Introduction
Gastric cancer (GC) incidence and mortality have
decreased. Yet, GC remains one of the major causes of
cancer-related mortality worldwide (1). Despite advances in diagnosis and
treatment, the 5-year survival rate of GC is only 20%, and
progressive behavior including invasion and metastasis remain major
contributors to GC-related morbidity and mortality (1). The progression of GC is a complex,
multistep process involving multiple genetic and epigenetic
alterations of oncogenes, tumor-suppressor genes, DNA repair genes,
cell cycle regulators and signaling molecules (2,3).
Apoptosis is an active mechanism of cell death
controlling many biologic events, including embryonic development,
differentiation and morphogenesis of tissues (4,5).
Normal tissue homeostasis requires a regulated balance between cell
proliferation and cell death (4,5). Loss
of apoptosis regulation can lead to a variety of diseases including
cancer. Increased resistance to apoptosis is an important hallmark
for the growth of many cancer cell types (6–8).
The inhibitor of apoptosis protein (IAP) family
consists of a group of intracellular proteins that are essential
for the regulation of apoptosis (9). IAPs bind directly and potentially
inhibit a complex array of cysteine aspartyl-specific proteases,
caspase-3, -7 and -9, which are responsible for apoptosis and which
are induced by diverse pro-apoptotic stimuli (9). Livin is one of the potent members of
the IAP family; it is undetectable in most normal tissues but is
upregulated in a wide variety of human cancers (10–13).
Livin promotes the invasion, growth and apoptotic resistance in a
variety of human cancer cells (10–13).
Livin overexpression is associated with poor prognosis and
resistance to radiotherapy and chemotherapy in several types of
human cancers (14–17). These findings have raised interest
in Livin as a potential novel therapeutic target for the treatment
of human cancers.
While still not completely resolved, several studies
have revealed various aspects of the biologic significance of Livin
in human GC (18–20). Livin is overexpressed in GC tissues,
when compared to its expression in normal gastric tissues adjacent
to cancer and benign gastric lesions, and its overexpression has
been associated with various prognostic variables (19,20).
Silencing of the Livin gene in human GC cells was reported to
induce apoptosis and render the cells more susceptible to
chemotherapeutic agents (20).
The present study applied small interfering RNA
(siRNA) targeting of the Livin gene to investigate the effect of
Livin knockdown on biologic behavior of human GC cell lines. In
addition, Livin expression was investigated in a well-defined
series of GC cases with long-term follow-up, with a focus on
patient survival.
Materials and methods
Patients and tissue samples
Twenty GC tissues and paired normal gastric tissues
were collected by endoscopic biopsy at Chonnam National University
Hwasun Hospital (Jeonnam, Korea) for use in the preparation of mRNA
and protein. For immunohistochemistry, tumor specimens were
collected from 149 consecutive patients who underwent surgery
between January 1998 and December 1999. None of the patients had
received preoperative radiotherapy or chemotherapy. All underwent
primary tumor resection with regional lymph node dissection.
Formalin-fixed and paraffin-embedded tissue blocks were selected by
viewing the original pathologic slides and choosing blocks that
showed the junction between normal gastric epithelium and the
tumor. The histologic grade was classified according to previously
established criteria (21,22). Tumor staging used the American Joint
Committee on Cancer (AJCC) staging system (23). Patient characteristics including
gender, age at time of surgery, tumor size, stage, survival and
follow-up information were obtained from hospital records and, when
necessary, by contact with attending pathologists and physicians.
The observation time was the interval between the time of surgery
and last contact (death or last follow-up). The study group
comprised 101 males and 48 females, with a mean ± standard
deviation (SD) age of 58.0±11.1 years (range, 25–83 years). The
mean ± SD size of the tumors was 4.5±2.9 cm (range, 0.2–20.0 cm).
The mean follow-up period was 100.1 months (range, 0–176.4 months).
All specimens were collected following the informed consent of
patients. The study was approved by the Ethics Committee of Chonnam
National University Hwasun Hospital.
Cell culture and siRNA transfection
Cell lines derived from AGS and SNU638 human GC
cells were obtained from the American Type Culture Collection
(Manassas, VA, USA). Cells were maintained in RPMI-1640
supplemented with 10% fetal bovine serum (both from Hyclone, Logan,
UT, USA) and 1% penicillin-streptomycin (Sigma-Aldrich, St. Louis,
MO, USA) in 5% CO2 at 37°C. Gene knockdown was performed
using the specific siRNA. Livin siRNA (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) and scramble siRNA (Qiagen, Valencia,
CA, USA) were transfected with Lipofectamine™ RNAiMAX (Invitrogen,
Carlsbad, CA, USA) for 48 h according to the manufacturer’s
recommendations.
Reverse transcription-polymerase chain
reaction (RT-PCR)
RNA isolation was performed using TRIzol reagent
(Invitrogen) following the instructions provided by the
manufacturer. Reverse transcription was carried out using 1 μg of
RNA and MMLV transcription reagents (Invitrogen) according to the
manufacturer’s recommendations. The amplification of specific DNA
was performed with Taq polymerase and specific primers for
Livin (5′-CACACAGGCCATCAGGACAAG-3′/5′-ACGGCACAAAGACGATGGAC-3′) and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (5′-ACCACAGTC
CATGCCATCAC-3′/5′-TCCACCACCCTGTTGCTGTA-3′, as an internal
control).
Western blotting
Total cell extracts were prepared using
Pierce® RIPA buffer (Thermo Scientific, Rockford, IL,
USA) with Halt™ protease and phosphatase inhibitor cocktail (Thermo
Scientific). Total cell extracts were separated on polyacrylamide
gels and then transferred to polyvinylidene fluoride membranes
(Millipore, Billerica, MA, USA). The specific proteins were blotted
with the primary antibody to Livin, Survivin and β-tubulin (Santa
Cruz Biotechnology, Inc.); extracellular signal-regulated kinase
(ERK), phospho-ERK, p38, phospho-p38, c-Jun NH2-terminal kinase
(JNK), phospho-JNK, cleaved caspase-3, -7, -9, cleaved
poly(ADP-ribose) polymerase (PARP), cyclin-dependent kinase 4
(CDK4), CDK6, cyclin D1, cyclin D3, cyclin B1, p21, p27, p57, p15
and p16 (Cell Signaling Technology, Danvers, MA, USA). The
membranes were developed using the enhanced chemiluminescence
detection system, horseradish peroxidase substrate (Millipore) and
a model LS-4000 luminescent image analyzer (FujiFilm, Tokyo,
Japan).
Cell invasion assay
The invasive ability was calculated as the number of
cells passing through the gelatin-coated Transwell filter chambers
(Corning, NY, USA). Viable cells (2×105) in 0.2% bovine
serum albumin (BSA) were seeded in the upper chambers of Transwell
units. Human plasma fibronectin (Calbiochem, La Jolla, CA, USA) as
a chemoattractant was added to 0.2% BSA located in the lower
chambers of the units. After 24 h of incubation in a 5%
CO2 humidified incubator, the cells on the upper surface
of each filter were carefully removed with a cotton swab, and cells
that had traversed the filter to invade the opposite surface of the
filter were stained with Diff-Quik (Sysmex, Kobe, Japan). The
number of invaded cells was determined in five random fields using
light microscopy, and the mean value was calculated from data
obtained from three separate chambers.
Cell migration assay
Cell migration was determined using the
Culture-Inserts (2×0.22 cm2; Ibidi, Regensburg,
Germany). To create a wound gap, cells were seeded on the
Culture-Inserts, which were gently removed using sterile tweezers
following a 24-h incubation. The progression of wound closure was
photographed using an inverted microscope. The distance between
gaps was normalized to 1 cm after capture of three random
sites.
Cell viability
Cell viability was determined by the EZ-CyTox
(tetrazolium salts, WST-1) cell viability assay kit (Daeil Lab
Service Co., Seoul, Korea). After application of WST-1 reagent at
37°C at determined times, cell viability was measured using an
Infinite M200 microplate reader with Magellan V6 data analysis
software (both from Tecan, Grödig, Austria). All assays were
performed three times in sets of three replicate wells.
Flow cytometric analysis
For Annexin V staining, live cells were washed in
phosphate-buffered saline (PBS) and then incubated with Annexin V
fluorescein isothiocyanate (FITC; R&D Systems, Minneapolis, MN,
USA). For cell cycle analysis, cells were incubated in 10 μg/ml
ribonuclease A (Sigma-Aldrich) and 50 μg/ml propidium iodide (PI)
at room temperature in the dark. BD Cell Quest® v3.3
(Becton-Dickinson, San Jose, CA, USA) and WinMDI v2.9 (The Scripps
Research Institute, San Diego, CA, USA) were used to analyze the
population of Annexin V-positive cells and sub-G1 phase.
Immunohistochemistry
Paraffin tissue sections from patients were
rehydrated through descending ethanol and were retrieved with
citrate buffer (pH 6.0). Thereafter, endogenous peroxidase activity
was quenched using Peroxidase-Blocking Solution (Dako, Carpinteria,
CA, USA) and the tissues were incubated with polyclonal rabbit
anti-human Livin in primary Diluent Solution (Invitrogen) overnight
at 4°C. After washing, antibody binding was visualized using a Dako
Real™ Envision horseradish peroxidase/3,3′-diaminobenzidine
detection system (Dako). Stained tissues were photographed using a
light microscope.
Evaluation of Livin expression
Assessment of immunostained specimens was performed
independently by two observers without knowledge of the
clinicopathological data. In the event of a discrepancy, a
consensus was reached after further evaluation. The intensity of
positive cancer cells was graded on a 4-point scale: 0, no staining
of cancer cells; 1, weak staining; 2, moderate staining; and 3,
strong staining. The percentage of staining of cancer cells was
rated on a 4-grade scale: 0, none; 1, <10%; 2, 10–50%; 3,
>50%. The intensity rating was multiplied by the percent
staining rating to obtain an overall score. The mean overall score
for 149 tumors analyzed was 4.0. This score was chosen as the
cut-off point for discrimination of Livin expression (>4,
positive expression; ≤4, negative expression).
Statistical analyses
For comparison of intergroups, data were derived
from at least three independent experiments. The data are presented
as means ± SD, and the Student’s t-test was used to determine
statistical significance. The χ2 test and Fisher’s exact
test, where appropriate, were used to compare expression of Livin
with various clinicopathological parameters. Actuarial survival
rates of patients with positive or negative Livin expression were
evaluated according to the Kaplan-Meier method and the differences
were tested with a log-rank test. The statistical software program
used was Statistical Package for the Social Sciences (SPSS/PC+
15.0; SPSS, Chicago, IL, USA). A P-value <0.05 indicated a
statistically significant difference.
Results
Inhibition of oncogenic behavior of GC
cells by Livin siRNA
We investigated the biologic roles of the Livin gene
on oncogenic behavior using siRNA in AGS and SNU638 human GC cell
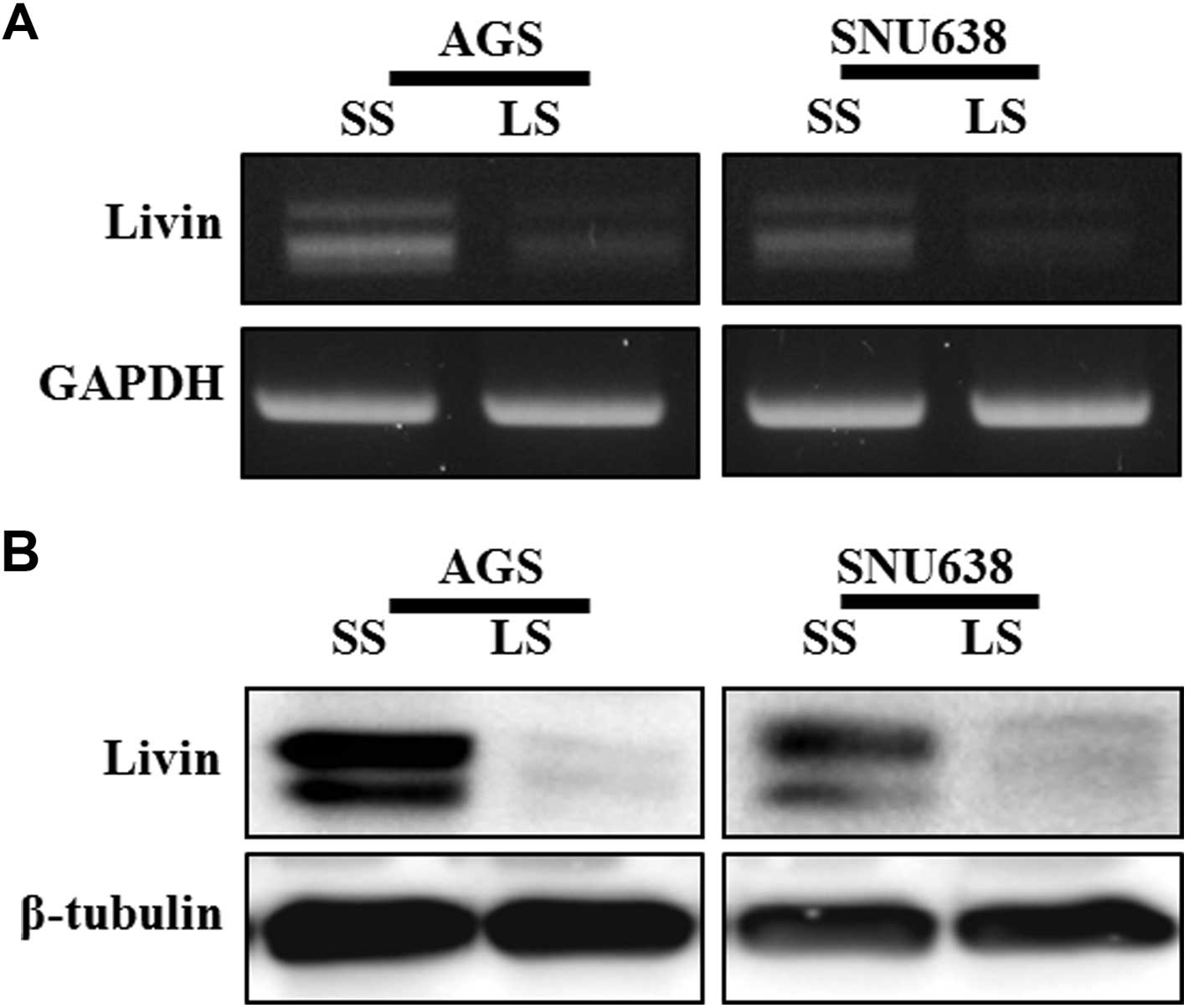
lines. Livin gene expression consistently showed a specific
reduction at the mRNA and protein levels in cells transfected with
Livin siRNA (Fig. 1). To evaluate
whether blocking Livin gene expression affects the oncogenic
behavior of GC cells, cell migration, invasion and proliferation
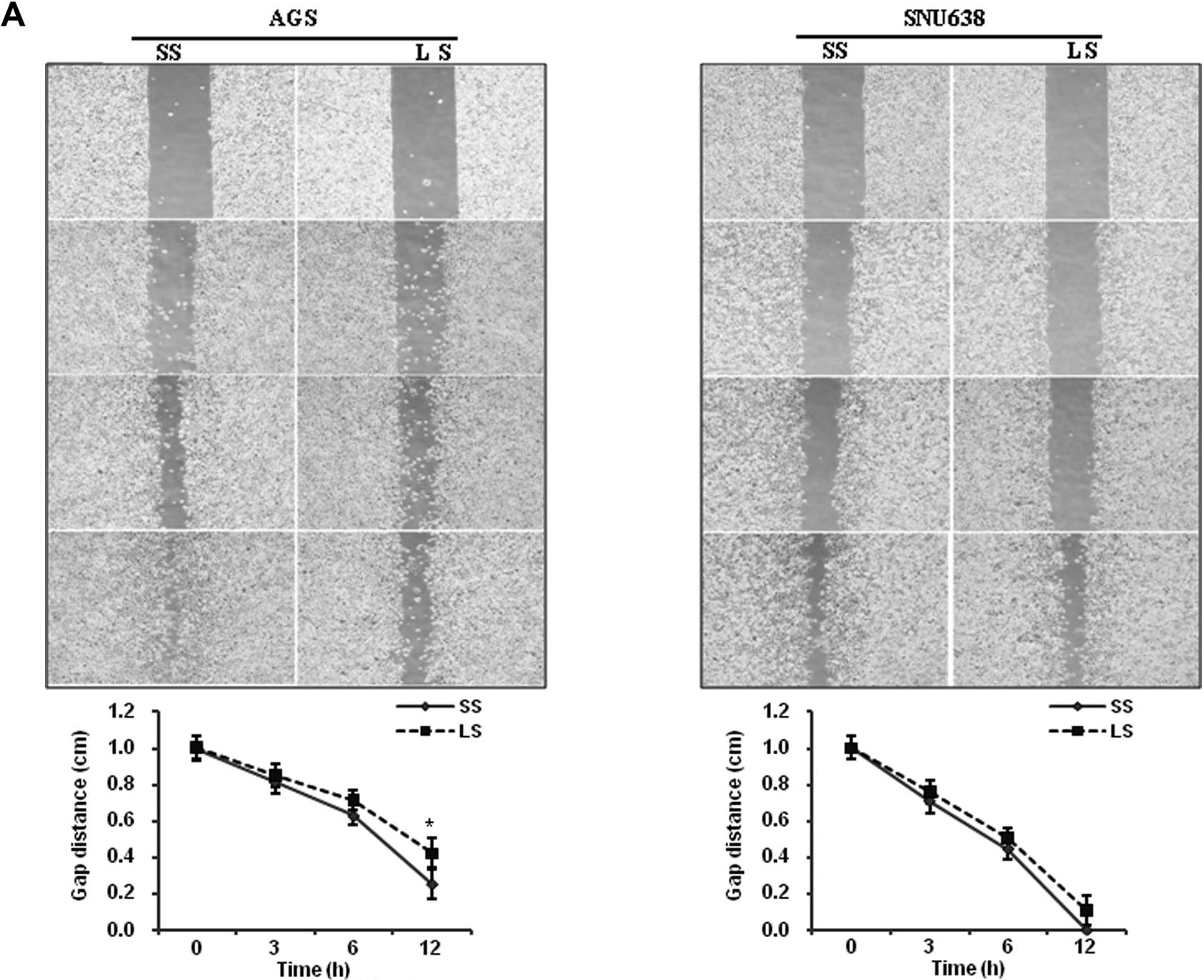
were assayed. The artificial wound gap in plates of the scramble
siRNA-transfected AGS cells was significantly narrower than that of
the Livin siRNA-transfected AGS cells at 12 h (P=0.049) (Fig. 2A). Transfection of Livin siRNA
inhibited AGS and SNU638 cell invasion from 1145.8±562.5 and
799.2±243.3 invaded cells/field (scramble siRNA) to 286.0±175.8 and
119.2±58.7 invaded cells/field (P=0.017 and P=0.002, respectively)
(Fig. 2B). Significant decreases in
cell proliferation were observed after 72 h in the Livin
siRNA-transfected AGS cells (P=0.022) and SNU638 cells (P=0.009),
when compared to the scramble siRNA-transfected cells (Fig. 2C).
Induction of apoptosis in GC cells by
Livin siRNA
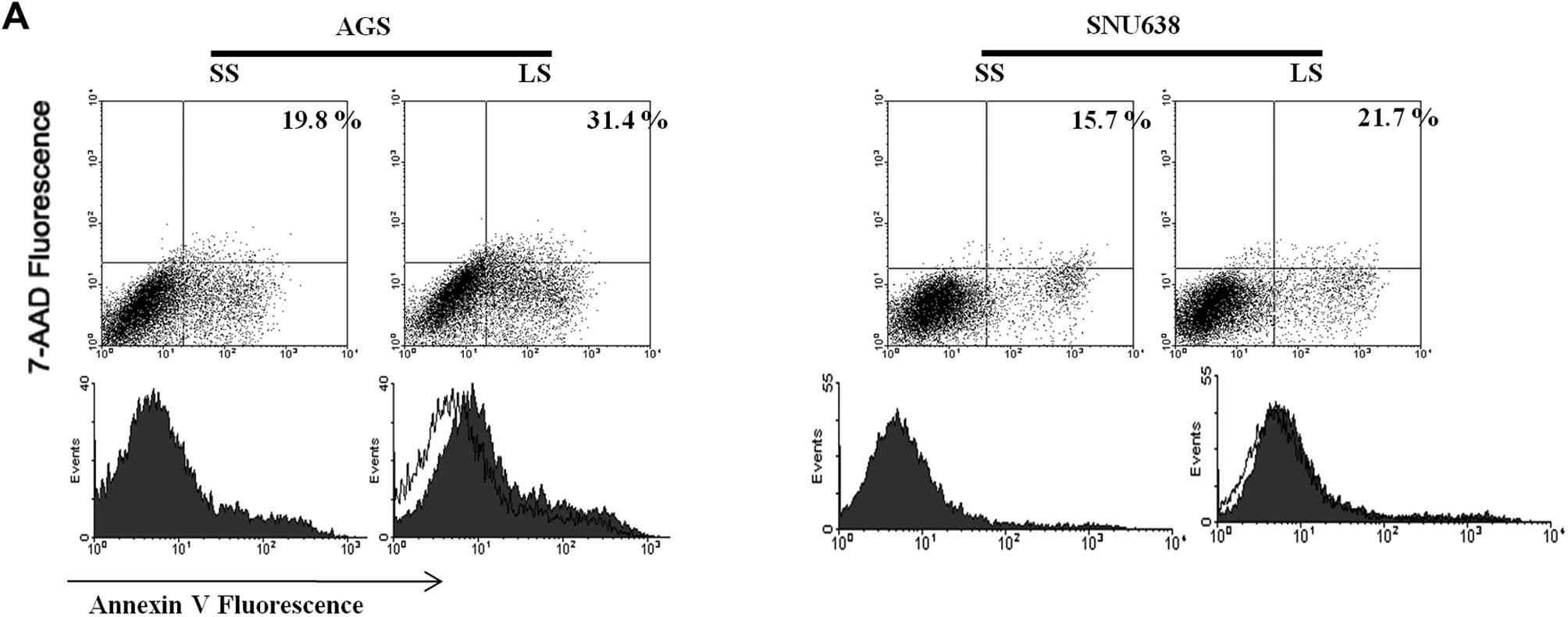
Apoptosis induced by transfection of siRNA was
assayed using flow cytometry. The cell apoptotic rate induced by
transfection of Livin siRNA was significantly increased, compared
with that induced by transfection of the scramble siRNA (19.8 vs.
31.4%) in AGS cells, but Livin knockdown had a minimal influence on
SNU638 cell apoptosis (15.7 vs. 21.7%) (Fig. 3A). Next, we investigated the
activation of caspases, which are critical mediators of apoptosis.
Expression of cleaved caspase-3, and -7 and PARP was upregulated in
the AGS and SNU638 cells after transfection with Livin siRNA. The
protein level of Survivin was reduced by transfection of Livin
siRNA in AGS and SNU638 cells (Fig.
3B).
Induction of cell cycle arrest in GC
cells by Livin siRNA
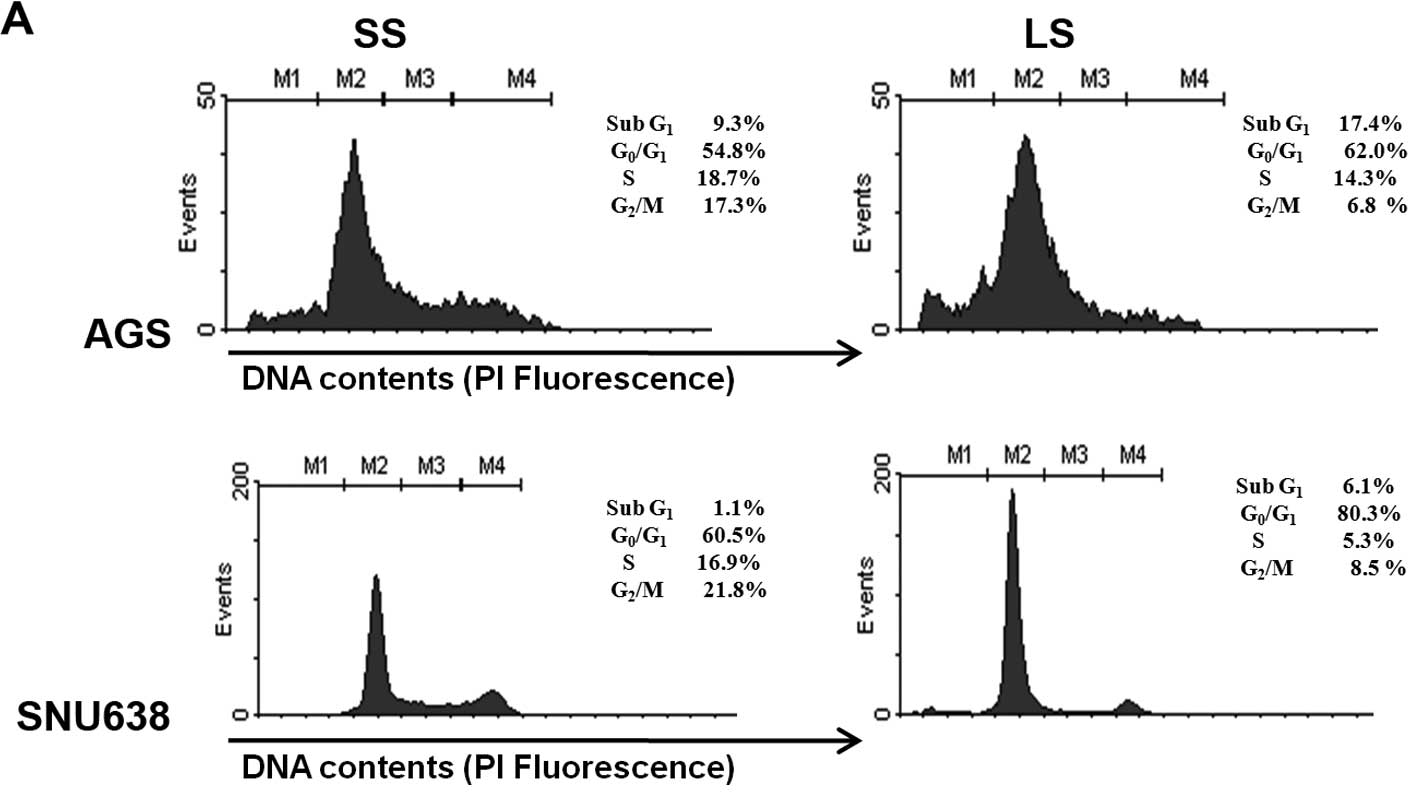
Flow cytometry was used to detect whether blocking
of Livin gene expression alters cell cycle distribution.
Transfection of Livin siRNA resulted in cell cycle arrest in the
G0/G1 phase of AGS and SNU638 cells (Fig. 4A). Next, the effects of Livin on
various CDK inhibitors (CDKIs), cyclins and CDKs, involved in cell
cycle progression were assessed. The cyclin D1, CDK4 and CDK6
protein levels were significantly decreased following transfection
of Livin siRNA in AGS and SNU638 cells, the p21 and p27 protein
levels were significantly increased, and the p57, p15 and p16
protein levels were not altered in response to Livin knockdown
(Fig. 4B).
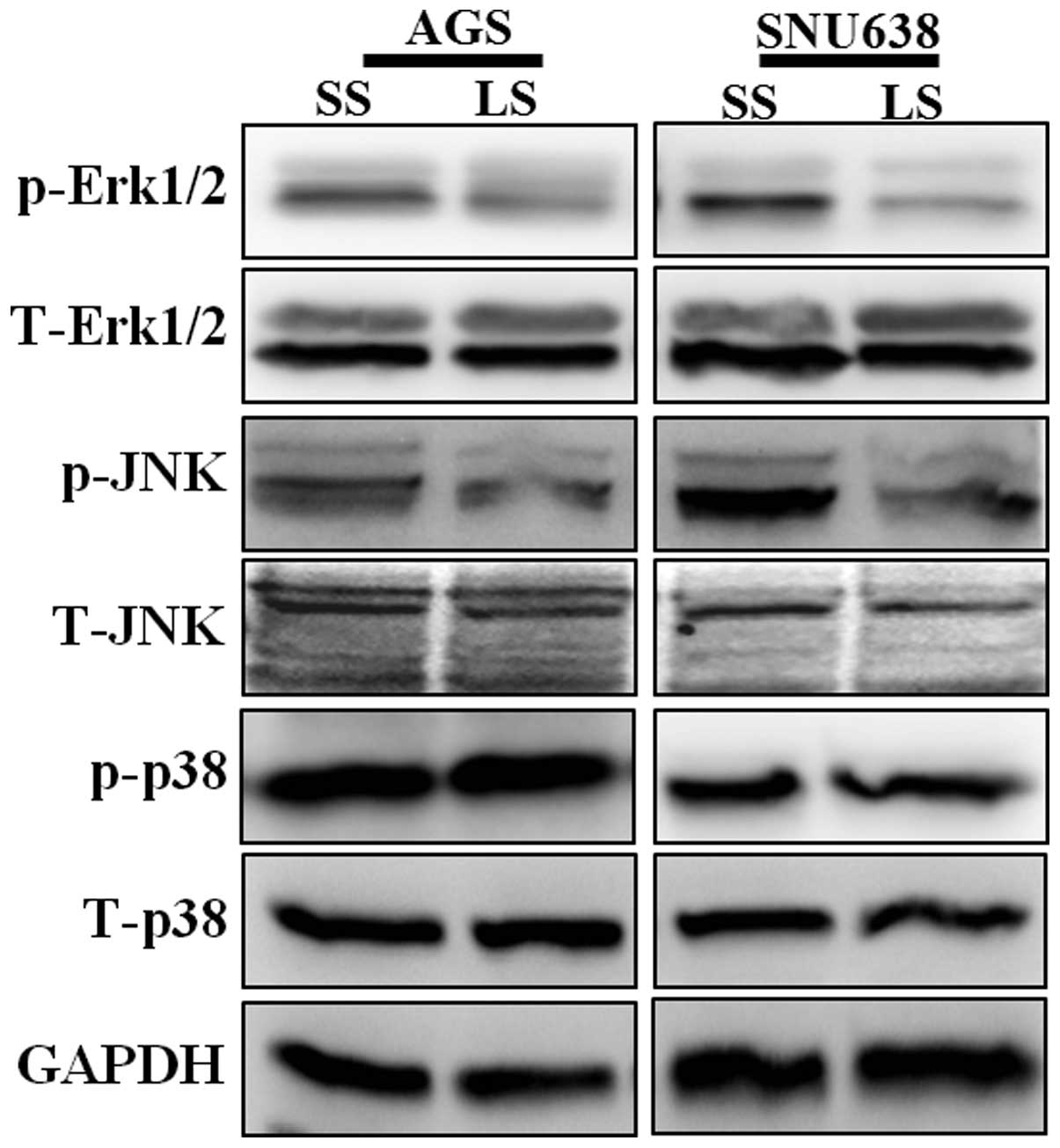
Impact of Livin knockdown on the
mitogen-activated protein kinase (MAPK) signaling pathway involved
in the apoptosis and cell cycle arrest of GC cells
The effect of Livin on stimulation of MAPK signaling
pathways leading to apoptosis and cell cycle arrest in AGS and
SNU638 cells was investigated. The phosphorylation levels of ERK1/2
and JNK were downregulated in Livin siRNA-transfected AGS and
SNU638 cells. The phosphorylation level of p38 was not altered by
transfection with Livin siRNA (Fig.
5).
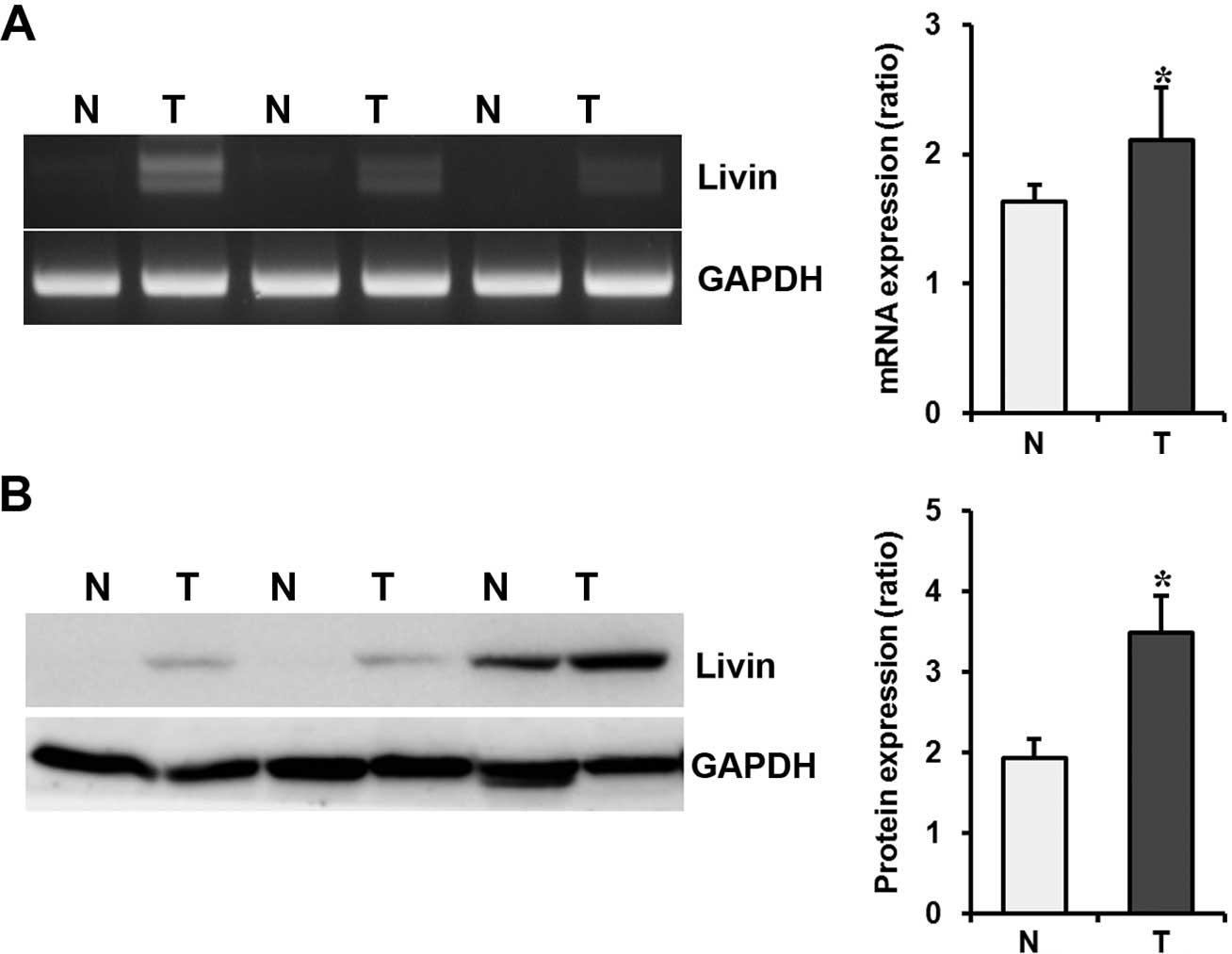
Livin mRNA and protein expression in GC
tissues
To confirm the results of the GC cell line studies,
the expression of Livin at the mRNA and protein levels was
evaluated by RT-PCR and Western blotting in human GC tissues,
paired normal gastric mucosa, and metastatic or non-metastatic
lymph node tissues of the same patients acquired by endoscopic
biopsy and from surgical specimens. In endoscopic biopsy specimens,
Livin expression was upregulated in cancer tissues when compared to
that in the paired normal mucosa at the mRNA and protein levels
(P=0.006 and P=0.040, respectively) (Fig. 6). In surgical specimens,
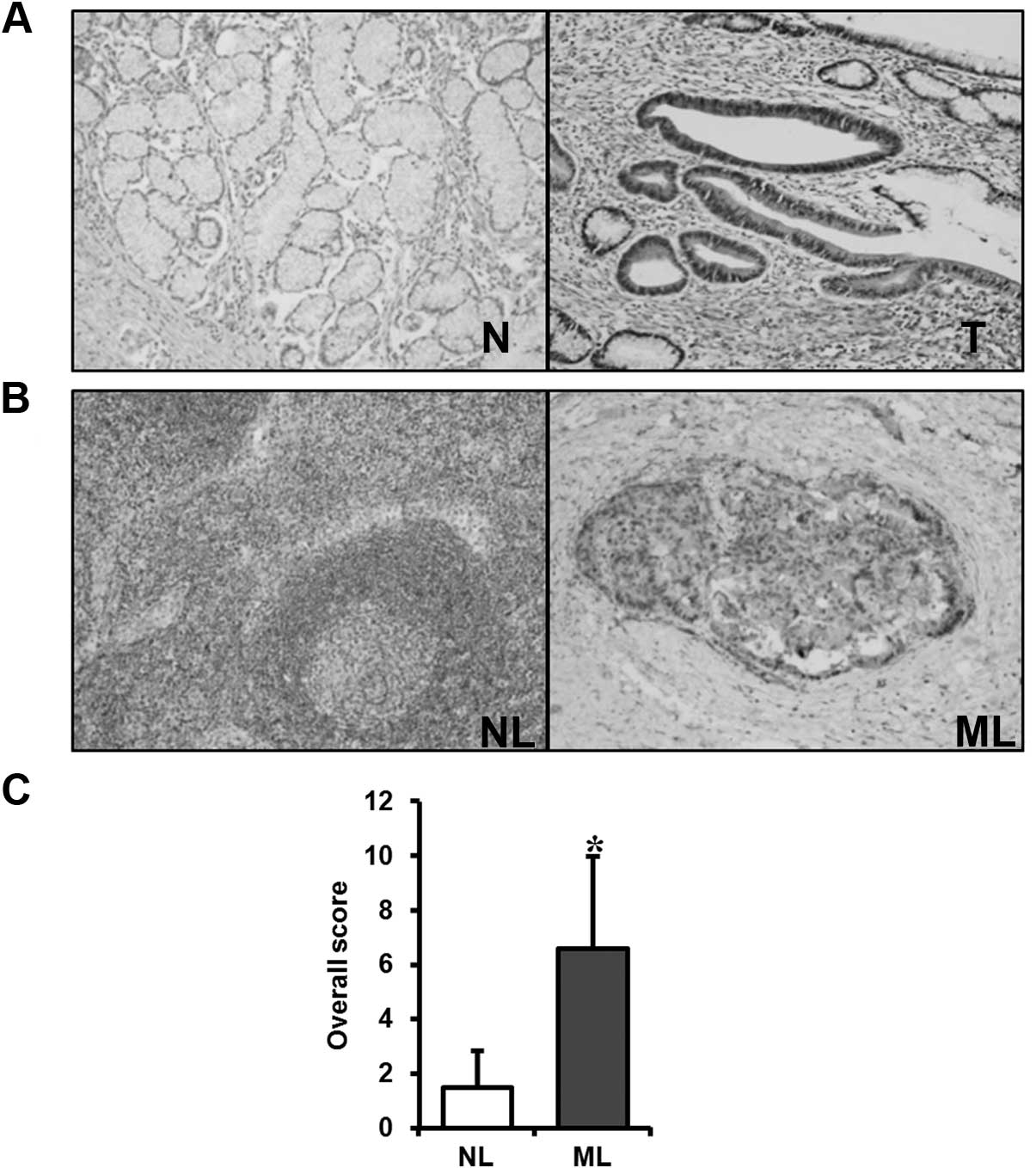
immunohistochemical staining of Livin protein was undetected or
only weakly stained in the normal gastric mucosa.
Immunohistochemical staining of the GC specimens localized Livin
expression to the cancer cells, with no expression evident in the
stromal compartment of the cancers (Fig. 7A). Immunohistochemical staining of
Livin in metastatic lymph node tissues was significantly stronger
than that in non-metastatic lymph node tissues (Fig. 7B). The score for immunohistochemical
staining of Livin in metastatic lymph node tissues was
significantly higher than that in non-metastatic lymph node tissues
(P<0.001) (Fig. 7C).
Correlation between Livin expression and
clinicopathological parameters in GCs
To study the prognostic role of Livin in GC
progression, we investigated the association between expression of
the Livin protein immunohistochemically in formalin-fixed,
paraffin-embedded tissue blocks obtained from 149 GC patients and
the clinicopathological data, including survival. Expression of
Livin protein was detected in 56 of the 149 (37.6%) GCs analyzed
(Table I). The correlation between
Livin expression and clinicopathological parameters is summarized
in Table I. No significant
correlation was found between Livin expression and various
clinicopathological parameters including age, gender, tumor size,
Lauren classification, histologic grade, depth of invasion, lymph
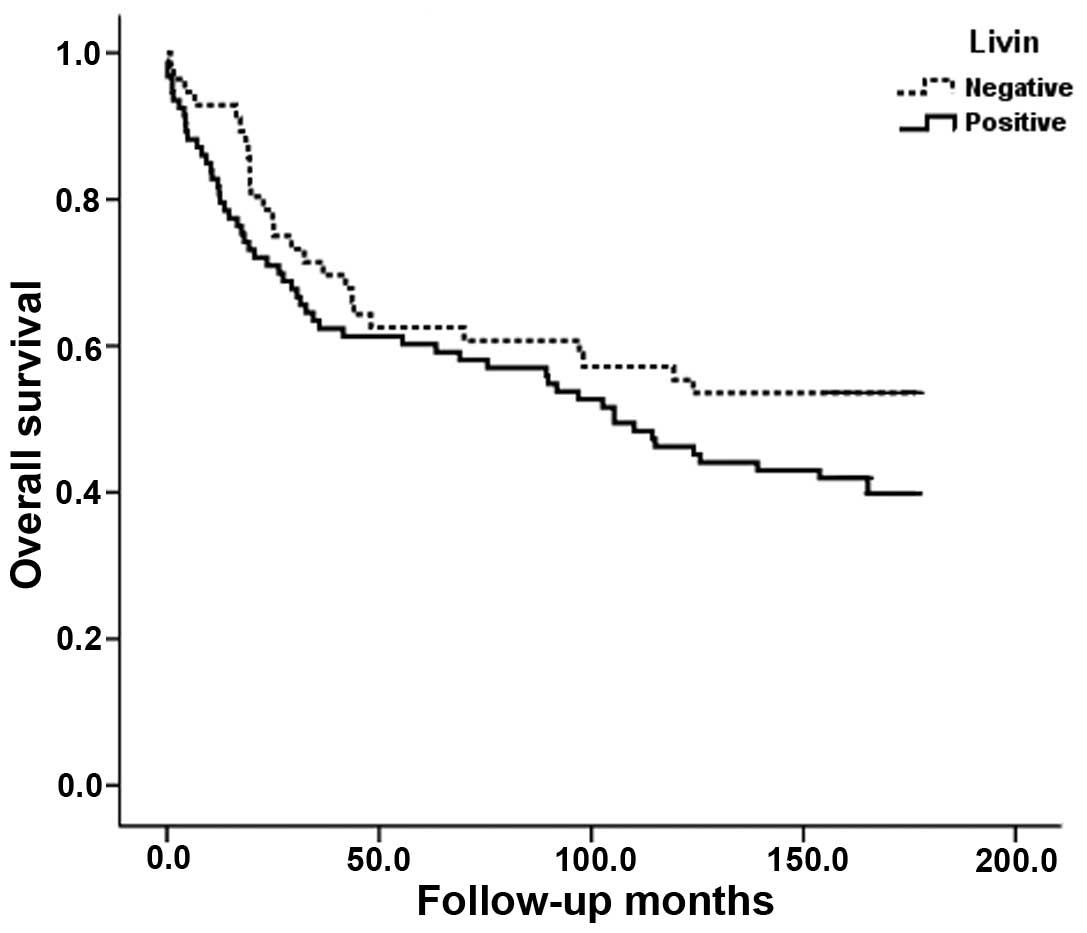
node metastasis, distant metastasis, or stage. Analysis of the
survival for all patients showed that Livin expression did not
correlate with survival (P=0.144) (Fig.
8).
 | Table ICorrelation between the Livin
expression and the clinicopathological parameters of gastric
cancer. |
Table I
Correlation between the Livin
expression and the clinicopathological parameters of gastric
cancer.
| | Livin | |
|---|
| |
| |
|---|
| Total (n=149) | Negative (n=93) | Positive (n=56) | P-value |
|---|
| Age (years) | | | | 0.457 |
| <58.0 | 62 | 36 | 26 | |
| ≥58.0 | 87 | 57 | 30 | |
| Gender | | | | 0.789 |
| Male | 101 | 63 | 38 | |
| Female | 48 | 30 | 18 | |
| Tumor size
(cm) | | | | 0.182 |
| <4.5 | 91 | 52 | 39 | |
| ≥4.5 | 58 | 41 | 17 | |
| Stage | | | | 0.255 |
| I | 73 | 41 | 32 | |
| II | 22 | 18 | 4 | |
| III | 34 | 19 | 15 | |
| IV | 20 | 15 | 5 | |
| Lauren
classfication | | | | 0.544 |
| Intestinal | 94 | 56 | 38 | |
| Diffuse | 55 | 37 | 18 | |
| Histologic
type | | | | 0.067 |
| WD | 45 | 27 | 18 | |
| MD | 15 | 8 | 7 | |
| PD | 89 | 58 | 31 | |
| Depth of invasion
(T) | | | | 0.863 |
| T1 | 65 | 38 | 27 | |
| T2 | 18 | 11 | 7 | |
| T3 | 54 | 36 | 18 | |
| T4 | 12 | 8 | 4 | |
| Lymph node
metastasis (N) | | | | 0.863 |
| N0 | 83 | 50 | 33 | |
| N1–3 | 66 | 43 | 23 | |
| Distant metastasis
(M) | | | | NA |
| M0 | 149 | 93 | 56 | |
| M1 | 0 | 0 | 0 | |
Discussion
Livin is a recently identified member of the IAP
family with a single baculovirus IAP repeat (BIR) domain and a
COOH-terminal ring domain, which plays an important role in
regulating apoptosis (10–13).
Many aspects of classical tumor biology research
have been investigated. Proposed hallmarks of cancer cells include
sustained proliferative signaling, selection of aggressive subtype
of cancer cells, replicative immortality, resistance to cell death
and deregulation of cellular energetics (6–8). In
the present study, Livin knockdown inhibited tumor cell migration,
invasion, proliferation, and induced apoptosis and cell cycle
arrest in GC cells. These results suggest that Livin may contribute
to GC cell invasion and metastasis.
Livin was previously found to inhibit apoptosis by
binding to caspase-3, -7 and -9, and its E3 ubiquitin-ligase
activity promotes the degradation of IAP antagonist SMAC/DIABLO
(10–13). In the present study, the expression
of cleaved caspase-3, -7 and PARP was upregulated in GC cells after
Livin knockdown. Therefore, Livin inhibits apoptosis by suppressing
the activity of caspases in GC cells.
Regulation of cell cycle progression appears to be
achieved principally by activity of cyclins, CDKs and CDKIs at the
G1/S and G2/M phase transitions (24,25).
Cell proliferation is achieved through the transition of cells from
G0/G1 arrest into the active cell cycle (24,25).
Dysregulation of cell cycle components may lead to tumor formation.
The formation of tumors occurs when genes such as cyclin, CDKs and
CDKIs mutate, causing cells to multiply uncontrollably (26–28).
In the present study, Livin knockdown induced cell cycle arrest in
the G0/G1 phase by decreasing the expression of cyclin D1, CDK4 and
CDK6, and by increasing p21 and p27 expression. Therefore, Livin
may contribute to GC progression via cell cycle dysregulation.
Livin was found to have anti-apoptotic potential
through the activation of JNK1 or MAPK signaling pathways (29–31).
Given this knowledge, we evaluated whether Livin knockdown induces
apoptosis and cell cycle arrest in GC cells via the regulation of
MAPK signaling pathways. We found that the ERK1/2 and JNK signaling
pathways were inhibited by Livin knockdown. These results suggest
that Livin may regulate GC cell behavior through the MAPK signaling
pathways.
Expression of Livin is pronounced in various human
cancer types including GC and has been linked with cancer
development and progression (14–20).
Appropriately, we evaluated the expression of Livin in GC tissues
and paired normal gastric mucosa of the same patients obtained by
endoscopic biopsy. Livin expression was significantly upregulated
in cancer tissues when compared to its expression in paired normal
mucosa at the mRNA and protein levels in fresh endoscopic biopsy
specimens, confirming previous findings (19,20).
These results suggest that Livin may play an important role in the
evolution of gastric carcinogenesis.
Livin expression was significantly upregulated in
metastatic lymph node tissues when compared to that in the
non-metastatic lymph node tissues in fresh surgical specimens.
These results suggest that Livin is associated with GC
progression.
Finally, we assessed the expression of Livin and its
prognostic relevance in a well-defined series of human GCs with
complete clinicopathological data including survival. No
significant correlation was found between Livin expression and
various clinicopathological parameters including age, gender, tumor
size, Lauren classification, histologic grade, depth of invasion,
lymph node metastasis, distant metastasis, or tumor stage.
Furthermore, Livin expression did not correlate with survival.
Previously, Livin expression was found to be associated with poor
differentiation and lymph node metastasis (19,20).
There are several possible explanations for this discrepancy.
First, the overall expression of Livin as reported in different
studies is difficult to compare due to different scoring systems
and different antibodies used. Second, the discrepancy may reflect,
in part, the relatively small sample size. Third, dysfunction of
genes is caused by a complex process of genetic mutation,
epigenetic alteration, and posttranscriptional modification.
Therefore, the expression of Livin as detected by
immunohistochemistry does not always imply its functional activity
in human cancers. Fourth, the steps involved in cancer development
and progression are not dependent on Livin-mediated apoptotic
regulation alone and are regulated by many biological processes
including growth, angiogenesis and invasion. Further studies are
warranted to clarify the impact of Livin on the biologic and
prognostic significance in GC.
Taken together, the data support the view that Livin
expression may play an important role in the evolution of gastric
carcinogenesis. The prognostic relevance of Livin in GC remains
unclear.
Acknowledgements
The present study was supported by a grant (0720570)
from the National R&D Program for Cancer Control, Ministry of
Health and Welfare, Korea.
References
|
1
|
Guggenheim DE and Shah MA: Gastric cancer
epidemiology and risk factors. J Surg Oncol. 107:230–236. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagini S: Carcinoma of the stomach: a
review of epidemiology, pathogenesis, molecular genetics and
chemoprevention. World J Gastrointest Oncol. 4:156–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tan IB, Ng I, Tai WM and Tan P:
Understanding the genetic basis of gastric cancer: recent advances.
Expert Rev Gastroenterol Hepatol. 6:335–341. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kiechle FL and Zhang X: Apoptosis:
biochemical aspects and clinical implications. Clin Chim Acta.
326:27–45. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schultz DR and Harrington WJ Jr:
Apoptosis: programmed cell death at a molecular level. Semin
Arthritis Rheum. 32:345–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brábek J, Mierke CT, Rösel D, Veselý P and
Fabry B: The role of the tissue microenvironment in the regulation
of cancer cell motility and invasion. Cell Commun Signal.
8:222010.PubMed/NCBI
|
|
8
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kenneth NS and Duckett CS: IAP proteins:
regulators of cell migration and development. Curr Opin Cell Biol.
24:871–875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan B: Research progress on Livin protein:
an inhibitor of apoptosis. Mol Cell Biochem. 357:39–45. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang L, Zhang Q, Liu B, Han M and Shan B:
Challenge and promise: roles for Livin in progression and therapy
of cancer. Mol Cancer Ther. 7:3661–3669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang H and Schimmer AD: Livin/melanoma
inhibitor of apoptosis protein as a potential therapeutic target
for the treatment of malignancy. Mol Cancer Ther. 6:24–30. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu B, Han M, Wen JK and Wang L:
Livin/ML-IAP as a new target for cancer treatment. Cancer Lett.
250:168–176. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gazzaniga P, Gradilone A, Giuliani L, et
al: Expression and prognostic significance of LIVIN, SURVIVIN and
other apoptosis-related genes in the progression of superficial
bladder cancer. Ann Oncol. 14:85–90. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hariu H, Hirohashi Y, Torigoe T, et al:
Aberrant expression and potency as a cancer immunotherapy target of
inhibitor of apoptosis protein family, Livin/ML-IAP in lung cancer.
Clin Cancer Res. 11:1000–1009. 2005.PubMed/NCBI
|
|
16
|
Kim DK, Alvarado CS, Abramowsky CR, et al:
Expression of inhibitor-of-apoptosis protein (IAP) livin by
neuroblastoma cells: correlation with prognostic factors and
outcome. Pediatr Devel Pathol. 8:621–629. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xi RC, Biao WS and Gang ZZ: Significant
elevation of survivin and livin expression in human colorectal
cancer: inverse correlation between expression and overall
survival. Onkologie. 34:428–432. 2011. View Article : Google Scholar
|
|
18
|
Yagihashi A, Asanuma K, Tsuji N, Torigoe
T, Sato N, Hirata K and Watanabe N: Detection of anti-livin
antibody in gastrointestinal cancer patients. Clin Chem.
49:1206–1208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang YZ, Fang TY, Xu HG and Zhuo ZQ:
Expression of CD44v6 and Livin in gastric cancer tissue. Chin Med J
(Engl). 125:3161–3165. 2012.PubMed/NCBI
|
|
20
|
Wang TS, Ding QQ, Guo RH, Shen H, Sun J,
Lu KH, You SH, Ge HM, Shu YQ and Liu P: Expression of livin in
gastric cancer and induction of apoptosis in SGC-7901 cells by
shRNA-mediated silencing of livin gene. Biomed Pharmacother.
64:333–338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lauren P: The two histologic main types of
gastric carcinoma: diffuse and so-called intestinal-type carcinoma.
An attempt at a histo-clinical classification. Acta Pathol
Microbiol Scand. 64:31–49. 1965.PubMed/NCBI
|
|
22
|
Watanabe H, Jass JR and Sobin LH: WHO
International Histologic Classification of Tumors: Histologic
Typing of Oesophageal and Gastric Tumors. 2nd edition.
Springer-Verlag; Berlin: 1990
|
|
23
|
American Joint Committee on Cancer. AJCC
Cancer Staging Manual: Stomach. Lippincott-Raven; Philadelphia: pp.
71–76. 1997
|
|
24
|
Morgan DO: Principles of CDK regulation.
Nature. 374:131–134. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Graña X and Reddy EP: Cell cycle control
in mammalian cells: role of cyclins, cyclin dependent kinases
(CDKs), growth suppressor genes and cyclin-dependent kinase
inhibitors (CKIs). Oncogene. 11:211–219. 1995.PubMed/NCBI
|
|
26
|
Tian Y, Wan H and Tan G: Cell
cycle-related kinase in carcinogenesis. Oncol Lett. 4:601–606.
2012.PubMed/NCBI
|
|
27
|
Curtin NJ: DNA repair dysregulation from
cancer driver to therapeutic target. Nat Rev Cancer. 12:801–817.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amoedo ND, El-Bacha T, Rodrigues MF and
Rumjanek FD: Cell cycle and energy metabolism in tumor cells:
strategies for drug therapy. Recent Pat Anticancer Drug Discov.
6:15–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen YS, Li HR, Lin M, Chen G, Xie BS, Xu
NL and Lin LF: Livin abrogates apoptosis of SPC-A1 cell by
regulating JNKI signaling pathway. Mol Biol Rep. 37:2241–2247.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sanna MG, Duckett CS, Richter BW, Thompson
CB and Ulevitch RJ: Selective activation of JNK1 is necessary for
the anti-apoptotic activity of hILP. Proc Natl Acad Sci USA.
95:6015–6020. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sanna MG, da Silva Correia J, Ducrey O,
Lee J, Nomoto K, Schrantz N, Deveraux QL and Ulevitch RJ: IAP
suppression of apoptosis involves distinct mechanisms: the
TAK1/JNK1 signaling cascade and caspase inhibition. Mol Cell Biol.
22:1754–1766. 2002. View Article : Google Scholar : PubMed/NCBI
|