Introduction
Neuroblastoma is a neuroendocrine tumor, arising
from any neural crest element of the sympathetic nervous system
(1). Neuroblastoma disease is very
heterogeneous and consists of high and low risk disease (2). Low-risk disease, infants aged 18
months and younger with favorable disease characteristics, which is
common with observation only or surgery and have a high likelihood
of long-term, has disease-free survival. Treating high-risk
patients, children aged 18 months and older with stage 4
neuroblastoma and unfavorable disease characteristics, is a bigger
challenge due to the presence of widespread metastatic disease at
presentation and a high degree of relapse despite multi-modality
treatment (3,4).
In neuroblastoma, ~50% of patients have metastatic
disease at diagnosis, thus creating major challenges for treatment
and cure (5,6). Moreover, high-risk patients of
neuroblastoma often metastasize and relapse despite initial
response to therapies. Frequently, recurrent and metastatic tumors
acquire drug resistance or aggressive phenotypes through the
selection of rare resistant clones from heterogeneous tumor
environment, which can result in major clinical obstacles in the
treatment of neuroblastoma (2).
Thus, it is imperative to identify novel therapeutic measures to
enhance the therapeutic effect and improve the survival of patients
with metastatic and recurrent high-risk neuroblastoma disease.
IL-24 is also known as melanoma
differentiation-associated 7 (mda-7) due to its first discovery
from human melanoma cells by combined treatment with IFN-β and MEZ
(7). Studies have shown that the
overproduction of IL-24 selectively inhibited cancer cell growth of
diverse origins by inducing apoptosis with minimal toxicity to
normal cells both in vitro and in vivo(8–14).
This broad-spectrum antitumor activity of IL-24 is distinct from
that of other extensively studied tumor-suppressor genes, and its
growth-inhibition properties are independent of the status of p53,
pRb, p21 or additional tumor-suppressor genes in cancer cells
(15–17).
We previously found the suppression of neuroblastoma
growth in response to overexpression of IL-24 in vitro and
in vivo. IL-24 exerts its tumor-suppressive effects by
multiple mechanisms, including the balance of Bcl-2 family proteins
toward the pro-apoptotic pathway and the activation of the caspase
cascade (18). Ramesh et
al(19) showed that IL-24
inhibited the migration and invasion of human lung cancer cells
in vitro. A phase I clinical trial was conducted by Fisher
et al(20), who showed IL-24
was safe and promoted significant clinical activity, particularly
in the context of patients with metastatic melanoma. We therefore
investigated the effects of the IL-24 on migration and invasion in
neuroblastoma cells in vitro and attempted to identify the
underlying mechanisms of metastasis suppression.
Materials and methods
Cell culture
As previously described, the human neuroblastoma
cell line SH-SY5Y was purchased from Shanghai Cell Bank of the
Chinese Academic of Sciences (Shanghai, China) (18). The SH-SY5Y cells were grown in EMEM
and Ham’s F12 (1:1 mixture) + 10% fetal bovine serum (FBS). Human
embryo kidney 293 cells were purchased from Canada Microbix
Biosystems Ltd. (Mississauga, Canada). The HEK293 cells were
cultured in DMEM medium + 10% FBS. All cells were maintained in a
humidified 37°C incubator with 5% CO2.
Virus production
The construction of replication-defective adenovirus
5 (Ad5) encoding IL-24 gene (Ad-IL24) was previously described
(18). The Ad-IL24 and Ad5 carrying
reporter gene Green Fluorescent Protein (Ad-GFP) were amplified in
HEK293 cells, purified by cesium chloride centrifugation, and
stored at −80°C prior to use.
Cell migration assay
The SH-SY5Y cells were seeded at a density of
6×105 cells/well in 6-well tissue culture plates. The
next day, cells were infected with Ad-IL24 and Ad-GFP [multiplicity
of infection (MOI)=10]. At 6 h after infection, the cells were
trypsinized, washed in PBS and resuspended in serum-free RPMI-1640
medium. A cell migration assay was performed in a 24-well Transwell
unit (cat no. CLS3398; Sigma-Aldrich, St. Louis, MO, USA). The
lower chambers of the Transwell units were filled with serum-free
medium, and the upper chambers were seeded with 1×104
cells from each treatment group in triplicate wells. After 24- and
48-h incubations, the cells that had passed through the filter into
the lower wells were counted, and the number was expressed as a
percentage of the sum of the cells in the upper and lower wells.
The experiments were performed 5 times and the results were
recorded as the means of these experiments.
In a parallel set of experiments, tumor cells
subjected to various treatments as described above were subjected
to cell viability assays at 24 and 48 h by MTT, as previously
described (18). These experiments
were performed to exclude the possibility that the inhibition of
cell migration by IL-24 was a result of cytotoxicity.
Cell invasion assay
The SH-SY5Y cells were seeded at a density of
6×105 cells/well in 6-well tissue culture plates. The
next day, cells were infected with Ad-IL24 and Ad-GFP (MOI=10) or
treated with 10 μM LY294002 (cat no. 9901; Cell Signaling
Technology, Inc., Danvers, MA, USA) or 1 μg/ml MMP-II inhibitor
(cat no. 203915-59-7; Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA). After treatment, cultures were replenished with complete
medium. At 6 h after treatment, cells were trypsinized, washed in
PBS, and resuspended in serum-free RPMI-1640 medium. A cell
invasion assay was performed in a 24-well Transwell unit coated
with Matrigel (cat no. 354578; Becton-Dickinson, Franklin Lakes,
NJ, USA). The lower chambers of the Matrigel-coated Transwell units
were filled with serum-free medium, and the upper chambers were
seeded with 1×104 cells from each treatment in
triplicate wells. After 24- and 48-h incubations, the cells that
had passed through the Matrigel-coated filter membrane into the
lower well were counted as a measure of invasion. The invading
cells were counted for each treatment and expressed as a percentage
of the sum of the cells in the upper and lower wells. Experiments
were performed at least 4 times and the results were recorded as
the mean of these experiments.
Immunofluorescence staining
The SH-SY5Y cells were seeded at a density of
6×105 cells/well in 6-well tissue culture plates. The
next day, the cells were infected with Ad-IL24 and Ad-GFP (MOI 10)
or treated with PBS. Following treatment, cultures were replenished
with complete medium. After 24 h treatment, cells were fixed in 2%
paraformaldehyde for 10 min, and blocked with 0.5% Tween-20 for 5
min. Thereafter, cells were incubated with a rabbit monoclonal
antibody to β-catenin (cat no. 9582S; Cell Signaling Technology,
Inc.) overnight at 4°C, followed by 1 h incubation with a
Cy3-conjugated goat anti-rabbit IgG (cat no. AP132C; Millipore,
Billerica, MA, USA) in the dark. An epifluorescence Leica
microscope was used to observe the samples and a digital camera
(Q-imaging) was used to capture the images.
Western blotting
Cells were harvested and lysed, and the cleared
lysates (30–50 μg/well) were separated on 10% Tris-glycine
polyacrylamide gel electrophoresis (PAGE) gels under standard
conditions. Proteins were then transferred to a nitrocellulose
membrane and incubated overnight at 4°C with the following primary
antibodies: anti-IL-24 (cat no. SAB1407085; Sigma-Aldrich),
anti-p85 PI3K (cat no. 4257S), anti-pJNK (cat no. 4668S),
anti-p38MAPK (cat no. 4631P, ), anti-pFAK (cat no. 8556S; all
reagents were from Cell Signaling Technology, Inc.) or anti-β-actin
(cat no. ab8229; Abcam, Cambridge, MA, USA). The membranes were
then washed and incubated with alkaline phosphatase-conjugated
secondary antibodies in Tris-buffered saline Tween-20 (TBST) for 2
h and developed using the nitro-blue tetrazolium
chloride/5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt
(NBT/BCIP) color substrate (Promega Corporation, Madison, WI, USA).
The density of the bands on the membrane was scanned and analyzed
using an image analyzer.
Gelatin zymography analysis
To determine the effect of Ad-IL24 treatment on MMP
production, a gelatin zymography assay was performed. The SH-SY5Y
cells were grown in low-serum (1% FBS) medium and seeded at
6×105 cells/well in 6-well tissue culture plates and
were infected with Ad-IL24 and Ad-GFP (MOI=10). Cells treated with
PBS served as a negative control in these experiments. At 6 h after
infection, the culture medium was removed and replaced with fresh
medium containing 1% FBS. At 24 and 48 h after infection, cell
culture supernatants were collected, clarified by centrifugation,
and subjected to electrophoresis in sodium dodecyl sulfate
(SDS)-PAGE. The gels were then washed and incubated with reaction
buffer [50 mM Tris-HCl (pH 7.4), 0.02% NaN3, and 10 mM
CaCl2] with constant shaking for 16 h at 37°C, stained
and destained. The protein concentration in the culture supernatant
was measured to confirm that equal amounts were used for the
assays. Relative activities of MMP-2 and MMP-9 were quantified and
analyzed using an image analyzer.
Statistical analysis
Values are expressed as the means ± standard
deviation (SD). The statistical analysis of the results was
performed using a one-way analysis of the variance (ANOVA) or
Student’s t-test. P-values <0.05 were considered to indicate
statistically significant differences.
Results
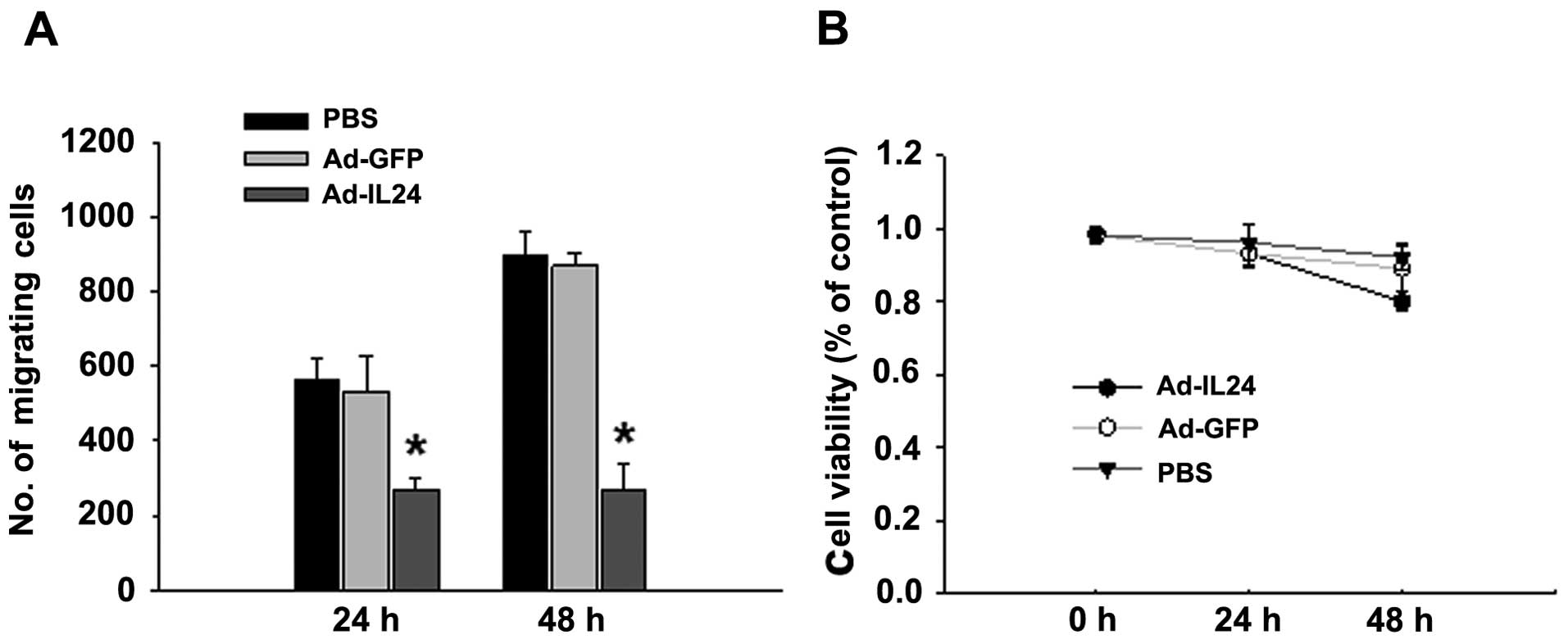
IL-24 inhibits neuroblastoma SH-SY5Y cell
migration
In the present study we tested the ability of
Ad-IL24 to inhibit cell migration in the neuroblastoma cell line
SH-SY5Y. Tumor cells treated with Ad-IL24 were significantly less
able to migrate than were cells treated with Ad-GFP or PBS
(Fig. 1A). The inhibitory effect on
cell migration occurred as early as 24 h in SH-SY5Y cells. To show
that the inhibition of cell migration was not due to IL-24-mediated
cell death, in a parallel set of experiments, we subjected cells
treated with PBS, Ad-GFP and Ad-IL24 to a cell viability assay at
24 and 48 h after infection. We observed no significant difference
in cell viability at 24 and 48 h after infection, indicating that
the inhibition of migration by IL-24 was not due to cell death
(Fig. 1B). Note that at 24 h
post-infection, all 3 experimental groups were superimposable,
indicating no significant cell death; by 48 h, some cell death
occurred; however, with this vector dose, significant
Ad-IL24-mediated death was observed only at 72 and 96 h
post-infection; the results are consistent with previous data
(18). These results show that
IL-24 inhibits tumor cell migration and that the inhibitory effect
is not due to cytotoxicity.
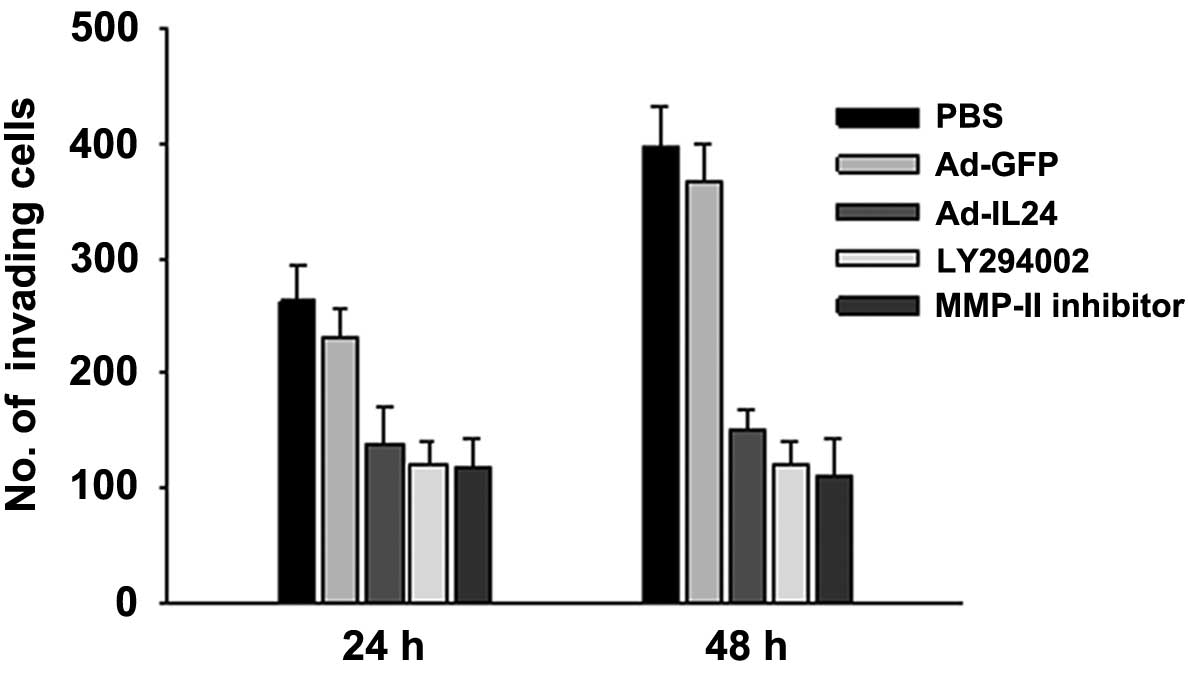
IL-24 inhibits neuroblastoma SH-SY5Y cell
invasion
SH-SY5Y cells treated with Ad-IL24 were much less
invasive, as indicated by the small number of cells on the outer
membrane of the Matrigel invasion assay filter, than cells treated
with PBS or Ad-GFP (Fig. 2). The
number of invading cells was significantly lower after treatment
with Ad-IL24 than with PBS or Ad-GFP. We observed the inhibitory
effect exerted by IL-24 as early as 24 h after Ad-IL24 treatment.
Furthermore, IL-24-mediated inhibitory effect was similar to the
inhibitory effect observed in cells treated with LY294002, a PI3K
inhibitor, or with MMP-II inhibitor. A cell viability assay showed
that the inhibition was not a result of IL-24-mediated cell death.
These results show that IL-24 effectively inhibits cell
invasion.
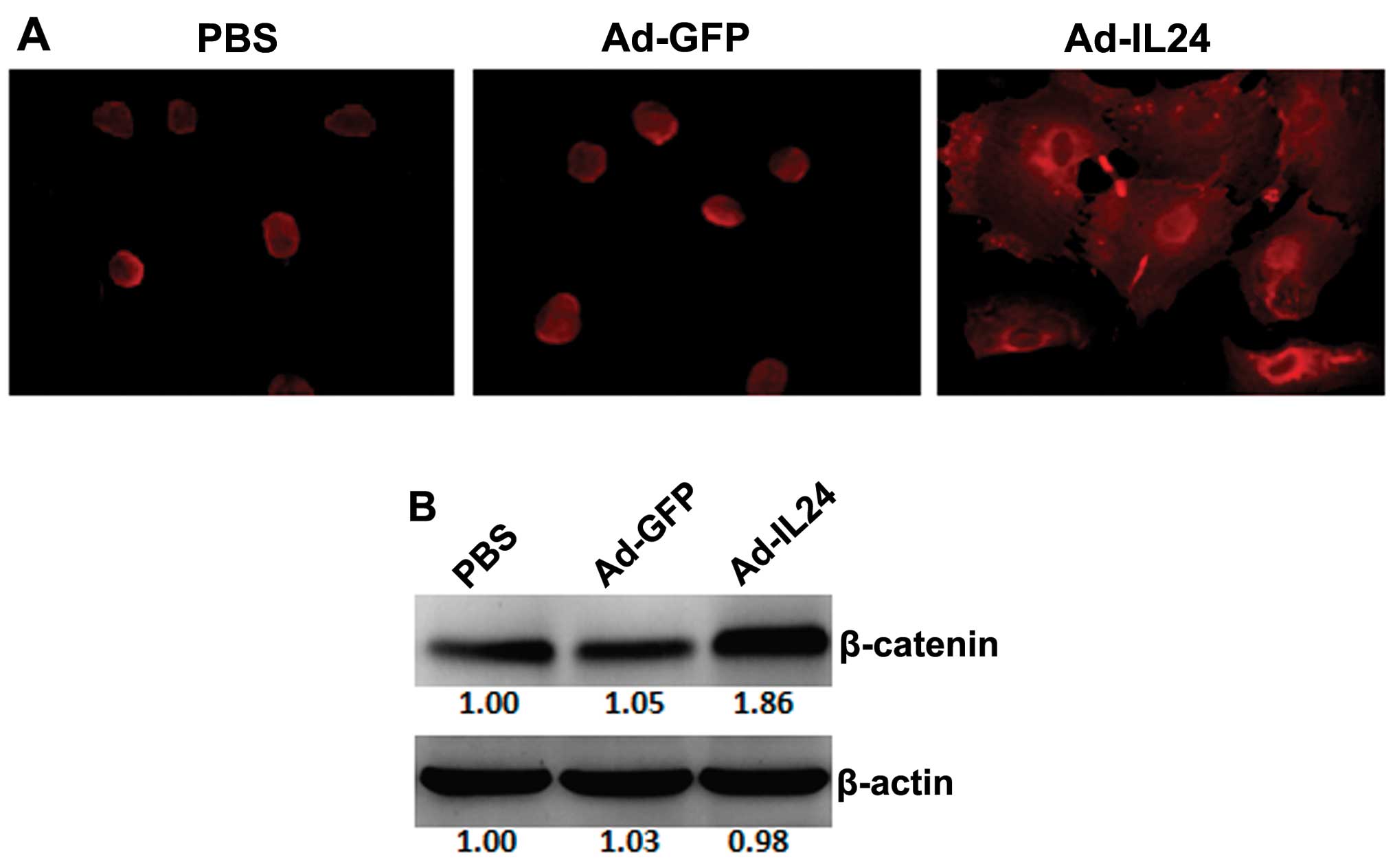
IL-24 affects subcellular localization
and upregulates cellular level of β-catenin
The subcellular localization of β-catenin was
examined by immunofluorescence. In the PBS- or Ad-GFP-treated
cells, positive β-catenin immunofluorescence staining was observed
in the cytoplasm and/or nucleus and was not observed at the plasma
membrane (Fig. 3A). In the
Ad-IL24-treated cells, positive β-catenin immunofluorescence
staining shifted in localization near the plasma membrane and was
not observed in the nucleus (Fig.
3A). The cellular level of β-catenin was examined by western
blotting. The intensity of the band corresponding to β-catenin was
stronger in the Ad-IL24-treated cells than in the PBS or Ad-GFP
cells (Fig. 3B), indicating that
Ad-IL24 increased the cellular level of β-catenin.
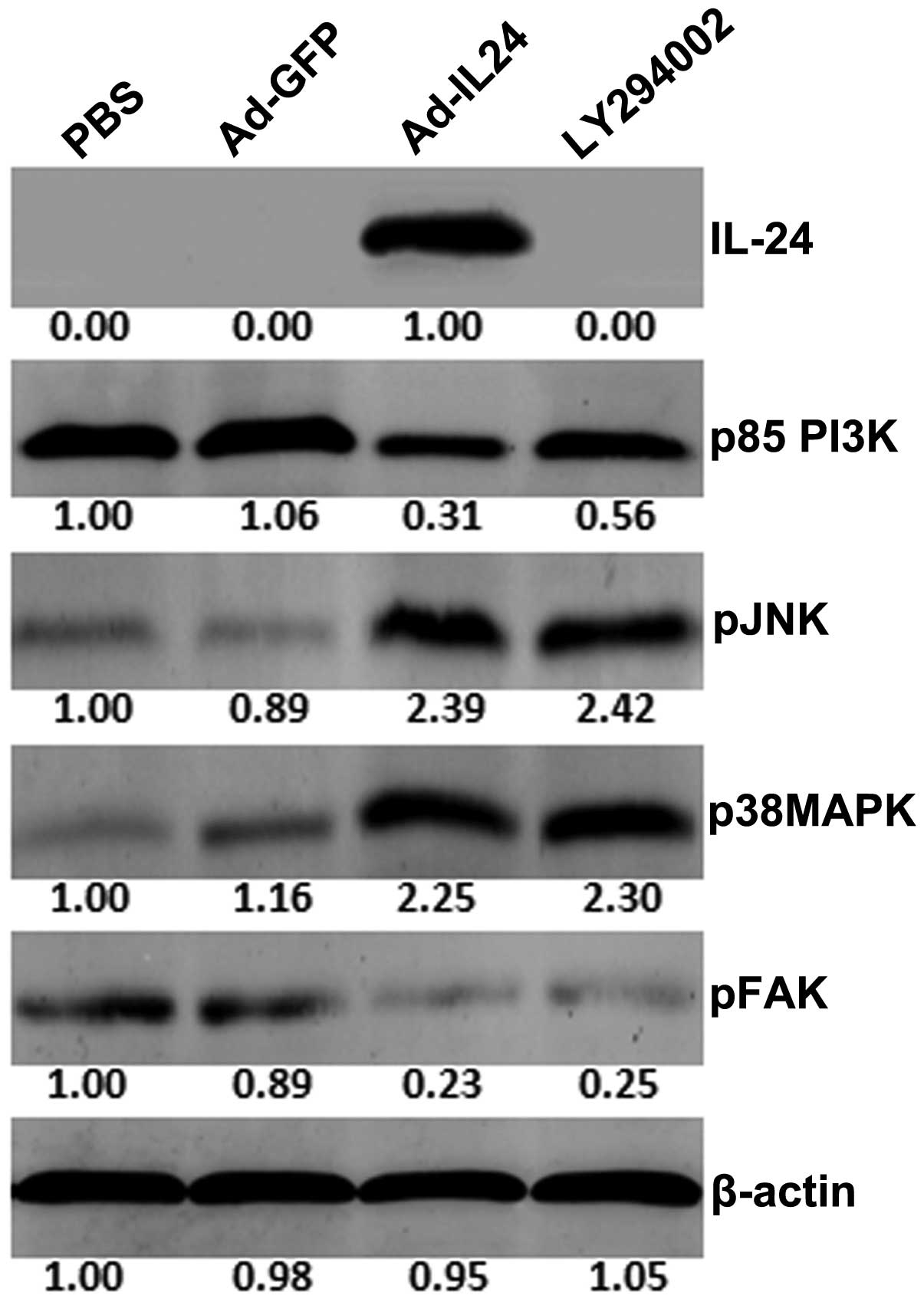
IL-24 regulates the proteins associated
with cell migration and invasion
We next examined the regulation of proteins that are
associated with cell migration and invasion signaling pathways by
western blot analysis. We did not observe IL-24 production in
Ad-GFP-, PBS- or LY294002-treated tumor cells, but high levels of
IL-24 production were found in Ad-IL24-treated cells (Fig. 4). Overproduction of the IL-24
protein in tumor cells resulted in decreased production of p85 PI3K
and pFAK and increased production of pJNK, and p38MAPK compared to
PBS- or Ad-GFP-treated cells. We also observed the inhibition of
p85 PI3K and pFAK in cells treated with LY294002. The decrease in
p85 PI3K expression in cells overproducing IL-24 was greater than
that observed with LY294002. Furthermore, LY294002 treatment
resulted in increased production of p38MAPK and pJNK. These results
indicate that IL-24, such as LY294002, effectively inhibits PI3K
and pFAK while increasing the expression of other signaling
proteins.
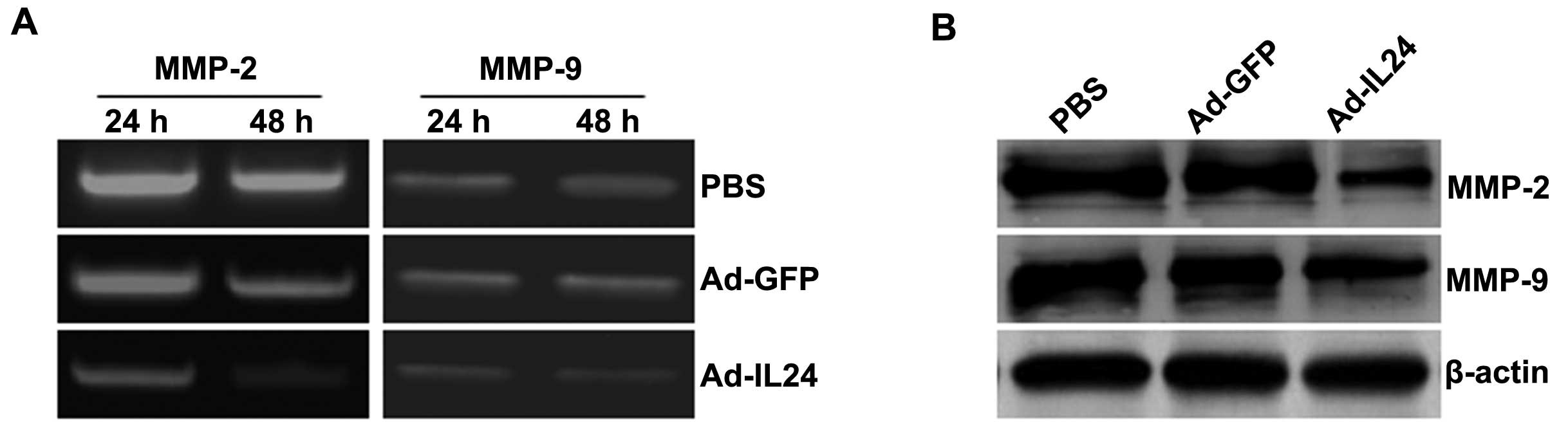
IL-24 inhibits matrix metalloproteinase
production in neuroblastoma SH-SY5Y cells
We next examined tumor cells overproducing IL-24 for
MMP regulation by zymography and western blot analysis. Zymography
analysis showed that the production of the MMP-2 and MMP-9 proteins
was decreased in tumor cells treated with Ad-IL24, compared with
cells that were treated with PBS or Ad-GFP (Fig. 5A). The results of zymography
analysis correlated with the results of the western blot analysis
(Fig. 5B). Thus, Ad-IL24 modulates
MMP expression and activity in neuroblastoma SH-SY5Y cells.
Discussion
In the present study, we showed that IL-24 can
inhibit the migration and invasion of human neuroblastoma cells. It
could be argued that the observed inhibition of cell migration was
due to the cytotoxicity of Ad-IL24; however, that was ruled out by
a cell proliferation assay. Our results of immunofluorescence
showed that IL-24 increases the expression of membranous β-catenin
to enhance cell-cell adhesiveness. Protein production, associated
with cell migration and invasion, analysis showed that the
inhibition of cell migration and invasion was due to IL-24. The
ability of IL-24 to inhibit neuroblastoma cell migration and
invasion supports the findings of Ramesh et al(19), who previously reported the
inhibition of lung tumor cell migration and invasion by
Ad-mda7.
Approximately 50% of neuroblastoma patients have
metastatic disease at diagnosis. The initial step of metastasis is
cell detachment from the primary tumor. β-catenin is essential for
cadherin-mediated cell-cell adhesion; the reducing expression of
β-catenin and cadherins at the cell surface is associated with
tumor metastasis (21). Loss of
membranous β-catenin occurs commonly in primary colorectal cancer
with metastatic potential and in the corresponding colorectal liver
metastases (22). Therefore, we
examined whether IL-24 affects the subcellular localization and
cellular level of β-catenin in neuroblastoma SH-SY5Y cells. In
IL-24-treated cells, immunofluorescence staining of β-catenin was
observed near the plasma membrane. The results of western blotting
showed that IL-24-treated cells contained higher levels of
β-catenin than cells that were treated with PBS or Ad-GFP. These
findings suggest that IL-24 may promote intracellular adhesion of
SH-SY5Y cells. This is the first report to focus on the subcellular
localization and cellular level of β-catenin following IL-24
treatment in tumor cells.
The next step of metastasis is migration of tumor
cells to a distant site. Cell migration has previously been shown
to be regulated by numerous molecules, including PI3K, p38MAPK,
pJNK and FAK (23–27). Inhibition of PI3K and MAPK activity
using specific inhibitors impaired epidermal growth factor
(EGF)-stimulated cell migration of ovarian tumor cells (28). In our study, we found that IL-24
inhibited cell migration by downregulating the production of the
p85 PI3K and FAK proteins. The inhibitory effect exerted by IL-24
was equivalent to that observed with the PI3K inhibitor LY294002.
Furthermore, we examined the regulation of additional signaling
molecules p38MAPK and JNK, that have previously been shown to
participate in cell migration, and demonstrated increased
production of these proteins upon IL-24 expression. Increased
expression of these molecules was also observed when cells were
treated with LY294002. Thus, IL-24, similar to LY294002,
selectively inhibits the PI3K pathway without affecting other
signaling pathways, resulting in reduced migration.
In addition to migration, tumor cells need to invade
into basement membranes to establish metastasis successfully at a
distant site. Tumor cell invasion involves the degradation of the
extracellular matrix, and this destruction has been attributed to
the activity of proteolytic enzymes (29). MMPs play an important role among the
proteases implicated in tumor cells (30). However, the production of MMPs has
been observed in many invasive tumor cell lines and during tumor
growth (31–33). Evidence that MMPs are involved in
invasion and angiogenesis in neuroblastoma comes from the
observation that MMP-2 and MMP-9 have been found in several
neuroblastoma cell lines and surgical specimens and that the extent
of MMP overproduction correlates with prognosis (34–36).
In the present study, we found that IL-24-treated cells were less
able to migrate and invade, exhibited lower expression and
secretion of MMP-2 and MMP-9 compared with PBS- or Ad-GFP-treated
cells.
In conclusion, we have shown for the first time that
IL-24 inhibits neuroblastoma cell migration and invasion in
vitro. Thus, IL-24 gene-based drugs may provide a novel
therapeutic strategy for neuroblastoma that can inhibit tumor
growth directly via induction of apoptosis and may also prevent
tumor invasion and metastasis.
Acknowledgements
The authors thank Professor Junnian Zheng for
providing the plasmid carrying the IL-24 cDNA (Laboratory of
Biological Cancer Therapy, Xuzhou Medical College, Xuzhou,
China).
References
|
1
|
Schor NF: Neuroblastoma as a
neurobiological disease. J Neurooncol. 41:159–166. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maris JM: Recent advances in
neuroblastoma. N Engl J Med. 362:2202–2211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gustafson WC and Matthay KK: Progress
towards personalized therapeutics: biologic- and risk-directed
therapy for neuroblastoma. Expert Rev Neurother. 11:1411–1423.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Isaacs H Jr: Fetal and neonatal
neuroblastoma: retrospective review of 271 cases. Fetal Pediatr
Pathol. 26:177–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maris JM, Hogarty MD, Bagatell R and Cohn
SL: Neuroblastoma. Lancet. 369:2106–2120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Øra I and Eggert A: Progress in treatment
and risk stratification of neuroblastoma: impact on future clinical
and basic research. Semin Cancer Biol. 21:217–228. 2011.PubMed/NCBI
|
|
7
|
Jiang H, Lin JJ, Su ZZ, Goldstein NI and
Fisher PB: Subtraction hybridization identifies a novel melanoma
differentiation associated gene, mda-7, modulated during human
melanoma differentiation, growth and progression. Oncogene.
11:2477–2486. 1995.
|
|
8
|
Dent P, Yacoub A, Hamed HA, et al: The
development of MDA-7/IL-24 as a cancer therapeutic. Pharmacol Ther.
128:375–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pataer A, Chada S, Roth JA, Hunt KK and
Swisher SG: Development of Ad-mda7/IL-24-resistant lung cancer cell
lines. Cancer Biol Ther. 7:103–108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng M, Bocangel D, Ramesh R, et al:
Interleukin-24 overcomes temozolomide resistance and enhances cell
death by down-regulation of O6-methylguanine-DNA
methyltransferase in human melanoma cells. Mol Cancer Ther.
7:3842–3851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lebedeva IV, Su ZZ, Vozhilla N, et al:
Mechanism of in vitro pancreatic cancer cell growth inhibition by
melanoma differentiation-associated gene-7/interleukin-24 and
perillyl alcohol. Cancer Res. 68:7439–7447. 2008. View Article : Google Scholar
|
|
12
|
Park MA, Yacoub A, Sarkar D, et al:
PERK-dependent regulation of MDA-7/IL-24-induced autophagy in
primary human glioma cells. Autophagy. 4:513–515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan S, Zhang H, Xie Y, et al: Recombinant
human interleukin-24 suppresses gastric carcinoma cell growth in
vitro and in vivo. Cancer Invest. 28:85–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Sheng W, Xie Y, et al: The in vitro
and in vivo antitumor activity of adenovirus-mediated
interleukin-24 expression for laryngocarcinoma. Cancer Biother
Radiopharm. 25:29–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang H, Su ZZ, Lin JJ, Goldstein NI,
Young CS and Fisher PB: The melanoma differentiation associated
gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci
USA. 93:9160–9165. 1996. View Article : Google Scholar
|
|
16
|
Lebedeva IV, Su ZZ, Chang Y, Kitada S,
Reed JC and Fisher PB: The cancer growth suppressing gene
mda-7 induces apoptosis selectively in human melanoma cells.
Oncogene. 21:708–718. 2002.PubMed/NCBI
|
|
17
|
Su ZZ, Lebedeva IV, Sarkar D, et al:
Melanoma differentiation associated gene-7, mda-7/IL-24,
selectively induces growth suppression, apoptosis and
radiosensitization in malignant gliomas in a p53-independent
manner. Oncogene. 22:1164–1180. 2003.PubMed/NCBI
|
|
18
|
Zhuo B, Wang R, Yin Y, et al: Adenovirus
arming human IL-24 inhibits neuroblastoma cell proliferation in
vitro and xenograft tumor growth in vivo. Tumour Biol.
34:2419–2426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ramesh R, Ito I, Gopalan B, Saito Y,
Mhashilkar AM and Chada S: Ectopic production of MDA-7/IL-24
inhibits invasion and migration of human lung cancer cells. Mol
Ther. 9:510–518. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fisher PB, Sarkar D, Lebedeva IV, et al:
Melanoma differentiation associated gene-7/interleukin-24
(mda-7/IL-24): novel gene therapeutic for metastatic
melanoma. Toxicol Appl Pharmacol. 224:300–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fujioka T, Takebayashi Y, Kihana T, et al:
Expression of E-cadherin and β-catenin in primary and peritoneal
metastatic ovarian carcinoma. Oncol Rep. 8:249–255. 2001.
|
|
22
|
Hugh TJ, Dillon SA, O’Dowd G, et al:
β-catenin expression in primary and metastatic colorectal
carcinoma. Int J Cancer. 82:504–511. 1999.
|
|
23
|
Wang F, Nohara K, Olivera A, Thompson EW
and Spiegel S: Involvement of focal adhesion kinase in inhibition
of motility of human breast cancer cells by sphingosine
1-phosphate. Exp Cell Res. 247:17–28. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Neudauer CL and McCarthy JB: Insulin-like
growth factor I-stimulated melanoma cell migration requires
phosphoinositide 3-kinase but not extracellular-regulated kinase
activation. Exp Cell Res. 286:128–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goncharova EA, Ammit AJ, Irani C, et al:
PI3K is required for proliferation and migration of human pulmonary
vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol.
283:L354–L363. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kadri Z, Petitfrère E, Boudot C, et al:
Erythropoietin induction of tissue inhibitors of
metalloproteinase-1 expression and secretion is mediated by
mitogen-activated protein kinase and phosphatidylinositol 3-kinase
pathways. Cell Growth Differ. 11:573–580. 2000.PubMed/NCBI
|
|
27
|
Lakka SS, Jasti SL, Kyritsis AP, et al:
Regulation of MMP-9 (type IV collagenase) production and
invasiveness in gliomas by the extracellular signal-regulated
kinase and jun amino-terminal kinase signaling cascades. Clin Exp
Metastasis. 18:245–252. 2000. View Article : Google Scholar
|
|
28
|
Ellerbroek SM, Halbleib JM, Benavidez M,
et al: Phosphatidylinositol 3-kinase activity in epidermal growth
factor-stimulated matrix metalloproteinase-9 production and cell
surface association. Cancer Res. 61:1855–1861. 2001.
|
|
29
|
Goldfarb RH and Liotta LA: Proteolytic
enzymes in cancer invasion and metastasis. Semin Thromb Hemost.
12:294–307. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matrisian LM: The matrix-degrading
metalloproteinases. Bioessays. 14:455–463. 1992. View Article : Google Scholar
|
|
31
|
Shapiro SD: Matrix metalloproteinase
degradation of extracellular matrix: biological consequences. Curr
Opin Cell Biol. 10:602–608. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pritchard SC, Nicolson MC, Lloret C, et
al: Expression of matrix metalloproteinases 1, 2, 9 and their
tissue inhibitors in stage II non-small cell lung cancer:
Implications for MMP inhibition therapy. Oncol Rep. 8:421–424.
2001.PubMed/NCBI
|
|
33
|
Nakopoulou L, Tsirmpa I, Alexandrou P, et
al: MMP-2 protein in invasive breast cancer and the impact of
MMP-2/TIMP-2 phenotype on overall survival. Breast Cancer Res
Treat. 77:145–155. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu HF, Lai KC, Hsu SC, et al: Involvement
of matrix metalloproteinases on the inhibition of cells invasion
and migration by emodin in human neuroblastoma SH-SY5Y cells.
Neurochem Res. 34:1575–1583. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ribatti D, Surico G, Vacca A, et al:
Angiogenesis extent and expression of matrix metalloproteinase-2
and -9 correlate with progression in human neuroblastoma. Life Sci.
68:1161–1168. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ara T, Fukuzawa M, Kusafuka T, et al:
Immunohistochemical expression of MMP-2, MMP-9, and TIMP-2 in
neuroblastoma: association with tumor progression and clinical
outcome. J Pediatr Surg. 33:1272–1278. 1998. View Article : Google Scholar : PubMed/NCBI
|