Introduction
Head and neck squamous cell carcinoma is the sixth
most frequent neoplasm worldwide (1,2). Even
with advances in adjuvant therapies, survival rates have not
improved significantly over the last 20 years (3). The major risk factors are tobacco and
alcohol (4), but some reports also
suggest human papilloma virus infections (5). Surgical excision, radiotherapy or a
combination of both are the treatments of choice (6,7).
Chemotherapy is occasionally used (6,7).
Although a combination of treatments has proven efficient, the
toxicity levels are high (8,9).
Carcinogenesis is a multifactorial process involving
several molecular events. Deregulation of cell cycle-related genes,
such as AKT, PTEN and CYCLINs, are common
among these events. AKT is a serine/threonine kinase whose
activation is highly correlated with several solid and hematologic
types of cancer (10–12); PTEN acts at the G1 phase blocking
the cell cycle that suppresses tumour progression (13–15).
CYCLINs are also essential for the correct cell cycle transition
(16,17). These genes have been associated with
head and neck squamous cell carcinomas.
Viscum album L. (VA), also called mistletoe,
is a hemi-parasitic plant, originally found in Europe. Composed of
the total plant extract, it implies a composition of great
complexity (18) and is found to
have a broad application in adjuvant therapy. Available clinical
evidence supporting anticancer effects and in vitro studies
using cancer cell lines have demonstrated cytotoxic effects that
might trigger apoptotic signals (18–23).
Over 200 host species are known, and it seems that distinct
chemical properties of VA are associated with the host (18,22).
The main components of the plant extract presenting therapeutic
application seem to be lectin, viscotoxins and polysaccharides
(20).
Since there are no reports to date on the effect of
this plant extract on oral squamous cell carcinomas, responses to
different commercially available VA formulas were evaluated in cell
lines derived from this cancer type. Results were assessed in terms
of cell growth, apoptosis and expression of AKT, PTEN and CYCLIN
D1.
Materials and methods
Cell lines and culture conditions
Human tongue cancer cell lines, purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA) SCC9
(CRL-1629) and SCC25 (CRL-1628), were cultivated in Dulbecco’s
modified Eagle’s medium and F-12 Nutrient Mixture (1:1)
supplemented with 10% bovine serum product, penicillin (100 U/ml),
streptomycin (100 μg/ml), amphotericin B (2.5 μg/ml) and glutamine
(4 mM) at 37ºC in a humidified atmosphere containing 5%
CO2. The cells were incubated for 24 and 48 h with the
drugs at a concentration of 0.3 mg/ml. This concentration
corresponded to the IC50 and was determined by the MTS
assay described below. These drugs were diluted in physiological
solution and added to the cell culture. This mixture could not
exceed 10% of the volume of the cell medium. Cytotoxicity of
physiological solution was previously tested and presented no
effect on cells (data not shown).
Drugs and reagents
The mistletoe extract Iscador is an aqueous sterile
preparation derived from VA grown on oak (Quercus), apple
tree (Mali) or pine (Pini), and fermented with
Lactobacillus plantarum. Iscador Qu Spezial (Quercus)
5 mg, Iscador P (Pini) 10 mg and Iscador M (Mali) 5
mg from Weleda AG, Arlesheim, Switzerland. Western blotting and
immunofluorescence were performed using anti-pAKT (sc7985; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), anti-CYCLIN D1
(sc47698; Santa Cruz Biotechnology, Inc.), goat anti-rabbit
(sc2004; Santa Cruz Biotechnology, Inc.), goat anti-mouse (sc2005;
Santa Cruz Biotechnology, Inc.); anti-PTEN (ab23694; Abcam,
Cambridge, MA, USA); mouse monoclonal anti-β-actin (clone AC-15;
Sigma-Aldrich, St. Louis, MO, USA), and the secondary antibody
conjugated with fluorescein (Novocastra). Mounting medium utilized
for the immunofluorescence assay was VectaShield (Vector
Laboratories).
IC50 determination using MTS
assay
Both cell lines were incubated in 96-well plates
(3×103/well) in the presence of different concentrations
of Iscador Qu Spezial. After 24, 48 and 72 h of cell culture, cell
viability was assessed using a
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) assay kit (CellTiter 96 Aqueous One Solution; Promega) at an
absorbance of 490 nm using a spectrophotometer. Considering that
the amount of the active principle of Iscador Qu Spezial is the
same as the Iscador M (5 mg/ml), and half of the Iscador P (10
mg/ml), IC50 obtained for Iscador Qu Spezial was used to
adjust drug volumes accordingly at the time of the experiments.
Flow cytometric analysis of
apoptosis
Apoptosis was assayed using an Annexin V-FITC/PI
Apoptosis Detection kit (BD Pharmingen, Franklin Lakes, NJ, USA)
following the manufacturer’s protocol. The results were analysed
using flow cytometry (BD FACSAria) with BD FACSDiva software,
version 6.1.3.U, in order to distinguish viable, early apoptotic,
late apoptotic or dead cells.
Relative quantification of gene
expression using real-time PCR (qPCR)
The qPCR was performed to determine the expression
level of genes: AKT1, AKT2, PTEN and CYCLIN
D1. GAPDH was used as internal control. Primer sequences are
shown in Table I. Total RNA was
extracted from cell lines using TRIzol reagent (Invitrogen,
Carlsbad, CA, USA). Samples of RNA (1.0 μg) were reverse
transcribed with MultiScribe™ (High Capacity cDNA Archive kit;
Applied Biosystems, Foster City, CA, USA), according to the
manufacturer’s protocol. qRT-PCR was carried out using SYBR-Green
PCR Master mix (Applied Biosystems) for 10 min at 95ºC, followed by
40 cycles at 95ºC for 10 sec and 60ºC for 1 min. Dissociation curve
analyses were performed at the end of cycling. Gene expression was
calculated using a relative expression software tool (REST method,
Pfaffl 2001).
 | Table IPrimer sequences. |
Table I
Primer sequences.
| | Primers
(5′→3′) |
|---|
| |
|
|---|
| Gene | Accession no. | Forward | Reverse |
|---|
| AKT1 | NM 001014431.1 |
TTTTTGAGCTCATCCTCATGG |
ACACGATACCGGCAAAGAAG |
| AKT2 | NM 001626.3 |
TGCCCTTCTACAACCAGGAC |
AACCTGTGCTCCATGACCTC |
| PTEN | NM 000314.4 |
AGTGGCACTGTTGTTTCACAAG |
GTGTGGGTCCTGAATTGGAG |
| CYCLIN
D1 | NM 53056.2 |
CGTGGCCTCTAAGATGAAGG |
CTGGCATTTTGGAGAGGAAG |
| GAPDH | NM 002046 |
GCATCCTGGGCTACACTGA |
CCACCACCCTGTTGCTGTA |
Western blotting
Ten micrograms of protein were boiled (5 min) and
subjected to SDS-PAGE. Western blotting was performed according to
a standard protocol using anti-human pAKT (1:700), PTEN (1:100) and
CYCLIN D1 (1:75) followed by incubation with secondary antibody
(goat anti-rabbit or goat anti-mouse, 1:3,000, respectively). Mouse
monoclonal anti-β-actin (1:5,000) was the loading control. The
bound antibodies were detected by colorimetric detection kit
Opti-4CN.
Immunofluorescence assay
Cells in the cover slip were fixed with methanol,
saturated in PBS/3% bovine serum albumin and incubated (1 h) with
anti-human pAKT (1:50), PTEN (1:50) and CYCLIN D1 (1:100).
Afterwards, washing cells were incubated (45 min) with a secondary
antibody (1:50) conjugated with fluorescein, at room temperature in
a dark chamber. Three random visual fields (magnification, ×400)
were observed under a fluorescent microscope, Zeiss Axiophot II
(Carl Zeiss, Oberkochen, Germany).
Statistical analysis
A Mann-Whitney test was used to determine the
IC50 and in the apoptosis assay. The qRT-PCR analysed
the drug effect over the gene expression after set time intervals.
Data analyses were performed using Statistical Package for Social
Sciences (SPSS), version 17.0. P-values ≤0.05 were considered
statistically significant.
Results
Iscador Qu Spezial induces cell death
dose- and time-dependently
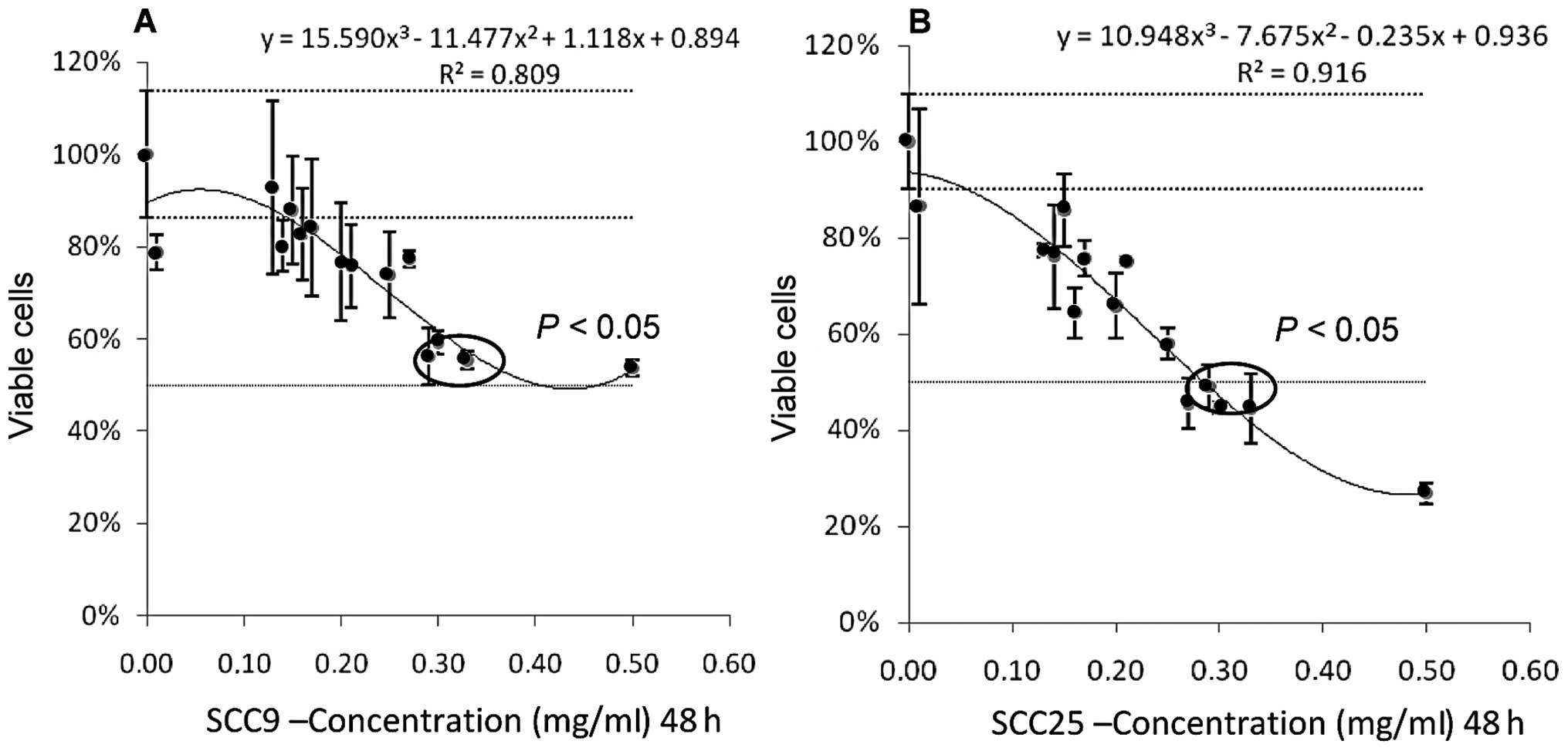
To determine whether the VA is capable of inducing
cell death, both cell lines were treated with Iscador Qu Spezial
for 24, 48 and 72 h at various concentrations. The cell viability
using MTS showed the time-dependent action of this drug. The
determined IC50 was 0.3 mg/ml after 48 h of exposure
(P=0.011) (Fig. 1). We then used
the same concentration (0.3 mg/ml) for assays using Iscador M and
Iscador P.
Iscador Qu Spezial and Iscador M are more
efficient than Iscador P in inducing cell death in SCC9 and
SCC25
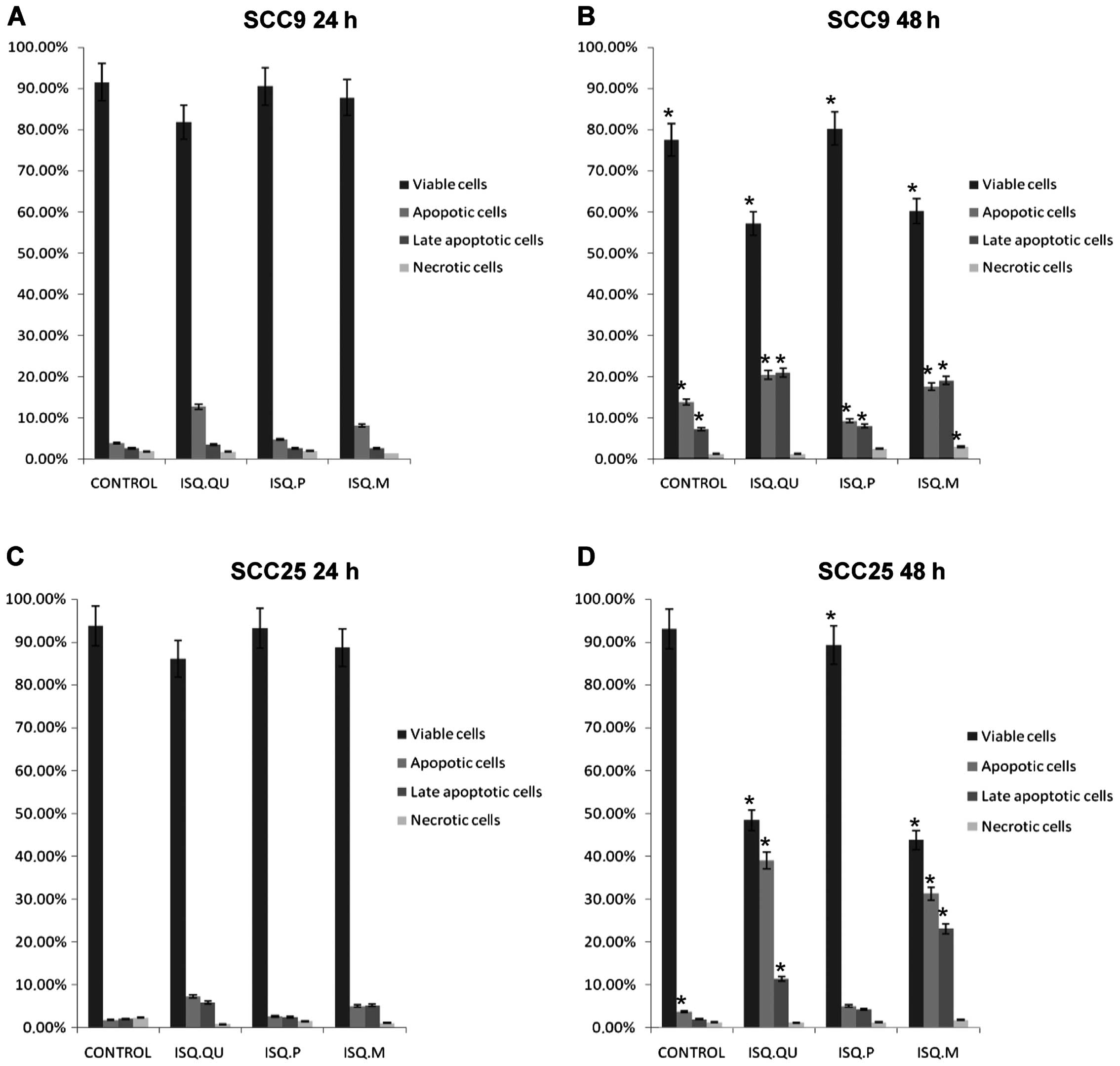
To confirm the apoptotic effect of Iscador Qu
Spezial, Iscador P and Iscador M in SCC9 and SCC25 cell lines, a
flow cytometric assay was performed. Iscador Qu Spezial and Iscador
M presented better apoptotic effect in both lineages after 48 h, as
compared to Iscador P (Fig. 2). The
results also corroborate the time-dependent action of these drugs
since we observed more apoptosis after 48 h than at 24 h of drug
exposure.
VA treatment alters the expression of
AKT, PTEN and CYCLIN D1 in SCC9 and SCC25
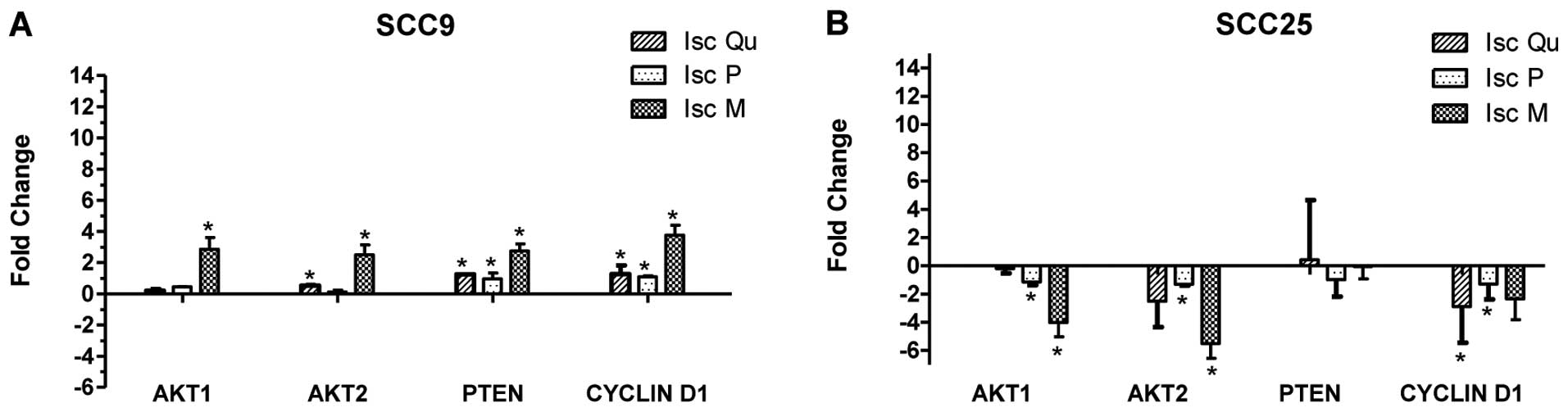
Real-time PCR was used in order to investigate the
impact of the drugs on the relative expression of AKT (AKT1,
AKT2), PTEN and CYCLIN D1, after 24 and 48 h
of treatment. The results were not uniform and varied depending on
the cell line, drug and gene (Fig.
3).
Iscador Qu Spezial and Iscador M
decreased the protein levels of CYCLIN D1 and pAKT
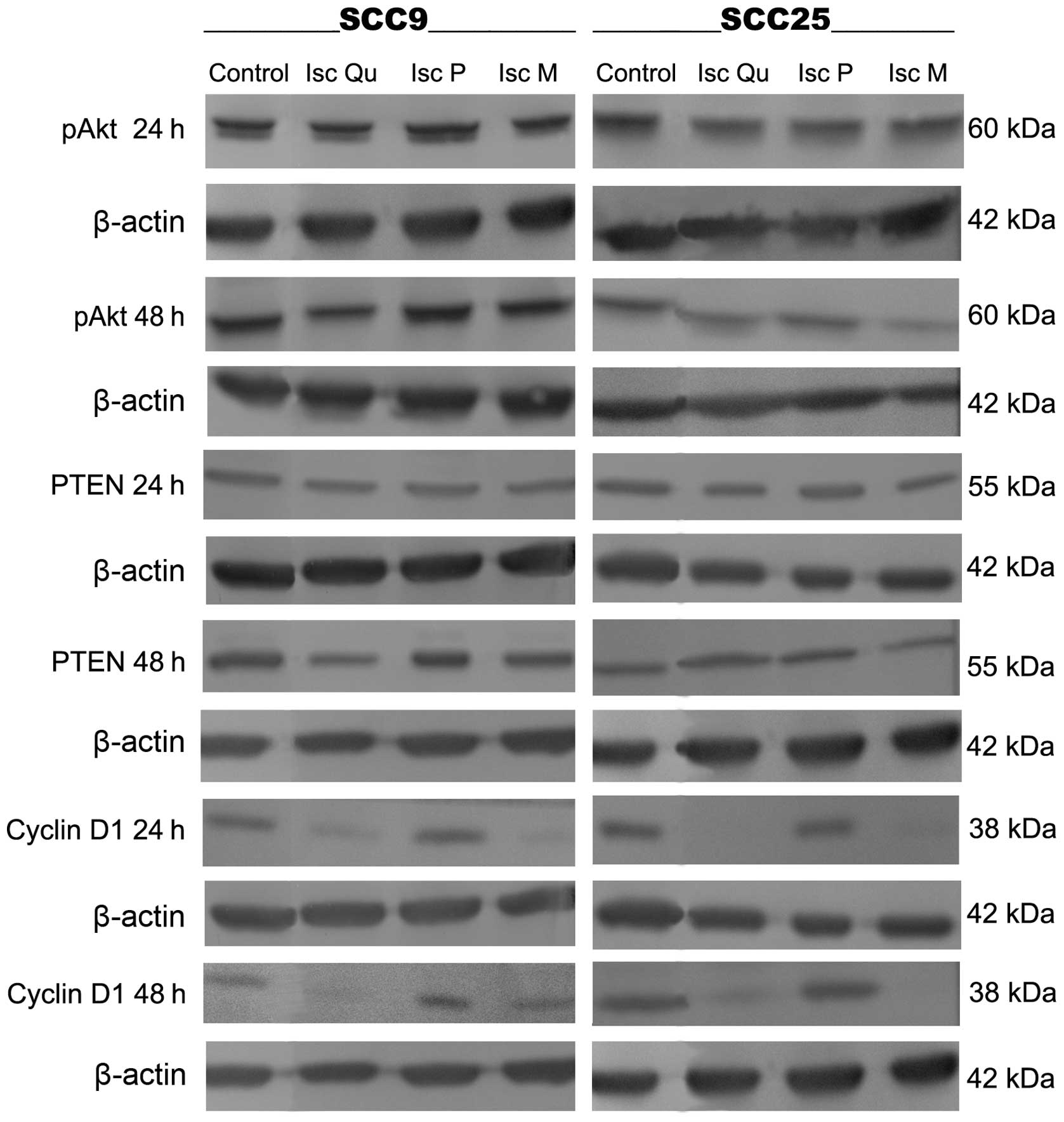
After 48 h of exposure to Iscador Qu and Iscador M,
but not with Iscador P, the expression of pAKT showed a slight
decrease in both cell lines (Fig.
4), while PTEN levels decreased only in SCC9, upon treatment
with Iscador Qu Spezial. CYCLIN D1 protein levels did not vary when
cells were treated with Iscador P whereas Iscador Qu Spezial and
Iscador M treatment lead to a substantial decrease in the
expression levels of CYCLIN D1 (Fig.
4).
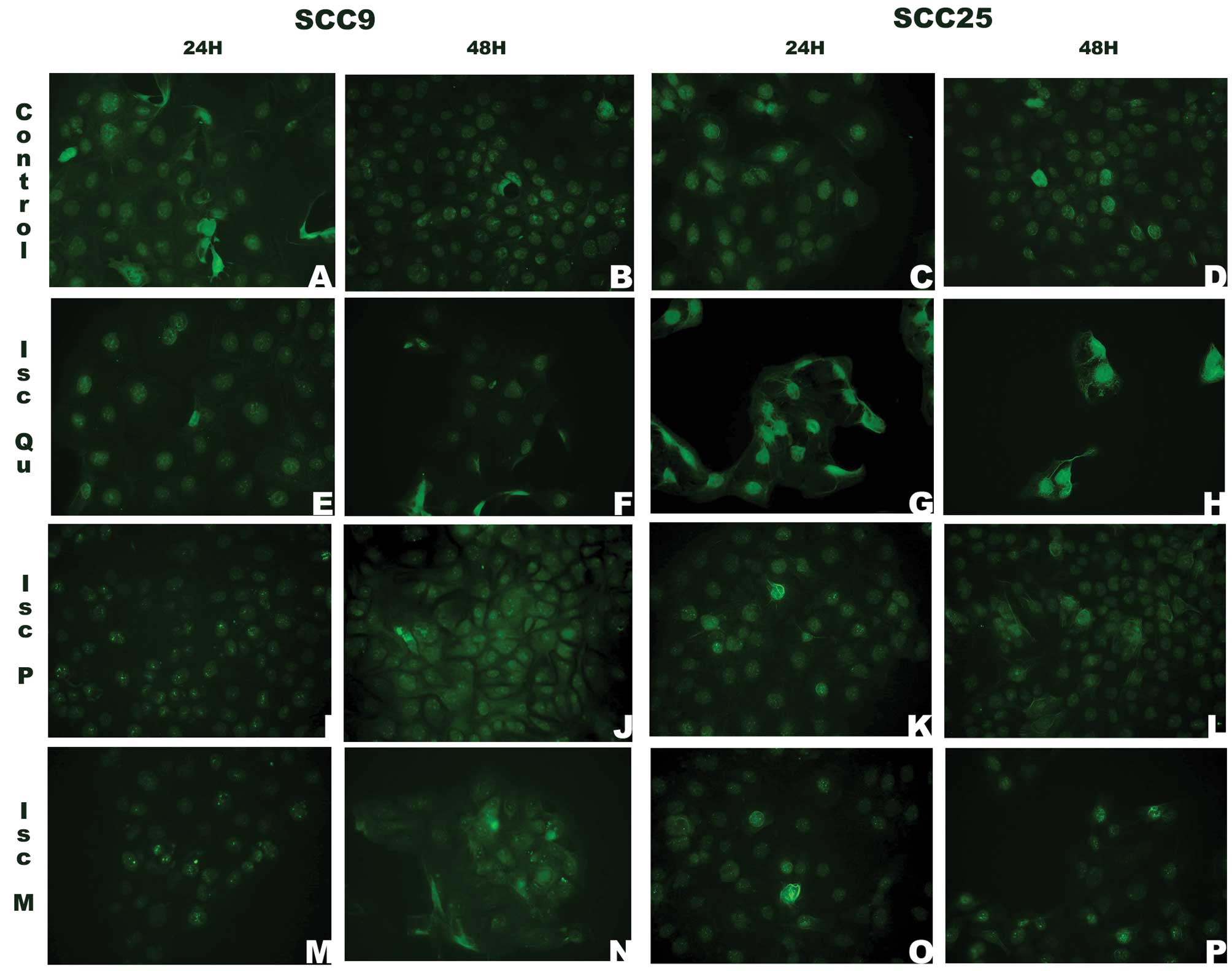
The cell compartment localization of AKT,
PTEN and CYCLIN D1 changed after VA treatment
The localization of the proteins pAKT, PTEN and
CYCLIN D1 in SCC9 and SCC25 cells upon VA treatment was assessed by
immunofluorescence assay. pAKT expression in the nucleus was
present in both cell lines regardless of the VA treatment (Fig. 5). However, SCC9 cells treated with
Iscador P and Iscador M for 48 h also showed a cytoplasmic pattern
of staining. The presence of pAKT in the cytoplasm of SCC25 cells
was seen only when they were treated with Iscador Qu Spezial
(Fig. 5F, J and N).
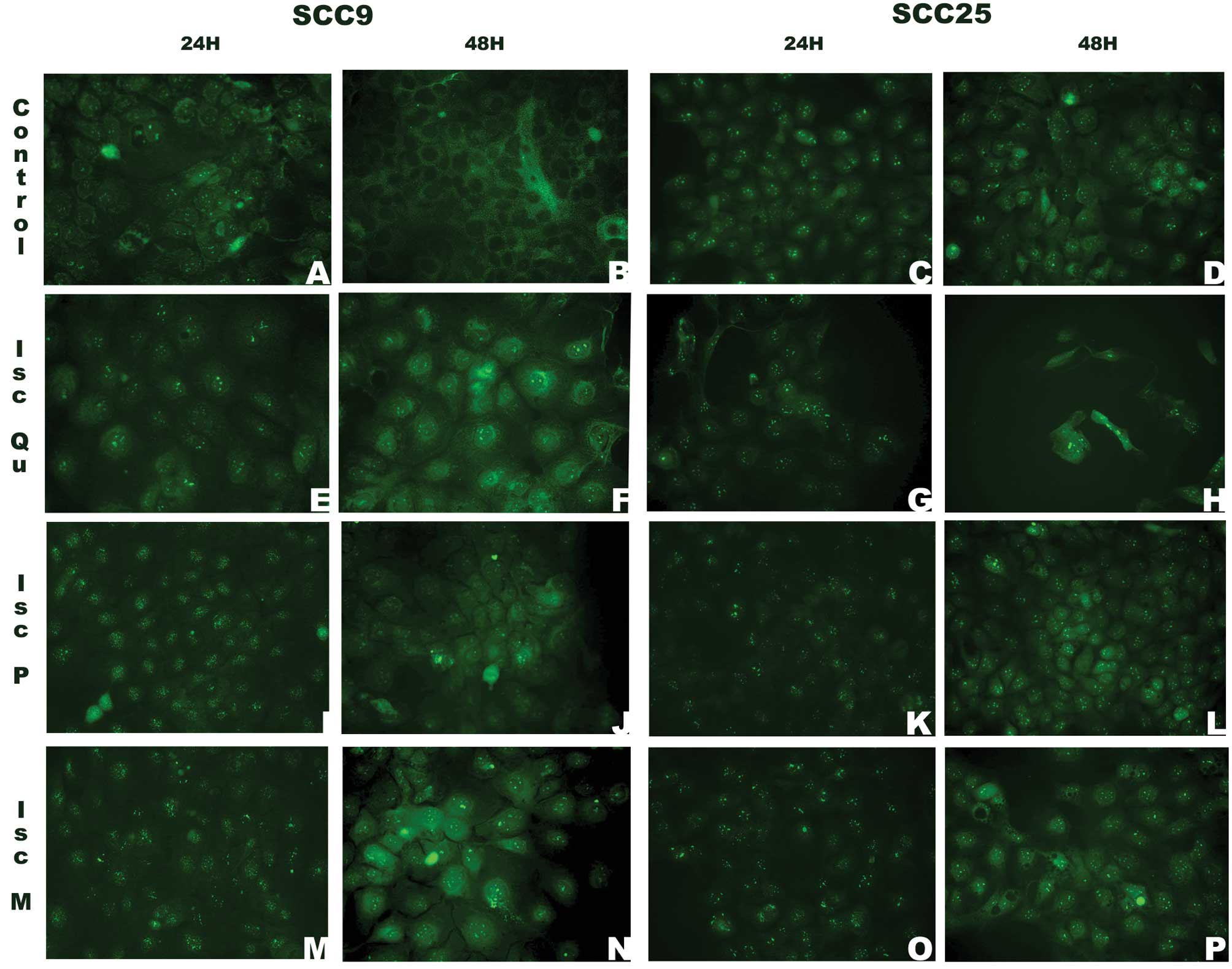
PTEN showed a predominantly nuclear pattern of
staining after 24 h of treatment. At 48 h, a cytoplasmic staining
could be seen for both lineages at all instances, while nuclear
staining disappeared for SCC9 control cells (Fig. 6B).
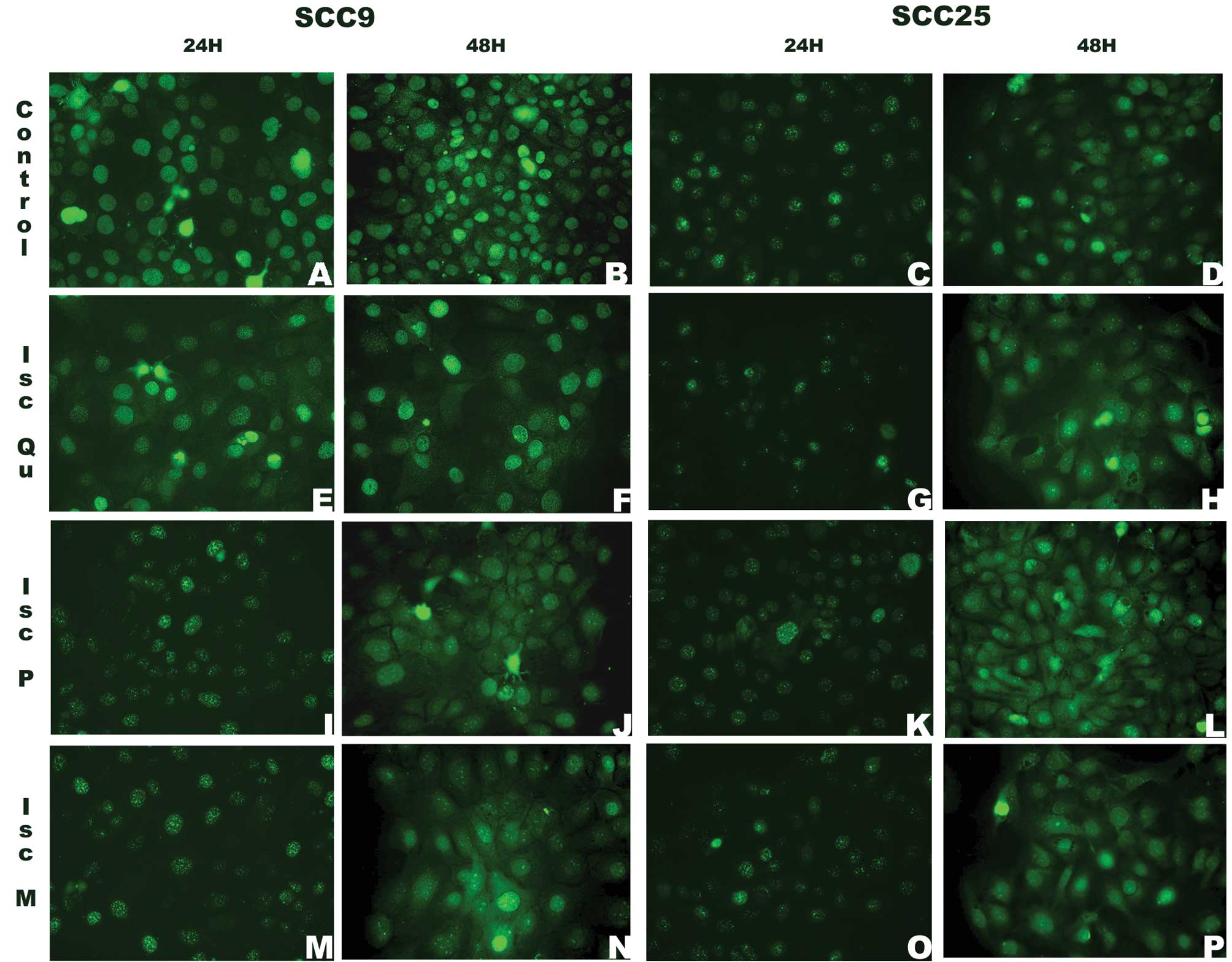
CYCLIN D1 location and pattern of expression was
independent from the treatment or cell line at 24 h. Cytoplasmic
staining was seen after 48 h of treatment, except in SCC9 treated
with Iscador Qu Spezial (Fig. 7E and
F).
Discussion
In vitro action of mistletoe extract Iscador
(Qu Spezial, Iscador P and Iscador M) was evaluated on cells of
tongue squamous cell carcinoma. The viability test showed a
time-dependent action of the drug. In agreement, an ampoule of
these drugs is used twice or three times a week in cancer treatment
(24). The drug effect was
different depending on the cell analysed, as previously observed by
other authors (18,19). Eggenschwiler et al(22) observed that cell lines derived from
the same type of tumor might have distinct behaviors in response to
the same drug. In fact, SCC9 cells seem to be more resistant to
apoptosis induced by mistletoe when compared with SCC25. On the
other hand, Iscador Qu Spezial, because of its higher concentration
of viscotoxins and lectins, provided a stronger effect compared
with the other extracts, as previously described (18,20,22,23,25).
We chose to analyse the effect of mistletoe on members of a pathway
frequently deregulated in oral squamous cell carcinoma, AKT, PTEN
and CYCLIN D1 (15,26,27).
Gene expression analysis by qRT-PCR of AKT1, AKT2,
PTEN and CYCLIN D1 was not consistent nor in
agreement with their respective protein levels. These results
suggest that mistletoe role on cell survival is not clearly related
to the regulation of this pathway at the gene expression level.
Nevertheless, Iscador Qu Spezial and Iscador M
decreased pAKT and CYCLIN D1 protein levels, leading to greater
potential to cause cellular death as previously described (18,22,23,25).
The apoptosis triggered by the cytotoxic effects of these drugs may
have been caused by inhibition of other signalling pathways also
altered in cancers, such as EGFR/Ras/Raf/MEK/ERK (28). It may also have been triggered by
the complex of STAT3 with NF-κB, which can lead to even more
significant proliferative potential, through the expression of
genes, such as c-myc and CYCLIN D1 (29). Studies of Sauter et al
demonstrated that silencing of CYCLIN D1 also has an effect on
inducing apoptosis in several cell lines of squamous cell carcinoma
of the head and neck as well as skin and cervix (30). Baldin et al explain that
CYCLIN D1 is detected in the nucleus during cell cycling; however,
when cells enter the S phase, the expression increase in cytoplasm
and decrease in nucleus (31). This
could clarify the changes in cell locations observed in this study.
Under normal conditions, CYCLIN D1 has nuclear localization in G1
and is phosphorylated, ubiquitinized and transported to the
cytoplasm in the S phase (29,31).
The alteration of chromatin structure can explain the agglutinated
aspect, which maintains the transcriptional repression of genes in
S during early G1. These promoters are accessible only in the
transition phase G1/S (31). pAKT
acts directly on cell cycle progression, survival and proliferation
(28,32,33).
The immunofluorescence analyses showed AKT at different cell
locations. After AKT activation (pAKT) in the cytoplasm, it can be
translocated to the nucleus. However, nuclear pAKT substrates have
not been elucidated yet (34,35).
PTEN immunolocalization was detected after incubation with the
drugs, although the quantitative analyses by western blotting were
not able to demonstrate this positivity. Nuclear PTEN is involved
with maintaining chromosome integrity and DNA repair (36,37). A
hypothetical action of the drugs analysed here could be the
maintenance of nuclear PTEN, because it is protected from
degradation by cytoplasmic proteasome. In the nucleus, it is still
able to antagonize AKT and provoke apoptosis (37). A theory claims that translocation of
PTEN from nucleus to cytoplasm may be associated with malignancy
(38,39). In the cytoplasm PTEN acts as a tumor
suppressor by blocking PI3K (40).
This study highlights the fact that Iscador Qu
Spezial and Iscador M are able to induce apoptosis in SCC9 and
SCC25 cell lines, while Iscador P was less efficient in both
lineages. Although the inhibition of CYCLIN D1 and pAKT were
detected at the protein level, and a possible effect on protein
location within the cell was detected, the nature of the
cytotoxicity remains to be identified. SCC9 cells seem to be more
resistant to apoptosis induced by mistletoe when compared with
SCC25 cells. Our results corroborate previously described effects
of VA and suggest that it could be potentially used as a drug in
OSCC therapy attempting to reverse a poor prognosis, leading to a
better management of the disease.
Acknowledgements
The authors thank Dr Michael Werner of the Institut
Hiscia Arlesheim (Switzerland) for providing all the ampoules of
the three Iscador utilized in this study.
References
|
1
|
Jemal A, Tiwari RC, Murray T, Ghafoor A,
Samuels A, Ward E, et al: Cancer statistics, 2004. CA Cancer J
Clin. 54:8–29. 2004. View Article : Google Scholar
|
|
2
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar
|
|
3
|
Sklenicka S, Gardiner S, Dierks EJ, Potter
BE and Bell RB: Survival analysis and risk factors for recurrence
in oral squamous cell carcinoma: does surgical salvage affect
outcome? J Oral Maxillofac Surg. 68:1270–1275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsantoulis PK, Kastrinakis NG, Tourvas AD,
Laskaris G and Gorgoulis VG: Advances in the biology of oral
cancer. Oral Oncol. 43:523–534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miller CS and Johnstone BM: Human
papillomavirus as a risk factor for oral squamous cell carcinoma: a
meta-analysis, 1982–1997. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 91:622–635. 2001.PubMed/NCBI
|
|
6
|
Scully C and Bagan J: Oral squamous cell
carcinoma overview. Oral Oncol. 45:301–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gold KA, Lee HY and Kim ES: Targeted
therapies in squamous cell carcinoma of the head and neck. Cancer.
115:922–935. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Corvò R: Evidence-based radiation oncology
in head and neck squamous cell carcinoma. Radiother Oncol.
85:156–170. 2007.PubMed/NCBI
|
|
9
|
Bernier J: Current state-of-the-art for
concurrent chemoradiation. Semin Radiat Oncol. 19:3–10. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bellacosa A, Kumar CC, Di Cristofano A and
Testa JR: Activation of AKT kinases in cancer: implications for
therapeutic targeting. Adv Cancer Res. 94:29–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cicenas J: The potential role of Akt
phosphorylation in human cancers. Int J Biol Markers. 23:1–9.
2008.PubMed/NCBI
|
|
12
|
Molinolo AA, Amornphimoltham P, Squarize
CH, Castilho RM, Patel V and Gutkind JS: Dysregulated molecular
networks in head and neck carcinogenesis. Oral Oncol. 45:324–334.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okumura K, Mendoza M, Bachoo RM, DePinho
RA, Cavenee WK and Furnari FB: PCAF modulates PTEN activity. J Biol
Chem. 281:26562–26568. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Steck PA, Pershouse MA, Jasser SA, Yung
WK, Lin H, Ligon AH, et al: Identification of a candidate tumour
suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in
multiple advanced cancers. Nat Genet. 15:356–362. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kurasawa Y, Shiiba M, Nakamura M, Fushimi
K, Ishigami T, Bukawa H, et al: PTEN expression and methylation
status in oral squamous cell carcinoma. Oncol Rep. 19:1429–1434.
2008.PubMed/NCBI
|
|
16
|
Lundberg AS and Weinberg RA: Control of
the cell cycle and apoptosis. Eur J Cancer. 35:531–539. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
el-Naggar AK, Steck K and Batsakis JG:
Heterogeneity of the proliferative fraction and cyclin D1/CCND1
gene amplification in head and neck squamous cell carcinoma.
Cytometry. 21:47–51. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zuzak TJ, Rist L, Eggenschwiler J, Grotzer
MA and Viviani A: Paediatric medulloblastoma cells are susceptible
to Viscum album (mistletoe) preparations. Anticancer Res.
26:3485–3492. 2006.PubMed/NCBI
|
|
19
|
Harmsma M, Grommé M, Ummelen M, Dignef W,
Tusenius KJ and Ramaekers FC: Differential effects of Viscum
album extract Iscador Qu on cell cycle progression and
apoptosis in cancer cells. Int J Oncol. 25:1521–1529. 2004.
|
|
20
|
Goedings P: Über die bildung von Viscum
album L. Element der Naturwissenschaft. 67:1–23. 1997.(In
German).
|
|
21
|
Molassiotis A, Scott JA, Kearney N, Pud D,
Magri MS, et al: Complementary and alternative medicine use in
breast cancer patients in Europe. Support Care Cancer. 14:260–267.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eggenschwiler J, Patrignani A, Wagner U,
Rehrauer H, Schlapbach R, Rist L, et al: Gene expression profiles
of different breast cancer cells compared with their responsiveness
to fermented mistletoe (Viscum album L.) extracts Iscador
from oak (Quercus), pine (Pinus), white fir
(Abies) and apple tree (Malus) in vitro.
Arzneimittelforschung. 56:483–496. 2006.PubMed/NCBI
|
|
23
|
Urech K, Buessing A, Thalmann G,
Schaefermeyer H and Heusser P: Antiproliferative effects of
mistletoe (Viscum album L.) extract in urinary bladder
carcinoma cell lines. Anticancer Res. 26:3049–3055. 2006.
|
|
24
|
Matthes H, Friedel WE, Bock PR and Zänker
KS: Molecular mistletoe therapy: friend or foe in established
anti-tumor protocols? A multicenter, controlled, retrospective
pharmaco-epidemiological study in pancreas cancer. Curr Mol Med.
10:430–439. 2010. View Article : Google Scholar
|
|
25
|
Maier G and Fiebig HH: Absence of tumor
growth stimulation in a panel of 16 human tumor cell lines by
mistletoe extracts in vitro. Anticancer Drugs. 13:373–379. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pedrero JM, Carracedo DG, Pinto CM,
Zapatero AH, Rodrigo JP, Nieto CS, et al: Frequent genetic and
biochemical alterations of the PI3K/Akt/PTEN pathway in head and
neck squamous cell carcinoma. Int J Cancer. 114:242–248. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng JQ, Ruggeri B, Klein WM, Sonoda G,
Altomare DA, Watson DK and Testa JR: Amplification of Akt2 in human
pancreatic cells and inhibition of Akt2 expression and
tumorigenicity by antisense RNA. Proc Natl Acad Sci USA.
93:3636–3641. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brazil DP, Yang ZZ and Hemmings BA:
Advances in protein kinase B signalling: AKTion on multiple fronts.
Trends Biochem Sci. 29:233–242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Diehl JA, Zindy F and Sherr CJ: Inhibition
of cyclin D1 phosphorylation on threonine-286 prevents its rapid
degradation via the ubiquitin-proteasome pathway. Genes Dev.
11:957–972. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sauter ER, Nesbit M, Litwin S,
Klein-Szanto AJ, Cheffetz S and Herlyn M: Antisense cyclin D1
induces apoptosis and tumor shrinkage in human squamous carcinoma.
Cancer Res. 59:4876–4881. 1999.PubMed/NCBI
|
|
31
|
Baldin V, Lukas J, Marcote MJ, Pagano M
and Draetta G: Cyclin D1 is a nuclear protein required for cell
cycle progression in G1. Genes Dev. 7:812–821. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Altomare DA and Testa JR: Perturbations of
the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Andjelkovic M, Alessi DR, Meier R,
Fernandez A, Lamb NJ, Frech M, et al: Role of translocation in the
activation and function of protein kinase B. J Biol Chem.
272:31515–31524. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nicholson KM and Anderson NG: The protein
kinase B/Akt signaling pathway in human malignancy. Cell Signal.
14:381–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shen WH, Balajee AS, Wang J, Wu H, Eng C,
Pandolfi PP, et al: Essential role for nuclear PTEN in maintaining
chromosomal integrity. Cell. 128:157–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Trotman LC, Wang X, Alimonti A, Chen Z,
Teruya-Feldstein J, Yang H, et al: Ubiquitination regulates PTEN
nuclear import and tumor suppression. Cell. 128:141–156. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Perren A, Komminoth P, Saremaslani P,
Matter C, Feurer S, Lees JA, et al: Mutation and expression
analyses reveal differential subcellular compartmentalization of
PTEN in endocrine pancreatic tumors compared to normal islet cells.
Am J Pathol. 157:1097–1103. 2000. View Article : Google Scholar
|
|
39
|
Gimm O, Perren A, Weng LP, Marsh DJ, Yeh
JJ, Ziebold U, et al: Differential nuclear and cytoplasmic
expression of PTEN in normal thyroid tissue, and benign and
malignant epithelial thyroid tumors. Am J Pathol. 156:1693–1700.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cully M, You H, Levine AJ and Mak TW:
Beyond PTEN mutations: the PI3K pathway as an integrator of
multiple inputs during tumorigenesis. Nat Rev Cancer. 6:184–192.
2006. View Article : Google Scholar : PubMed/NCBI
|