Introduction
Lung cancer is the leading type of cancer worldwide
with high mortality rates in men and women alike, at 29 and 26%,
respectively (1). Non-small cell
lung carcinoma (NSCLC) is the most frequent subtype, representing
~85% of all cases, and most patients have locally advanced or
distant metastatic disease at the time of presentation (2). Although NSCLCs are relatively
insensitive to chemotherapy, compared to small cell carcinoma, and
are primarily treated by surgical resection with curative intent
(3), chemotherapy is increasingly
being used both pre-operatively (neoadjuvant chemotherapy) and
post-operatively (adjuvant chemotherapy) (3,4). Most
researchers believed that anticancer drugs for NSCLC may be found
from natural plants (5). Several
natural compounds have been found to be effective in inhibiting
NSCLC in the lab and in the clinic (5). Among them, paclitaxel, the most
typical compound from yew has been approved to be used clinically
for cancer patients. A number of other herbal compounds such as
xanthatin, lycobetaine, resveratrol, ursolic acid and lycorine are
also being investigated for NSCLC (5).
Sophora species (Leguminosae), an important
source of Chinese herbal drugs, has been used widely throughout
China for thousands of years. As a traditional Chinese herb, the
root of Sophora flavescens Ait. (Kushen) has long been
applied for the therapy of numerous diseases, such as hepatitis
(6), cardiac diseases (7) and skin diseases (8). Quinolizidine alkaloids have been found
to be its chief active components in Sophora flavescens
including matrine, oxymatrine, sophocarpine, sophoridine and other
alkaloids (9). Basic and clinical
studies have shown that these alkaloids possess a variety of
pharmacological effects including anti-inflammation (10,11),
immunity-regulation (12),
antivirus (6) and antitumor action
(13–15).
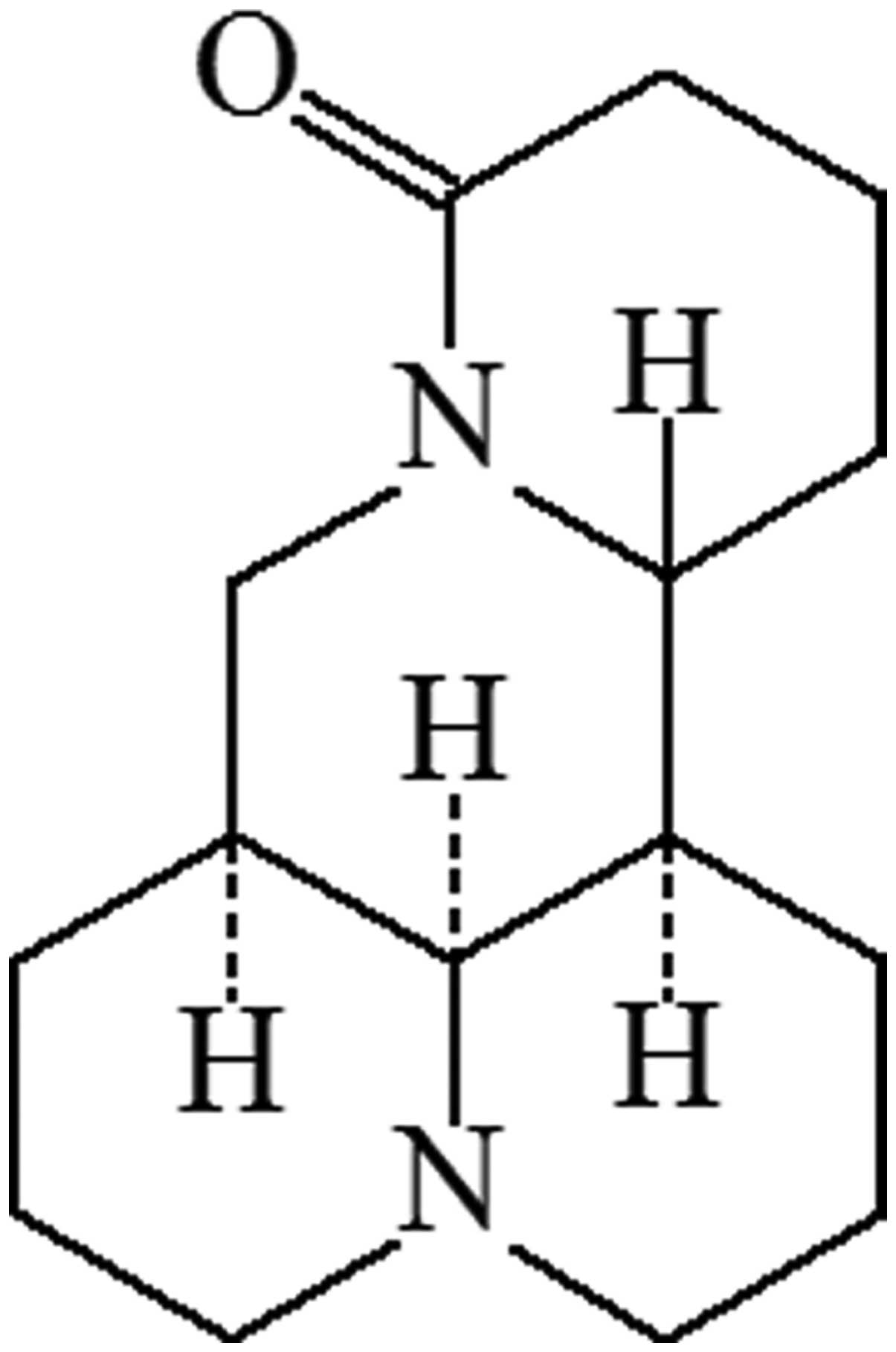
Matrine, with a molecular weight of 248.4 (Fig. 1), is the major quinolizidine
alkaloid and has been considered a major bioactive component of the
dried roots of Sophora flavescens. It has been found that
matrine possesses a wide range of pharmacological activities, and
its antitumor activity has attracted considerable attention in
recent years. Cumulative data have demonstrated that matrine exerts
anticancer effect on many series of human cancer cells including
lung cancer (16), hepatoma
(16), breast cancer (17), pancreatic cancer (18), prostate cancer (19,20)
and colon cancer (21).
Upregulation of protein E2F-1 and activation of caspases contribute
to matrine-induced leukemia cell proliferation inhibition and
apoptosis (15). For MDA-MB-231
breast cancer cells, matrine has been demonstrated to inhibit cell
proliferation by reducing the ratios of Bcl-2/Bax protein and mRNA
levels and to reduce cancer cell invasion by inhibiting the
VEGF-AKT-NF-κB signaling pathway, as well as by inactivating MMPs
(22).
In particular, several efforts focused on how
matrine inhibits lung cancer (16,23,24),
suggesting that it may be a potential anticancer agent for lung
cancer. Additionally, the results that matrine in combination with
anticancer drugs significantly inhibited SPCA-1 (24) or A549 cells (23) indicated its potent inhibitory effect
on NSCLC. However, although suppressing MAPK/ERK signal
transduction was demonstrated to be involved in matrine resisting
NSCLC (23), no other mechanisms
were confirmed. In this study, we found that matrine caused NSCLC
apoptosis due to induction of ROS generation and subsequent
activation of MAPK/p38 signaling pathway.
Materials and methods
Materials
Matrine was from Tianyuan Biological Agent Plant
(Xi’an, Shaanxi, China; purity, 98%). RPMI-1640 was purchased from
Mediatech (Herndon, VA, USA). Fetal bovine serum (FBS) was supplied
by HyClone (Logan, UT, USA), and 0.05% Trypsin-EDTA was from
Invitrogen Life Technologies (Grand Island, NY, USA).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was from Sigma-Aldrich (St. Louis, MO, USA). Annexin V-FITC
Apoptosis Detection kit I was from BD Biosciences (San Diego, CA,
USA). Enhanced chemiluminescence solution was from PerkinElmer Life
Sciences (Boston, MA, USA).
Carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone
(Z-VAD-FMK) was purchased from ALEXIS Biochemicals Corporation (San
Diego, CA, USA). The MAPK/p38 pathway inhibitor SB202190 was
obtained from LC Laboratories (Woburn, MA, USA). The following
antibodies were used: p38, phospho-p38, caspase-3, PARP, Bcl2, BAD
(all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
β-actin (Sigma).
Cell lines and culture
Human NSCLC cells A549, NCI-H358 were obtained from
the cell bank of the Chinese Academy of Sciences (Shanghai, China).
A549 and NCI-H358 cells were grown in antibiotic-free RPMI-1640
supplemented with 10% FBS and incubated in a humid incubator (37°C,
5% CO2).
Cell proliferation assay
Cells dispersed evenly in medium were seeded in a
6-well plate with a density of 1×105 cells/well. Next
day, cells were treated with various concentrations of matrine
(0–100 μM) for 48 h. After incubation, MTT (5 mg/ml) was added to
each well, followed by a 4 h incubation. The medium was discarded
and 150 μl of dimethyl sulfoxide (DMSO) was added into each well,
and incubated for 20 min. The optical density (OD) in 570 nm was
measured by a BioTek multilabel counter. The cell proliferation
inhibition index was calculated according to the formula:
(ODcontrol − ODmatrine/ODcontrol)
× 100%. The experiments were repeated 3 times.
Cell apoptosis analysis
A549 cells were seeded in 6-well plates at a density
of 2×106 cells/dish in RPMI-1640 supplemented with 10%
FBS and were grown overnight at 37°C in a humidified incubator with
5% CO2. Cells were treated with matrine (0–50 μM) for 48
h, followed by apoptosis assay using the Annexin V-FITC Apoptosis
Detection kit I. Cells without treatment were used as a
control.
Caspase-3/7 activity assay
Caspase-3/7 activity was measured by the Sensolyte
Homogeneous AMC caspase-3/7 assay kit (AnaSpec Systems, San Jose,
CA, USA) according to the manufacturer’s instructions. Briefly,
cells were seeded in black 96-well plates and cultured for 24 h
followed by treatment with matrine (0–50 μM) for 24 h. Then, 50
μl/well of caspase-3/7 substrate solution was added into each well.
The reagents were mixed completely by shaking and the reaction was
incubated at room temperature for 1 h. Finally, fluorescence
intensity was measured at Ex/Em=354/442 nm using a BioTek
multilabel counter.
ROS assay
A549 and NCI-H358 cells were respectively seeded at
a density of 1×104 cells/well in 96-well plates. Cells
were incubated in the presence of various concentrations of matrine
(0–50 μM) for 24 h or 30 μM matrine for the indicated time (0–24 h)
with 6 replicates of each treatment followed by loading with
CM-H2DCFDA following the manufacturer’s protocol. In
some cases, cells were pretreated with N-acetyl-L-cysteine (NAC; 5
mM) for 1 h, and then treated with/without matrine (30 and 50 μM)
for 24 h, followed by loading with CM-H2DCFDA for 2 h.
Fluorescent intensity was measured at Ex/Em=485/535 nm using a
BioTek multilabel counter.
Western blot analysis
Treated cells were briefly washed 2 times with cold
PBS. Cells were lysed in the lysis buffer [50 mM Tris, pH 7.2; 150
mM NaCl; 1% sodium deoxycholate; 0.1% sodium dodecyl sulfate (SDS);
1% Triton X-100; 10 mM NaF; 1 mM Na3VO4;
protease inhibitor cocktail (1:1,000; Sigma)]. Lysates were
sonicated for 2×15 sec and centrifuged at 13,000 × g for 2 min at
4°C. Protein concentration was determined by bicinchoninic acid
assay with bovine serum albumin as standard (Pierce Biotechnology,
Inc., Rockford, IL, USA). Western blotting was carried out as
previously described. The antibodies used were described above.
Statistical analysis
The results are expressed as the mean values ±
standard error (means ± SE). A one-way analysis of variance (ANOVA)
was used to examine differences among the matrine groups.
Differences were considered statistically significant when
P<0.05.
Results
Matrine inhibits proliferation and
induces apoptosis in NSCLC cells
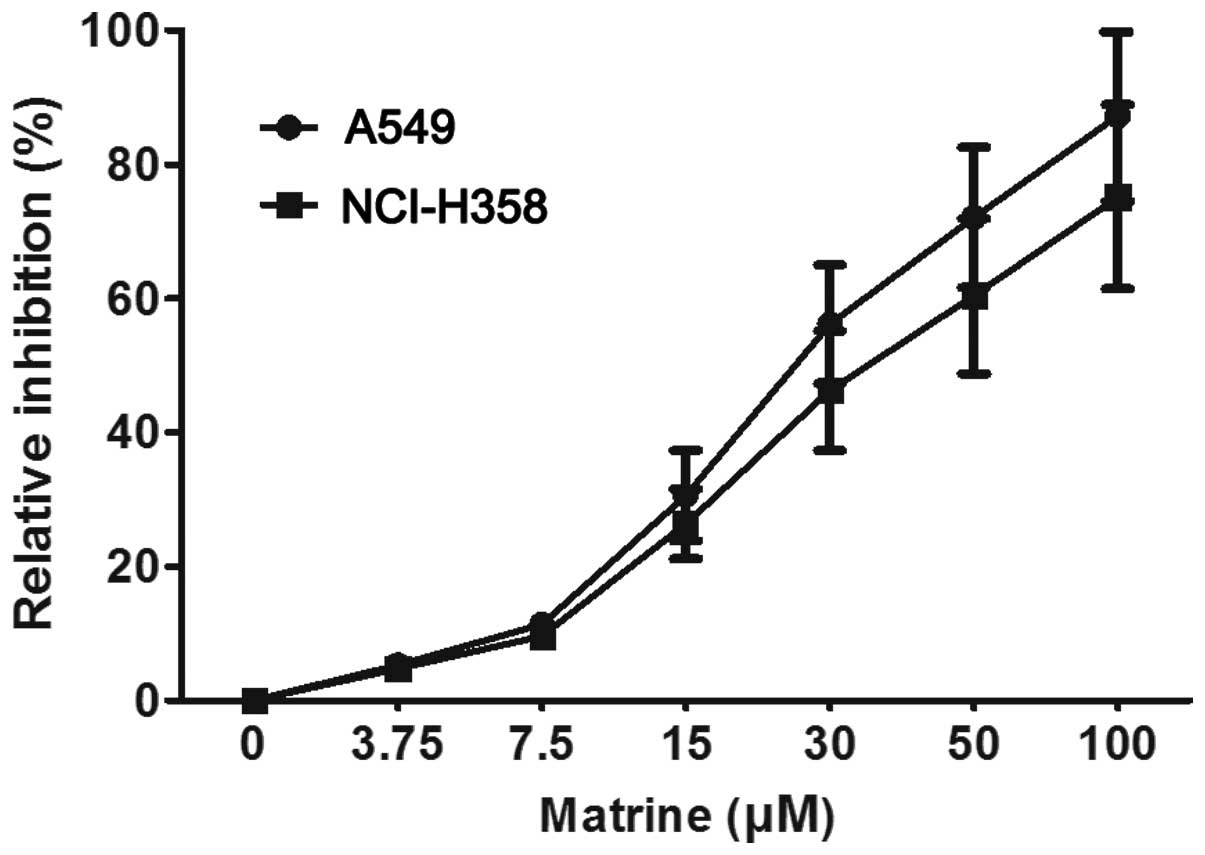
As shown in Fig. 2,
relative inhibition curves induced by matrine in two lung cancer
cells increased in a dose-dependent manner. With a logarithmic
regression analysis, the concentration of matrine which results in
50% of maximal proliferation inhibition (IC50) of cells
was calculated. The IC50 value was ~25.0 μM for A549
cells, and ~34.6 μM for NCI-H358 cells.
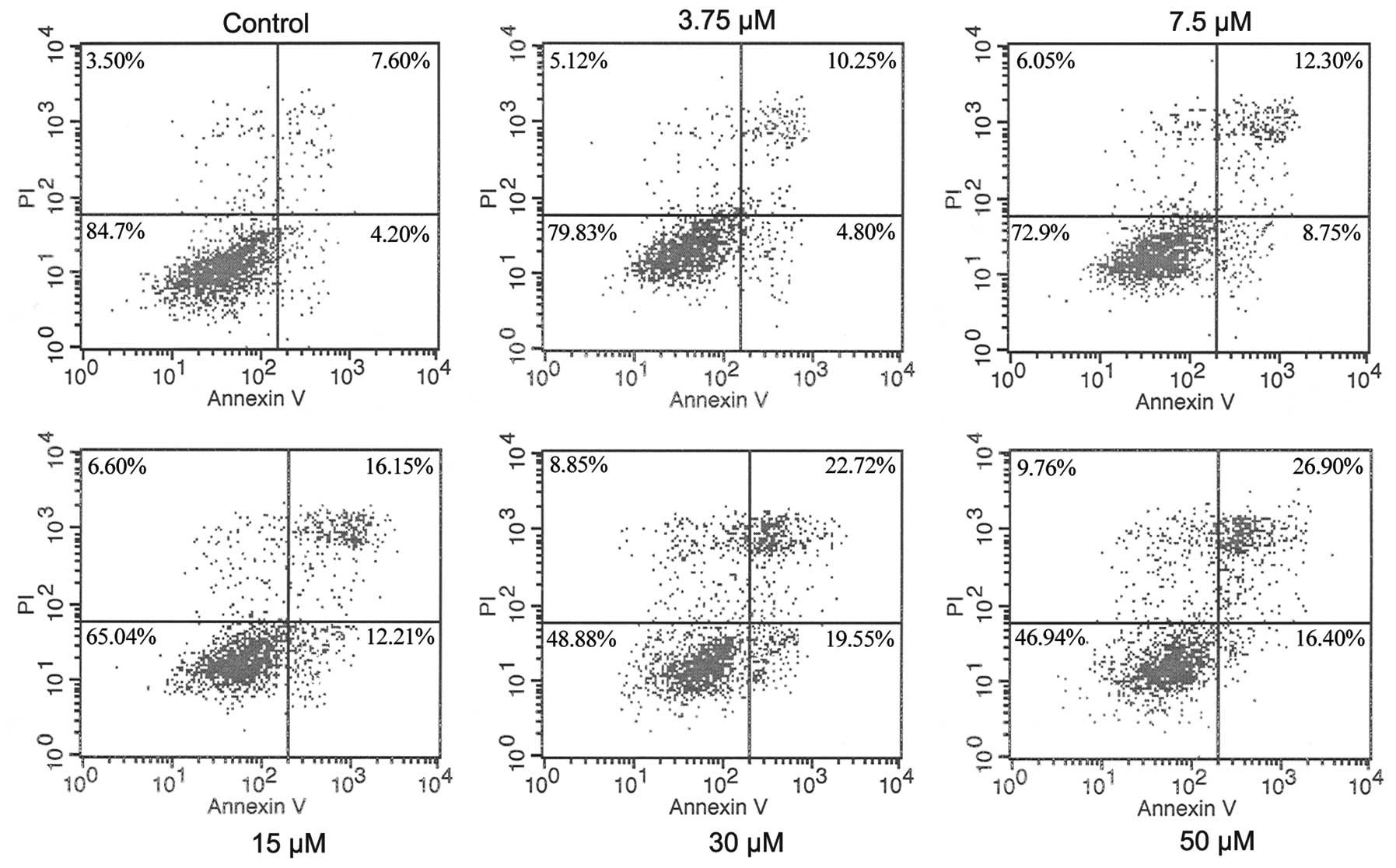
To examine whether matrine-inhibited cell
proliferation is related to induction of cell apoptosis, Annexin
V-FITC and PI staining were used. As indicated in Fig. 3, the rate of apoptotic cells
increased significantly (more than ~40%) from 11.8% of control to
28.4, 42.3 and 43.3%, in A549 cells treated with matrine (15, 30
and 50 μM) (Fig. 3). Matrine
clearly induced cell apoptosis.
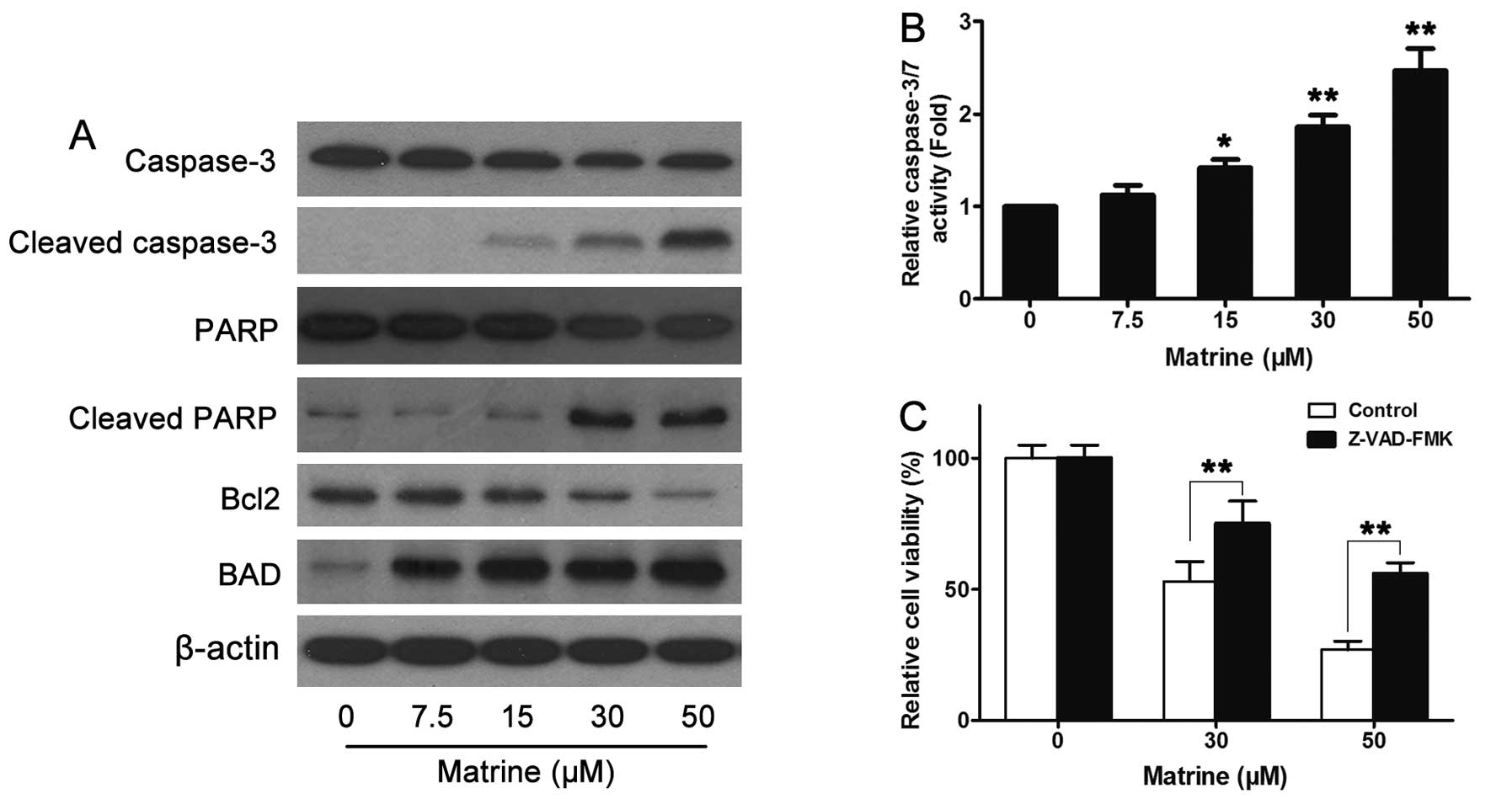
Caspase and apoptotic factors involved in
matrine-induced cell apoptosis
Apoptosis is a complicated process involving several
factors, and is divided into 2 different types; one is dependent on
caspase, the other is not. To understand which type of apoptosis
matrine induces, two key molecular proteins indicating apoptosis
were detected including caspase-3 and PARP by western blot assay.
The results indicated that the 17 kD cleaved-caspase-3, regarded as
the marker of caspase activation in apoptosis, was expressed, as
well as the 89 kD cleaved-PARP (Fig.
4A). This suggested that matrine-induced apoptosis was
dependent on caspase. To further confirm this, the caspase-3/7
activity was examined using caspase-3/7 assay kit. As shown in
Fig. 4B, the caspase-3/7 activity
showed significant change in A549 cells with the treatment of the
indicated concentration of matrine. Additionally, Z-VAD-FMK, a
caspase inhibitor, pretreated for half an hour in A549 cells also
reversed matrine-induced inhibition (Fig. 4C). Bcl2, exerting an anti-apoptotic
function in response to a wide range of apoptotic stimuli, was also
inhibited in A549 cells by matrine, while BAD, a pro-apoptotic
factor of the Bcl-2 family that promotes cell death by displacing
Bax from binding to Bcl-2 and Bcl-xL, expressed more by induction
of matrine than the control. Taken together, matrine-induced cell
apoptosis was dependent on caspase.
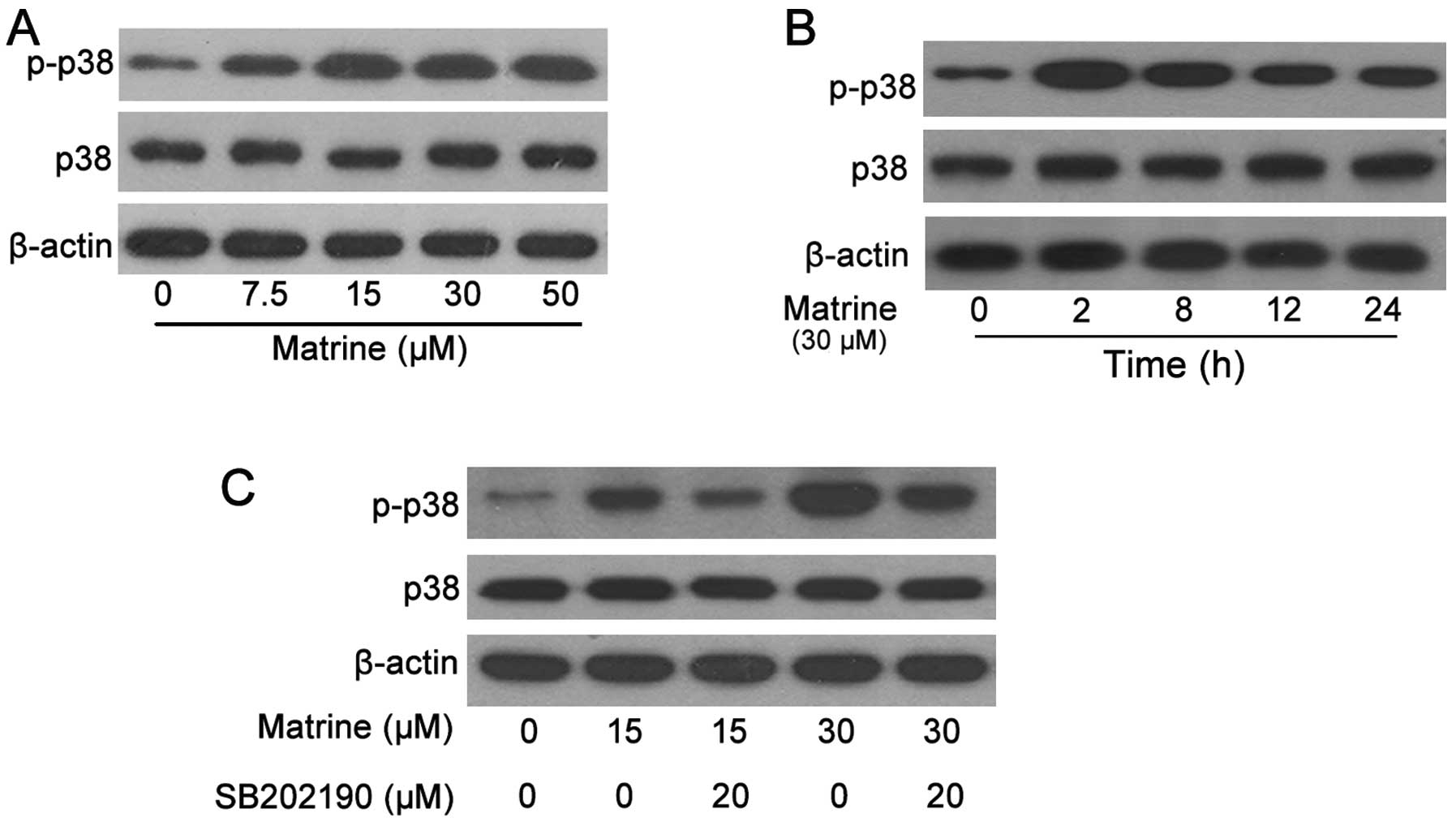
Matrine activates MAPK/p38
Since matrine triggered a caspase-dependent
apoptosis course, it is necessary to further indicate which
upstream pathways regulate it. Here, we found p38 was an important
pathway to mediate matrine-induced cell apoptosis. After A549 cells
were exposed to matrine (0–50 μM) for 8 h, the cellular lysates
were subjected to western blot analysis and the results revealed
that matrine significantly increased the phosphorylation of p38
starting from 7.5 μM (Fig. 5A).
With the treatment of 30 μM matrine for the indicated time (0–24
h), the phosphorylation of p38 was also upregulated and was
expressed the highest at 2 h, before decreasing gradually to 24 h
(Fig. 5B). Therefore, matrine
activates the p38 pathway.
The MAPK inhibitors were generally used to further
ascertain whether the tested samples functioned via this pathway.
SB202190, blocking p38, pretreated the A549 cells for 1 h following
the 8-h treatment of matrine, the upregulated phosphorylation of
p38 was partially reversed by the inhibitors (Fig. 5C). From the cell counting data, A549
cells treated with matrine plus SB202190 showed more viability than
those without inhibitors. This indicated that activation of the p38
pathway is necessary for matrine inhibition of cell apoptosis.
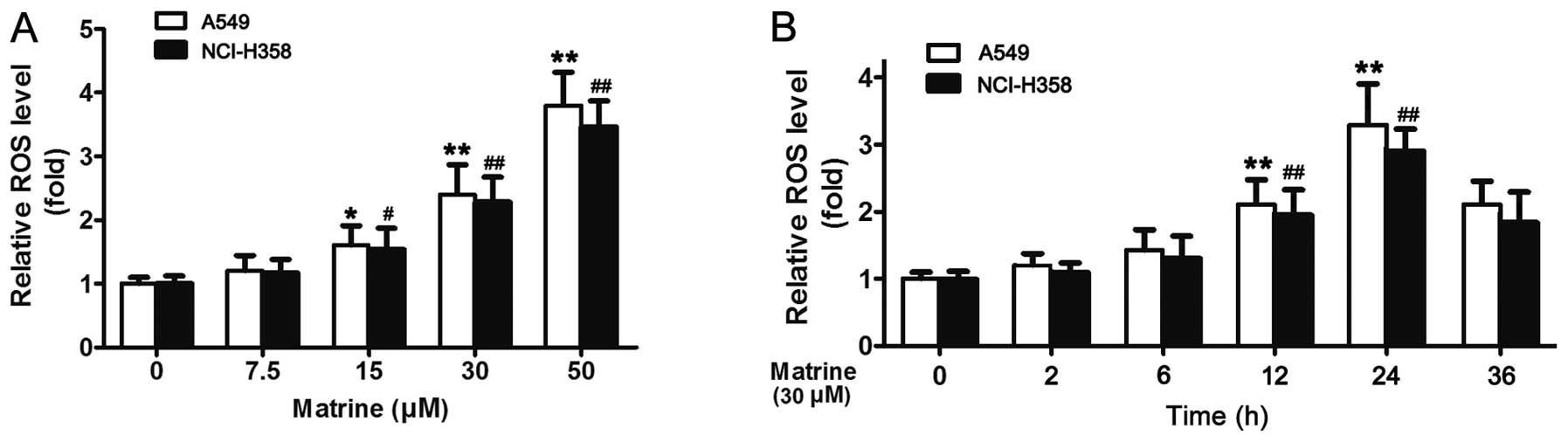
Matrine induces ROS generation
It is well known that ROS have a cellular signaling
role in several biological systems, both in animals and plants, and
in most stress-related cell progress. ROS induce programmed cell
death or necrosis, induce or suppress the expression of many genes,
and activate cell signaling cascades, such as the mitogen-activated
protein kinases. Therefore, whether matrine activating MAPK is also
due to inducing ROS or related to other factors, remains to be
confirmed. CM-H2DCFDA, a stable non-fluorescent
molecule, specially designed to detect ROS through oxidized by
oxygen radicals to form fluorescent molecule excitated by specific
wavelength lights was used to measure the level of ROS in A549
cells treated with matrine; the data showed that matrine induced
ROS generation in a dose- and time-dependent manner (Fig. 6A and B).
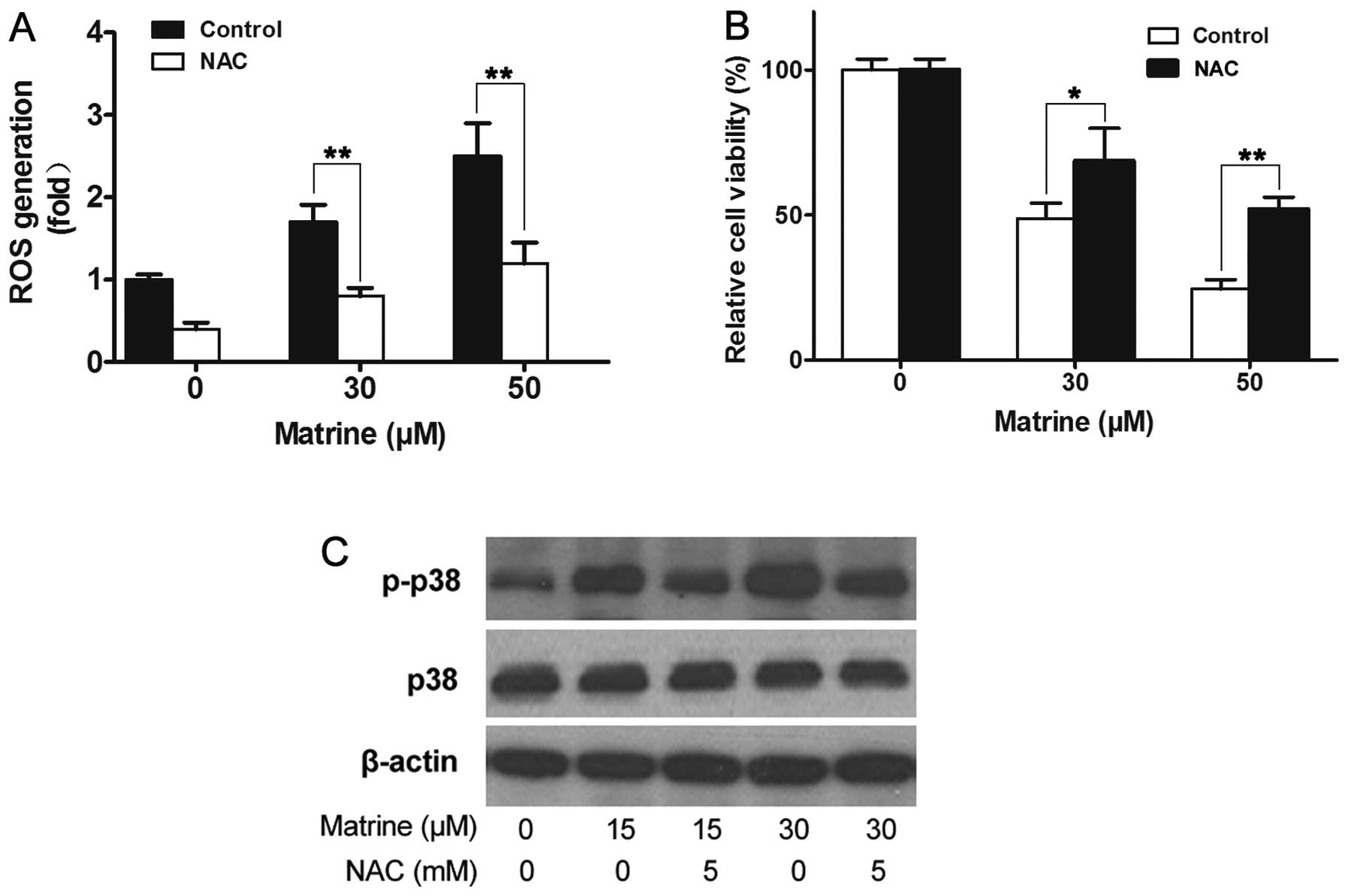
NAC reverses the effect of matrine on
ROS, p38 and cell viability
With the exception of ROS, several other factors
including stress, EGF, radicals, IL-1, integrin can act on the MAPK
pathway leading to cell proliferation, apoptosis, necrosis. Hence,
it would be confirmed that matrine activating p38 pathway is
mediated through inducing ROS generation if clearing ROS can
reverse all or at least part of the effects mediated by matrine.
NAC, a powerful antioxidant to clear the ROS, was used, then ROS
generation, MAPKs pathway and cell viability were revaluated. The
results of Fig. 7 suggest that ROS
generation induced by matrine was completely reversed by NAC (5 mM)
(Fig. 7A); second, matrine-induced
increased expression of phosphorylation of p38 was also
significantly reversed by NAC (Fig.
7B); third, most of the A549 cells were alive after treatment
with matrine plus NAC (Fig. 7C).
Based on the above results, matrine acting on the MAPK pathway is
inevitably dependent on ROS.
Discussion
Non-small cell lung cancer (NSCLC) is any type of
epithelial lung cancer other than small cell lung carcinoma (SCLC).
It is further distinguished into three subtypes: squamous cell
carcinoma, large cell carcinoma and adenocarcinoma (2,25).
Since the early 2000s, a greater understanding of the molecular
biology of NSCLC has led to revolutionary treatment of these
carcinomas (26).
Most SCLCs acquire multi-drug resistance, while
NSCLCs tend to be intrinsically resistant to chemotherapy (27). Less than 5% of SCLC patients
currently survive five years past initial diagnosis, but 15% of
patients with NSCLC survive 5 years (27). Historically, response rates rarely
exceeded 20% prior to the development of cisplatin, with an
increase to 20–40% during the cisplatin-combination era. In the
post-cisplatin era, new antitumor drugs such as gemcitabine,
vinorelbine, paclitaxel and docetaxel have been improved with the
intention of increasing response rates to as high as 50–60%
(28,29). Except for the above-mentioned
chemicals, several compounds from natural herbs including
lycobetaine, resveratrol, indirubin, ursolic acid have been
considered potential anticancer drugs for NSCLC (5). Matrine, an alkaloid from Sophora
flavescens Ait., was also found to be an anticancer agent, and
possibly showed higher inhibition on lung cancer cells than any
other cancer cells. Additionally, matrine inhibition of lung
carcinoma angiogenesis via suppression of MAPK/ERK signal
transduction suggested that the MAPK signaling pathway plays a
critical role in it (23).
It is well known that MAPKs contain three main
family members, respectively known as the c-Jun NH2-terminal kinase
(JNK), the p38 and the extracellular signal-regulated kinase (ERK).
Each of the MAPK cascade pathways works by the same three-tier
manner. The MAPK kinase kinase (MAPKKK), activated by environmental
or extracellular stimulus, activates MAPK kinase (MAPKK) which
sequentially activates MAPK through gradient phosphorylation.
Phosphorylation of the MAPK leads to activation of the
corresponding substrates, which regulate the transcription factor
and control cell proliferation, differentiation, motility and
apoptosis (30,31).
Previously, MAPK/ERK was demonstrated to be involved
in matrine suppressing HUVEC cell migration induced by A549 cancer
cells. Matrine inhibition of phosphorylation of ERK induces
antiangiogenic effects leading to the elimination of lung carcinoma
(23). Thus, in the present study,
we focused on elucidating whether MAPK/p38 also involves matrine
induction of apoptosis in NSCLC and how it acts.
p38, known as stress-activated protein kinases
(SAPKs), is widely expressed in most tissues and participates in
several different stress signaling pathways that control a spectrum
of cellular processes (31,32). Most of the data demonstrated that
activation of the p38-MAPK signaling induced cell apoptosis and
death in cancer cells (33,34). Increase of caspase-3/7 activity and
overexpression of cleaved caspase-3 and cleaved PARP make it clear
that matrine induces a caspase-dependent apoptosis in NSCLC cells,
as well as Z-VAD-FMK, a cell-permeant caspase inhibitor that
irreversibly binds to the catalytic site of caspase, can reverse
inhibition of proliferation in part. Then, matrine significantly
increasing phosphorylation of p38 disclosed p38 also mediates
matrine induction of apoptosis.
Free radicals and reactive molecules containing
oxygen are collectively known as ROS and induce oxidative stress in
cells (35). ROS, such as hydrogen
peroxide (H2O2) and superoxide
(O2−), are found higher in most tumors and cancer cells
than in normal tissues and cells (36). Thus, ROS are conventionally regarded
as cytotoxic and mutagenic, and induce cell death, apoptosis and
senescence in high levels (35). On
the contrary, ROS also function as signaling molecules to mediate
cell growth, migration, differentiation and gene expression in low
levels (35). In the present study,
matrine was demonstrated to induce cell apoptosis in NSCLC cells,
then found to significantly induce ROS generation. These two
combined with the finding that cell death and ROS generation were
reversed by NAC, suggested that induction of ROS was one of the
critical reasons causing cancer cell apoptosis by matrine.
Several downstream signaling pathways mediated by
ROS were tracked, including MAP kinases (37), JAK/STAT (38), NF-κB (39), ion channels (40) and angiogenesis (41). Therefore, we also tried to explain
whether matrine activates p38 by inducing ROS. As expected, using
NAC to clear ROS could partially inhibit the level of
phosphorylation of p38, indicating that MAPK/p38 was involved in
matrine-induced cell apoptosis.
Collectively, the mechanism of matrine-induced NSCLC
cell apoptosis was presented in this study. Matrine could stimulate
ROS generation in NSCLC cells and subsequently activated p38,
resulting in a caspase-dependent cell apoptosis by the indication
of inhibition of Bcl2 and by activating caspase-3 and PARP. Matrine
may be a promising agent for chemoprevention and treatment in NSCLC
patients.
Acknowledgements
This study was supported by the Jiangsu University
development foundation for clinical medicine (No. JLY20120171).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Reungwetwattana T, Weroha SJ and Molina
JR: Oncogenic pathways, molecularly targeted therapies, and
highlighted clinical trials in non-small-cell lung cancer (NSCLC).
Clin Lung Cancer. 13:252–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ioannidis G, Georgoulias V and Souglakos
J: How close are we to customizing chemotherapy in early non-small
cell lung cancer? Ther Adv Med Oncol. 3:185–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sadowska AM, Nowé V, Janssens A, Boeykens
E, De Backer WA and Germonpré PR: Customizing systemic therapy in
patients with advanced non-small cell lung cancer. Ther Adv Med
Oncol. 3:207–218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ancuceanu RV and Istudor V:
Pharmacologically active natural compounds for lung cancer. Altern
Med Rev. 9:402–419. 2004.PubMed/NCBI
|
|
6
|
Azzam HS, Goertz C, Fritts M and Jonas WB:
Natural products and chronic hepatitis C virus. Liver Int.
27:17–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu W, Tang JH and Wang YD: The research
progress on Sophora flavescens Ait. Lishizhen Medicine and
Materia Medica Research. 829–830. 2006.(In Chinese).
|
|
8
|
Zhang YL: Clinical study on matrine for
the treatment of psoriasis. Hebei J Med Sci. 69:590–591. 1996.(In
Chinese).
|
|
9
|
Liu M, Liu XY and Cheng JF: Advance in the
pharmacological research on matrine. Zhongguo Zhong Yao Za Zhi.
28:801–804. 2003.(In Chinese).
|
|
10
|
Tan HR and Zhang BH: Experimental study of
the anti-inflammatory effect of matrine. Zhong Xi Yi Jie He Za Zhi.
5:108–110. 691985.(In Chinese).
|
|
11
|
Chuang CY, Xiao JG and Chiou GC: Ocular
anti-inflammatory actions of matrine. J Ocul Pharmacol. 3:129–134.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu JY, Hu JH, Zhu QG, Li FQ, Wang J and
Sun HJ: Effect of matrine on the expression of substance P receptor
and inflammatory cytokines production in human skin keratinocytes
and fibroblasts. Int Immunopharmacol. 7:816–823. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma L, Wen S, Zhan Y, He Y, Liu X and Jiang
J: Anticancer effects of the Chinese medicine matrine on murine
hepatocellular carcinoma cells. Planta Med. 74:245–251. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ho JW, Ngan Hon PL and Chim WO: Effects of
oxymatrine from Ku Shen on cancer cells. Anticancer Agents Med
Chem. 9:823–826. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang H, Hou C, Zhang S, et al: Matrine
upregulates the cell cycle protein E2F-1 and triggers apoptosis via
the mitochondrial pathway in K562 cells. Eur J Pharmacol.
559:98–108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Zhang H, Yu P, et al: Effects of
matrine against the growth of human lung cancer and hepatoma cells
as well as lung cancer cell migration. Cytotechnology. 59:191–200.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li LQ, Li XL, Wang L, et al: Matrine
inhibits breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7
cells. Cell Physiol Biochem. 30:631–641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu T, Song Y, Chen H, Pan S and Sun X:
Matrine inhibits proliferation and induces apoptosis of pancreatic
cancer cells in vitro and in vivo. Biol Pharm Bull. 33:1740–1745.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen K, Hu ZQ, Wang T, Guo JL, Guo H and
Ye ZQ: Matrine inhibits the proliferation of prostate cancer cells
and the activity of androgen receptor. Zhonghua Nan Ke Xue.
14:719–722. 2008.(In Chinese).
|
|
20
|
Chen K, Hu Z, Wang T, Guo H and Ye Z:
Inhibitory effect of matrine on the expression of PSA and AR in
prostate cancer cell line LNCaP. J Huazhong Univ Sci Technolog Med
Sci. 28:697–699. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou XH, Wei X, Huang ZS, et al: Effects
of matrine on proliferation and telomerase activity of colon cancer
SW1116 cells. Zhong Yao Cai. 32:923–925. 2009.(In Chinese).
|
|
22
|
Yu P, Liu Q, Liu K, Yagasaki K, Wu E and
Zhang G: Matrine suppresses breast cancer cell proliferation and
invasion via VEGF-Akt-NF-κB signaling. Cytotechnology. 59:219–229.
2009.PubMed/NCBI
|
|
23
|
Lu J, Luo Q, Cheng P, Liu X, Bai M and Tu
M: The role of matrine and mitogen-activated protein
kinase/extracellular signal-regulated kinase signal transduction in
the inhibition of proliferation and migration of human umbilical
vein endothelial cells induced by lung cancer cells. Zhongguo Fei
Ai Za Zhi. 12:747–752. 2009.(In Chinese).
|
|
24
|
Zhu MY, Jiang ZH, Lu YW, Guo Y and Gan JJ:
Matrine and anti-tumor drugs in inhibiting the growth of human lung
cancer cell line. Zhong Xi Yi Jie He Xue Bao. 6:163–165. 2008.(In
Chinese).
|
|
25
|
Saintigny P and Burger JA: Recent advances
in non-small cell lung cancer biology and clinical management.
Discov Med. 13:287–297. 2012.PubMed/NCBI
|
|
26
|
Aisner DL and Marshall CB: Molecular
pathology of non-small cell lung cancer: a practical guide. Am J
Clin Pathol. 138:332–346. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Young LC, Campling BG, Cole SP, Deeley RG
and Gerlach JH: Multidrug resistance proteins MRP3, MRP1, and MRP2
in lung cancer: correlation of protein levels with drug response
and messenger RNA levels. Clin Cancer Res. 7:1798–1804.
2001.PubMed/NCBI
|
|
28
|
Bunn PJ and Kelly K: New combinations in
the treatment of lung cancer: a time for optimism. Chest. 117(Suppl
1): S138–S143. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gori B, Ricciardi S, del Signore E, Fulvi
A and de Marinis F: Oral tyrosine kinase inhibitors in the
first-line treatment of advanced non-small cell lung cancer. Expert
Opin Ther Targets. 16(Suppl 2): S55–S60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bradham C and McClay DR: p38 MAPK in
development and cancer. Cell Cycle. 5:824–828. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kumar P, Miller AI and Polverini PJ: p38
MAPK mediates γ-irradiation-induced endothelial cell apoptosis, and
vascular endothelial growth factor protects endothelial cells
through the phosphoinositide 3-kinase-Akt-Bcl-2 pathway. J Biol
Chem. 279:43352–43360. 2004.
|
|
34
|
Park MT, Choi JA, Kim MJ, et al:
Suppression of extracellular signal-related kinase and activation
of p38 MAPK are two critical events leading to caspase-8- and
mitochondria-mediated cell death in phytosphingosine-treated human
cancer cells. J Biol Chem. 278:50624–50634. 2003. View Article : Google Scholar
|
|
35
|
Hancock JT, Desikan R and Neill SJ: Role
of reactive oxygen species in cell signalling pathways. Biochem Soc
Trans. 29:345–350. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liou GY and Storz P: Reactive oxygen
species in cancer. Free Radic Res. 44:479–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McCubrey JA, Lahair MM and Franklin RA:
Reactive oxygen species-induced activation of the MAP kinase
signaling pathways. Antioxid Redox Signal. 8:1775–1789. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dixit D, Sharma V, Ghosh S, Koul N, Mishra
PK and Sen E: Manumycin inhibits STAT3, telomerase activity, and
growth of glioma cells by elevating intracellular reactive oxygen
species generation. Free Radic Biol Med. 47:364–374. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Satriano J and Schlondorff D: Activation
and attenuation of transcription factor NF-κB in mouse glomerular
mesangial cells in response to tumor necrosis factor-alpha,
immunoglobulin G, and adenosine 3′:5′-cyclic monophosphate.
Evidence for involvement of reactive oxygen species. J Clin Invest.
94:1629–1636. 1994.
|
|
40
|
Matalon S, Hardiman KM, Jain L, et al:
Regulation of ion channel structure and function by reactive
oxygen-nitrogen species. Am J Physiol Lung Cell Mol Physiol.
285:L1184–L1189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ushio-Fukai M and Nakamura Y: Reactive
oxygen species and angiogenesis: NADPH oxidase as target for cancer
therapy. Cancer Lett. 266:37–52. 2008. View Article : Google Scholar : PubMed/NCBI
|