: Specific inhibition of SRC kinase impairs malignant glioma
growth in vitro and in vivo. Mol Ther Nucleic Acids. 1:e192012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Creedon H and Brunton VG: Src kinase
inhibitors: promising cancer therapeutics? Crit Rev Oncog.
17:145–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sheppard K, Kinross KM, Solomon B, Pearson
RB and Phillips WA: Targeting PI3 kinase/AKT/mTOR signaling in
cancer. Crit Rev Oncog. 17:69–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hemmings BA and Restuccia DF: PI3K-PKB/Akt
pathway. Cold Spring Harb Perspect Biol. 4:a0111892012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mamidipudi V and Cartwright CA: A novel
pro-apoptotic function of RACK1: suppression of Src activity in the
intrinsic and Akt pathways. Oncogene. 28:4421–4433. 2009.
View Article : Google Scholar : PubMed/NCBI
|