Introduction
Human esophageal squamous cell carcinoma (ESCC) is
one of the most aggressive malignancies. Prominent gender
differences in the prevalence of ESCC have been demonstrated by
most epidemiological studies. Esophageal cancer is generally more
common among males than females, with a male to female ratio
exceeding 3–4:1 (1). The prognosis
of ESCC female patients is significantly better than that of male
patients (2). While these data
indicate the potential involvement of sex hormone-related pathways
in the pathogenesis of ESCC, the relevant molecular events remain
unclear.
Previous studies suggest a possible correlation
between the estrogenic pathway and tumorigenesis and/or progression
of ESCC (3,4). Esophageal cancer in females tends to
occur postmenopausally, and the incidence rate for females
increases with age (5). Sex
steroids, such as estrogen, are well-known for their role(s) in the
control of cell differentiation and proliferation in
estrogen-dependent tissues such as breast and endometrial tissues.
However, it is noteworthy that estrogens appear to play a pivotal
role in several types of human malignancies that are not considered
as classical, estrogen-dependent, neoplasms, such as lung (6,7),
urinary bladder (8) and
gastrointestinal tract malignancies (9,10).
Consequently, estrogenic pathways may be involved in the regulation
of the biological behavior of ESCC.
Human tissues contain two isoforms of ER, ERα and
ERβ, which are generated by genes located on human chromosome 6q25
(11) and chromosome 14q22–24
(12), respectively. While several
studies have identified the expression of ERs in ESCC (4,13–19),
the distribution patterns of ERα and ERβ as well as their clinical
implication remain highly controversial (15,16,18,20).
Accumulated data indicate a functional interaction between ERα and
ERβ in human cells (21,22). Comprehensive comparison of the
available data on ERα and ERβ expression in ESCC is difficult since
they are determined separately in different studies using different
methods. We considered that simultaneous determination of both ERα
and ERβ expression will allow us to analyze the quantitative
relationship between ERα and ERβ, which may have prognostic value
for ESCC and aid in the better understanding of their involvement
in the development of the malignant phenotype of ESCC.
The aim of this study was to determine the
expression of ER protein and its correlation with clinical
variables of ESCC patients.
Materials and methods
Ethics statement
The study was approved by the Ethics Committee of
the Shantou University Medical College (Shantou, China), and only
patients who provided written informed consent were included in the
study.
Patients and tissues
Eighty-nine ESCC tissues were obtained from patients
who underwent potentially curative esophagectomy from 2000 to 2006
at the Shantou Central Hospital (Shantou, China). These patients
had received neither chemotherapy nor irradiation therapy prior to
surgery. From these 89 cases, a total of 7 specimens of
non-neoplastic epithelium were obtained for evaluating the
expression of ERα, and 9 specimens were obtained for ERβ
evaluation. These specimens had undergone tissue microarray (TMA)
construction before immunohistochemical staining. Relevant clinical
data were retrieved from careful review of the medical records. All
of the tumors were confirmed as ESCC by pathologists in the
Clinical Pathology Department of the Shantou Central Hospital. The
pathological stage of each cancer was determined according to the
TNM system, and each lesion was graded histologically according to
World Health Organization classification. The median follow-up time
was 30.8 months (range, 1.4–54.4 months).
Immunohistochemistry
Mouse monoclonal antibodies for ERα (F-10; dilution,
1:50) and ERβ (14C8; dilution, 1:100) were purchased from Santa
Cruz Biotechnology, Inc. and GeneTex Inc., respectively.
Immunohistochemical staining was performed using the biotin
streptavidin-peroxidase procedure as described previously (23).
Image analysis
Images were acquired using a Nikon microscope
coupled to a Nikon DS-Fi1 digital camera (Nikon, Tokyo, Japan). Ten
randomly selected discontinuous fields (x200) per slide were
evaluated for each specimen. The positive area was stained yellow,
and Image-Pro Plus software was used to quantify the integrated
optical density (IOD). When evaluating the possible correlation
between ER status and clinical outcome among individual patients,
the cases were tentatively classified into two groups according to
their ER IOD. A value of ERα IOD (range, 84–14,665) ≥5,200 was
considered as positive expression and a value <5,200 was
considered as negative expression. A value of ERβ IOD (range,
3,877–31,923) ≥18,000 was considered as high expression and a value
<18,000 was considered as low expression.
Statistical analysis
Results are expressed as means ± SD. The statistical
analyses between ER IOD and clinicopathological parameters of
individual patients were carried out with the use of the Student’s
t-test, one-way ANOVA test, the Mann-Whitney U test, the
Kruskal-Wallis test and paired t-test. Statistical analyses between
gender and other clinicopathological characteristics were assessed
with the Fisher’s exact test. Correlation of ERα with ERβ was
performed using Pearson’s correlation. Overall survival (OS) curves
of the patients were generated according to the Kaplan-Meier
method, and statistical significance was calculated using the
Breslow test. Statistical differences were examined using SPSS
software. Each P-value is two-tailed, and the significance level
was set at P=0.05.
Results
Association between gender and
clinicopathological parameters of the ESCC patients
The relationship between the gender of the patients
and clinicopathological parameters are summarized in Table I. Females had a significantly older
age at the onset of ESCC than that of males (P=0.0005). No
significant association was detected between gender and tumor size,
differentiation, depth of invasion, presence of lymph node
metastasis or TNM classification of tumors.
 | Table IComparison of the clinicopathologic
features between male and female esophageal squamous cell carcinoma
patients. |
Table I
Comparison of the clinicopathologic
features between male and female esophageal squamous cell carcinoma
patients.
| Gender, n (%) | |
|---|
|
| |
|---|
| Clinical
parameters | Male | Female | P-value |
|---|
| Age (years)a | 56.5±8.010 | 63.8±7.229 | 0.0005 |
| Tumor size
(cm)b | | | 0.129 |
| ≤3 | 37 (53) | 15 (79) | |
| >3 – ≤5 | 25 (36) | 3 (16) | |
| >5 | 8 (11) | 1 (5) | |
| Histologic
gradeb | | | 0.398 |
| G1 | 24 (34) | 4 (21) | |
| G2 | 40 (57) | 12 (63) | |
| G3 | 6 (9) | 3 (16) | |
| Invasive
depthb | | | 0.289 |
| T2 | 3 (4) | 2 (11) | |
| T3+T4 | 67 (96) | 17 (89) | |
| LN metastasisb | | | 0.797 |
| N0 | 37 (53) | 9 (47) | |
| N1+N2+N3 | 33 (47) | 10 (53) | |
| TNM
classificationb | | | 0.615 |
| IB+IIA+IIB | 38 (54) | 9 (47) | |
|
IIIA+IIIB+IIIC+IV | 32 (46) | 10 (53) | |
Expression of ER protein in human
esophageal non-neoplastic epithelium and carcinoma
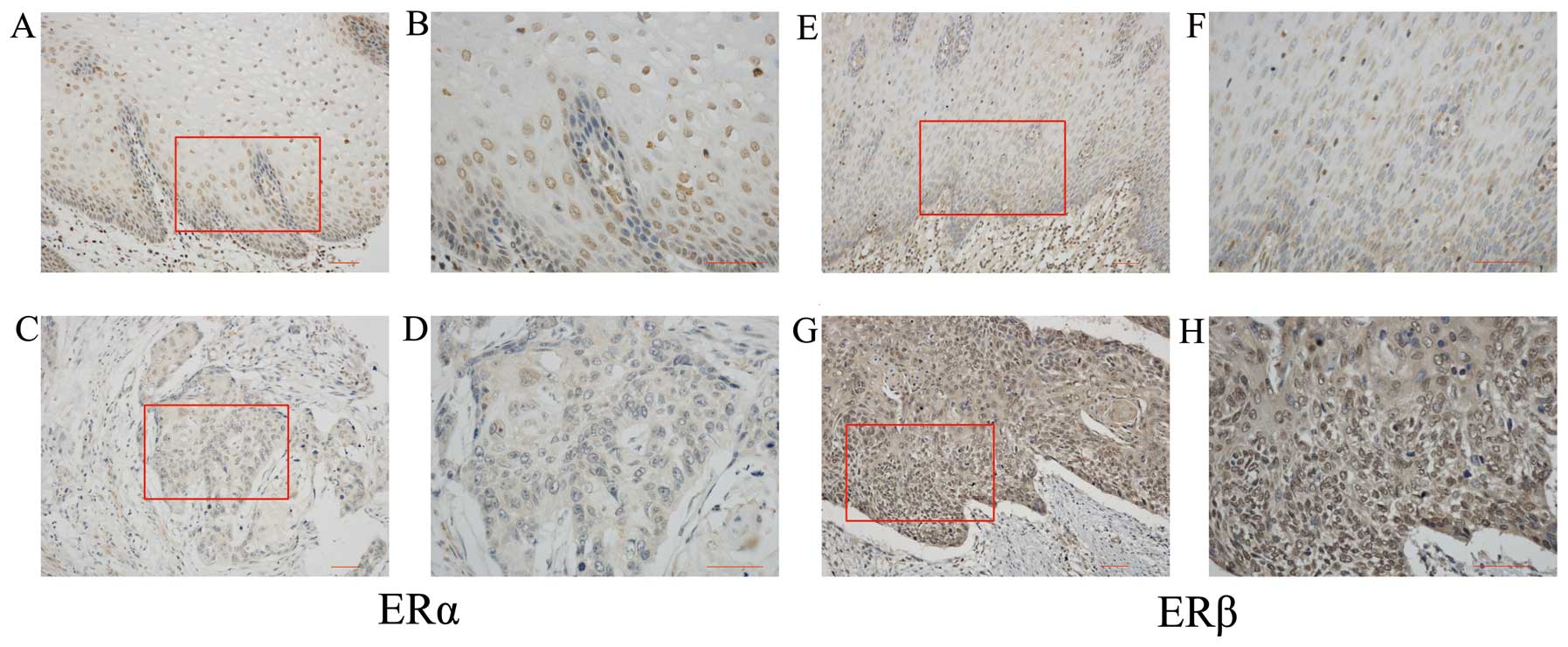
Both ERα and ERβ were expressed in human esophageal
non-neoplastic epithelium (Fig. 1A, B,
E and F) with ratios of 3/7 and 6/9, respectively. ERα
immunoreactivity was detected in the nuclei of carcinoma cells in
21/89 ESCC tissue samples. The mean value of ERα IOD in the 89 ESCC
tissue samples was 3,741.0±2,978.6. ERβ immunoreactivity was
detected in the nuclei of carcinoma cells in 87/89 ESCC tissue
samples. The mean value of ERβ IOD in 89 ESCC tissue samples was
17,998.7±6,664.5.
Based on the staining intensity, ERα and ERβ
displayed opposite immunostaining patterns. For ERα, most of the
cells showed moderate immunostaining in non-neoplastic epithelium
(Fig. 1A and B), whereas negative
to weak signals were observed in ESCC (Fig. 1C and D). However, ERβ
immunoreactivity was detected to be much weaker in the
non-neoplastic epithelium (Fig. 1E and
F) than in ESCC (Fig. 1G and
H). The expression difference between non-neoplastic epithelium
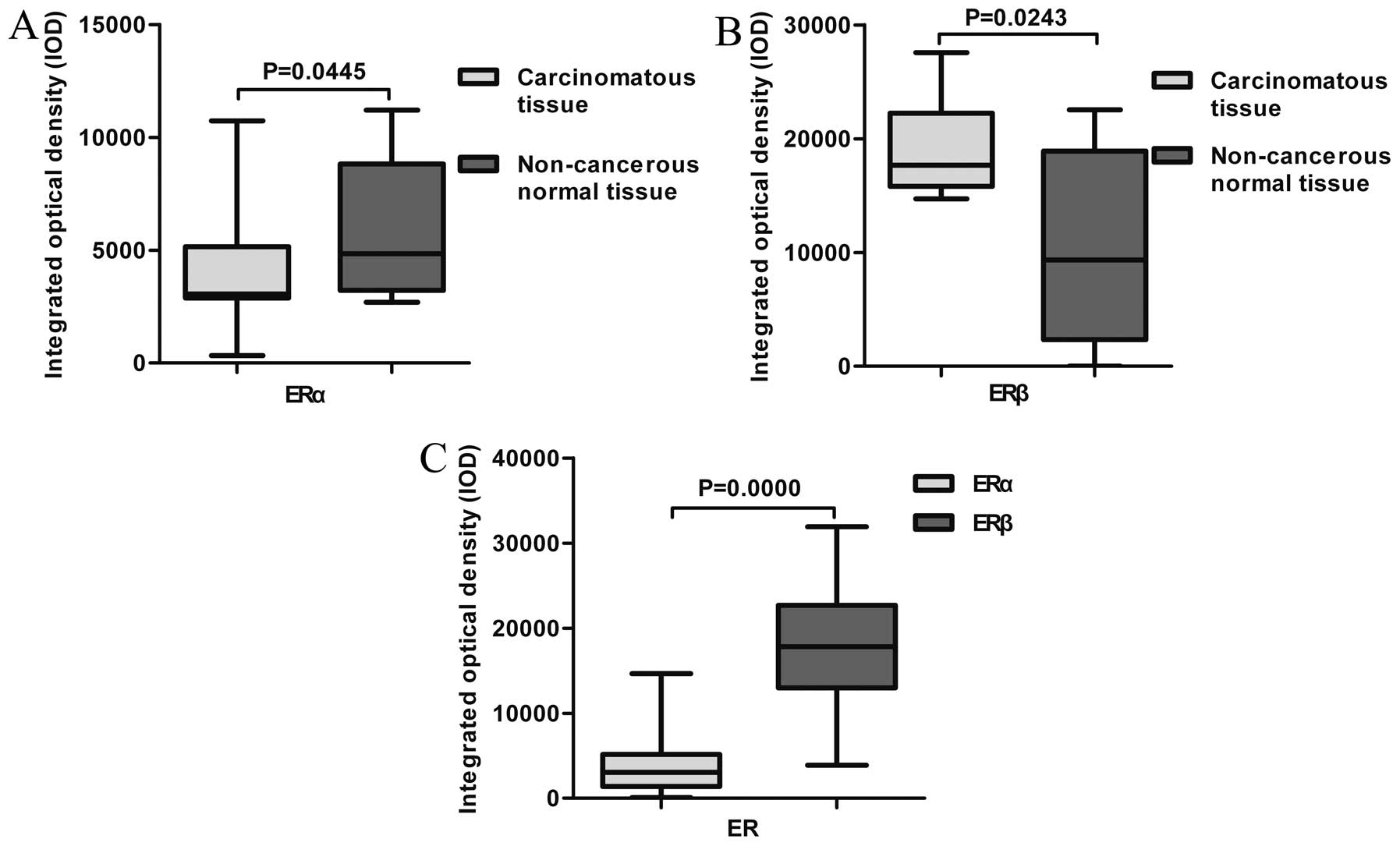
and ESCC was statistically significant for both ERα (P=0.0445) and
ERβ (P=0.0243; Fig. 2A and B).
Association between the expression levels
of the ERs and clinicopathological parameters of the ESCC
patients
Associations between ER expression levels and
clinicopathological parameters of the patients are summarized in
Table II. There was a
statistically significant inverse correlation between ERα IOD and
invasive depth of the tumor (P=0.0426). No significant association
was detected between ERα status and age, gender, tumor size,
differentiation, presence of lymph node metastasis or TNM
classification of tumors.
 | Table IIAssociation between ERα and ERβ and
clinicopathological variables in 89 esophageal squamous cell
carcinoma patients. |
Table II
Association between ERα and ERβ and
clinicopathological variables in 89 esophageal squamous cell
carcinoma patients.
| | IOD |
|---|
| |
|
|---|
| | ERα | ERβ |
|---|
| |
|
|
|---|
| Clinical
parameters | N | Mean ± SD | P-valuea | Mean ± SD | P-valueb |
|---|
| Age (years) | | | 0.5823 | | 0.7437 |
| ≤58 | 43 |
3,671.2±2,432.4 | |
17,757.8±6,303.0 | |
| >58 | 46 |
3,806.3±3,437.9 | |
18,223.9±7,047.7 | |
| Gender | | | 0.7264 | | 0.7158 |
| Male | 70 |
3,557.7±2,702.0 | |
17,863.6±6,842.6 | |
| Female | 19 |
3,831.9±3,004.4 | |
18,496.5±6,109.7 | |
| Tumor size
(cm) | | | 0.3405 | | 0.9545 |
| ≤3 | 52 |
3,573.8±3,068.8 | |
17,899.4±6,768.9 | |
| >3 – ≤5 | 28 |
3,388.4±2,183.5 | |
18,299.6±6,599.9 | |
| >5 | 9 |
4,551.4±2,408.9 | |
17,636.0±6,995.5 | |
|
Differentiation | | | 0.4880 | | 0.7710 |
| G1 | 28 |
3,249.8±2,501.5 | |
18,034.6±6,696.4 | |
| G2 | 52 |
3,729.6±2,735.5 | |
18,259.8±6,509.5 | |
| G3 | 9 |
4,480.7±3,646.0 | |
16,495.1±8,010.6 | |
| Invasive depth | | | 0.0426 | | 0.2677 |
| T2 | 5 |
5,373.8±1,291.0 | |
14,773.2±7,422.8 | |
| T3+T4 | 84 |
3,511.1±2,787.2 | |
18,190.7±6,616.0 | |
| LN metastasis | | | 0.9368 | | 0.2964 |
| N0 | 46 |
3,593.9±2,508.5 | |
17,281.6±6,602.0 | |
| N1+N2+N3 | 43 |
3,640.9±3,020.3 | |
18,765.8±6,722.8 | |
| TNM
classification | | | 0.6823 | | 0.1914 |
| I+II | 47 |
3,613.9±2,484.1 | |
17,123.0±6,619.7 | |
| III+IV | 42 |
3,620.2±3,053.8 | |
18,978.6±6,656.1 | |
No significant association was detected between ERβ
status and age, gender, tumor size, differentiation, invasive
depth, presence of lymph node metastasis or TNM classification of
tumors.
ERα expression is inversely correlated
with ERβ expression in ESCC
Paired t-test showed that the expression of ERβ was
much stronger than that of ERα (P=0.0000; Fig. 2C). There was also a statistically
significant inverse correlation between the expression of ERα and
ERβ in ESCC (r=−0.2902, P=0.0058).
ERα and ERβ expression is of prognostic
value for ESCC patients
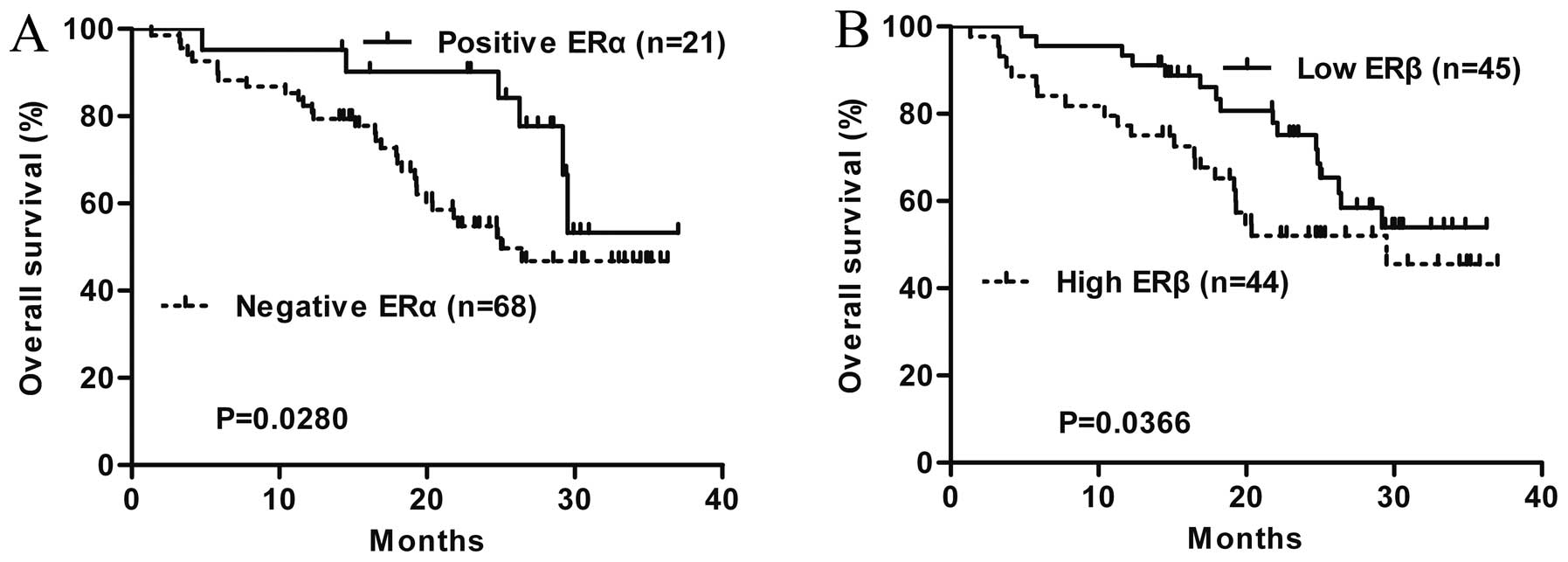
Patients with ERα expression had a more favorable
outcome than that of patients without ERα expression (P=0.0280;
Fig. 3A). However, high ERβ levels
were found to be associated with unfavorable outcome (P=0.0366;
Fig. 3B).
Discussion
In the present study, a significantly lower level of
ERα immunoreactivity was detected in ESCC tissues than that in the
normal esophageal tissues, which was consistent with the finding of
Zuguchi et al(18). ER
signaling via the PI3K/AKT signaling pathway phosphorylates EZH2 at
S21, reducing levels of H3K27me3 in uterine myometrial cells
(24). Meanwhile, the expression
frequency and expression levels of H3K27me3 were significantly
higher in ESCCs than in normal tissues, and high expression of EZH2
was correlated with tumor aggressiveness and adverse patient
outcome in ESCC (25,26). Therefore, estrogen might exert a
protective effect through ERα in esophageal squamous tissue. As for
ERβ, Wang et al(20)
reported that ERβ expression was correlated with lower malignant
potential of ESCC. Nevertheless, we found a high frequency of ERβ
expression in carcinomatous tissues than that in non-cancerous
normal tissues. This is consistent with the results of Kalayarasan
et al(16) showing that ERβ
was overexpressed in poorly differentiated SCC and adenocarcinoma
when compared to normal esophageal mucosa. In lung adenocarcinoma,
ERβ-mediated estradiol was found to enhance epithelial mesenchymal
transition through increased transcription of midkine (27). Thus, ERβ might also promote the
process of ESCC by inducing epithelial mesenchymal transition.
Meanwhile, several studies have demonstrated that the estrogenic
action through ER signaling promotes the proliferation of carcinoma
cells (6,28). For example, treatment with
ERβ-specific agonist DPN led to a significant increase in the cell
proliferation in primary urothelial cells, which predominantly
expressed ERβ (8). From this point
of view, our findings concerning the altered expression of ERα and
ERβ may carry clinical implication for the treatment of ESCC.
Reagents that upregulate the expression of ERα and/or downregulate
the expression of ERβ may be useful for the chemotherapy of ESCC
patients.
In this study, carcinomatous and non-cancerous
normal tissues of the esophagus were used, and all of the ESCC
tissues were obtained from patients who underwent esophagectomy.
The majority of cases were at a relatively high pathological stage
(T3). Therefore, T1 cases were lacked, and only 5 cases were
classified as T2. The dynamic changes in ER expression throughout
the progression of ESCC could not be monitored. Future
investigations should include normal esophageal tissues, low-grade
dysplasia and high-grade dysplastic tissues, particularly T1 and T2
cases, to elucidate the potential function of ERs in the
progression of ESCC.
We addressed the prognostic value of ER protein
expression for patients with ESCC in the present study. A
significant inverse correlation between the status of ERα and the
invasive depth of tumor was detected. The patients with
ERα-positive expression had a more favorable outcome than patients
without ERα expression. As for ERβ, Kaplan-Meier survival analysis
showed that high expression of ERβ in ESCC was significantly
associated with unfavorable clinical outcome of patients. These
results indicate that negativity for ERα and high expression of ERβ
may be an unfavorable prognostic factor for ESCC patients. Since
only immunohistochemistry was used in this study, further research
using quantitative molecular methods are obviously required to
clarify the prognostic value of ERs in ESCC.
McDonnell and Norris (21) reported that estrogenic signals
through ERβ can inhibit ERα-dependent transcription when both ERα
and ERβ are present in cells and are bound to estrogen. An inverse
correlation between expression of ERα and that of ERβ was also
demonstrated in lung cancer (22).
In this study, we found that the expression of ERα was inversely
correlated with that of ERβ, which indicates that there may be some
functional interactions between ERα and ERβ in ESCC. To the best of
our knowledge, this represents a novel observation that has not
been previously reported.
Prominent gender differences in the prevalence of
ESCC should not be ignored. In the present study, the age of onset
for female ESCCpatients was significantly older than that of male
patients. Zuguchi et al(18)
postulated that the gender differences in the prevalence of ESCC
might be due to lifestyle differences between male and female
patients. However, the difference in the onset age and the fact
that all of the 19 female ESCC patients were older than the average
postmenopausal age could not be explained by lifestyle differences.
Rather, the estrogen levels as well as differential distribution of
ERα and ERβ may contribute to the age- and menopause-related
changes in risk for the female population.
In conclusion, our study revealed that ERα was
downregulated and ERβ was upregulated, and the expression of ERα
was negatively associated with that of ERβ in ESCC. The absence of
ERα and high expression of ERβ indicate a poorer prognosis of ESCC.
The use of selective ER modulators may be clinically effective for
the treatment of ESCC patients. Future investigations are needed to
delineate the mechanisms that contribute to the loss of ERα
expression and the overexpression of ERβ during the progression of
ESCC.
Acknowledgements
We thank Dr Sima Sarvari for assistance with the
manuscript preparation.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Sugimachi K, Matsuoka H, Matsufuji H,
Maekawa S, Kai H and Okudaira Y: Survival rates of women with
carcinoma of the esophagus exceed those of men. Surg Gynecol
Obstet. 164:541–544. 1987.PubMed/NCBI
|
|
3
|
Matsuoka H, Sugimachi K, Ueo H, Kuwano H,
Nakano S and Nakayama M: Sex hormone response of a newly
established squamous cell line derived from clinical esophageal
carcinoma. Cancer Res. 47:4134–4140. 1987.PubMed/NCBI
|
|
4
|
Ueo H, Matsuoka H, Sugimachi K, Kuwano H,
Mori M and Akiyoshi T: Inhibitory effects of estrogen on the growth
of a human esophageal carcinoma cell line. Cancer Res.
50:7212–7215. 1990.PubMed/NCBI
|
|
5
|
Derakhshan MH, Liptrot S, Paul J, Brown
IL, Morrison D and McColl KE: Oesophageal and gastric
intestinal-type adenocarcinomas show the same male predominance due
to a 17 year delayed development in females. Gut. 58:16–23.
2009.PubMed/NCBI
|
|
6
|
Niikawa H, Suzuki T, Miki Y, et al:
Intratumoral estrogens and estrogen receptors in human non-small
cell lung carcinoma. Clin Cancer Res. 14:4417–4426. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mauro LV, Dalurzo M, Carlini MJ, et al:
Estrogen receptor β and epidermal growth factor receptor as
early-stage prognostic biomarkers of non-small cell lung cancer.
Oncol Rep. 24:1331–1338. 2010.
|
|
8
|
Teng J, Wang ZY, Jarrard DF and Bjorling
DE: Roles of estrogen receptor alpha and beta in modulating
urothelial cell proliferation. Endocr Relat Cancer. 15:351–364.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rath-Wolfson L, Purim O, Ram E,
Morgenstern S, Koren R and Brenner B: Expression of estrogen
receptor β1 in colorectal cancer: Correlation with
clinicopathological variables. Oncol Rep. 27:2017–2022. 2012.
|
|
10
|
Hogan AM, Collins D, Baird AW and Winter
DC: Estrogen and gastrointestinal malignancy. Mol Cell Endocrinol.
307:19–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Woo IS, Park MJ, Choi SW, et al: Loss of
estrogen receptor-α expression is associated with hypermethylation
near its ATG start codon in gastric cancer cell lines. Oncol Rep.
11:617–622. 2004.
|
|
12
|
Peng B, Lu B, Leygue E and Murphy LC:
Putative functional characteristics of human estrogen receptor-beta
isoforms. J Mol Endocrinol. 30:13–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Utsumi Y, Nakamura T, Nagasue N, Kubota H
and Morikawa S: Role of estrogen receptors in the growth of human
esophageal carcinoma. Cancer. 64:88–93. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Utsumi Y, Nakamura T, Nagasue N, Kubota H,
Harada T and Morikawa S: Effect of 17 beta-estradiol on the growth
of an estrogen receptor-positive human esophageal carcinoma cell
line. Cancer. 67:2284–2289. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nozoe T, Oyama T, Takenoyama M, Hanagiri
T, Sugio K and Yasumoto K: Significance of immunohistochemical
expression of estrogen receptors alpha and beta in squamous cell
carcinoma of the esophagus. Clin Cancer Res. 13:4046–4050. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kalayarasan R, Ananthakrishnan N, Kate V
and Basu D: Estrogen and progesterone receptors in esophageal
carcinoma. Dis Esophagus. 21:298–303. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang QM, Yuan L, Qi YJ, Ma ZY and Wang LD:
Estrogen analogues: promising target for prevention and treatment
of esophageal squamous cell carcinoma in high risk areas. Med Sci
Monit. 16:HY19–HY22. 2010.PubMed/NCBI
|
|
18
|
Zuguchi M, Miki Y, Onodera Y, et al:
Estrogen receptor alpha and beta in esophageal squamous cell
carcinoma. Cancer Sci. 103:1348–1355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang H, Sukocheva OA, Hussey DJ and Watson
DI: Estrogen, male dominance and esophageal adenocarcinoma: is
there a link? World J Gastroenterol. 18:393–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang QM, Qi YJ, Jiang Q, Ma YF and Wang
LD: Relevance of serum estradiol and estrogen receptor beta
expression from a high-incidence area for esophageal squamous cell
carcinoma in China. Med Oncol. 28:188–193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McDonnell DP and Norris JD: Connections
and regulation of the human estrogen receptor. Science.
296:1642–1644. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kawai H, Ishii A, Washiya K, et al:
Estrogen receptor alpha and beta are prognostic factors in
non-small cell lung cancer. Clin Cancer Res. 11:5084–5089. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu S, Zhu N and Chen H: Expression
patterns of human DAB2IP protein in fetal tissues. Biotech
Histochem. 87:350–359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bredfeldt TG, Greathouse KL, Safe SH, Hung
MC, Bedford MT and Walker CL: Xenoestrogen-induced regulation of
EZH2 and histone methylation via estrogen receptor signaling to
PI3K/AKT. Mol Endocrinol. 24:993–1006. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He LR, Liu MZ, Li BK, et al: Prognostic
impact of H3K27me3 expression on locoregional progression after
chemoradiotherapy in esophageal squamous cell carcinoma. BMC
Cancer. 9:4612009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He LR, Liu MZ, Li BK, et al: High
expression of EZH2 is associated with tumor aggressiveness and poor
prognosis in patients with esophageal squamous cell carcinoma
treated with definitive chemoradiotherapy. Int J Cancer.
127:138–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao G, Nie Y, Lv M, et al:
ERbeta-mediated estradiol enhances epithelial mesenchymal
transition of lung adenocarcinoma through increasing transcription
of midkine. Mol Endocrinol. 26:1304–1315. 2012. View Article : Google Scholar
|
|
28
|
Zhao G, Zhao S, Wang T, Zhang S, Lu K, Yu
L and Hou Y: Estrogen receptor beta signaling regulates the
progression of Chinese non-small cell lung cancer. J Steroid
Biochem Mol Biol. 124:47–57. 2011. View Article : Google Scholar : PubMed/NCBI
|