Introduction
Oral squamous cell carcinoma (OSCC) is the sixth
most common cancer internationally, accounting for ~5% of all
malignant tumours worldwide (1).
OSCCs commonly metastasize to cervical lymph nodes. Each tumour
generally metastasizes in a particular group of cervical lymph
nodes and the principles and criteria governing neck dissection
(ND) are based on the primary tumour characteristics, according to
the cTNM staging and its primary site (2,3).
Cervical lymph node metastases (LNMs) are key
malignancy criteria in OSCC (4).
Their presence influences the therapeutic plan and prognosis, since
it is associated with a 50% decrease in survival (5,6).
The selection of OSCC cases requiring ND depends
mainly on the clinical TNM staging (cTNM). cTNM provides a stage
grouping based on the extent of the primary tumour (T score), the
involvement of the regional, cervical, lymph nodes (N score) and
the detection of distant metastases (M score) (2,3,7). These
parameters are accurately quantified after performing a series of
clinical-instrumental examinations such as PET, total body CT scan,
neck echo-color Doppler, fibrolaryngoscopy,
esophageal-gastric-duodenoscopy, bronchoscopy and, if any doubts
persist, fine needle aspiration biopsy (FNAB) and biopsy must be
also performed.
Once clinical T, N and M parameters have been
defined, a ND is mandatory for the OSCCs showing cervical LNMs (any
cT/N+) and for locally advanced primary tumours (cT3 or cT4) with
clinically undetectable LNMs (cN0).
Based on the intent or purpose, NDs have been also
classified into therapeutic and elective. Therapeutic NDs are
performed in OSCCs with cervical metastases detected in clinical
preoperative setting (any T/N+). Elective NDs are selected for
locally advanced primary tumours (T3 or T4) with clinically
undetectable LNMs (cN0) (8). NDs
are also performed in the cases of small primary tumours clinically
negative to node involvement (cT1-T2/N0) that during intraoperative
assessment of their sentinel lymph node reveal positivity to
metastasis (9).
Both ND anatomical extent and involvement of
surrounding structures are related to the node levels involved in
the dissection and they are planned on the basis of the OSCC
primary site; correlations between primary site of the cancer and
level of metastasis have been demonstrated and, to date, they aid
the surgeon in the surgical ND management (10).
Currently, NDs are classified into four basic
procedures according to the extent of different cervical lymph node
groups and surrounding structures: radical ND, modified radical ND,
(MRND) extended ND and selective ND (8,11).
Following histopathological assessment of the tumour
margins of excision and after the evaluation of the involvement of
the surrounding structures, adjuvant therapy may also be performed:
radiotherapy for T4 tumours with free surgical margins and/or ≥N2;
and both radiotherapy and chemotherapy for tumours with any N+ plus
extracapsular spread (ECS) and ones with any T and positive or
close margins or perineural invasion and/or neoplastic vascular
embolization. T1–3 tumours with free margins and pN0/pN1 do not
require adjuvant treatment (12,13).
Despite the progress in pre-surgical instrumental
examinations (head and neck CT scan, neck echo-color Doppler,
fibrolaryngoscopy, esophageal-gastric-duodenoscopy, bronchoscopy
and PET), clinical lymph node staging is not completely error-free
due to false positivity in the presence of reactive lymph nodes,
non metastatic lymph node enlargement and false negativity for
small- or micrometastases clinically undetectable (14,15).
Finally, the controversial role of sentinel lymph node positivity
and the surgical morbidity after ND have led to the evaluation of
alternative and super-selective surgeries in order to reduce the
overtreatments (15,16–18).
For these reasons, further in-depth studies
regarding the behaviour of lymph node cervical metastases may be
useful to refine therapeutic management, thereby decreasing the
overtreatment-related morbidity and mortality.
The aim of the present study was to define LNM
frequency, topographic distribution, size (micrometastases vs.
macrometastases) and histological pattern correlating them with the
clinical features of primary tumour in 121 OSCC patients who had
undergone ND, considering the survivals related to their
presence/absence and the morbidity related to the negative ND and
due to the neck surgery, comparing our results with the literature
and suggesting an evidence-based re-evaluation of the therapeutic
approach to ND.
Materials and methods
Study population and clinical
pathological data
Resection specimens from 121 patients who had
undergone ND surgery for OSCC at the National Cancer Institute of
Naples, ‘G. Pascale’, Italy, between the years 1993–2004, formed
the basis of the present retrospective analysis. All patients
underwent MRND for OSCC (11,19).
Patients who had had previous surgery (other than diagnostic
biopsy) were excluded. Throughout the time period of the study, the
resection specimens were performed by the same surgical team and
were assessed by the same pathological team. Pathological report
and topography of the extent and location of the metastatic disease
for each patient were reviewed and number, size and histological
patterns of LNM-positive cases (pN+) were re-evaluated according to
previous literature (14,15,20,21) in
order to calculate frequency, distribution and other significant
statistical correlations existing between primary tumour features
and pN+.
The series comprised 80 males (mean age of
63.10±10.79 years; range, 30–83 years, median 63 years) and 41
females (mean age, 63.12 ±15.19 years; range, 25–86 years, median,
65 years). The cTNM staging was assessed according to the 6th
edition AJCC (2) since data refer
to the period between 1999–2004. Patient demographic and clinical
characteristics of the 121 cases are summarized in Table I.
 | Table IPatient demographics and clinical
characteristics. |
Table I
Patient demographics and clinical
characteristics.
| N (%) |
|---|
| Gender
(male/female) | 80/41 (66/34) |
| Male mean age,
years (range) | 63.10 (30–83) |
| Female mean age,
years (range) | 63.12 (25–86) |
| Primary T site |
| Tongue | 53 (44) |
| Floor of the
mouth | 23 (19) |
| Cheek | 4 (3) |
| Trigone | 17 (14) |
| Oropharynx | 8 (7) |
| Palate | 3 (2) |
| Fornix | 10 (9) |
| Not specified | 3 (2) |
| Histologic
grade |
| Low | 35 (29) |
| Intermediate | 65 (54) |
| High | 21 (17) |
| AJCC stage |
| I | 11 (9) |
| II | 30 (25) |
| III | 28 (23) |
| IV | 52 (43) |
Pre-operative, operative and
post-operative protocols
At the National Cancer Institute of Naples ‘G.
Pascale’, Italy, the selection of OSCC cases requiring ND depends
on the cTNM (2). cTNM parameters
are accurately quantified after performing a series of
clinical-instrumental examinations such as PET, total body CT scan,
neck echo-color Doppler, fibrolaryngoscopy,
esophageal-gastric-duodenoscopy, bronchoscopy and, if any doubts
persist, fine needle aspiration biopsy (FNAB) and biopsy.
According to the literature, MRNDs are considered
mandatory for the OSCCs clinically showing cervical LNMs (any cT/N+
and therapeutic ND), for locally advanced primary tumours with
clinically undetectable LNMS (cT3/N0 and cT4/cN0, elective ND) and
in the cases of small primary tumours clinically negative to node
involvement (cT1-T2/N0) that during intraoperative assessment of
their sentinel lymph node reveal a positivity to metastasis (SLN+)
(8,9).
For the ND surgical approach, we adopted MRND
(10), thus involving levels I–IV
for all oral sub-sites, except for trigone, posterior tongue and
anterior pillar where ND extended until level V; level IIB was
always comprised.
After surgery and histopathological assessment of
the margins of excision of the primary tumour and after the
evaluation of the involvement of the surroundings structures,
adjuvant therapy was performed; radiotherapy for T4 tumours with
free-surgical margins and/or ≥N2, and both radiotherapy and
chemotherapy both for tumours with any N+ plus ECS, tumours with
any T and positive or close margins or perineural invasion and/or
neoplastic vascular embolization tumours T1–3 with free margins and
pN0/pN1 were not enrolled for adjuvant treatments (12,13).
Since data refer to patients recruited from 1999 to
2004, prior to the last Cancer Staging Atlas publication, we
considered the previous edition (2); hence, T4 tumours were not
distinguished in T4a and T4b, consequently, stage IV has not been
subclassified into stages IVa, IVb and IVc (7).
Histopathological node metastasis
classification
All 121 lymph node specimens dissected were
histopathologically re-evaluated for the present study. In order to
detect micrometastases and to confirm the involvement of lymph
nodes, the standardized sectioning protocol was performed. One
H&E stained section was prepared from each block and examined
for the presence of nodal involvement by tumour. If present,
metastatic disease was reported. If node was negative or equivocal
for metastatic disease, or positive for micrometastases, the
removed lymph nodes were also serially sectioned at 25-μm intervals
4 μm thick and alternately stained with H&E and
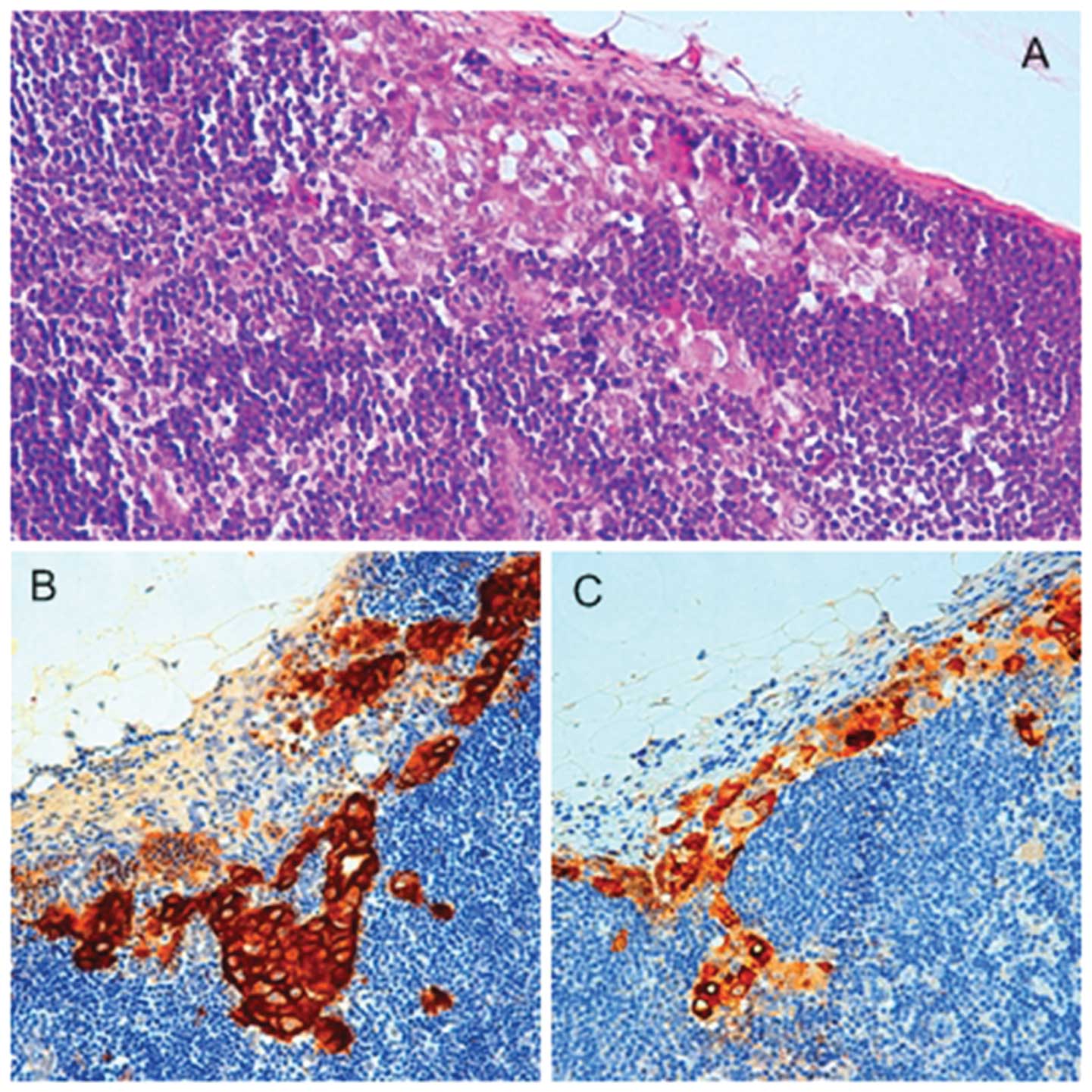
immunohistochemical staining (IHC) using anti-cytokeratin and EMA
antibodies (Fig. 1) as described
below. This pattern was continued throughout the entire block. Each
immunostained component was always compared with adjacent sections
stained by H&E.
Woolgar’s classification criteria were considered in
order to classify the type of metastasis (21,22).
Woolgar distinguished metastatic lymph nodes into two groups with
different prognosis and features: typical metastatic pattern as
‘orderly involvement of successive anatomical nodal levels,
creating an inverted cone with maximum volume and maximum ECS at
levels I or II and a gradual reduction in the volume/extent of
metastasis at the numerically higher levels’ (21), vs. atypical metastatic pattern,
termed ‘aberrant’ by Woolgar, and characterized by various
features. The ones referring to the atypical pattern and considered
in the present study were the involvement of ‘other’ anatomical
groups of nodes (including parapharyngeal, facial, buccal, lingual
and sublingual nodes), involvement of controlateral cervical lymph
nodes, skipping of anatomical levels other than level I and the
presence of a single micrometastasis (21).
Once defined, the histopathological features of node
metastases were correlated with the clinical and histopathological
features of the primary tumour in order to establish statistical
and prognostic correlations.
Immunohistochemistry
Histological and immunohistochemical analyses were
performed on formalin-fixed, paraffin-embedded tissue samples.
Immunostaining was performed using the linked streptavidin-biotin
horseradish peroxidase technique (LSAB-HRP). Antigen retrieval was
performed by microwave heating, a first time for 3 min at 650 W, a
second and a third time for 3 min at 350 W, the slides immersed in
10 mM citrate buffer pH 6.0. After heating, the sections were
blocked for 60 min with 1.5% horse serum (Santa Cruz Biotechnology)
diluted in PBS buffer before reaction with the primary antibody
(Ab). The primary monoclonal antibodies anti CK AE1/AE3 (dilution
1:50, pH 6.0; Dako, Carpinteria, CA, USA) and EMA (dilution 1:75,
with protein K; Dako) were incubated overnight. After two washes
with PBS, the slides were treated with biotinylated
species-specific secondary antibodies and streptavidin-biotin
enzyme reagent (Dako, Glostrup, Denmark), and the colour developed
by 3,3′-diaminobenzidine tetrahydrochloride. Sections were
counterstained with Mayer’s hematoxylin and mounted using
xylene-based mounting medium. In negative controls, the primary
antibody was omitted. The results of the IHC were separately
evaluated by two independent observers by carefully examining the
entire section with an optical microscope (Olympus BX41). For each
case, the presence and the extent of positive cells in all sections
examined was determined. Isolated tumour cells (ITCs) are defined
as tumour cell clusters that are not >0.2 mm in largest diameter
and are denoted as lymph node negative (pN0[i+]). Micrometastases
are defined as metastases that are >0.2 mm in diameter but ≤2
mm, denoted as lymph node positive (pN1mi). Carcinoma
macrometastases measured >2 mm in maximum extent. Two
investigators experienced in oral pathology blindly and
independently examined the study sections initially, and then they
evaluated together the histopathological and immunostained sections
until they reached an agreement.
Statistical analysis
Data were analysed by the GraphPad Prism software
version 5.0 for Windows (GraphPad Software, San Diego, CA, USA;
www.graphpad.com) and Excel Microsoft Office.
Differences among the groups were estimated using the one-way
analysis of variance (ANOVA) and the Student-Newman-Keuls test.
Only P-values <0.05 were considered significant. Overall
survivals in the different groups were calculated by the
Kaplan-Meier curves and log-rank (Mantel-Cox) test was applied for
comparing survival probabilities. The pathological positivity of
the ND (pN+) and the type of LNMs were correlated with the site of
primary tumour. The percentages of positive node metastases (pN+)
in elective, therapeutic and secondary to SLN+ NDs and the number
of lymph nodes harvested were also evaluated.
Results
Differentiation degree, cTNM staging and
pN status of 121 cases
Patient demographics and characteristics such as
gender, OSCC site, primary tumour differentiation grading and cTNM
staging according to the international guidelines (2) of the 121 OSCCs considered are shown in
Table I.
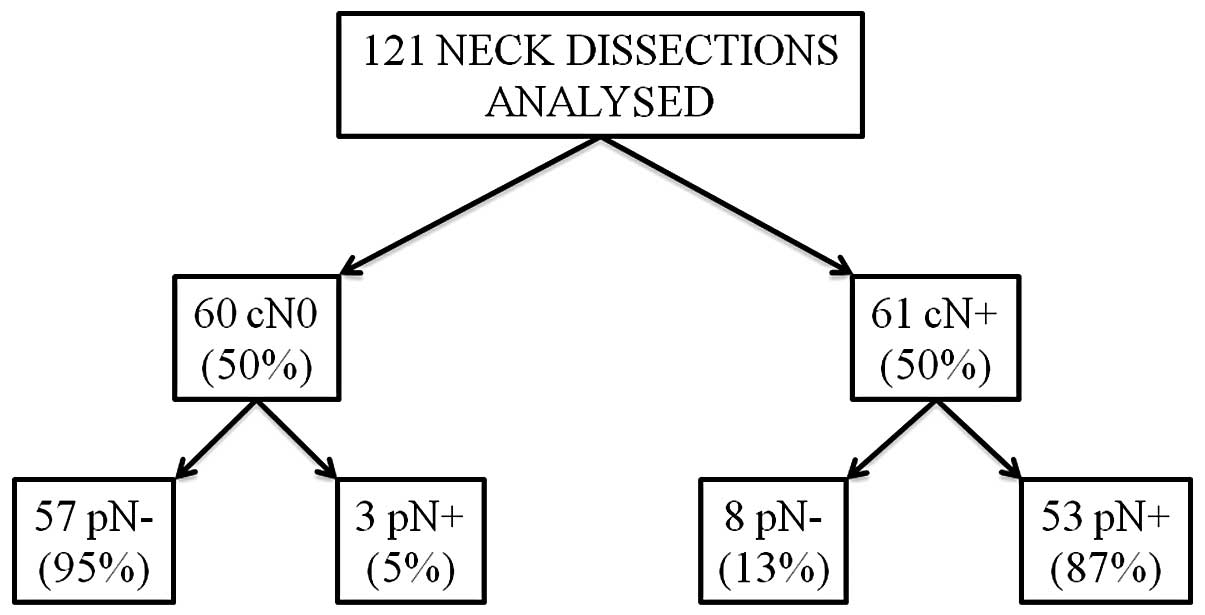
At pre-surgical clinical-instrumental evaluation,
61/121 cases (50.4%) were considered positive (cN+) and 60/121
(49.6%) negative (cN0) to node metastases. The histopathological
lymph node assessment subsequent to ND revealed at least one node
metastasis in 56/121 NDs, thus considered pathologically positive
lymph nodes (pN+) and no node metastasis in the remaining 65 cases,
which were considered pathologically negative lymph nodes (pN0).
The percentages of true positive (cN+ and pN+), true negative (cN0
and pN0), false positive (cN+ and pN0) and false negative (cN0 and
pN+) were 87, 95, 13 and 5% respectively, as reported with details
of accuracy, sensitivity, specificity, and positive and negative
predictive values in Fig. 2.
The pN+ distribution according to the grading,
staging MRND intent and, conversely, the pN+ frequency in each
group are reported in Table
II.
 | Table IIpN+ distribution and frequency
according to histological grading, AJCC staging and MRND
intent. |
Table II
pN+ distribution and frequency
according to histological grading, AJCC staging and MRND
intent.
| Histological
grade | Total ND cases
(%) | pN+ distribution
according to grade | Total pN+ cases in
each group |
|---|
| Low | 35 (29) | 11/56 (20) | 11/35 (31) |
| Intermediate | 65 (54) | 32/56 (57) | 32/65 (49) |
| High | 21 (17) | 13/56 (23) | 13/21 (62) |
|
| cTNM | Total ND cases
(%) | pN+
distribution according to cTNM | Total
pN+ cases in each group |
|
| Stage I | 11 (9) | 0/56 (0) | 0/11 (0) |
| Stage II | 30 (25) | 2/56 (4) | 2/30 (6.6) |
| Stage III | 28 (23) | 13/56 (23) | 13/28 (46.4) |
| Stage IV | 52 (43) | 41/56 (73) | 41/52 (79) |
|
| cT status | cN status | MRND clinically
accorded | Total ND cases
(%) | pN+
according to MRND intent | Total
pN+ cases in each group |
|
| Any T | cN+ | Therapeutic | 61/121 (50) | 53/56 (94) | 53/61 (88.3) |
| T3 or T4 | cN0 | Elective | 19/121 (16) | 1/56 (2) | 1/19 (5.3) |
| T1 or T2 | cN0 | Secondary to
SLN+ | 41/121 (34) | 2/56 (4) | 2/42 (4.8) |
Distribution of 121 MRNDs according to
intent to surgery and related pN+ frequencies
Among the 121 NDs considered, 61/121 (50%),
presenting at least a clinical node involvement (cN+), were
therapeutic NDs; 19/121 (16%), presenting a cT3/cT4 and cN0 were
elective NDs; the remaining 41/121 (34%), presenting primary
tumours small in size (cT1/cT2) and no evident clinical node
metastases (cN0) but positive to sentinel lymph node metastases
(SLN+) during the intraoperative assessment of sentinel lymph node,
were NDs performed to establish the presence of other node
metastases in addition to SLN+ (Table
II). The distribution of 56 pN+ according to
clinical-instrumental indications and, conversely, the pN+
frequency in each indication are reported in Table II.
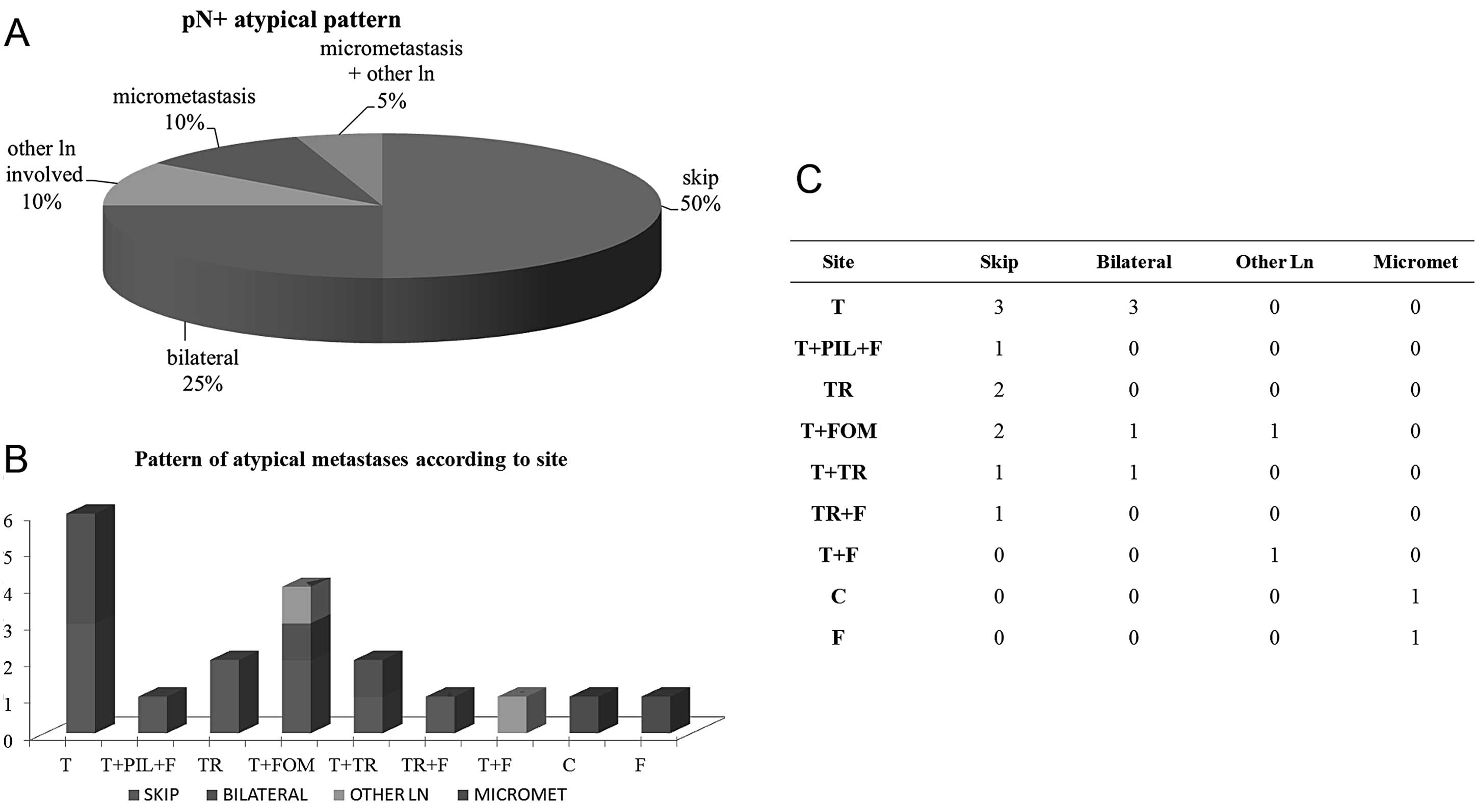
pN+ pattern
After excluding 19 pN+ cases due to lack of data
useful to classify the pN+ histopathological pattern, the remaining
37 pN+ NDs were distinguished into 17/37 with typical (46%) and
20/37 with atypical (54%) node metastases according to Woolgar’s
classification (21). The atypical
pattern was largely represented by skip metastases [10/20 (50%)],
followed by the frequency of bilateral metastases [5/20 (25%)],
micrometastases [2/20 (10%)], involvement of other lymph nodes
alone [2/20 (10%)], and 1/20 (5%) showing both micrometastases and
other lymph nodes co-interested (Fig.
3A). According to primary tumour site, skip metastases
indiscriminately involved any site of the oral cavity, except cheek
and fornix, which, on the contrary, preferentially showed
micrometastases; bilateral metastases were frequently detected in
OSCCs affecting tongue as a single site or in association with
floor of the mouth or trigone; ‘other different lymph nodes’ were
involved in floor of the mouth tumours (Fig. 3B and C).
Among 56 pN+ cases, 15/56 pN+ (26.8%) involved
levels I–III, 11/56 pN+ (19.6%) involved levels IV–V, and 30/56
(53.6%) pN+ were censored due to level not reported.
ND anatomical levels I–III were typically involved
by node metastases while levels IV–V showed pN+ only for primary
tumours of trigone, floor of the mouth plus tongue, tongue alone
and multiple sites (data not shown).
Number of lymph nodes removed
A total of 3,390 lymph nodes were harvested in 121
NDs, mean 18.5±22.7 per ND, range 1–110. One hundred and sixty-two
lymph nodes were pN+ in 56 NDs, mean 2.9±0.03, range 1–10.
Recurrence
Recurrence was observed in 8 cases, whose clinical,
histological and lymph node features are shown in Table III.
 | Table IIIClinical, histological and lymph node
features in the 8 cases with recurrence. |
Table III
Clinical, histological and lymph node
features in the 8 cases with recurrence.
| Gender | Age (years) | T site | Histological
grade | AJCC stage | pN status | Outcome |
|---|
| Female | 80 | FOM | Low | II | pN0 | Alive |
| Female | 83 | FOM | Intermediate | III | pN+ | Alive |
| Female | 48 | Tongue | Intermediate | IV | pN+ | Alive |
| Female | 70 | Tongue | Low | I | pN0 | Deceased |
| Male | 64 | Tongue | Intermediate | II | pN0 | Deceased |
| Male | 57 | FOM, Tongue | Intermediate | IV | pN+ | Alive |
| Male | 70 | Fornix | Intermediate | III | pN+ | Deceased |
| Male | 76 | Trigone | Intermediate | IV | pN0 | Deceased |
Survival curves
Overall survival statistical significance and
percentages of subjects alive at 1, 2 and 5 years from the
diagnosis are reported in Table
IV.
 | Table IVOne-, 2- and 5-year overall survival
and the statistical significance. |
Table IV
One-, 2- and 5-year overall survival
and the statistical significance.
| 1-year (%) | 2-year (%) | 5-year (%) | P<0.05 |
|---|
| Stage I–II | 90.00 | 87.50 | 78.34 | Yes |
| Stage III–IV | 76.16 | 50.78 | 46.16 | |
| Low grade | 83.24 | 68.65 | 68.65 | No |
| Intermediate-high
grade | 81.11 | 64.95 | 51.45 | |
| pN0 | 93.54 | 94.54 | 69.11 | Yes |
| pN+ | 66.03 | 45.41 | 45.41 | |
| pN+/Stage
I–IIa | 0 | 0 | 0 | Yes |
| pN+/Stage
III–IV | 67.46 | 46.20 | 46.20 | |
| pN+/Low grade | 50.00 | 41.66 | 41.66 | No |
|
pN+/Intermediate-high grade | 69.35 | 44.56 | 44.56 | |
| Typical pN+ | 50.89 | 44.53 | 44.53 | No |
| Atypical pN+ | 72.22 | 41.27 | 41.27 | |
| Typical pN+/Low
grade | 50.00 | 50.00 | 50.00 | No |
| Typical
pN+/Intermediate-high grade | 51.56 | 51.56 | 51.56 | |
| Atypical pN+/Low
grade | 78.57 | 34.37 | 34.37 | |
| Atypical
pN+/Intermediate-high grade | 50.00 | 25.00 | 25.00 | |
| pN+ with
ECS/macrometastases | 80.67 | 28.68 | 28.68 | Yes |
| pN+ with
micrometastases | 80.00 | 40.00 | 40.00 | |
| pN0 | 93.54 | 81.50 | 69.10 | |
| LN<20 | 72.38 | 52.36 | 46.93 | Yes |
| LN 20–30 | 88.80 | 66.67 | 66.67 | |
| LN>30 | 96.29 | 87.59 | 80.60 | |
| pN0/Elective | 87.50 | 79.54 | 62.64 | Yes |
|
pN0/Therapeutic | 87.50 | 30.00 | 15.00 | |
| pN0/SLN+ | 100.00 | 94.51 | 84.63 | |
| pN+/Elective | 100.00 | 100.00 | 100.00 | |
|
pN+/Therapeutic | 68.10 | 45.71 | 45.71 | |
| pN+/SLN+b | 0 | 0 | 0 | |
| SLN+ | 95.00 | 89.79 | 80.40 | Yes |
| E | 88.23 | 80.88 | 65.36 | |
| T | 70.745 | 42.86 | 39.56 | |
| pN+/SLN+ | 100.00 | 95.52 | 84.63 | Yes |
| pN+/E | 100.00 | 100.00 | 100.00 | |
| pN+/T | 68.11 | 45.72 | 45.72 | |
| pN0/SLN+ | 100.00 | 94.52 | 84.63 | Yes |
| pN0/E | 87.50 | 71.59 | 62.64 | |
| pN0/T | 87.50 | 30.00 | 15.00 | |
Overall survival was statistically different
according to early AJCC vs. late AJCC stages (P=0.0004) with a
5-year survival of 78.34 and 46.16%, respectively, and according to
absence/presence of nodal metastases (pN0 vs. pN+, P=0.0003) with a
1–5 year overall survival ranging between 97–74% in pN0 cases and
between 69–54% in pN+ cases.
Overall survival according to histological grade and
typical vs. atypical lymph nodal metastatic pattern did not reach
statistical significance when independently considered or when
together. When comparing pN0 subjects, pN+ with macrometastases or
ECS and pN+ with micrometastases, patients reporting pN+ with
micrometastases showed an intermediate probability of survival
after 5 years and these data were statistically significant
(P=0.004). With regard to the number of lymph nodes harvested,
statistically significant different survivals (P=0.005) were found
in cases with >30 lymph nodes harvested, whose 5-year survival
was >80% compared to 46.93% in cases with <20 lymph nodes and
66.67% in the 20–30 lymph node group, independently of the pN
status.
With regard to intent to surgery, overall survival
among subjects who had undergone elective, therapeutic and after
SLN+ NDs were always statistically significant with the worst
prognosis in patients who had undergone therapeutic ND and the most
positive one in patients who had undergone ND after SLN+. pN status
did not appear to be responsible for differences in survival since
patients with pN0/therapeutic MRND showed a 15% 5-year survival
compared to pN+/therapeutic MRND patients with a 45.72% 5-year
survival.
Discussion
In the present study, a descriptive and statistical
retrospective study on 121 OSCCs who had undergone neck dissection
(ND) was conducted, focusing on metastatic pattern (typical vs.
atypical), number of lymph nodes harvested and node metastasis
features in terms of size, anatomical extent and surgical decision
orienting the ND (elective, therapeutic and after SLN+).
Correlation among histological malignancy grading in
OSCC and different clinical parameters such as clinical staging,
recurrence and prognosis have been published in different studies
and a close relationship between the degree of histological
differentiation and the incidence of lymph node metastasis (LNM)
has been reported by several investigators in an attempt to
identify a better prognosis. Our results are partly in accordance
with previous literature (23).
Descriptive data showed, as expected, that the more
the primitive tumour was towards undifferentiation and AJCC
advanced staging, the more frequent the nodal metastases. Despite
these findings, statistically significant differences in the
overall survival were observed only in relation to AJCC staging and
not according to histological grading.
Since 88.3% of NDs performed for therapeutic intent
presented at least one pN+, the therapeutic value was confirmed. On
the contrary, the low pN+ frequencies in elective NDs (5.3%) and in
NDs secondary to SLN+ (4.8%) revealed an overtreatment in the
remaining 94.7 and 95.2% of cases, respectively. pN+ was found in 2
out of 42 NDs performed after SLN+ (0/11, 0%, pN+ in cT1-cN0M0 NDs
and 2/31, 6.45%, in cT2-cN0M0 NDs), thus confuting previous
literature supporting its role as a diagnostic marker for other
nodal metastases (24,25) and bringing the predictive role of
SLN positivity into discussion, leading us to conclude that a ND
secondary to SLN+ is an overtreatment in 100% of cT1-cN0M0 NDs and
in 93.55% of cT2-cN0M0 NDs. We suggest conducting further
biomolecular studies focusing on molecular markers able to predict
occult metastatic disease in SLN biopsies, thereby improving the
quality of the treatments (26).
The significant association between clinical staging
and histopathological report, confirmed by high accuracy (90.9%)
revealed the quality of the pre-surgical clinical and instrumental
staging of the tumours.
With regard to the anatomical lymph nodal levels
involved, results showing involvement of levels IV–V only for
primary tumours of trigone, floor of the mouth plus tongue, tongue
alone and multiple sites, express the major morbidity of the OSCCs
of these primary sites, requesting an ND extended until level V and
lead us to re-consider the extended ND in the cases of OSCC
affecting different oral sites such as cheek, which never showed
levels of IV–V involvement in the present study. Shah (27) provided evidence that the pattern of
neck metastasis of carcinomas from upper aerodigestive tract is
predictable based on the location of the primary lesion. Moreover,
it has been shown that in patients with oral carcinoma and clinical
evidence of neck disease, the rate of pathologic involvement of
level V nodes was only 4%. In the present study, the percentage of
pathologic involvement of level IV–V nodes was 19.6%, higher when
compared with the 4% value reported by Shah (27).
Recurrences, observed in 8 cases, mainly occurred in
floor of the mouth and tongue OSCCs, at intermediate grade of
differentiation and independently from the pN status.
The survival probability was in accordance with the
number of lymph nodes harvested, as previously demonstrated by Amar
et al(6), who found the
larger number of lymph nodes dissected in the ND related to the
group of better prognoses among pN0 cases, while we found this
result independently from the pN status.
We also confirmed the statistically significant
different survival probability of patients with micrometastases
(5-year, 40%), intermediate among pN0 (5-year, 69.10%) and pN+ with
macrometastases patients (5-year, 28.68%), as previously reported
by Broglie et al(20) and
Han et al(28).
Statistically significant difference in the overall
survival was also found according to clinical intent to surgery
independently from the pN0, with a better prognosis in patients who
had undergone elective ND or after SLN+ when compared
with the ones who had undergone therapeutic ND, independently from
pN status.
In conclusion, the surgical management of regional
metastatic neck disease in patients with oral and oropharyngeal
cancer remains a topic of debate and controversy. For several years
classical comprehensive ND has been the mainstay of treatment.
Selective and super-selective neck treatments were recently
introduced and widely applied in order to reduce morbidity and
mortality related to extensive ND, thus improving the postoperative
quality of life after preservation of level V and the surrounding
anatomical structures.
With regard to atypical metastases according to
Woolgar’s definitions, no statistically significant differences
were found related to the overall survival of this group vs.
typical metastatic pattern. However, we noted different atypical
features related to different primary tumour sites. Our data
suggest that enhancing our knowledge of these types of atypical
patterns site-related, and further evaluations such as novel
imaging techniques (29) and
molecular analyses (30) may help
us to understand if any behaviour and biomolecular differences
exist among OSCCs according to site.
References
|
1
|
Saman DM: A review of the epidemiology of
oral and pharyngeal carcinoma: update. Head Neck Oncol. 4:1–7.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Greene FL, Page DL, Fleming ID, Fritz AG,
Balch CM, Haller DG and Morrow M: AJCC Cancer Staging Handbook.
From the AJCC Cancer Staging Manual. 6th edition. Springer-Verlag;
2002, View Article : Google Scholar
|
|
3
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: the 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wenzel S, Sagowski C, Kehrl W and
Metternich FU: The prognostic impact of metastatic pattern of lymph
nodes in patients with oral and oropharyngeal squamous cell
carcinomas. Eur Arch Otorhinolaryngol. 261:270–275. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kowalski LP and Medina JE: Nodal
metastases: predictive factors. Otolaryngol Clin North Am.
31:621–637. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amar A, Chedid HM, Rapoport A, Cernea CR,
Dedivitis RA, Curioni OA and Brandão LG: Prognostic significance of
the number of lymph nodes in elective neck dissection for tongue
and mouth floor cancers. Braz J Otorhinolaryngol. 78:22–26.
2012.PubMed/NCBI
|
|
7
|
Compton CC and Byrd DR: Lip and oral
cavity. 2nd AJCC Cancer Staging Atlas. Compton CC, Byrd DR,
Garcia-Aguilar J, Kurtzman SH, Olawaiye A and Washington MK:
Springer; New York: pp. 41–53. 2012, View Article : Google Scholar
|
|
8
|
Ferlito A, Robbins KT, Shah JP, Medina JE,
Silver CE, Al-Tamimi S, Fagan JJ, Paleri V, Takes RP, Bradford CR,
Devaney KO, Stoeckli SJ, Weber RS, Bradley PJ, Suárez C, Leemans
CR, Coskun HH, Pitman KT, Shaha AR, de Bree R, Hartl DM, Haigentz M
Jr, Rodrigo JP, Hamoir M, Khafif A, Langendijk JA, Owen RP,
Sanabria A, Strojan P, Vander Poorten V, Werner JA, Bień S, Woolgar
JA, Zbären P, Betka J, Folz BJ, Genden EM, Talmi YP, Strome M,
González Botas JH, Olofsson J, Kowalski LP, Holmes JD, Hisa Y and
Rinaldo A: Proposal for a rational classification of neck
dissections. Head Neck. 33:445–450. 2011.PubMed/NCBI
|
|
9
|
Byers RM, Wolf PF and Ballantyne AJ:
Rationale for elective modified neck dissection. Head Neck Surg.
10:160–167. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pagedar NA and Gilbert RW: Selective neck
dissection: a review of the evidence. Oral Oncol. 45:416–420. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferlito A, Robbins KT, Silver CE, Hasegawa
Y and Rinaldo A: Classification of neck dissections: an evolving
system. Auris Nasus Larynx. 36:127–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bernier J, Domenge C, Ozsahin M,
Matuszewska K, Lefèbvre JL, Greiner RH, Giralt J, Maingon P,
Rolland F, Bolla M, Cognetti F, Bourhis J, Kirkpatrick A and van
Glabbeke M: Postoperative irradiation with or without concomitant
chemotherapy for locally advanced head and neck cancer. N Engl J
Med. 350:1945–1952. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cooper JS, Pajak TF, Forastiere AA, Jacobs
J, Campbell BH, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M,
Machtay M, Ensley JF, Chao KS, Schultz CJ, Lee N and Fu KK:
Postoperative concurrent radiotherapy and chemotherapy for
high-risk squamous-cell carcinoma of the head and neck. N Engl J
Med. 350:1937–1944. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van den Brekel MW, van der Waal I, Meijer
CJ, Freeman JL, Castelijns JA and Snow GB: The incidence of
micrometastases in neck dissection specimens obtained from elective
neck dissections. Laryngoscope. 106:987–991. 1996.PubMed/NCBI
|
|
15
|
Murer K, Huber GF, Haile SR and Stoeckli
SJ: Comparison of morbidity between sentinel node biopsy and
elective neck dissection for treatment of the n0 neck in patients
with oral squamous cell carcinoma. Head Neck. 33:1260–1264. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schiefke F, Akdemir M, Weber A, Akdemir D,
Singer S and Frerich B: Function, postoperative morbidity, and
quality of life after cervical sentinel node biopsy and after
selective neck dissection. Head Neck. 31:503–512. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Broglie MA, Haile SR and Stoeckli SJ:
Long-term experience in sentinel node biopsy for early oral and
oropharyngeal squamous cell carcinoma. Ann Surg Oncol.
18:2732–2738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teymoortash A, Hoch S, Eivazi B and Werner
JA: Postoperative morbidity after different types of selective neck
dissection. Laryngoscope. 120:924–929. 2010.PubMed/NCBI
|
|
19
|
Robbins KT, Shaha AR, Medina JE, Califano
JA, Wolf GT, Ferlito A, Som PM and Day TA; Committee for Neck
Dissection Classification, American Head and Neck Society.
Consensus statement on the classification and terminology of neck
dissection. Arch Otolaryngol Head Neck Surg. 134:536–538. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Broglie MA, Haerle SK, Huber GF, Haile SR
and Stoeckli SJ: Occult metastases detected by sentinel node biopsy
in patients with early oral and oropharyngeal squamous cell
carcinomas: impact on survival. Head Neck. 35:660–666. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Woolgar JA: The topography of cervical
lymph node metastases revisited: the histological findings in 526
sides of neck dissection from 439 previously untreated patients.
Int J Oral Maxillofac Surg. 36:219–225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Woolgar JA, Scott J, Vaughan ED, Brown JS,
West CR and Rogers S: Survival, metastasis and recurrence of oral
cancer in relation to pathological features. Ann R Coll Surg Engl.
77:325–331. 1995.PubMed/NCBI
|
|
23
|
Shah JP: Surgical approaches to the oral
cavity primary and neck. Int J Radiat Oncol Biol Phys. 69:S15–S18.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stoeckli SJ, Alkureishi LW and Ross GL:
Sentinel node biopsy for early oral and oropharyngeal squamous cell
carcinoma. Eur Arch Otorhinolaryngol. 266:787–793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ross GL, Soutar DS, MacDonald DG, Shoaib
T, Camilleri IG and Robertson AG: Improved staging of cervical
metastases in clinically node-negative patients with head and neck
squamous cell carcinoma. Ann Surg Oncol. 11:213–218. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huber GF, Züllig L, Soltermann A, Roessle
M, Graf N, Haerle SK, Studer G, Jochum W, Moch H and Stoeckli SJ:
Down regulation of E-Cadherin (ECAD) - a predictor for occult
metastatic disease in sentinel node biopsy of early squamous cell
carcinomas of the oral cavity and oropharynx. BMC Cancer.
11:2172011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shah JP: Pattern of cervical node
metastasis from squamous carcinomas of the upper aerodigestive
tract. Am J Surg. 160:405–409. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han MW, Cho KJ, Roh JL, Choi SH, Nam SY
and Kim SY: Patterns of lymph node metastasis and their influence
on outcomes in patients with submandibular gland carcinoma. J Surg
Oncol. 106:475–480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Contaldo M, Agozzino M, Moscarella E,
Esposito S, Serpico R and Ardigò M: In vivo characterization of
healthy oral mucosa by reflectance confocal microscopy: a
translational research for optical biopsy. Ultrastruct Pathol.
37:151–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Di Domenico M, Pierantoni GM, Feola A,
Esposito F, Laino L, DE Rosa A, Rullo R, Mazzotta M, Martano M,
Sanguedolce F, Perillo L, D’Angelo L, Papagerakis S, Tortorella S,
Bufo P, Lo Muzio L, Pannone G and Santoro A: Prognostic
significance of N-Cadherin expression in oral squamous cell
carcinoma. Anticancer Res. 31:4211–4218. 2011.PubMed/NCBI
|