Introduction
ACC is a malignant neoplasm that originates in both
the minor and major salivary glands, the naso-pharyngeal secretory
gland and the lacrimal gland with some other rare primary sites,
and is characterized by slow growth, diffuse invasion, a high
tendency towards mass formation and local recurrence, and the
potential to produce distant metastases, mainly to the lungs and
bones (1,2). Late distant metastases are, together
with local recurrences, responsible for the rather low long-term
survival rate (3). ACC of the
salivary gland is an uncommon malignancy, comprising only 1% of
head and neck tumors, 10% of salivary malignancies and 22% of all
salivary gland malignancies (4,5). Many
types of combination chemotherapy and molecular target therapy have
been investigated; however, only limited responses have been shown
(5).
The telomerase-specific replication-competent
adenovirus (Telomelysin, OBP-301), in which the human telomerase
reverse transcriptase (hTERT) promoter element drives expression of
the E1A and E1B genes linked with an internal ribosomal entry site
(IRES), induces selective E1 expression (6–9).
Moreover, >85% of human cancers display telomerase activity
(10), and both telomerase activity
and hTERT expression are increased in human salivary gland
carcinomas. Intratumoral injection of OBP-301 in combination with a
replication-deficient adenovirus expressing the green fluorescent
protein (GFP) gene (OBP-401, TelomeScan) causes viral spreading
into the regional lymphatic area with subsequent selective
replication in metastatic lymph nodes in BALB/c nu/nu mice
(11). Furthermore, we showed that
OBP-301 induces a selective antitumor effect in OSCC cells in
vitro and in an in vivo orthotopic graft model (12). However, the antitumor effects of
these telomerase-specific replication-selective oncolytic viruses
are not yet fully understood in the framework of ACC. In the
present study, we investigated the in vitro and in
vivo inhibitive efficacy of OBP-301 and OBP-401 against
ACC.
Materials and methods
Cell lines and cell culture
Human salivary gland adenoid cystic carcinoma cell
lines (Acc2 and AccM) were kindly provided by Dr W.L. Qiu. The Acc2
cell line was derived from a 28-year-old female patient, and AccM
was established from Acc2 as a highly metastatic subclone (13,14).
Acc2, AccM and the OSCC cell line SAS were maintained in
vitro as monolayers at 37°C with 5% CO2 in
Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10%
fetal bovine serum, 100 units/ml penicillin and 100 mg/ml
streptomycin.
Adenoviruses
The telomerase-specific replication-selective
oncolytic virus vector OBP-301 (Telomelysin) was constructed and
previously described; the hTERT promoter element drives expression
of the E1A and E1B genes linked with an internal
ribosome entry site (IRES). A GFP gene driven by the
cytomegalovirus (CMV) promoter was inserted into OBP-301, so that
the virus would express GFP during efficient replication in
infected cells (7,9,15).
OBP-401 (TelomeScan) is a telomerase-specific
replication-selective oncolytic virus variant, in which a
GFP gene under control of the cytomegalovirus promoter is
inserted into the deleted E3 region of OBP-301 for monitoring viral
replication (11). The viruses were
purified by CsCl2 linear gradient ultracentrifugation.
The viral titers were determined by plaque-forming assay using 293
cells and then stored at −80°C.
Western blot analysis
Protein was extracted from collected cells with
Triton X-100 lysis buffer (50 mM Tris pH 8.0, 300 mM NaCl, 0.5%
Triton X-100, 5 mM EDTA, 1 mM sodium o-vanadate)
supplemented with Complete Mini (Roche Diagnostics, Basel,
Switzerland) protease inhibitor tablets. Cells were lysed after 48
h of cell seeding, and protein concentrations were subsequently
measured using the Bio-Rad Quick Start protein assay (Bio-Rad
Laboratories, Hercules, CA, USA). Western blot analysis of fraction
markers was carried out according to the method previously
described (16). The proteins in
the SDS sample buffer (25 μg protein) were heated, separated on
4–15% Mini Protean TGX precast gels (Bio-Rad Laboratories) and
transferred to a polyvinylidene difluoride (PVDF) membrane (Life
Technologies, Billerica, MA, USA). After the membrane was blocked
with 0.3% skim milk, the primary antibodies against hTERT (Merck
Millipore, Billerica, MA, USA), CAR, GAPDH (Santa Cruz
Biotechnology, CA, USA) and subsequently, peroxidase-linked
secondary antibodies (GE Healthcare, Buckinghamshire, UK) were
used. The blots were visualized using the ECL Western blotting
detection system (GE Healthcare).
Cell viability assay
Acc2, AccM and SAS cells were seeded on 96-well
plates at a density of 2.5×103 cells/well 18–24 h before
viral infection. Cells were infected with OBP-301 at multiplicity
of infection (MOI) of 1, 10, 50 and 100 plaque-forming units
(PFU)/cell. Cell viability was determined by an XTT assay at 24,
48, 72 and 120 h using the Cell proliferation kit II (Roche
Diagnostics) according to the protocol recommended by the
manufacturer; the particular XTT was sodium
3′-[1-(phenylamino-carbonyl)-3,4-tetrazolium]-bis
(4-methoxy-6-nitro)benzene-sulfonic acid hydrate.
Quantitative real-time RT PCR
analysis
Acc2 and AccM cells, seeded on 100-mm dishes at a
density of 4×105 cells 18–24 h before viral infection,
were infected with OBP-301 at 50 PFU/cells. Following the removal
of the virus, cells were further incubated and harvested 2, 24, 48
and 72 h later, and total RNA was extracted using TRIzol (Life
Technologies). cDNA was generated from 500 ng of total RNA using
the iScript cDNA synthesis kit (Bio-Rad Laboratories). Quantitative
real-time PCR for the E1A gene was performed using the iCycler iQ
Real-time PCR detection system (Bio-Rad Laboratories) and the iQ
SYBR-Green Supermix (Bio-Rad Laboratories), according to the
manufacturer’s instructions. The primer sequences of E1A used in
the present study were as follows: forward, 5′-GCT GAT CGA AGA GGT
ACT GC-3′ and reverse, 5′-ACC GCC AAC ATT ACA GAG TC-3′. The cDNA
amplification program for E1A genes consisted of denaturation at
95°C for 2 min, followed by 45 cycles at 95°C for 5 sec and
annealing and extension at 60°C for 20 sec (17).
Fluorescence microscopy
Acc2 and AccM cells were infected with OBP-401 at
the indicated MOI values in a 6-well flat-bottomed culture plate
and further incubated for the indicated time periods. The
expression of GFP fluorescence was measured and photographed at the
magnification of ×20 using a fluorescence microplate reader
(BioStation CT; Nikon, Corp., Tokyo, Japan).
Animal experiments
Acc2 and AccM cells (3×106 cells/mouse)
suspended in Matrigel were injected into the submandibular glands
of female 6-week-old BALB/c nu/nu nude mice to generate a
subcutaneous tumor model. When tumors reached 3–5 mm in diameter,
OBP-301 or OBP-401 at a dose of 1×108 plaque-forming
units (PFU)/tumor or PBS was injected into the tumor (12).
The perpendicular diameter of the tumors was
measured every 3 days, and the tumor volume was calculated using
the following formula: Tumor volume (mm3) = a ×
b2 × 0.5, where a is the longest diameter, b is the
shortest diameter and 0.5 is a constant to calculate the volume of
an ellipsoid. The body weights of mice were monitored and recorded.
The experimental protocol was approved by the Ethics Review
Committee for Animal Experimentation at Showa University.
Results
In vitro cytopathic effect of OBP-301 on
ACC cell lines and the SCC cell line, CAR and hTERT protein
expression and E1A RNA expression
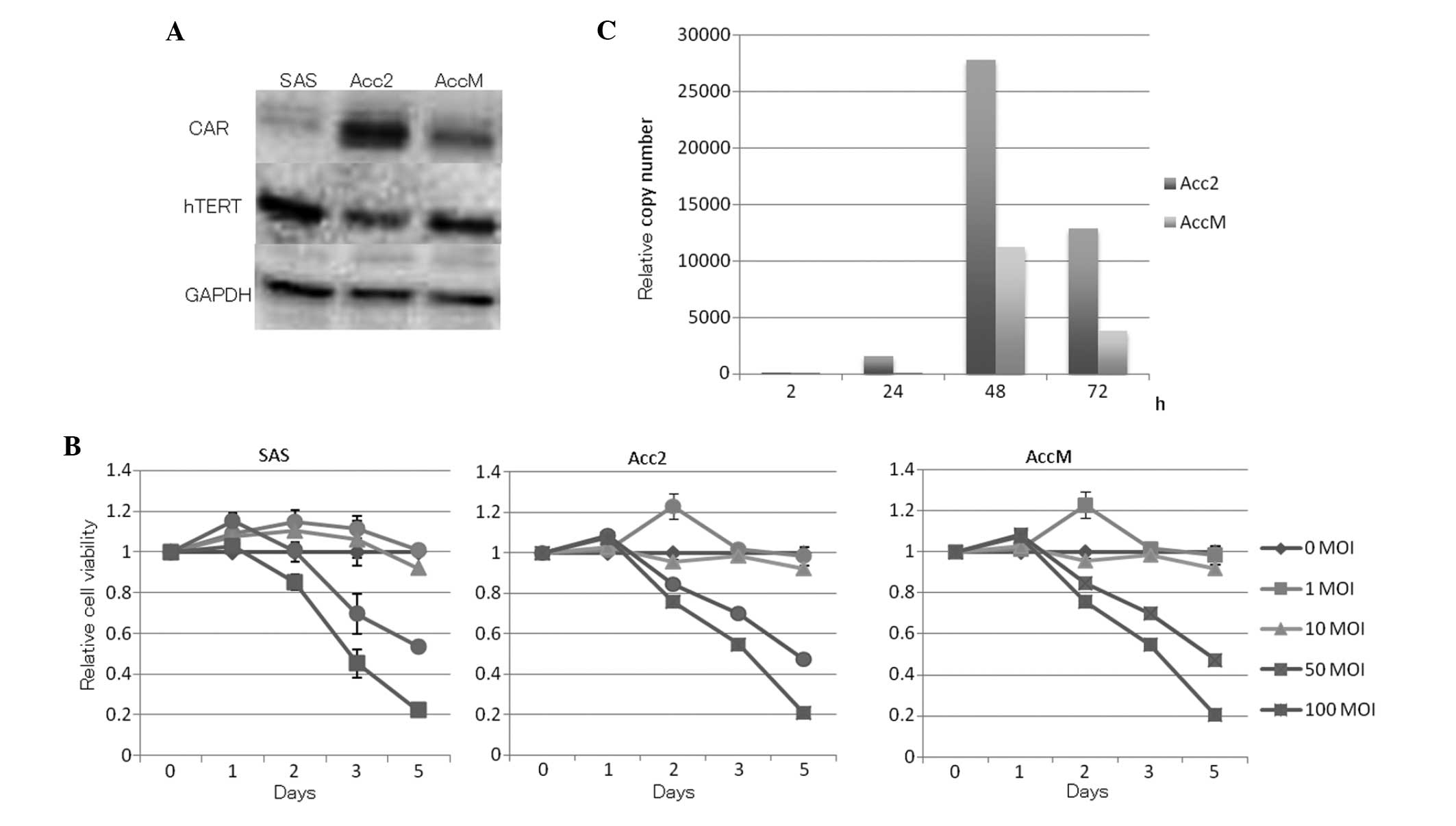
Western blot analysis revealed the protein
expression levels of CAR and hTERT in ACC and SCC cell lines
(Fig. 1A). The in vitro
cytopathic effect of OBP-301 on ACC cell lines and the human SCC
cell line by XTT assay is shown (Fig.
1B). ACC and SCC cell lines were infected with OBP-301 at
various MOIs for 5 days. OBP-301 infection induced cell death in a
time-dependent manner. Acc2 showed the highest expression levels of
CAR and AccM showed the highest expression of hTERT. E1A expression
in OBP-301-infected Acc2 and AccM cell lines multiplied after 48 h
when OBP-301 at a MOI of 50 was used (Fig. 1C).
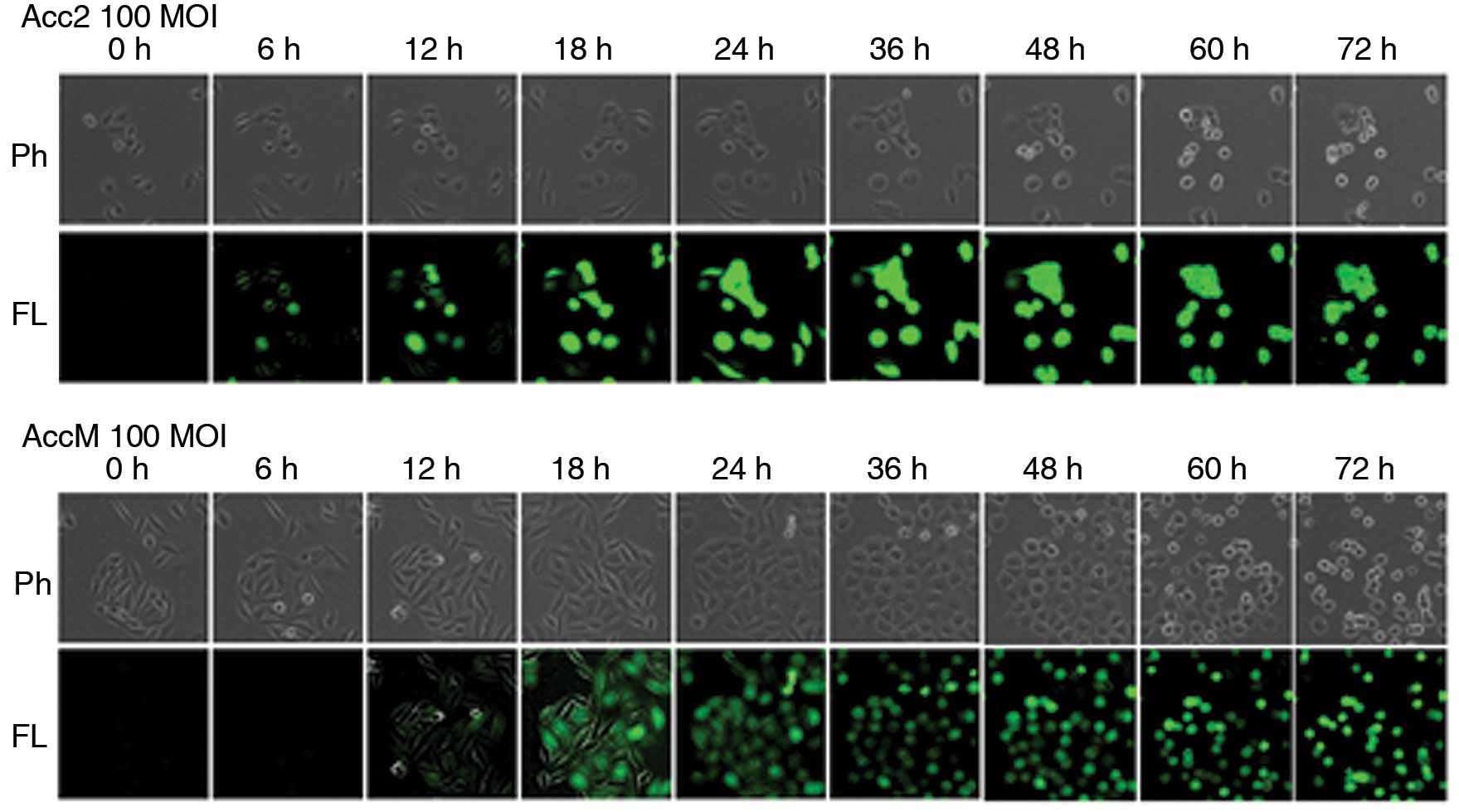
Visualization of OBP-401 in ACC cell
lines in vitro
Tumor-specific replication-competent adenovirus
OBP-401 contains the GFP gene under control of the CMV promoter at
the deleted E3 region of the telomerase-specific
replication-selective type 5 adenovirus, OBP-301. To determine
whether OBP-401 replication is associated with selective GFP
expression, ACC cell lines were analyzed by fluorescence microscopy
after OBP-401 infection. Acc2 and AccM cells expressed bright GFP
fluorescence as early as 6 and 12 h, respectively, after OBP-401
infection at a MOI of 100. The fluorescence intensity gradually
increased in a dose-dependent manner (Fig. 2).
In vivo studies of antitumor effects of
intratumoral injection of OBP-401 in a xenograft mouse model
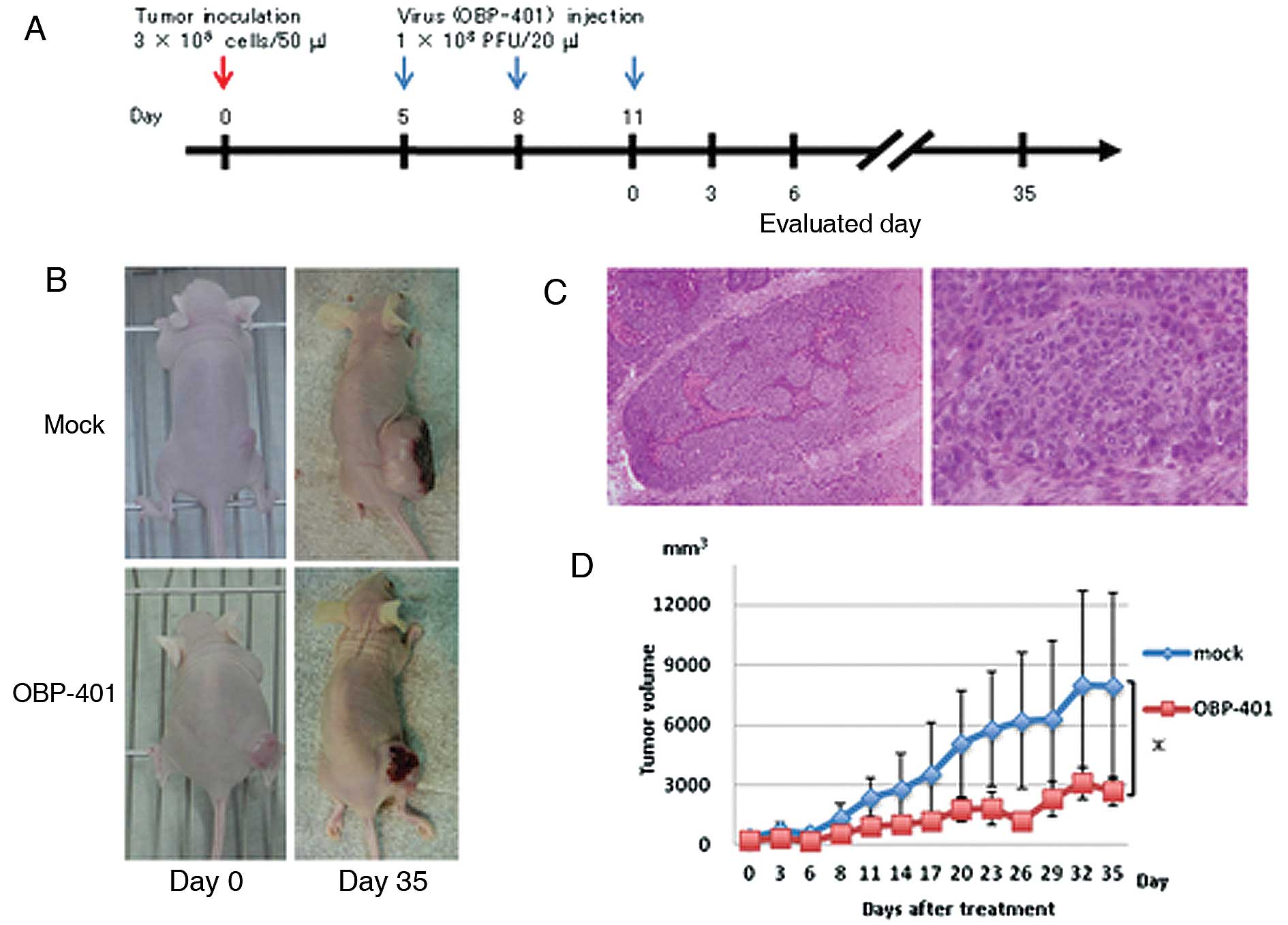
We investigated the antitumor activity of OBP-401
against Acc2 cells in vivo. We used an animal model for ACC
in which Acc2 cells were implanted into the flank of BALB/c
nu/nu mice. Mice bearing palpable Acc2 tumors with diameters
of 5–10 mm received three courses of intratumoral injections of
108 PFU of OBP-401 or PBS (mock treatment) every 3 days
after the initial tumor inoculation (Fig. 3A). Representative images from each
group showed that tumors treated with OBP-401 three times with
starting day 11 after tumor inoculation were smaller than those of
mock-treated mice 35 days after the third viral infection (Fig. 3B). Histopathological examination of
the excised primary tumors showed a tumor composed of implanted
Acc2 cells with a solid architecture (Fig. 3C). Representative images from each
group showed that tumors treated with OBP-401 with starting day 5
after tumor inoculation were consistently smaller than those of
mock-treated mice 35 days after the last viral injection
(P<0.05) (Fig. 3D).
Selective visualization of infected
viruses in subcutaneous tumors in vivo
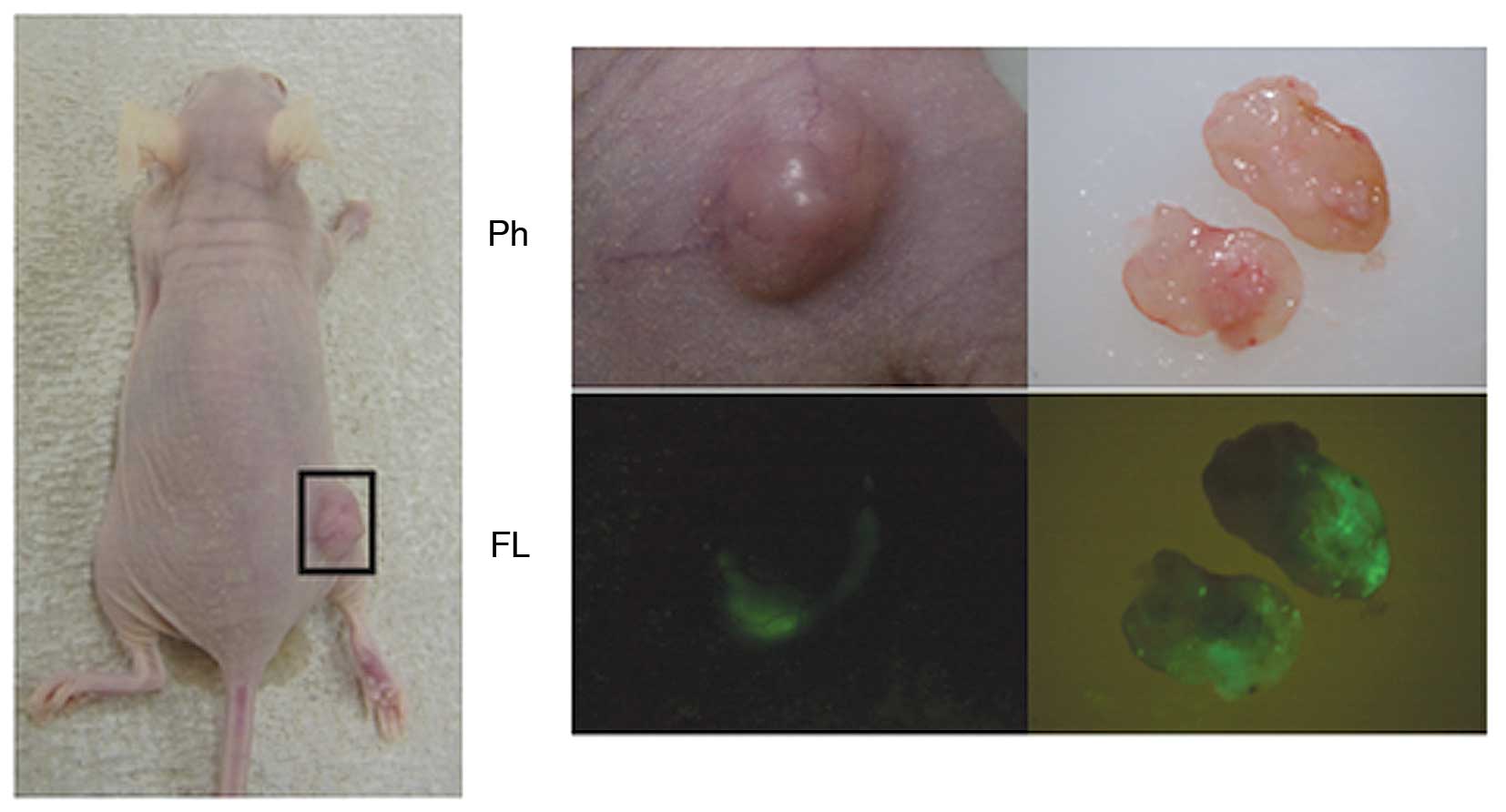
We visualized OBP-401-infected Acc2 cells
intratumorally in BALB/c nu/nu mice. Tumor-bearing mice,
inoculated subcutaneously with 3×106 Acc2 cells, were
observed 14 days after treatment with OBP-401 for the third time
(Fig. 4, left panel). GFP signals
were detected through the skin (Fig.
4, middle panel), showing that adenoviruses had spread into the
surgically removed tissue (Fig. 4,
right panel).
Discussion
Telomerase is expressed in a majority of human
cancers, and its activation plays a critical role in tumorigenesis
by sustaining cell immortality (18). Telomerase activity is detected in
about 85% of human cancers (19),
whereas telomerase is absent in most normal somatic tissues
(20). The adenovirus E1B gene is
expressed early in viral infection and its gene product inhibits
E1A-induced p53-dependent apoptosis, which in turn promotes the
cytoplasmic accumulation of late viral mRNA, leading to a shutdown
of host cell protein synthesis. In most vectors that replicate
under the transcriptional control of the E1A gene, including
hTERT-specific oncolytic adenoviruses, the E1B gene is
driven by the endogenous adenovirus E1B promoter (15). In OBP-301, the tumor-specific hTERT
promoter regulates both the E1A and E1B genes.
OBP-301 is expected to control its replication more stringently,
thereby providing better therapeutic effects in tumor cells as well
as attenuated toxicity in normal tissue (6).
The sensitivity varied greatly between cells despite
human telomerase reverse transcriptase and CAR protein expression.
The hTERT promoter is reported to be inactive in normal cells. It
may provide transcriptional activity in some cell types with
telomerase activity, such as stem or germline cells (21,22).
In fact, protein expression of CAR was higher in SAS than in Acc2
or AccM, and AccM protein expression of hTERT was higher than that
of Acc2 (Fig. 1A). Expression
levels of CAR and hTERT showed no significant difference between
AccM and SAS cells; nevertheless, the anticancer effect of OBP-301
on the SAS cell line was better than that on AccM cells by the XTT
assay. Thus, neither hTERT nor CAR protein expression levels appear
to be useful for predicting the outcome of OBP-301 treatment. We
reported that OBP-301 showed an antitumor effect on Acc2 cells in a
dose-dependent manner (Fig. 1B).
In vitro, OBP-301 has been shown to kill various human
cancer cell lines, as determined by cell proliferation assay
(6,9,17,23,24);
moreover, a previous study showed no significant toxicity of
OBP-301 to normal human cells (11,25,26).
E1A is the first adenoviral gene to be expressed; therefore,
quantitative real-time RT-PCR analysis of E1A reveals intracellular
amplification of OBP-301 (Fig. 1C).
As E1A expression correlates directly with overall virus efficacy,
the ability of the host cell to permit expression would profoundly
influence all subsequent parts of the life cycle. However, previous
studies found that E1A expression does not correspond with CAR and
hTERT expression; hence, the levels of expression vary widely and
do not correlate with OBP-301 sensitivity (12,27).
Furthermore, a recent report showed that OBP-702, in which a human
wild-type p53 gene expression cassette was inserted into the
E3 region of OBP-301 suppressed the viability of cancer
cells more efficiently than OBP-301, inducing dual apoptotic and
autophagic cell death (28,29). This novel apoptotic mechanism
suggests that the p53-expressing OBP-702 also has a possibility of
antitumor efficacy for adenoid cystic carcinoma cells.
In the present study, we inoculated tumor cells
subcutaneously into BALB/c nu/nu mice and confirmed the
formation of tumors with diameters of 5–9 mm after 5 days. We
showed that OBP-401 has antitumor activity against ACC tumors in
vivo (Fig. 3B and D). When
tumors of OBP-401 treated mice (2,694 mm3) were compared
with those of non-treated mice (7,945 mm3; P<0.05),
the results indicated that their size clearly decreased after 35
days. Acc2 cell lines examined in our preliminary experiments were
susceptible to telomerase-specific replication-selective oncolytic
virus infection in vitro and in vivo, suggesting that
the proportion of potentially treatable cancer is high. Recently,
OBP-301 has been developed as an oncolytic viral agent for the
treatment of human cancer and is currently being evaluated in a
Phase-I clinical trial (30). On
the basis of these results, and with future clinical applications
in mind, we established a therapeutic strategy for the use of
telomerase-specific oncolytic adenoviruses to treat patients with
adenoid cystic carcinoma. This strategy involves assessment of the
expression levels of CAR and hTERT in human ACC cells, which would
then allow easy selection of the most effective protocol for the
treatment of patients with oncolytic adenoviruses.
We demonstrated GFP expression following OBP-401
in vitro and in vivo (Figs. 2 and 4). Administration of OBP-401 can provide
an additional advantage in cancer therapy. OBP-401, similar to
OBP-301, is an oncolytic virus and selectively kills human tumor
cells by viral replication. Tumor cells infected with OBP-401
express GFP fluorescence and then lose viability, allowing the
timing of detection. We speculate that OBP-401 would spread into
the regional lymph nodes after intratumoral injection (11,12),
express GFP signals in tumor cells by virus replication and finally
kill tumor cells even if the surgeon failed to remove all nodes
containing micrometastasis. Thus, the oncolytic activity of OBP-401
may function as a backup safety antitumor program. Further
prospective clinical studies are required to confirm the direct
correlation between GFP expression in biopsy samples following
ex vivo OBP-401 infection and the clinical response to
OBP-401 in patients with ACC.
In conclusion, our data clearly indicate that
telomerase-specific replication-selective oncolytic adenoviruses
can significantly inhibit ACC cell growth in vitro and in
vivo. Moreover, these viruses can be used in an ex vivo
diagnostic assay to predict the therapeutic potential of viruses in
ACC patients (31). This novel
technology will affect and contribute to the minimum operative
procedure for ACC cancer patients.
Acknowledgements
The authors wish to thank Drs Yoshiki Mukudai and
Sunao Shiogama for helpful suggestions and Ms. Miho Yoshihara for
her secretarial assistance. The present study was supported by
grants-in-aid for Scientific Research from the Japan Society for
the Promotion of Science (to S.K. and Y.M.) and the High-Technology
Research Center Project from the Ministry of Education, Culture,
Sports, Science and Technology (to S.S.).
References
|
1
|
Umeda M, Komatsubara H, Nishimura N, Oku
N, Shibuya Y, Yokoo S and Komori T: Establishment and
characterization of a human adenoid cystic carcinoma line of the
salivary gland which is serially transplantable and spontaneously
metastasizes to the lung in nude mice. Oral Oncol. 38:30–34. 2002.
View Article : Google Scholar
|
|
2
|
Alleyne CH, Bakay RA, Costigan D, Thomas B
and Joseph GJ: Intracranial adenoid cystic carcinoma: case report
and review of the literature. Surg Neurol. 45:265–271. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van der wal JE, Becking AG, Snow GB and
van der Wall I: Distant metastases of adenoid cystic carcinoma of
the salivary glands and the value of diagnostic examinations during
follow-up. Head Neck. 24:779–783. 2002.PubMed/NCBI
|
|
4
|
Mithani SK, Shao C, Tan M, Smith IM,
Califano JA, El-Naggar AK and Ha PK: Mitochondrial mutations in
adenoid cystic carcinoma of the salivary glands. PLoS One.
4:e84932009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dodd RL and Slevin NJ: Salivary gland
adenoid cystic carcinoma: a review of chemotherapy and molecular
therapies. Oral Oncol. 42:759–769. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawashima T, Kagawa S, Kobayashi N, et al:
Telomerase-specific replication-selective virotherapy for human
cancer. Clin Cancer Res. 10:285–292. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taki M, Kagawa S, Nishizaki M, et al:
Enhanced oncolysis by a tropism-modified telomerase-specific
replication-selective adenoviral agent OBP-405 (‘Telomelysin-RGD’).
Oncogene. 24:3130–3140. 2005.PubMed/NCBI
|
|
8
|
Umeoka T, Kawashima T, Kagawa S, et al:
Visualization of intrathoracically disseminated solid tumors in
mice with optical imaging by telomerase-specific amplification of a
transferred green fluorescent protein gene. Cancer Res.
64:6259–6265. 2004. View Article : Google Scholar
|
|
9
|
Hashimoto Y, Watanabe Y, Shirakiya Y, et
al: Establishment of biological and pharmacokinetic assays of
telomerase-specific replication-selective adenovirus. Cancer Sci.
99:385–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bischoff JR, Kirn DH, Williams A, et al:
An adenovirus mutant that replicates selectively in p53-deficient
human tumor cells. Science. 274:373–376. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kishimoto H, Kojima T, Watanabe Y, et al:
In vivo imaging of lymph node metastasis with
telomerase-specific replication-selective adenovirus. Nat Med.
12:1213–1219. 2006. View
Article : Google Scholar
|
|
12
|
Kurihara Y, Watanabe Y, Onimatsu H, et al:
Telomerase-specific virotheranostics for human head and neck
cancer. Clin Cancer Res. 15:2335–2343. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He RG, Qiu WL and Zhou XJ: The
establishment of Acc-2 and Acc-3 and their morphological
observation. J West China Stromatol. 6:1–3. 1986.
|
|
14
|
Guan XF, Qiu WL, He RG and Zhou XJ:
Selection of adenoid cystic carcinoma cell clone highly metastatic
to the lung: an experimental study. Int J Oral Maxillofac Surg.
26:116–119. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujiwara T: A novel molecular therapy
using bioengineered adenovirus for human gastrointestinal cancer.
Acta Med Okayama. 65:151–162. 2011.PubMed/NCBI
|
|
16
|
Yasuda Y, Kondo S, Nagumo T, et al:
Anti-tumor activity of dehydroxymethylepoxyquinomicin against human
oral squamous cell carcinoma cell lines in vitro and in
vivo. Oral Oncol. 47:334–339. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakakibara A, Tsukuda M, Kondo N,
Tsukamoto H, Mukudai Y, Umezawa K and Shintani S: Examination of
the optimal condition on the in vitro sensitivity to telomelysin in
head and neck cancer cell lines. Auris Nasus Larynx. 38:589–599.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim NW, Piatyszek MA, Prowse KR, et al:
Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shay JW and Bacchetti S: A survey of
telomerase activity in human cancer. Eur J Cancer. 33:787–791.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong CK, Masutomi K and Hahn WC:
Telomerase: regulation, function and transformation. Crit Rev Oncol
Hematol. 54:85–93. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kyo S, Takakura M, Kohama T and Inoue M:
Telomerase activity in human endometrium. Cancer Res. 57:610–614.
1997.PubMed/NCBI
|
|
22
|
Kyo S and Inoue M: Complex regulatory
mechanisms of telomerase activity in normal and cancer cells: how
can we apply them for cancer therapy? Oncogene. 21:688–697. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sasaki T, Tazawa H, Hasei J, et al:
Preclinical evaluation of telomerase-specific oncolytic virotherapy
for human bone and soft tissue sarcomas. Clin Cancer Res.
17:1828–1838. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li G, Kawashima H, Ogose A, et al:
Efficient virotherapy for osteosarcoma by telomerase-specific
oncolytic adenovirus. J Cancer Res Clin Oncol. 137:1037–1051. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fujiwara T, Kagawa S, Kishimoto H, et al:
Enhanced antitumor efficacy of telomerase-selective oncolytic
adenoviral agent OBP-401 with docetaxel: preclinical evaluation of
chemovirotherapy. Int J Cancer. 119:432–440. 2006. View Article : Google Scholar
|
|
26
|
Hioki M, Kagawa S, Fujiwara T, et al:
Combination of oncolytic adenovirotherapy and Bax gene therapy in
human cancer xenografted models. Potential merits and hurdles for
combination therapy. Int J Cancer. 122:2628–2633. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakajima O, Matsunaga A, Ichimaru D, Urata
Y, Fujiwara T and Kawakami K: Telomerase-specific virotherapy in an
animal model of human head and neck cancer. Mol Cancer Ther.
8:171–177. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamasaki Y, Tazawa H, Hashimoto Y, et al:
A novel apoptotic mechanism of genetically engineered
adenovirus-mediated tumour-specific p53 overexpression through
E1A-dependent p21 and MDM2 suppression. Eur J Cancer. 48:2282–2291.
2012. View Article : Google Scholar
|
|
29
|
Hasei J, Sasaki T, Tazawa H, et al: Dual
programmed cell death pathways induced by p53 transactivation
overcome resistance to oncolytic adenovirus in human osteosarcoma
cells. Mol Cancer Ther. 12:314–325. 2013. View Article : Google Scholar
|
|
30
|
Nemunaitis J, Tong AW, Nemunaitis M, et
al: A phase I study of telomerase-specific replication competent
oncolytic adenovirus (telomelysin) for various solid tumors. Mol
Ther. 18:429–434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Del Vecchio S, Zannetti A, Fonti R, Pace L
and Salvatore M: Nuclear imaging in cancer theranostics. Q J Nucl
Med Mol Imaging. 51:152–163. 2007.
|