Introduction
Colorectal cancer (CRC) is the third most prevalent
cancer in the world with more than 940,000 cases and nearly 500,000
deaths occurring annually worldwide (1). Almost 20–25% of colorectal cancer
patients present with distant metastases at the time of diagnosis
and the median overall survival (OS) does not exceed 2 years
(2). The liver is involved in
80–90% of the cases and, in almost 50%, it is the only site of
metastasis. Integration of chemotherapy and surgery in the
treatment of hepatic metastases represents the best choice for
improving survival in this subset of patients (3). Notably, 10–30% of newly diagnosed
colorectal cancer patients with liver-only deposits have their
metastases resectable at presentation. In such instances, the
European Colorectal Metastases Treatment Group strongly recommends
pre-operative chemotherapy and liver resection (4). However, an estimated 70–90% of these
patients have unresectable hepatic metastases at presentation
(5). Until a few years ago, the
limited clinical efficacy of available anticancer drugs coupled
with no substantial improvements in liver surgery, which
constituted a critical procedure with high postoperative mortality
and morbidity rates, did not allow for a consistent chance of cure
to these patients. In the last decade, there has been a shift in
this paradigm mainly due to a more anatomic and surgical approach
since the successful introduction of liver resection, as well as
the clinical development of more effective drugs, such as
oxaliplatin and irinotecan, and more recently, of targeted
monoclonal antibodies. There is increasing evidence that the best
management choice in colorectal patients with unresectable
liver-only metastases should be represented by integrated
treatments in which the critical aim of chemotherapy is to reduce
hepatic cancer deposits, thus permitting potentially curative liver
surgery (known as conversion chemotherapy), rather than to
represent a palliative support program (6). In particular, standard 5-fluorouracil
plus irinotecan or oxaliplatin-based chemotherapy offers conversion
rates ranging from 9 to 22% according to the GERCOR trial with a
potentially curative liver resection (known as R0 resection) rate
ranging from 7 to 13% (7). However,
although there is a strong correlation between response rate and
resection rate both in patients selected for liver metastases only
and in non-selected patients, data on conversion rates with
biological regimens are scarce and conflicting, and no randomized
trials focusing on a liver-limited disease population are available
(8). In addition, duration of
treatment, drug toxicity, particularly related to liver functions,
and optimal timing for liver surgery remain unclear (6).
In the present study, we report on treatment of 48
colorectal cancer patients with unresectable metastases confined to
the liver, with conversion chemotherapy with or without
anti-epidermal growth factor receptor (EGFR-cetuximab) or
anti-vascular endothelial growth factor (VEGF-bevacizumab)
monoclonal antibody. The study end-points included assessment of:
(i) conversion and liver R0 resection rates; (ii) relationship
between conversion rate and other prognostic factors including
different chemotherapeutic regimens and (iii) OS rates in converted
and non-converted patients.
Patients and methods
Clinical records from 48 consecutive patients with
stage IV colorectal cancer and unresectable liver-only metastases
were retrospectively selected from the database of our Department
in the period from December, 2006 to February, 2011. All tumors
were staged upon evaluation of findings of physical examination,
routine laboratory tests and diagnostic imaging [complete
colonoscopy with biopsy, abdominal ultrasound, chest and abdomen
CT-scan, scintigraphic bone scan, whole body magnetic resonance
imaging and F-18 fluorodeoxyglucose positron emission tomography
(FDG-PET) scan, and contrast enhanced ultrasonography of the liver
when appropriate].
All patients had hepatic metastases judged
unresectable due to invasion of major liver pedicles (metastatic
disease adjacent or involving all 3 hepatic veins, and/or the
portal vein bifurcation, and/or the retrohepatic vena cava, and/or
the vascular structures of the opposite lobe) or intrahepatic
dissemination (bilobar disease) requiring a liver resection
potentially capable of jeopardizing postoperative liver function.
The inoperability was defined by a multidisciplinary team including
two independent skillful liver surgeons blinded each other. All
cases were independently reviewed by each surgeon; discrepancies
between investigators (<10% of the cases) required a third joint
observation with conclusive agreement.
Thirteen patients (27.1%) showed metachronous
liver-limited metastases following a potentially curative
colorectal cancer resection (means 20±8, range 13–42, median 17
months from colorectal surgery). The remaining 35 patients
presented synchronous liver metastases; all but 2 underwent
immediate surgery of the primary colorectal cancer due to
significant tumor symptoms (20 patients), or according to our own
policy in asymptomatic patients (9). Overall, 46/48 patients (96%) underwent
resection of the primary colorectal cancer.
Before the start of conversion chemotherapy,
patients were screened for K-Ras status; 24 (50%) harbored a
wild-type and 21 (43.7%) a mutated protein. In 3 cases (6.3%),
K-Ras status was not evaluated. All 24 wild-type K-Ras cancer
patients were treated with irinotecan or oxaliplatin-based
chemotherapy and anti-EGFR antibody cetuximab. Sixteen patients
received as first-line treatment irinotecan or oxaliplatin and
5-fluorouracil or capecitabine-based chemotherapy and anti-VEGF
antibody bevacizumab. Finally, 8 patients received chemotherapy
without biological drugs. Response to systemic therapy was
evaluated every two months according to RECIST guidelines;
metastases were restaged with regard to their resectability and,
when appropriate, patients were referred to liver surgery as soon
as possible, albeit not sooner than 4 weeks after the last cycle of
chemotherapy. A switch to second-line chemotherapy was decided in
cases of disease progression (PD). No patient was lost to follow-up
and it was completed by May 31, 2011. All patients gave their
informed consent and the study was approved by the Department of
Clinical and Experimental Medicine and Surgery of the Second
University of Naples.
Statistical analysis
Statistical analysis was carried out using the SPSS
statistical package (SPSS Inc., Chicago, IL, USA) integrated by the
MedCalc® software version 9.4.2.0 (Mariakerke, Belgium).
In all analyses, the significance level was specified as p<0.05.
Logistic regression analysis was performed to determine the
relationship between conversion rate and other potentially
influential covariables. The following prognostic factors were
considered: age, gender, site and tumor (T) and nodes (N) of the
colorectal tumor, time of liver metastasis appearance (synchronous
or metachronous), K-Ras status, type of conversion chemotherapy and
best response. The OS was calculated from the date of initiation of
the conversion chemotherapy to the date of mortality or the end of
follow-up, marking alive patients as censored. Univariate
statistical analysis was determined by long-rank test (Mantel-Cox).
Curves were plotted using the product-limit method (Kaplan-Meier);
p-values and hazard ratios (HR) with 95% confidence interval (CI)
were provided. The independent significance of prognostic
variables, showing a p-value <0.1 on univariate analysis, was
determined by multivariate analysis using the Cox’s proportional
hazards model. The model was also tested for possible interaction
effects between covariates correlated with each other, to determine
if these variables could independently influence survival. Finally,
a stepwise multivariate analysis was performed in order to generate
a model of the best linear combination of variables able to predict
OS rate.
Results
Objective responses to conversion chemotherapy were
seen in 27 patients (56.2%; 95% CI 42.1–70.2%). Four patients
(8.3%) achieved radiological complete response (CR) of liver
metastases. Twenty-three patients (47.9%) showed a radiological
partial response (PR). On the contrary, 13/48 patients (27.1%) were
considered stable (SD) and continued chemotherapy until progression
or unacceptable toxicity, and 8 patients (16.7%) progressed during
first-line treatment. Fig. 1 shows
the flow-chart of all eligible patients. Overall, 17 patients
showed a significant reduction in liver metastases so as to become
amenable to resection (conversion rate 35.4%; 95% CI 21.8–48.9%).
However, the 4 complete response patients refused liver surgery (2
are alive without cancer at 8 and 21 months from the end of
chemotherapy, and the remaining 2 patients relapsed in the liver at
12 and 13 months after chemotherapy, and are currently awaiting
liver resection). The remaining 13 patients (all partial response
patients) underwent hepatic resection after shrinkage of initially
unresectable liver metastases (liver resection rate 27.1%; 95% CI
14.5–39.6%). Liver operations, including 9 major hepatectomies (≥3
liver segments) and 4 bilateral and multiple metastasectomies, were
performed through an exclusive abdominal incision, with
lymphadenectomy of the hepatic pedicle followed by liver
transection performed by Kelly clamp crushing method or, more
recently, using a new fully automated radiofrequency generator
supplying a comb-shaped bipolar multi-electrode device
[SURTRON® SB, LED SpA, Aprilia (LT, Italy)] (10). Inflow vascular occlusion was never
necessary. All procedures were judged potentially curative (R0
resection), with removal of all macroscopic tumoral mass(es),
absence of microscopic residual tumor, and histology-negative
resection margins. No postoperative hemorrhage, biliary leakage or
abscess formation were observed. One patient suffered from slight
liver impairment function successfully treated with medical
therapy. All resected patients underwent postoperative chemotherapy
according to the previous regimen permitting liver surgery.
 | Figure 1Flow-chart of 48 eligible patients.
CR, complete response; PR, partial response; SD, stable disease;
PD, progressive disease FOLFIRI, (day 1, irinotecan 180
mg/m2, folinic acid 400 mg/m2, fluorouracil
400 mg/m2 i.v. bolus, then 2,400 mg/m2 over
46 h continuous infusion, every 2 weeks); XELIRI, (day 1,
irinotecan 250 mg/m2, capecitabine 1,700
mg/m2/day bid os for 14 days followed by 7 days rest,
every 3 weeks); FOLFOX, day 1, oxaliplatin 85 mg/m2,
folinic acid 400 mg/m2, fluorouracil 400
mg/m2 i.v. bolus, then 2,400 mg/m2 over 46 h
continuous infusion, every 2 weeks); XELOX, (day 1, oxaliplatin 130
mg/m2, capecitabine 1,700 mg/m2/day bid os
for 14 days followed by 7 days rest, every 3 weeks); CETUXIMAB,
initial dose of 400 mg/m2 in 2 h i.v. infusion, and then
250 mg/m2 in 1 h i.v. infusion weekly; BEVACIZUMAB, 5
mg/kg i.v. infusion on day 1 every 2 weeks and stopped 4 weeks
before surgery. |
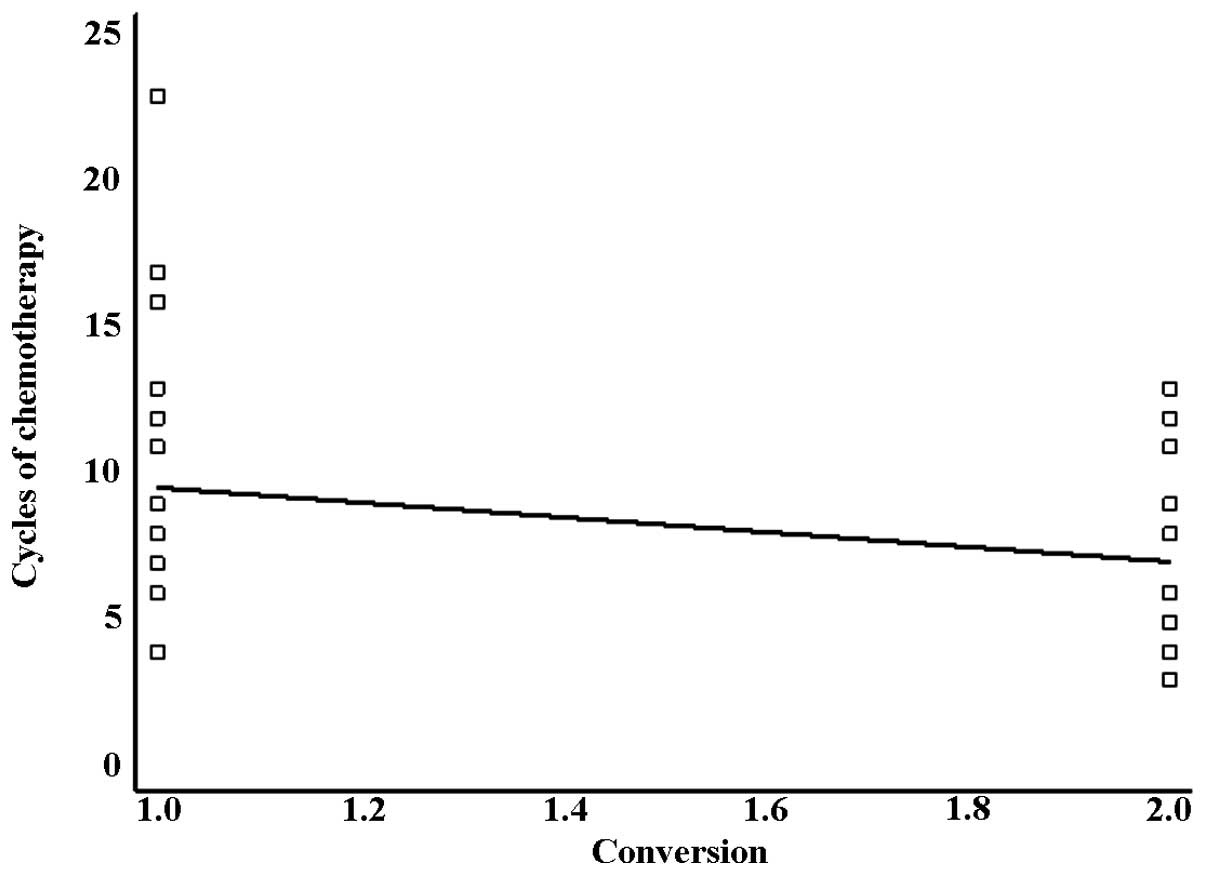
The median number of chemotherapy cycles was 8
(means 8.7±4.1; range 3–23). In converted patients, the number of
cycles of chemotherapy (means 7.0±3.2; range 3–13, median 6) was
lower than in non-converted patients (means 9.6±4.2; range 4–23,
median 8). The difference was statistically significant in logistic
regression analysis, as shown in Fig.
2 (r=0.3022, slope=−2.5541, p=0.0370).
The likelihood of a successful conversion
chemotherapy was not related to age, gender, site of the primary
colorectal tumor, T and N parameters, time of liver metastasis
appearance and type of chemotherapy. On the contrary, it appeared
significantly related to the best response [all complete and 13/23
partial response patients showed conversion (r=0.6467,
slope=−1.1708, p<0.0001)], and to the K-Ras status. Indeed,
12/24 wild-type K-Ras patients (50%) were suitable for liver
resection at the end of conversion chemotherapy, while only 5
(20.8%) of the remaining 24 patients (21 mutated and 3 not
evaluable K-Ras patients) had a successful response (r=0.3047,
slope=−0.3188, p=0.0350).
Analysis related to overall survival
The mean follow-up time was 17±10 months (range
3–54, median 13 months). During this time period, 21 patients
(43.7%) succumbed to the disease. One to four-year OS rates were
78.8, 50.1, 29.2 and 19.5%, respectively (means 27±3, median 24
months). In univariate analysis, age, gender, stage of the primary
colorctal cancer, and type of chemotherapy were not significantly
associated with OS. On the contrary, the right site of the primary
colorectal tumor, metachronous metastases, wild-type K-Ras, and
objective response to chemotherapy were demonstrated to be
significantly associated with an improved survival rate (Table I). Liver metastasis shrinkage and a
further chance of potentially curative liver surgery as well as
complete response were significantly correlated with better
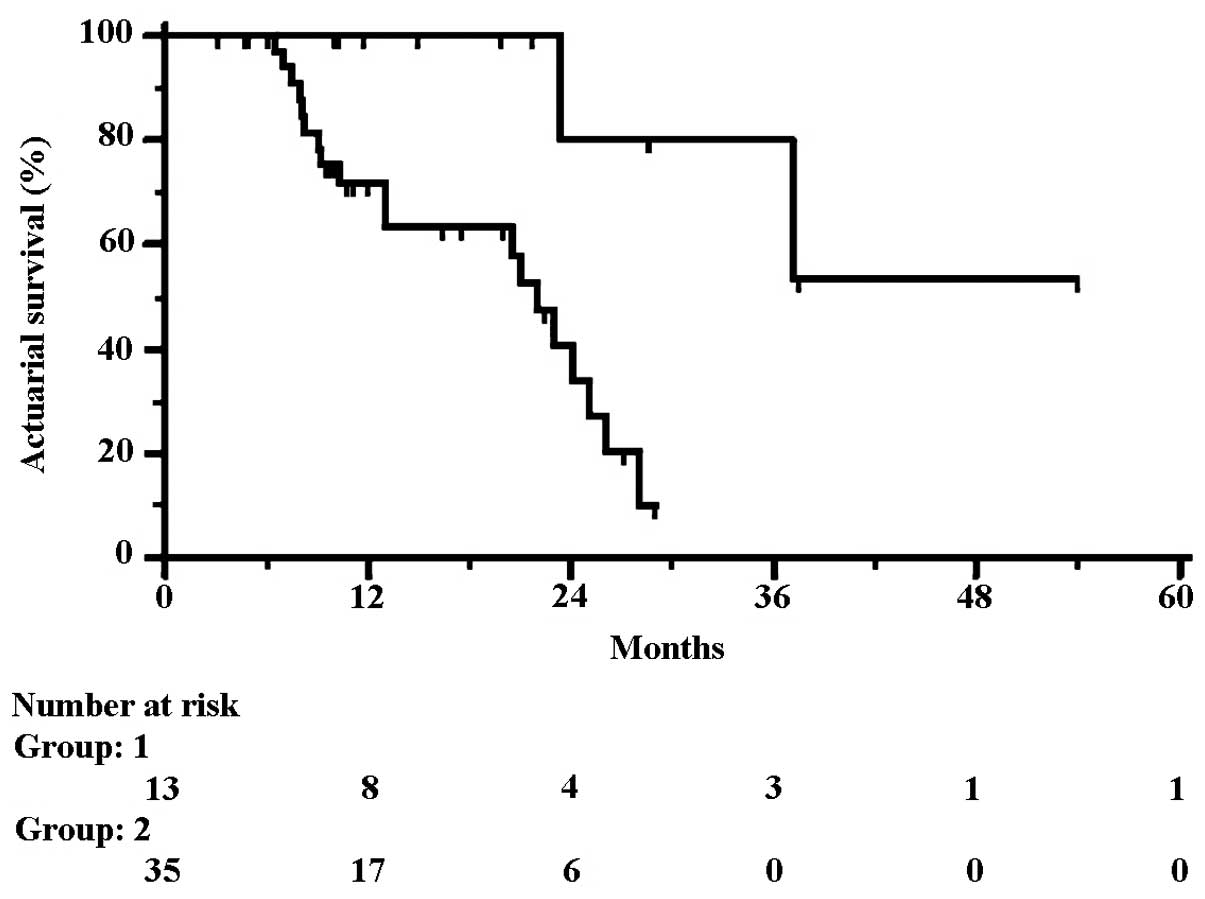
survival rates. One to four-year OS rates in 17 converted patients
were 100, 85.7, 85.7 and 57.1%, which were significantly different
from the rates recorded in non-converted patients (67.4, 32.4 and
0% at 3 years, respectively). Although 4 converted patients did not
undergo liver resection and are living, the curves of 13 resected
patients plotted against non-resected patients still showed a
significant difference. One to four-year OS rates were 71.6, 40.6,
10.1 and 10.1% in non-resected and 100, 80, 80 and 53.3% in
resected patients, respectively. The HR for OS was 0.16 (95% CI
0.09–0.63; p=0.0037), corresponding to a 70% increase in the rate
of survival at 28 months from 10.1% (1.4–18.8%) to 80% (62.1–97.9%)
with liver resection, and to an increase in the median survival
from 22 to 54 months (Fig. 3). Of
note, resected patients had an estimated 16% relative risk of
cancer mortality compared to non-resected patients.
 | Table ICharacteristics of the series and
univariate analysis related to survival rate. |
Table I
Characteristics of the series and
univariate analysis related to survival rate.
| Characteristics | No. of pts | Deceased | 48-month survival
(%) | Hazard ratio | 95% CIa Hazard ratio | P-value |
|---|
| Age, (<69/>69
years)b (range, 37–82 years) | 24/24 | 12/9 | 19/0 | 1.40 | 0.59–3.33 | 0.4346 |
| Gender
(male/female) | 27/21 | 14/7 | 13/34 | 0.85 | 0.32–2.19 | 0.7342 |
| Site
(right/left/rectum)c | 18/23/7 | 3/14/4 | 63/12/0 | - | - | 0.1667 |
| Right vs. no
right | 18/30 | 3/18 | 63/8 | 0.32 | 0.16–1.02 | 0.0567 |
|
Synchronous/metachronous | 35/13 | 16/5 | 19/51 | 0.43 | 0.08–1.20 | 0.0917 |
| Tumord |
| 2 | 4 | 0 | 100 | - | - | 0.2856 |
| 3 | 28 | 12 | 0 | | | |
| 4 | 14 | 8 | 19 | | | |
| Nodesd |
| 0 | 14 | 5 | 0 | - | - | 0.1021 |
| 1 | 19 | 7 | 23 | | | |
| 2 | 13 | 8 | 19 | | | |
| K-Ras status |
| Wild-type | 24 | 6 | 48 | - | - |
<0.0001 |
| Mutated | 21 | 13 | 9 | | | |
| Not
determined | 3 | 2 | 0 | | | |
| WT vs. no WT | 24/24 | 6/15 | 48/8 | 0.41 | 0.18–1.01 | 0.0554 |
|
Chemotherapye | 8 | 5 | 0 | - | - | 0.1388 |
| Chemo |
| Chemo +
cetuximab | 24 | 6 | 48 | | | |
| Chemo +
bevacizumab | 16 | 10 | 12 | | | |
| Best response to
chemo |
| Complete | 4 | 0 | 100 | - | - | 0.0033 |
| Partial | 23 | 7 | 35 | | | |
| Stable
disease | 13 | 8 | 0 | | | |
| Progression
disease | 8 | 6 | 0 | | | |
| Conversion |
| Yes | 17 | 2 | 57 | 0.09 | 0.06–0.37 |
<0.0001 |
| No | 31 | 19 | 0 | | | |
| Liver
resection |
| Yes | 13 | 2 | 53 | 0.16 | 0.09–0.63 | 0.0037 |
| No | 35 | 19 | 10 | | | |
In multivariate analysis, metachronous metastases
and conversion were shown to be the only covariates independently
associated with a longer survival rate (Table II). Since conversion rate
correlated with K-Ras status and response to chemotherapy (see
above), thus questioning the true significance of this factor in
statistical analyses, Cox’s test was performed with activation of
an interaction term. Independently of K-Ras status and response,
possibility of conversion came out as an independent variable for
long-term outcome. After backward elimination, stepwise regression
selected again synchronous metastases (HR=5.67, 95% CI 1.59–20.19,
p=0.0077) and no conversion (HR=35.29, 95% CI 4.22–295.08,
p=0.0010) as the best combination of variables capable of
predicting poor long-term OS.
 | Table IIMultivariate analysis related to
overall survival in 48 colon cancer patients treated with
conversion chemotherapy for liver metastases. |
Table II
Multivariate analysis related to
overall survival in 48 colon cancer patients treated with
conversion chemotherapy for liver metastases.
| Variables | Coefficient | Standard error | Hazard ratio | 95% CIa Hazard ratio | P-value |
|---|
| Left colon and
rectum | 0.9163 | 0.7514 | 2.49 | 0.57–10.82 | 0.2227 |
| Synchronous
metastases | 1.9543 | 0.7649 | 7.05 | 1.58–31.37 | 0.0106 |
| K-Ras mutated | 0.6741 | 0.4931 | 1.96 | 0.75–5.13 | 0.1716 |
| No response to
chemo | 0.0534 | 0.4102 | 1.05 | 0.47–2.34 | 0.8963 |
| No conversion | 2.7426 | 1.2724 | 15.5 | 1.29–185.6 | 0.0311 |
| Conversion/K-Ras
statusb | 0.4252 | 0.5088 | 2.31 | 0.54–9.95 | 0.4034 |
|
Conversion/responseb | 0.0106 | 0.4073 | 7.11 | 1.60–31.51 | 0.9792 |
Discussion
The standard of care for metastatic colorectal
cancer consists of a combination of 5-fluorouracil, leucovorin and
oxaliplatin or irinotecan-based chemotherapy; the different
sequences of drugs were equivalent in terms of efficacy in a phase
III randomized trial (7).
Furthermore, exposure to all cytotoxic agents was shown to
correlate with increase in survival (11). Recently, combination of conventional
chemotherapy with agents targeting the vascular endothelial growth
factor and epidermal growth factor receptor in the wild-type K-Ras
population was also shown to add improvement in the
chemotherapeutic efficacy (12–15).
However, hepatic resection remains the only procedure to cure stage
IV colorectal cancer patients with liver-limited metastases and,
for immediately resectable disease, preoperative and perioperative
chemotherapy has been shown to offer a longer progression-free and
overall survival (4,16,17).
Management of unresectable metastases of colorectal
cancer is a significant challenge. Tumor response to chemotherapy
has been clearly demonstrated to correlate with resectability rate.
The efficacy of new regimens in this context may therefore be
primarily evaluated with regard to their ability to induce tumor
shrinkage, thus allowing surgery, rather than to long-term control
of disease (18). Standard
chemotherapy regimens offer conversion rates of between 9 and 22%
with R0 resection rates of 7–13%, and intensified chemotherapy with
triplet combination, known as FOLFOXIRI, has been associated with
overall response rates of 70% and 19% of R0 hepatic surgery in
phase III randomized trials (19).
Despite these relevant results, this regimen has not entered
clinical practice due to substantial toxicity. Furthermore, several
trials including an anti-EGFR antibody resulted in an increased
response rate and a higher R0 resection rate. In particular, in the
phase II randomized CELIM study, which combined cetuximab with
FOLFIRI or FOLFOX, complete or partial responses in 62% of patients
with a 34% of R0 resection were recorded (20). Furthermore, recently published phase
II trials reported notable data on the ability of chemotherapy plus
anti-VEGF antibody bevacizumab to shrink inoperable liver
metastases (21–24).
In our series, we registered a rate of major
responses equal to 56.2%, which was not very distant from response
rates from other trials in which chemotherapy and biologic drugs
were delivered in selected populations of colorectal cancer with
liver limited disease. However, best response was not an
independent prognostic variable correlated with overall survival.
This was not unexpected, since the main factor influencing
long-term outcome was not related to tumor response, but to the
likelihood to resect hepatic deposits. Among 27 responder patients,
substantial shrinkage of liver metastases was obtained in 17
patients (35.4%), and 13 of them (27.1%) who underwent potentially
curative surgery showed the best long-term outcome. Of note, 2 of 4
complete responder patients refusing surgery had liver recurrence,
suggesting that complete radiological response was not able to
predict complete tumoral necrosis, thus confirming the need for an
aggressive surgical approach (25,26).
As per the policy of our treating institution, all
patients with unresectable metastases from colorectal cancer are
treated with chemotherapy and anti-EGFR antibody if harboring
wild-type K-Ras. Of note, although no different activity in terms
of radiological response was seen between the addition of cetuximab
(in wild-type K-Ras population) or bevacizumab (in mutated
patients), 9 (37.5%) of 24 patients treated with chemotherapy plus
cetuximab could be subjected to resection, as opposed to only 12%
in the chemotherapy plus bevacizumab population.
For patients with liver metastases that are
resectable at presentation, perioperative chemotherapy has become
the standard treatment in several institutions, with the
recommendation that surgery be performed after a maximum of 6
cycles of systemic therapy (17).
On the contrary, in the case of patients with initially
unresectable liver metastases, there is no general agreement
regarding the number of cycles of chemotherapy to be delivered.
Therefore, it is recommended that patients be carefully monitored
and surgery performed as soon as the metastases become resectable
(18). However, excessive duration
of chemotherapy aimed at shrinking hepatic deposits may produce
substantial liver damage, as shown by several series reporting on
associations between irinotecan and steatohepatitis and between
oxaliplatin and sinusoidal dilatation (27), with significantly increased
post-operative morbidity (28).
However, it was recently shown that liver surgery can be safely
performed in patients undergoing preoperative chemotherapy, and
that addition of biologic drugs does not appear to increase the
morbidity rates of hepatic resection for colorectal liver
metastases (29). In our series, no
significant increase in postoperative complications was observed,
with only 1 patient suffering from transient liver function
impairment and no postoperative deaths. However, it has to be
emphasized that, in this study, converted patients received a
significantly lower number of cycles of chemotherapy than
non-converted patients. Thus, conversion to surgery may be achieved
after relatively few cycles of chemotherapy, thus preventing liver
injury and severe post-operative complications.
The prognostic significance of synchronous versus
metachronous disease remains controversial. Synchronous
presentation has been reported to negatively affect long-term
survival in some studies, whereas no differences in survival were
found between colorectal cancer patients with early or delayed
unresectable liver-only metastases in other investigations
(30,31). In our series, the relative risk of
cancer mortality in metachronous metastases was 43% with respect to
synchronous metastases. In addition, along with no chance of
conversion, synchronous metastases were shown to be an independent
prognostic variable of poor long-term outcome, as also confirmed by
stepwise analysis.
In conclusion, our data support the role of
anticancer treatments to shrink unresectable liver metastases from
colorectal cancer. Surgery of liver metastases treated with
conversion chemotherapy is the only way to significantly prolong
outcome for this subset of patients. Prospective randomized trials
are required to define the precise contribution of new therapeutic
agents in these settings.
References
|
1
|
Poston GJ, Adam R, Alberts S, et al:
OncoSurge: a strategy for improving resectability with curative
intent in metastatic colorectal cancer. J Clin Oncol. 23:7125–7134.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Manfredi S, Lepage C, Hatem C, Coatmeur O,
Faivre J and Bouvier AM: Epidemiology and management of liver
metastases from colorectal cancer. Ann Surg. 244:254–259. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sundermeyer ML, Meropol NJ, Rogatko A,
Wang H and Cohen SJ: Changing patterns of bone and brain metastases
in patients with colorectal cancer. Clin Colorectal Cancer.
5:108–113. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nordlinger B, Van Cutsem E, Rougier P, et
al: Does chemotherapy prior to liver resection increase the
potential for cure in patients with metastatic colorectal cancer? A
report from the European Colorectal Metastases Treatment Group. Eur
J Cancer. 43:2037–2045. 2007. View Article : Google Scholar
|
|
5
|
Cook AD, Single R and McCahill LE:
Surgical resection of primary tumors in patients who present with
stage IV colorectal cancer: an analysis of surveillance,
epidemiology, and end results data, 1988 to 2000. Ann Surg Oncol.
12:637–645. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nordlinger B, Vauthey JN, Poston G,
Benoist S, Rougier P and Van Cutsem E: The timing of chemotherapy
and surgery for the treatment of colorectal liver metastases. Clin
Colorectal Cancer. 9:212–218. 2010. View Article : Google Scholar
|
|
7
|
Tournigand C, André T, Achille E, et al:
FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: a randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Geva R, Prenen H, Topal B, Aerts R,
Vannoote J and Van Cutsem E: Biologic modulation of chemotherapy in
patients with hepatic colorectal metastases: the role of anti-VEGF
and anti-EGFR antibodies. J Surg Oncol. 102:937–945. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Galizia G, Lieto E, Orditura M, et al:
First line chemotherapy vs bowel tumor resection plus chemotherapy
for patients with unresectable synchronous colorectal hepatic
metastases. Arch Surg. 143:352–358. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Galizia G, Castellano P, Pinto M, et al:
Radiofrequency-assisted liver resection with a comb-shaped bipolar
device versus clamp crushing: a clinical study. Surg Innov.
19:407–414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grothey A, Sargent D, Goldberg RM and
Schmoll HJ: Survival of patients with advanced colorectal cancer
improves with the availability of fluorouracil-leucovorin,
irinotecan, and oxaliplatin in the course of treatment. J Clin
Oncol. 22:1209–1214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van Cutsem E, Rivera F, Berry S, et al:
Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX,
FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the
BEAT study. Ann Oncol. 20:1842–1847. 2009.PubMed/NCBI
|
|
13
|
Van Cutsem E, Köhne CH, Láng I, et al:
Cetuximab plus irinotecan, fluorouracil, and leucovorin as
first-line treatment for metastatic colorectal cancer: updated
analysis of overall survival according to tumor KRAS and
BRAF mutation status. J Clin Oncol. 29:2011–2019.
2011.PubMed/NCBI
|
|
14
|
Bokemeyer C, Bondarenko I, Hartmann JT, et
al: Efficacy according to biomarker status of cetuximab plus
FOLFOX-4 as first-line treatment for metastatic colorectal cancer:
the OPUS study. Ann Oncol. 22:1535–1546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Douillard JY, Siena S, Cassidy J, et al:
Randomized, phase III trial of panitumumab with infusional
fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4
alone as first-line treatment in patients with previously untreated
metastatic colorectal cancer: the PRIME study. J Clin Oncol.
28:4697–4705. 2010. View Article : Google Scholar
|
|
16
|
Mitry E, Fields AL, Bleiberg H, et al:
Adjuvant chemotherapy after potentially curative resection of
metastases from colorectal cancer: a pooled analysis of two
randomized trials. J Clin Oncol. 26:4906–4911. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nordlinger B, Sorbye H, Glimelius B, et
al: Perioperative chemotherapy with FOLFOX4 and surgery versus
surgery alone for resectable liver metastases from colorectal
cancer (EORTC Intergroup trial 40983): a randomised controlled
trial. Lancet. 371:1007–1016. 2008. View Article : Google Scholar
|
|
18
|
Folprecht G, Grothey A, Alberts S, Raab HR
and Köhne CH: Neoadjuvant treatment of unresectable colorectal
liver metastases: correlation between tumour response and resection
rates. Ann Oncol. 16:1311–1319. 2005. View Article : Google Scholar
|
|
19
|
Masi G, Vasile E, Loupakis F, et al:
Randomized trial of two induction chemotherapy regimens in
metastatic colorectal cancer: an updated analysis. J Natl Cancer
Inst. 103:21–30. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Folprecht G, Gruenberger T, Bechstein WO,
et al: Tumour response and secondary resectability of colorectal
liver metastases following neoadjuvant chemotherapy with cetuximab:
the CELIM randomised phase 2 trial. Lancet Oncol. 11:38–47. 2010.
View Article : Google Scholar
|
|
21
|
Klinger M, Tamandl D, Eipeldauer S, et al:
Bevacizumab improves pathological response of colorectal cancer
liver metastases treated with XELOX/FOLFOX. Ann Surg Oncol.
17:2059–2065. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Masi G, Loupakis F, Salvatore L, et al:
Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil,
and folinate) as first-line treatment for metastatic colorectal
cancer: a phase 2 trial. Lancet Oncol. 11:845–852. 2010. View Article : Google Scholar
|
|
23
|
Wong R, Cunningham D, Barbachano Y, et al:
A multicentre study of capecitabine, oxaliplatin plus bevacizumab
as perioperative treatment of patients with poor-risk colorectal
liver-only metastases not selected for upfront resection. Ann
Oncol. 22:2042–2048. 2011. View Article : Google Scholar
|
|
24
|
Bertolini F, Malavasi N, Scarabelli L, et
al: FOLFOX6 and bevacizumab in non-optimally resectable liver
metastases from colorectal cancer. Br J Cancer. 104:1079–1084.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adam R, Wicherts DA, de Haas RJ, et al:
Complete pathologic response after preoperative chemotherapy for
colorectal liver metastases: myth or reality? J Clin Oncol.
26:1635–1641. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goèré D, Gaujoux S, Deschamp F, et al:
Patients operated on for initially unresectable colorectal liver
metastases with missing metastases experience a favorable long-term
outcome. Ann Surg. 254:114–118. 2011.
|
|
27
|
Cleary JM, Tanabe KT, Lauwers GY and Zhu
AX: Hepatic toxicities associated with the use of preoperative
systemic therapy in patients with metastatic colorectal
adenocarcinoma to the liver. Oncologist. 14:1095–1105. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Welsh FK, Tilney HS, Tekkis PP, John TG
and Rees M: Safe liver resection following chemotherapy for
colorectal metastases is a matter of timing. Br J Cancer.
96:1037–1042. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pessaux P, Panaro F, Casnedi S, et al:
Targeted molecular therapies (cetuximab and bevacizumab) do not
induce additional hepatotoxicity: preliminary results of a
case-control study. Eur J Surg Oncol. 36:575–582. 2010. View Article : Google Scholar
|
|
30
|
Robertson DJ, Stukel TA, Gottlieb DJ,
Sutherland JM and Fisher ES: Survival after hepatic resection of
colorectal cancer metastases: a national experience. Cancer.
115:752–759. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rees M, Tekkis PP, Welsh FK, O’Rourke T
and John TG: Evaluation of long-term survival after hepatic
resection for metastatic colorectal cancer: a multifactorial model
of 929 patients. Ann Surg. 247:125–135. 2008. View Article : Google Scholar : PubMed/NCBI
|