Introduction
Nasopharyngeal carcinoma (NPC) is a squamous-cell
carcinoma of the head and neck region, most common in Southern
China and Southeast Asia (1).
Radiotherapy and chemotherapy are typical conventional treatment
for NPC.However, the 5-year survival rate of NPC is ~70% and local
recurrence and distant metastases are the main causes of treatment
failures, indicating that new therapeutic targets must be explored
and developed (2).
An elevated rate of glucose consumption and the
dependency on aerobic glycolysis for ATP generation are noticeable
hallmarks of cancer cells, a phenomenon known as the Warburg
effect. In recent years, it was also observed that glycolysis was
promoted in NPC cells, and a higher 18F-FDG uptake was
associated with distant failure and poor prognosis in NPC patients
(3,4). Since the metabolic alteration provides
cancer cells with enough energy and biosynthetic precursors,
metabolic enzymes involved in glycolysis have become therapeutic
targets with great potential (5).
Lactate dehydrogenase (LDH), including two subunits
LDH-A and LDH-B, is an enzyme widely existing in human cells and
tissues. It catalyzes the transformation of pyruvate to lactate
accompanied by conversion of NADH to NAD+, which plays a
vital role in the process of glycolysis. It has long been noted
that LDH-A expression is upregulated in human neoplastic tissues
(6). Recently, an increasing number
of studies indicate that LDH-A plays an essential role in tumor
maintenance, growth and progression (7–10).
Furthermore, relevant studies have demonstrated that inhibition of
LDH-A induces oxidative stress and suppresses tumor growth in a
variety of cancer cell lines (9,11–13).
Regarding NPC, LDH-A was reported to be highly expressed in head
and neck cancer cells and was found to be associated with local
relapse, worse survival and distant metastasis (14). In addition, several studies have
also demonstrated that an increased LDH serum level is a poor
prognostic factor in NPC patients (15,16).
However, to date, there is no research on whether inhibition of
LDH-A impairs the growth of NPC cancer cells.
In previous studies, oxamate, a competitive LDH-A
inhibitor, has been shown to inhibit the growth of cervical, breast
and liver cancer cells in vitro(17–19).
Moreover, oxamate also significantly enhanced the sensitivity of
cancer cells to several chemotherapy agents (20–22).
Here, in the present study, the effect of LDH-A inhibition by
oxamate on NPC cells was explored, and the involved mechanisms were
evaluated. Moreover, the influence of oxamate on the
radiosensitivity of NPC cells was examined since radiotherapy is
the main treatment strategy for NPC. Finally, a tumor mouse model
was employed to test the inhibitive effect of oxamate in
vivo.
Materials and methods
Reagents and cell lines
Oxamate sodium was purchased from Sigma-Aldrich
Corp. (St. Louis, MO, USA). CNE-1 and CNE-2 cells were obtained
from the American Type Culture Collection (ATCC, Manassas, VA,
USA). Both cell types were cultured in Dulbecco’s modified Eagle’s
medium (DMEM; Gibco), supplemented with 10% fetal calf serum (FCS),
5 mmol/l L-glutamine, 5 mmol/l non-essential amino acids, 100 U/ml
penicillin and 100 U/ml streptomycin (Invitrogen, Carlsbad, CA,
USA) at 37ºC under 5% CO2.
MTT assay
A
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)
assay was performed in 96-well plates to test cell viability. Cells
were seeded at 104/well and treated with different
concentrations of oxamate sodium (0–100 mmol/l) for 24, 48 and 72
h, respectively. Then 20 μl of MTT solution (5 mg/l) was added to
each well, and the plates were incubated at 37ºC for another 4 h.
The supernatant was discarded, and 150 μl dimethyl sulfoxide was
added for 10 min. The absorbance was measured using a microplate
reader (Bio-Tek Instruments, Inc., Winooski, VT, USA) at 570 nm.
The cell viability is expressed as the percentage of untreated
control cells.
Colony formation assay
Cells pretreated with 20 mmol/l oxamate sodium for
24 h were irradiated at 0–9 Gy with 6 MV X-rays from a linear
accelerator (Siemens, Munich, Germany), with a dose rate of 200
cGy/min. The cells were then seeded at different densities
according to the IR dose. After 14 days of incubation at 37ºC, the
cells were fixed, stained with Giemsa and counted. Colonies were
scored only when containing ≥50 cells. Experiments were conducted
in triplicate. Survival fractions were fitted into a linear
quadratic model using GraphPad Prism software (version 5.0).
Detection of LDH activity
Intracellular LDH activity was determined using an
LDH activity assay kit (BioVision, Tucson, AZ, USA). The assay is
based on an enzymatic coupling reaction in which LDH reduces NAD to
NADH, which then reacts with a specific probe to generate a color
(λmax = 450 nm). Cells treated with different
concentrations of oxamate were harvested and lysed for protein to
measure the LDH activity. Results were normalized to the protein
concentrations of the cell lysate, and the protein concentrations
were determined using a BCA protein assay kit (Beyotime, Haimen,
China).
Measurement of intracellular ATP
Intracellular ATP was measured using a firefly
luciferase-based ATP assay kit (Beyotime) following the
manufacturer’s instructions. Briefly, cells were cultured in DMEM
with different concentrations of oxamate sodium (0, 20, 50 and 100
mmol/l). After 24 h, cells were lysed and centrifuged at 12,000 × g
for 5 min. The supernatant (100 μl) was transferred to a 24-well
plate mixed with 100 μl ATP detection working dilution.
Luminescence was measured by a microplate reader (Bio-Tek
Instruments, Inc.). The protein concentration of each group was
also determined using a BCA protein assay kit. The relative ATP
level is expressed as ATP value/protein value.
Analysis of cell cycle distribution
Cells were collected at 24 h after treatment with
different concentrations of oxamate sodium, then fixed with 70%
ethanol and stored overnight at 4ºC. After centrifugation and
washing with 1X PBS twice, the fixed cells were stained with 500 μl
of propidium iodine (PI) (10 μg/ml; Sigma-Aldrich) for 30 min in
the dark. Cell cycle distribution was analyzed using 10,000 cells
by flow cytometry with the FACSCalibur system (Becton-Dickinson,
San Jose, CA, USA).
Analysis of apoptosis
Apoptosis was tested using the Annexin V-FITC
apoptosis kit (BD Biosciences, San Jose, CA, USA). Cells treated
with different concentrations of oxamate sodium for 48 h were
harvested and stained with Annexin V/PI for 30 min. The apoptotic
fraction was detected by a flow cytometer with the FACSCalibur
system.
Assay of reactive oxygen species
(ROS)
ROS content was tested using an ROS detection kit
(Beyotime), based on the intracellular peroxide-dependent oxidation
of DCFH-DA to form the fluorescent compound
2′,7′-dichlorofluorescein (DCF). Cells treated with 0, 20, 50 and
100 mmol/l oxamate sodium were collected and incubated with 1 ml
DCFH-DA (20 μM) for 30 min. Fluorescence intensity was detected by
flow cytometry.
Western blot analysis
Cells were harvested and suspended in IP lysing
buffer (Beyotime) containing protease inhibitor (25 mg/ml; Roche,
Mannheim, Germany). The protein concentration was measured using
the BCA protein assay kit. Then 50 μg of total protein was
separated on SDS-PAGE gel and transferred to polyvinylidene
difluoride (PVDF) membranes. After blocking in PBS with 5% non-fat
dry milk for 45 min, the membranes were incubated with relevant
antibodies for 1 h. Signals were detected using an ECL western
blotting kit (Beyotime). The following antibodies were utilized:
anti-cyclin B1 mouse antibody (1:500; Santa Cruz Biotechnology),
anti-CDK1 mouse antibody (1:500; Santa Cruz Biotechnology),
anti-Bcl-2 mouse antibody (1:500; Santa Cruz Biotechnology,
anti-Bax mouse antibody (1:500; Santa Cruz Biotechnology),
anti-caspase-3 rabbit antibody (1:1,000; Cell Signaling
Technology), anti-cleaved-caspase-3 rabbit antibody (1:1,000; Cell
Signaling Technology), anti-β-actin antibody (1:2,000; Santa Cruz
Biotechnology), anti-mouse antibody (1:1,000; Santa Cruz
Biotechnology) and anti-rabbit antibody (1:1,000; Santa Cruz
Biotechnology).
Animal experiments
Female Balb/c nude mice weighing 18–22 g at 4–6
weeks of age were purchased from the Institute of Laboratory Animal
Sciences (Shanghai, China) and were raised under specific
pathogen-free environments. CNE-1 cells (1×106)
suspended in 100 μl of 1:1 DMEM culture and Matrigel (BD
Biosciences) were injected subcutaneously into the flanks of the
nude mice. When tumor sizes reached ~100 mm3, 24 mice
were randomly divided into 4 groups (n=6 mice/group) and treated
with PBS, oxamate, irradiation, or oxamate combined with
irradiation, respectively. Oxamate was intraperitoneally injected
at 750 mg/kg daily for 3 weeks, and mice were irradiated with 3.3
Gy X-rays for a consecutive 3 days (total dose of 9.9 Gy) 2 h after
injection of oxamate from the second day of drug administration
using a 6-MV linear accelerator (Siemens). Tumor sizes and mouse
body weights were measured every 3 days for 5 weeks. Tumor volumes
were calculated using the formula: Volume = length ×
width2/2.
Statistical analysis
Each experiment was repeated three times and
performed in triplicate. Data are expressed as the means ± standard
deviation (SD). Differences between two groups were assessed by the
two-tailed Student’s t-test. All of the statistical analyses were
performed using SPSS version 17.0. A P-value <0.05 was
considered to indicate a statistically significant result.
Results
Inhibition of LDH by oxamate suppresses
cell viability and energy metabolism in NPC cells
First, to explore the effect of oxamate on cell
proliferation in NPC cells, MTT assays were conducted. CNE-1 and
CNE-2 cancer cells were treated with different doses of oxamate for
24–72 h. As shown in Fig. 1A,
treatment with increasing concentrations of oxamate inhibited cell
proliferation in a dose- and time-dependent manner in both NPC
cancer cell lines. The IC50 values were 74.6, 32.4 and
17.8 mmol/l and 62.3, 44.5, 31.6 mmol/l at 24, 48 and 72 h in the
CNE-1 and CNE-2 cancer cells, respectively. Then, to confirm the
LDH inhibition effect of oxamate as previously reported (18,19), a
commercially available kit was used to determine the intracellular
LDH enzyme activity after treatment with different doses of
oxamate. The results showed that oxamate significantly decreased
LDH activity (Fig. 1B), which
indicated that oxamate was an inhibitor of human LDH enzyme and
provided evidence of the LDH inhibitory effect by oxamate for the
subsequent experiments. Fig. 1C
shows that oxamate markedly reduced the intracellular ATP levels.
Relative ATP levels in the 20, 50 and 100 mmol/l oxamate-treated
groups were 87.3±5.2, 51.3±8.5 and 32.7±4.1%, respectively, when
compared to the untreated control group (100±7.0%) (P=0.21, 0.025
and 0.007) in the CNE-1 cell line, and 84.3±5.0, 47.6±8.3 and
27±5.3%, when compared to the untreated control group (100±6.2%)
(P=0.002, 0.001 and <0.001) in the CNE-2 cell line. The results
demonstrated that LDH inhibition by oxamate disturbed energy
metabolism and decreased ATP production in the NPC cells.
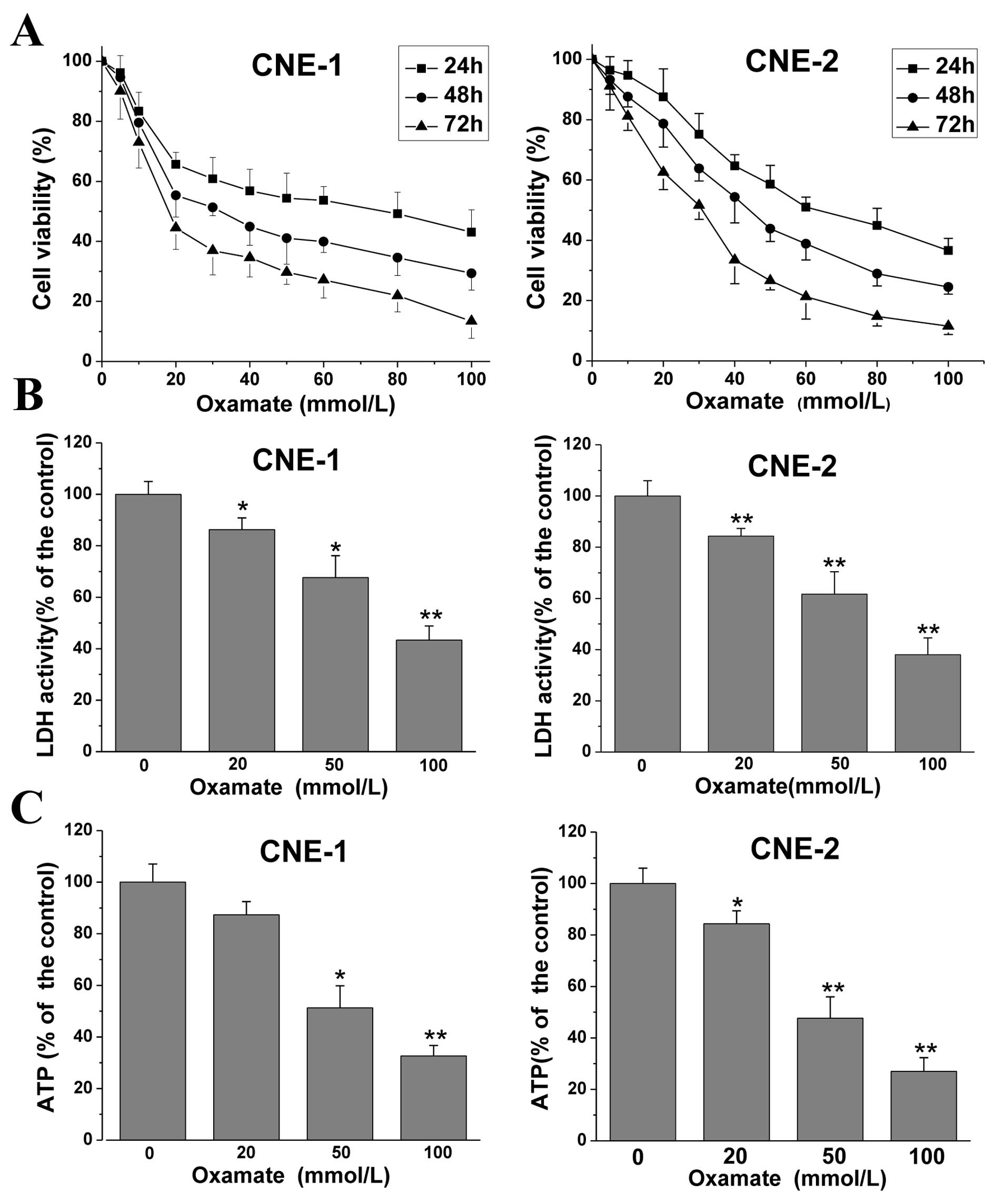 | Figure 1Oxamate inhibits cell growth, LDH
enzyme activity and decreases ATP levels in CNE-1 and CNE-2 cells.
(A) Cells were exposed to different concentrations of oxamate for
24, 48 and 72 h, in 96-well plates, and the effects on cell
viability were tested by MTT assay. Cell viability was calculated
as a percentage of untreated cells (100%); values are represented
as means ± SD; n=6. (B) Cells were treated with 0, 20, 50 and 100
mM oxamate for 24 h, then intracellular LDH enzyme activity was
determined using a commercially available kit. Values are
represented as means ± SD; n=3. *P<0.05,
**P<0.01 vs. the untreated control. (C) Cells were
treated with 0, 20, 50 and 100 mM for 24 h, and intracellular ATP
levels were assayed by a firefly luciferase-based ATP assay kit.
Values are represented as means ± SD; n=3. *P<0.05,
**P<0.01 vs. the untreated control. Each experiment
was repeated three times and performed in triplicate. LDH, lactate
dehydrogenase. |
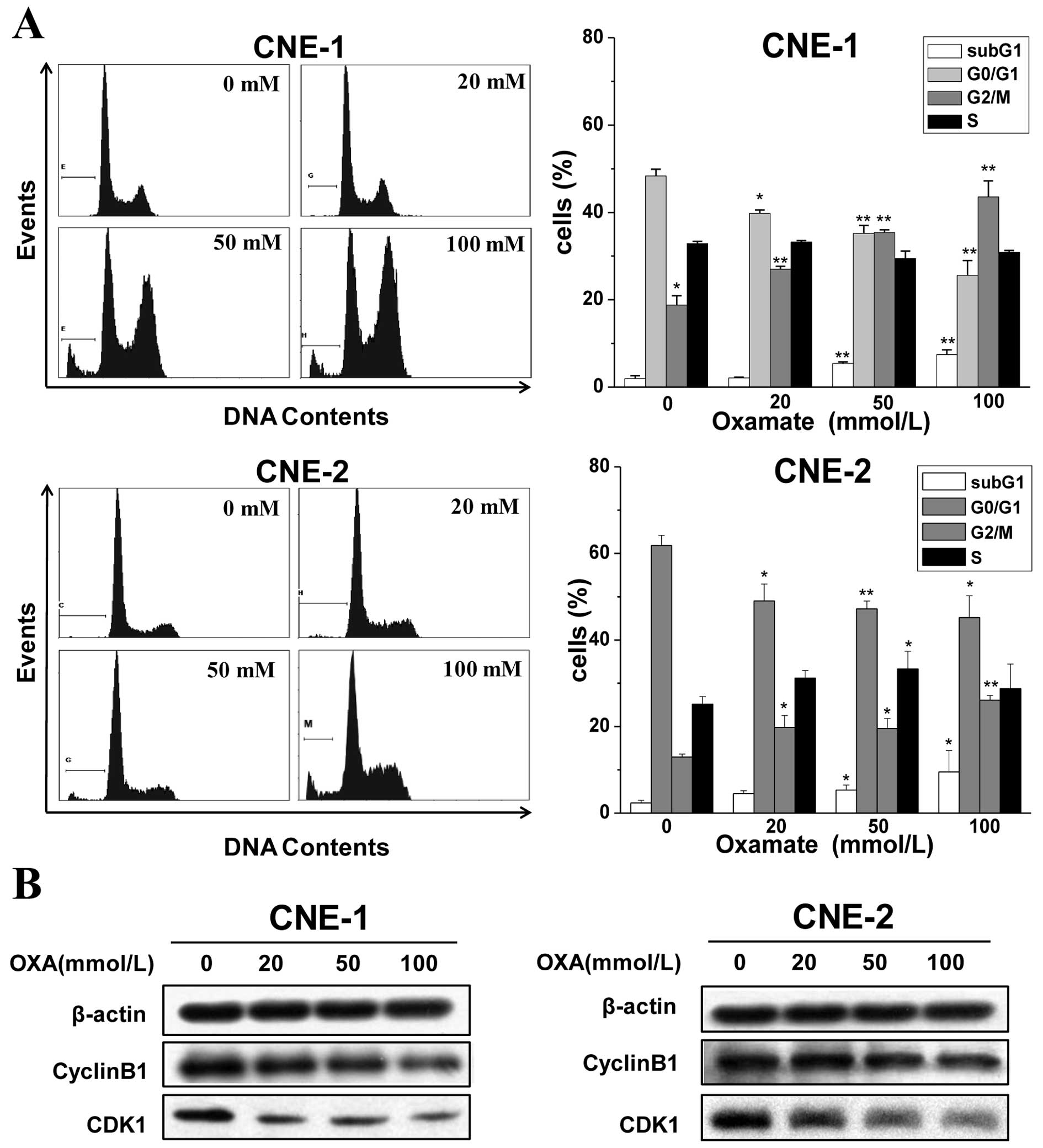
Oxamate induces G2/M arrest
via downregulation of CDK1/cyclin B1 in NPC cells
Since a great majority of anticancer agents act on
the cell cycle and its checkpoint (23), alterations in cell cycle
distribution after oxamate treatment were investigated in NPC
cancer cells. Cells were exposed to 0, 20, 50 and 100 mmol/l
oxamate for 24 h, and flow cytometry was used to analyze the cell
cycle changes after PI staining. As shown in Fig. 2A, there was a dose-dependent
increase in the numbers of CNE-1 and CNE-2 cells in the G2/M phase
after a 24-h treatment with oxamate at concentrations of 0, 20, 50
and 100 mmol/l. The percentages of cells in the G2/M phase were
18.8±2.1, 26.9±1.6, 35.4±2.3, 43.6±3.6, respectively, in the CNE-1
cells and 13.0±3.2, 19.7±2.7, 21.5±2.3 and 26.1±1.1%, in the CNE-2
cells, respectively. Meanwhile, the proportions of cells in the
G0/G1 phase were decreased. However, the S
phase fractions did not change significantly in both cell lines. In
addition, it was noteworthy that there was a significant increase
in the percentage of cells in the sub-G1 phase after
treatment with oxamate.
To further certify the G2/M arrest
induced by oxamate and to understand the mechanisms, western blot
analysis was used to examine the changes in expression of proteins
related to G2/M transition. As shown in Fig. 2B, the expression levels of cyclin B1
and CDK1 significantly decreased in both the CNE-1 and CNE-2 cells
after treatment with oxamate, which suggests that oxamate induces
G2/M arrest by modulating the expression of cyclin B1
and CDK1.
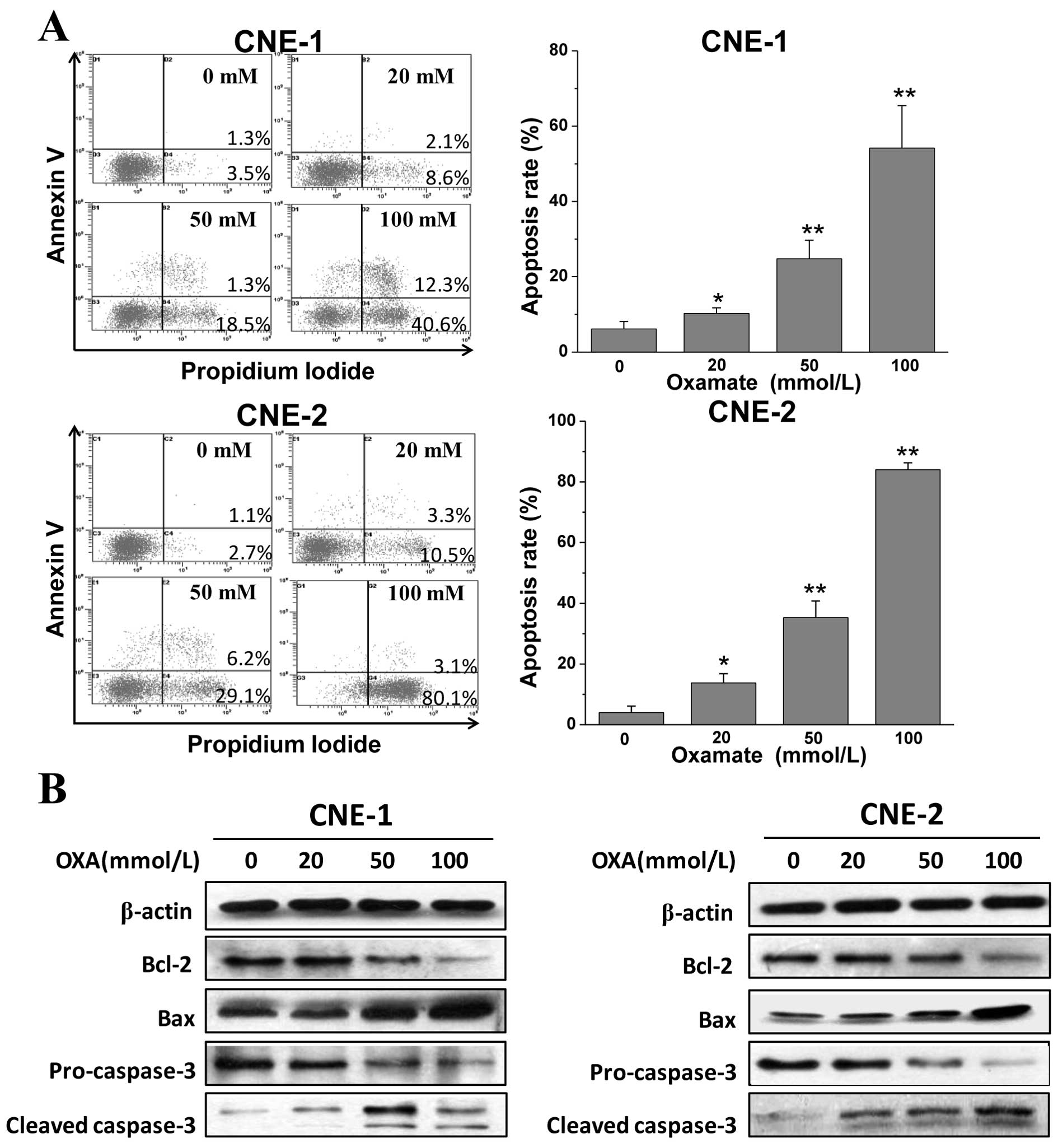
Oxamate induces apoptosis via the
mitochondrial pathway
We demonstrated that LDH inhibition by oxamate
impaired cell growth and increased the sub-G1 fraction
in both cell lines. To confirm that oxmate treatment induces
apoptosis, cells were exposed to different concentrations of
oxamate for the prolonged time of 48 h, and then Annexin V/PI
staining and flow cytometric analysis were performed. After the
48-h treatment with oxamate, the percentages of early and late
apoptotic cells were increased in a dose-dependent manner (Fig. 3A). Next, western blot analysis was
utilized to determine the changes in expression of
apoptosis-related proteins. As shown in Fig. 3B, the expression of pro-apoptotic
Bax and cleaved-caspase-3 was significantly enhanced, while the
anti-apoptotic signals of Bcl-2 and pro-caspase-3 were reduced
notably after treatment with different concentrations of oxamate
for 48 h. The results indicate that oxamate induces apoptosis via
caspase-3 activation and the mitochondrial pathway in NPC
cells.
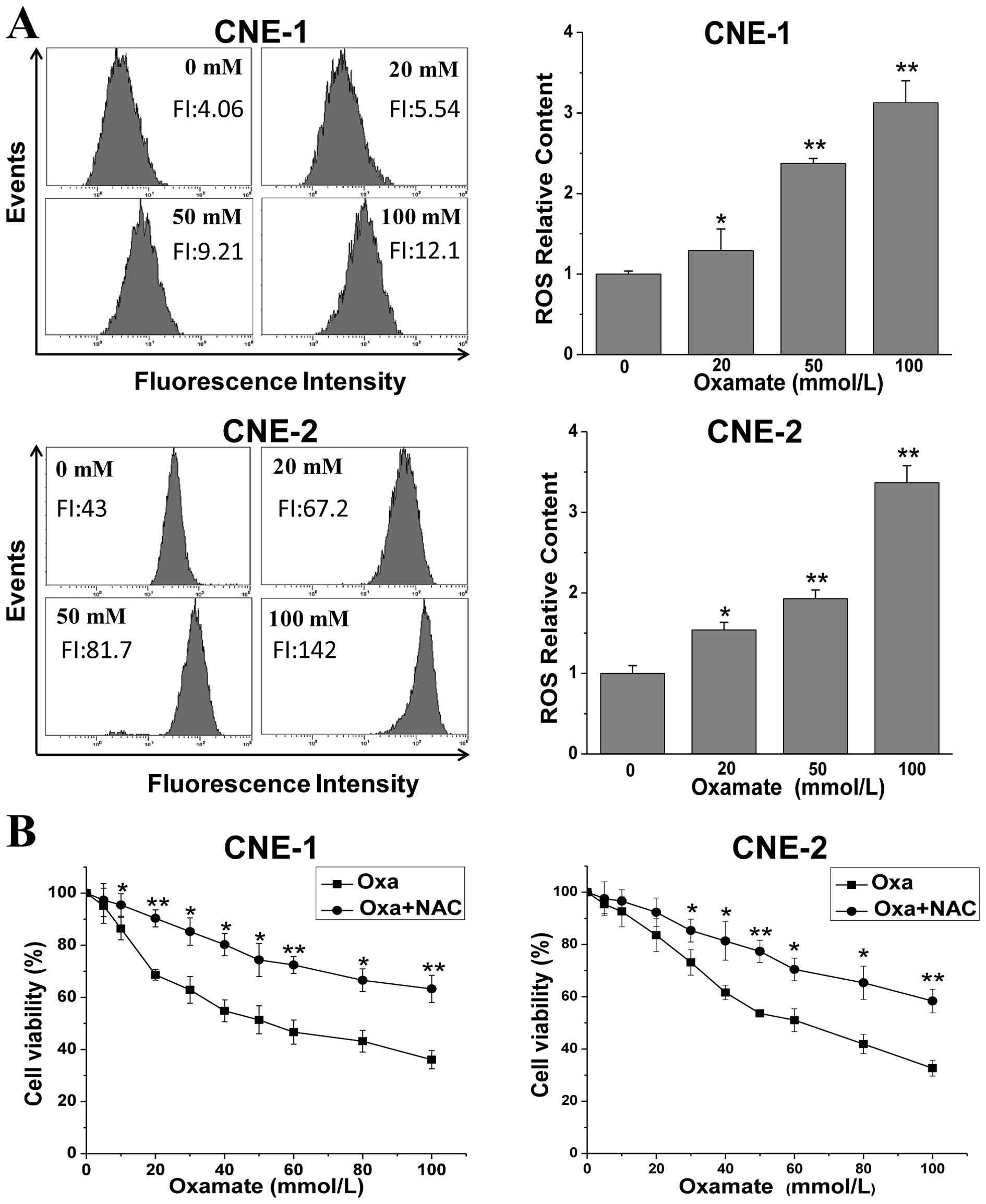
Oxamate increases ROS levels in NPC
cells
To further explore the mechanisms involving the
inhibitory effect induced by oxamate in NPC cells, we determined
the changes in ROS levels after oxamate treatment, which play an
important role in the mitochondrial apoptotic pathway. As shown in
Fig. 4A, oxamate dose-dependently
enhanced ROS levels in both NPC cell lines. ROS levels were
increased to 1.3-, 2.4- and 3.1-fold (P<0.01) after treatment
with 20, 50 and 100 mmol/l oxamate for 24 h, when compared to the
untreated control group. Similarly, there was an 1.5- to 3.3-fold
increase (P<0.01) in the CNE-2 cells. To examine the role of ROS
generation in the oxamate-induced growth inhibitory effect,
oxamate-treated cells were incubated simultaneously with 10 mM
N-acetylcysteine, a specific scavenger of ROS. As shown in Fig. 4B, pre-treatment with NAC
significantly blocked the growth inhibitory effect in both CNE-1
and CNE-2 cell lines, indicating that ROS generation contributes
partially to the anti-proliferative effect induced by oxamate.
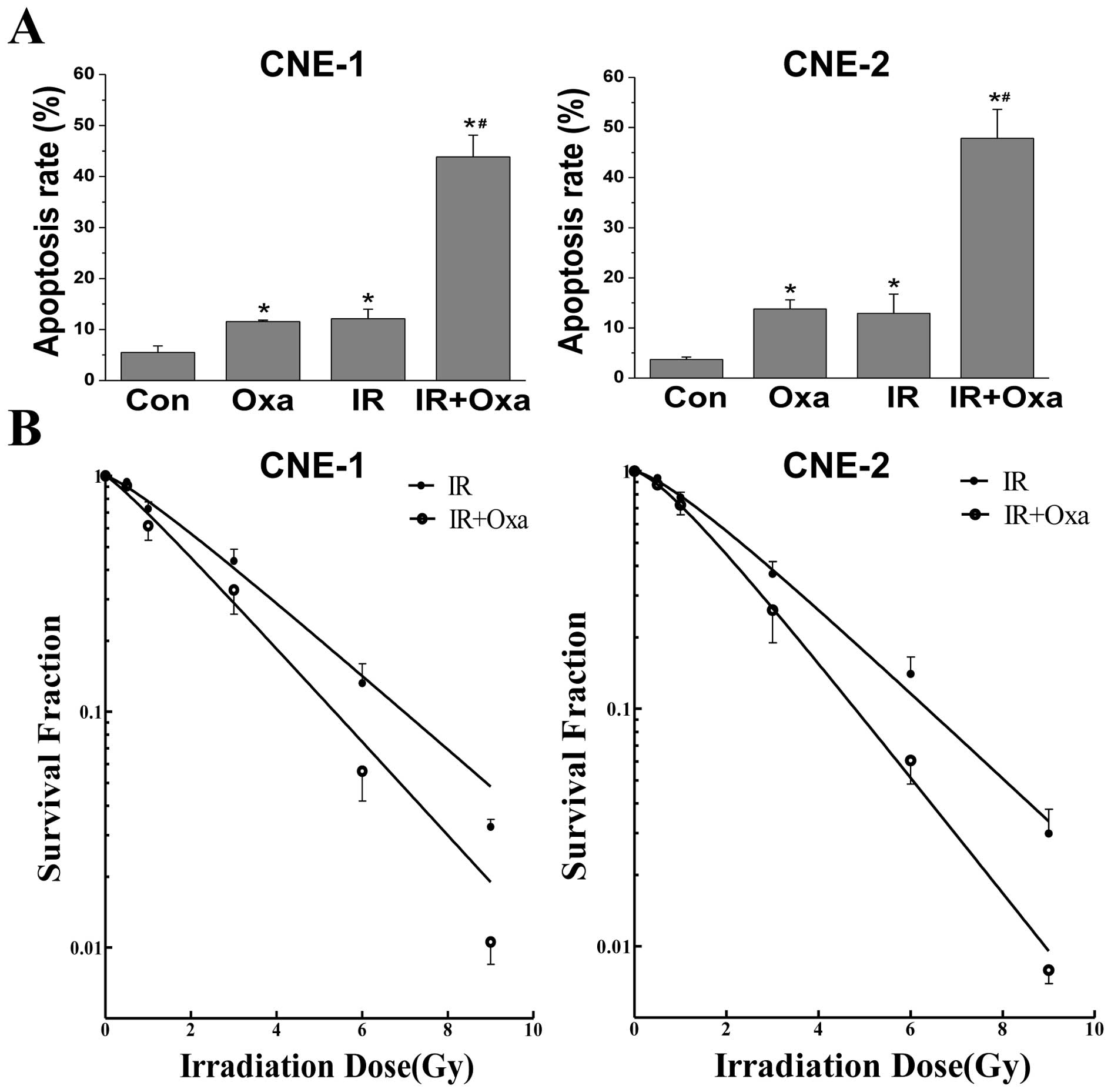
Oxamate increases sensitivity to ionizing
radiation in NPC cells
Radiotherapy is the main treatment for NPC patients
at present. Thus, we examined whether LDH inhibition by oxamate
influences the sensitivity of NPC cells to ionizing radiation.
Firstly, Annexin-V/PI staining was conducted to observe the effect
of oxamate on the apoptosis induced by irradiation within a short
period of time, and then colony formation assays were performed to
evaluate the long-term effects of the combined treatment. As shown
in Fig. 5A the combination of
irradiation and oxamate synergistically enhanced the apoptosis
rates in both NPC cancer cells, when compared to either treatment
alone. Furthermore, oxamate enhanced the IR-induced inhibition of
clonogenic survival in NPC cells (Fig.
5B). The sensitivity enhancement ratios (SERs) were 1.26 and
1.35 in CNE-1 and CNE-2 cells, respectively, as determined by
analyzing the linear quadratic models. These results revealed that
oxamate increased the radiosensitivity in NPC cells in
vitro, and the effects were similar in both CNE-1 and CNE-2
cells.
Oxamate improves the efficacy of
irradiation in vivo
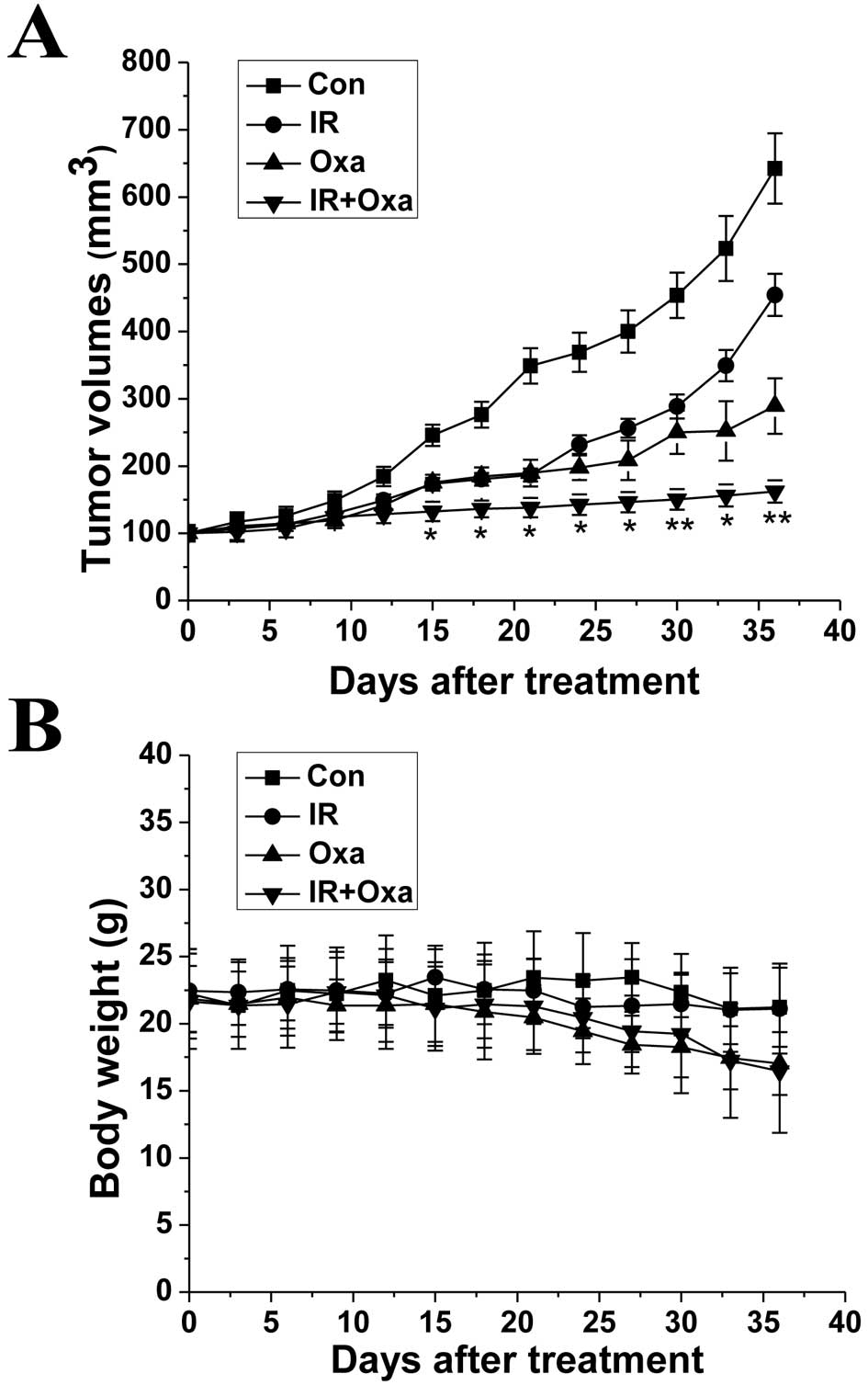
Finally, we examined whether oxamate inhibits tumor
growth in vivo and its effects when combined with
irradiation treatment. As shown in Fig.
6A, oxamate effectively delayed tumor growth in vivo.
Moreover, combined treatment with oxamate and irradiation
significantly improved the growth inhibitory effect when compared
to either oxamate alone or irradiation alone. As shown in Fig. 6B that inhibition by oxamate had
little influence on the body weights of the mice. A small decrease
in body weights of the mice was noted following treatment with
oxamate and irradiation. However, the decrease had no statistical
significance when compared to the mice treated with oxamate
alone.
Discussion
In the present study, we investigated the role of
LDH inhibition by oxamate and its effect on radiosensitivity in two
human NPC cell lines. We found that oxamate induced G2/M
cell cycle arrest via downregulation of the CDK1/cyclin B1 pathway
and promoted apoptosis through enhancing mitochondrial ROS
generation. We also determined that oxamate increased sensitivity
to irradiation. Furthermore, we verified similar results in the
tumor xenograft model. These results indicate that LHA may serve as
an attractive therapeutic target for NPC treatment.
Although the exact molecular mechanism involved in
the Warburg effect remains to be explored, the altered energy
metabolism in cancer cells provides an opportunity for developing
novel cancer therapeutic strategies by targeting the glycolytic
pathway (5). Previous studies
revealed that LDH-A expression is elevated both in squamous cell
head and neck cancer cells and the blood serum of NPC patients and
is associated with poor prognosis (4,15,16).
As anticipated, LDH inhibition by oxamate was found to impair cell
growth and increase ROS production in NPC cells. Reactive oxygen
species (ROS) are highly reactive molecules and free radicals
containing oxygen. ROS play an important role in
mitochondrial-mediated apoptosis. Moreover, mitochondria can be
both a source and a target of ROS (24). As previously described, LDH is a
crucial enzyme transforming pyruvate to lactate in the anaerobic
glycolysis pathway. More pyruvates are forced to enter into the
tricarboxylic acid (TCA) cycle when LDH is inhibited by oxamate.
However, mitochondrial dysfunction and impaired oxidative
phosphorylation are quite common in cancer cells, as demonstrated
in many studies (25–27). In this case, the OXPHOS chains are
abnormally activated and more ROS are generated, resulting in
mitochondrial membrane destruction and subsequent apoptosis. In the
present study, caspase-3 activation was observed by western blot
analysis, and the growth inhibitory effect induced by oxamate was
blocked by the ROS scavenger NAC. These data indicate that LDH
inhibition by oxamate induces ROS-mediated intrinsic mitochondrial
apoptosis in NPC cells.
The cell cycle involves a series of events driven by
cyclins and subsequent cyclin-dependent kinases (CDKs), which
further activate transcription factor proteins for cell cycle
progression from one phase to another. The CDK1/cyclin B1 kinase
complex plays a major role during G2/M transition. A
reduction in CDK1/cyclin B1 kinase activity triggers
G2/M cell cycle arrest (28). In the present study, LDH inhibition
by oxamate induced G2/M arrest and also a decrease in
the protein levels of cyclin B1 and CDK1 in NPC cells. Thus, it is
rational to postulate that G2/M arrest induced by
oxamate was mediated by reducing the activity of the CDK1/cyclin B1
kinase complex. In the present study, cell cycle progression was
correlated closely with the status of energy metabolism, although
upstream signals regulating the G2/M+related proteins
remain to be further investigated. Notably, dichloroacetate (DCA),
an inhibitor of pyruvate dehydrogenase kinase also induced
G2/M arrest in colorectal cancer cells in a previous
study (29).
Radiotherapy plays a vitally important role in NPC
treatment. Unfortunately, radiation resistance always results in
subsequent recurrence and metastasis of cancer, which leads to
treatment failure. It has been reported that inhibition of LDH
sensitizes cancer cells to chemotherapy (18–20).
However, the relationship between LDH inhibition and
radiosensitivity is still unclear. In the present study, we found
that oxamate significantly increased sensitivity to X-ray
irradiation in NPC cells both in vitro and in vivo.
As known, the ‘oxygen effect’ is an important phenomenon in
radiation biology, which refers to the enhanced killing effect of
radiation in the presence of oxic conditions. Irradiation exposure
can cause DNA damage as well as mitochondrial-dependent ROS
generation. In addition, ROS are also critical mediators of
radiation-induced cellular toxicity (30,31).
Therefore, ROS play an important role in regulating the
radiosensitivity of tumor cells (32). The results in the present study
showed that LDH inhibition by oxamate induced increased ROS
production and mitochondrial apoptosis in NPC cells. Thus, it is
reasonable to assume that the increased ROS levels induced by
oxamate synergistically enhanced the DNA damage effect and toxicity
of irradiation in NPC cells. Other studies also found that
glycolysis inhibitors increased radiosensitivity in cancer cells
(33–35). Sharma et al(34) reported that non-coordinated
expression of antioxidant enzymes was another essential factor that
led to selective radiosensitization in malignant cells, in addition
to redox status.
Oxamate is a conventional competitive inhibitor of
LDH-A at high concentrations, which limits its therapeutic
potential in clinical practice (18,36,37).
However, the present study indicates that targeting LDH-A may be a
feasible therapeutic strategy for the treatment of NPC. New
effective small molecules specifically targeting LDH-A are being
developed, including several active compounds from Chinese
traditional herbal medicine. Some have shown promising clinical
utility (38–40).
In conclusion, the present study provides evidence
for LDH-A inhibition in the treatment of NPC alone or combined with
irradiation both in vitro and in vivo. Targeting
glycolysis may be an effective strategy for NPC therapy. Further
studies are required to explore whether inhibition of other key
enzymes in the glycolytic pathway has similar effects as the
inhibition of LDH-A. Further efforts are needed to develop highly
effective novel inhibitors of the LDH-A enzyme.
Acknowledgements
The present study was supported by grants from the
National Science Foundation of China (no. 81202149) and the Open
Program of the Key Laboratory of Nuclear Medicine, Ministry of
Health and the Jiangsu Key Laboratory of Molecular Nuclear Medicine
(KF2011).
References
|
1
|
Wei WI and Sham JS: Nasopharyngeal
carcinoma. Lancet. 365:2041–2054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guigay J: Advances in nasopharyngeal
carcinoma. Curr Opin Oncol. 20:264–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie P, Yue JB, Fu Z, Feng R and Yu JM:
Prognostic value of 18F-FDG PET/CT before and after
radiotherapy for locally advanced nasopharyngeal carcinoma. Ann
Oncol. 21:1078–1082. 2010.
|
|
4
|
Chan SC, Chang JT, Wang HM, et al:
Prediction for distant failure in patients with stage M0
nasopharyngeal carcinoma: the role of standardized uptake value.
Oral Oncol. 45:52–58. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pelicano H, Martin DS, Xu RH and Huang P:
Glycolysis inhibition for anticancer treatment. Oncogene.
25:4633–4646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldman RD, Kaplan NO and Hall TC: Lactic
dehydrogenase in human neoplastic tissues. Cancer Res. 24:389–399.
1964.PubMed/NCBI
|
|
7
|
Fantin VR, St-Pierre J and Leder P:
Attenuation of LDH-A expression uncovers a link between glycolysis,
mitochondrial physiology, and tumor maintenance. Cancer Cell.
9:425–434. 2006. View Article : Google Scholar
|
|
8
|
Zhao YH, Zhou M, Liu H, et al:
Upregulation of lactate dehydrogenase A by ErbB2 through heat shock
factor 1 promotes breast cancer cell glycolysis and growth.
Oncogene. 28:3689–3701. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Le A, Cooper CR, Gouw AM, et al:
Inhibition of lactate dehydrogenase A induces oxidative stress and
inhibits tumor progression. Proc Natl Acad Sci USA. 107:2037–2042.
2010.PubMed/NCBI
|
|
10
|
Serganova I, Rizwan A, Ni XH, et al:
Metabolic imaging: a link between lactate dehydrogenase A, lactate,
and tumor phenotype. Clin Cancer Res. 17:6250–6261. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang ZY, Loo TY, Shen JG, et al: LDH-A
silencing suppresses breast cancer tumorigenicity through induction
of oxidative stress mediated mitochondrial pathway apoptosis.
Breast Cancer Res Treat. 131:791–800. 2012. View Article : Google Scholar
|
|
12
|
Xie H, Valera VA, Merino MJ, et al: LDH-A
inhibition, a therapeutic strategy for treatment of hereditary
leiomyomatosis and renal cell cancer. Mol Cancer Ther. 8:626–635.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sheng SL, Liu JJ, Dai YH, Sun XG, Xiong XP
and Huang G: Knockdown of lactate dehydrogenase A suppresses tumor
growth and metastasis of human hepatocellular carcinoma. FEBS J.
279:3898–3910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koukourakis MI, Giatromanolaki A, Winter
S, Leek R, Sivridis E and Harris AL: Lactate dehydrogenase 5
expression in squamous cell head and neck cancer relates to
prognosis following radical or postoperative radiotherapy.
Oncology. 77:285–292. 2009. View Article : Google Scholar
|
|
15
|
Zhou GQ, Tang LL, Mao YP, et al: Baseline
serum lactate dehydrogenase levels for patients treated with
intensity-modulated radiotherapy for nasopharyngeal carcinoma: a
predictor of poor prognosis and subsequent liver metastasis. Int J
Radiat Oncol Biol Phys. 82:e359–e365. 2012. View Article : Google Scholar
|
|
16
|
Wan XB, Wei L, Li H, et al: High
pretreatment serum lactate dehydrogenase level correlates with
disease relapse and predicts an inferior outcome in locally
advanced nasopharyngeal carcinoma. Eur J Cancer. 9:2356–2364. 2013.
View Article : Google Scholar
|
|
17
|
Papaconstantinou J and Colowick SP: The
role of glycolysis in the growth of tumor cells. II. The effect of
oxamic acid on the growth of HeLa cells in tissue culture. J Biol
Chem. 236:285–288. 1961.PubMed/NCBI
|
|
18
|
Thornburg JM, Nelson KK, Clem BF, et al:
Targeting aspartate aminotransferase in breast cancer. Breast
Cancer Res. 10:R842008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fiume L, Manerba M, Vettraino M and Di
Stefano G: Impairment of aerobic glycolysis by inhibitors of lactic
dehydrogenase hinders the growth of human hepatocellular carcinoma
cell lines. Pharmacology. 86:157–162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou M, Zhao YH, Ding Y, et al: Warburg
effect in chemosensitivity: targeting lactate dehydrogenase-A
re-sensitizes Taxol-resistant cancer cells to Taxol. Mol Cancer.
9:332010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fiume L, Vettraino M, Manerba M and Di
Stefano G: Inhibition of lactic dehydrogenase as a way to increase
the anti-proliferative effect of multi-targeted kinase inhibitors.
Pharmacol Res. 63:328–334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao YH, Liu H, Liu ZX, et al: Overcoming
trastuzumab resistance in breast cancer by targeting dysregulated
glucose metabolism. Cancer Res. 71:4585–4597. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shapiro GI and Harper JW: Anticancer drug
targets: cell cycle and checkpoint control. J Clin Invest.
104:1645–1653. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000.PubMed/NCBI
|
|
25
|
Modica-Napolitano JS and Singh KK:
Mitochondrial dysfunction in cancer. Mitochondrion. 4:755–762.
2004.
|
|
26
|
Pelicano H, Lu W, Zhou Y, et al:
Mitochondrial dysfunction and reactive oxygen species imbalance
promote breast cancer cell motility through a CXCL14-mediated
mechanism. Cancer Res. 69:2375–2383. 2009. View Article : Google Scholar
|
|
27
|
Hung WY, Huang KH, Wu CW, et al:
Mitochondrial dysfunction promotes cell migration via reactive
oxygen species-enhanced β5-integrin expression in human gastric
cancer SC-M1 cells. Biochim Biophys Acta. 1820:1102–1110.
2012.PubMed/NCBI
|
|
28
|
Lindqvist A, van Zon W, Karlsson Rosenthal
C and Wolthuis RM: Cyclin B1-Cdk1 activation continues after
centrosome separation to control mitotic progression. PLoS Biol.
5:e1232007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Madhok BM, Yeluri S, Perry SL, Hughes TA
and Jayne DG: Dichloroacetate induces apoptosis and cell-cycle
arrest in colorectal cancer cells. Br J Cancer. 102:1746–1752.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leach JK, Van Tuyle G, Lin PS,
Schmidt-Ullrich R and Mikkelsen RB: Ionizing radiation-induced,
mitochondria-dependent generation of reactive oxygen/nitrogen.
Cancer Res. 61:3894–3901. 2001.PubMed/NCBI
|
|
31
|
Tominaga H, Kodama S, Matsuda N, Suzuki K
and Watanabe M: Involvement of reactive oxygen species (ROS) in the
induction of genetic instability by radiation. J Radiat Res.
45:181–188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Diehn M, Cho RW, Lobo NA, et al:
Association of reactive oxygen species levels and radioresistance
in cancer stem cells. Nature. 458:780–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cao WG, Yacoub S, Shiverick KT, et al:
Dichloroacetate (DCA) sensitizes both wild-type and overexpressing
Bcl-2 prostate cancer cells in vitro to radiation. Prostate.
68:1223–1231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sharma PK, Bhardwaj R, Dwarakanath BS and
Varshney R: Metabolic oxidative stress induced by a combination of
2-DG and 6-AN enhances radiation damage selectively in malignant
cells via non-coordinated expression of antioxidant enzymes. Cancer
Lett. 295:154–166. 2010. View Article : Google Scholar
|
|
35
|
Sandulache VC, Skinner HD, Wang Y, et al:
Glycolytic inhibition alters anaplastic thyroid carcinoma tumor
metabolism and improves response to conventional chemotherapy and
radiation. Mol Cancer Ther. 11:1373–1380. 2012. View Article : Google Scholar
|
|
36
|
Goldberg EB and Colowick SP: The role of
glycolysis in the growth of tumor cells. 3. Lactic dehydrogenase as
the site of action of oxamate on the growth of cultured cells. J
Biol Chem. 240:2786–2790. 1965.PubMed/NCBI
|
|
37
|
Elwood JC: Effect of oxamate on glycolysis
and respiration in sarcoma 37 ascites cells. Cancer Res.
28:2056–2060. 1968.PubMed/NCBI
|
|
38
|
Granchi C, Bertini S, Macchia M and
Minutolo F: Inhibitors of lactate dehydrogenase isoforms and their
therapeutic potentials. Curr Med Chem. 17:672–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Granchi C, Roy S, Giacomelli C, et al:
Discovery of N-hydroxyindole-based inhibitors of human lactate
dehydrogenase isoform A (LDH-A) as starvation agents against cancer
cells. J Med Chem. 54:1599–1612. 2011. View Article : Google Scholar
|
|
40
|
Wang Z, Wang N, Chen J and Shen J:
Emerging glycolysis targeting and drug discovery from Chinese
medicine in cancer therapy. Evid Based Complement Alternat Med.
2012:8731752012.PubMed/NCBI
|