Introduction
Malignant pleural mesothelioma (MPM) is an asbestos
related neoplasm, associated with poor prognosis. Its incidence is
projected to increase in Europe with a peak around 2020 and with
approximately 250,000 expected deaths in the next 40 years
(1). Although the MARS trial
(2), despite its questionable
methodology, recently showed disappointing results of surgery, some
multimodal treatment studies, evaluating surgery [extrapleural
pneumonectomy (EPP) or pleurectomy/decortication (P/D)] plus
chemoradiotherapy showed satisfactory results in terms of survival,
with a median ranging from 9.4 to 27.5 months (3).
Prognostic assessment is especially based on stages
of disease. Different staging systems have been used over the past
years for MPM (4), the most current
being the TNM staging system proposed in 1995 by the International
Mesothelioma Interest Group (IMIG) (5). In this classification the nodal
staging is the same as for lung cancer, despite the differences in
lymph spread pattern between MPM [which involves almost uniformly
the parietal pleura (6)] and lung
carcinoma (7). The prognostic value
of nodal involvement in MPM is debated. Many authors (8–11)
showed the N+ status to be associated with poor outcome, whereas
others (12,13) observed no significant difference
between N+ and N0 patients. Moreover, the N+ group is
heterogeneous, with respect to topography and extent. Adjustments
of pathological staging, taking differently into account the N
factor, have been recently proposed (14). In particular, the N1 status remains
unsettled as some authors suggest classifying it as lower stage
(8) and others suggest upgrading it
to higher stage (15). Multiple N2
disease and involvement of ≥4 nodes are also considered as negative
prognostic factors (8,9).
In several cancers, including lung and breast
carcinoma (16), the number of
involved lymph nodes has been shown to be associated with survival.
Given the known anatomic variation in lymph node numbers between
patients, according to BMI and preoperative treatments, the
metastatic lymph node ratio (LNR), which is more reliable as not
affected by these variations, has been proposed as a prognostic
factor in rectal, esophageal and lung cancer (16–18).
The aim of this study was to evaluate the nodal involvement impact
on survival and to assess the role of metastatic LNR as a
prognostic factor in MPM. This study was conducted studying
patients from four French centers with similar strategy in
management of patients with MPM.
Materials and methods
Patient population
This retrospective study was conducted by analyzing
data from patients treated between December, 1997 and September,
2010 in four French Thoracic Surgery Departments of University
Hospitals [Cochin-Hôtel-Dieu Hospital (Paris), Georges Pompidou
European Hospital (Paris), University Hospital of Nice (Nice) and
University Hospital of Lille (Lille)]. During this period, 99
patients underwent extrapleural pneumonectomy (EPP) in a curative
intent for MPM formally diagnosed by biopsy. Individual data were
assessed from clinical and pathological charts. Information
regarding the pattern of nodal involvement was analyzed from the
final histological findings. The pTNM IMIG stage classification
(5) was used. This study was
approved by the Institutional Review Board of the French Society of
Thoracic and Cardiovascular Surgery
(CERC-SFCTCV-2012-9-25-11-19-52-HyIl) and patients consent
requirement was waived.
Preoperative work-up
Patients were considered to be operable after a
thorough work-up including physical examination, physiological
evaluation and imaging. All of them underwent contrast-enhanced CT
scans of the thorax and upper abdomen. At the beginning of 2002 a
PET-CT scan was progressively performed in a standard manner. When
axial mediastinal nodes involvement was uncertain (enlarged or
hypermetabolic), a mediastinoscopy was carried out to ascertain
that these nodes were free of invasion. Cerebral CT scan was
performed according to the surgeons and preference of the centers
in a non-systematic manner. Neither laparoscopy nor contralateral
thoracoscopy was performed for staging purposes.
Surgical approach
EPP was performed through a posterolateral
thoracotomy as part of a multimodal treatment including
chemotherapy, radiotherapy and in one center hyperthermic
intrathoracic chemotherapy either as neoadjuvant or adjuvant
treatment. The lung, pleura, pericardium and diaphragm were excised
en bloc and reconstruction of the pericardium and diaphragm was
performed as described by Wolf et al(19). A systematic nodal dissection was
performed according to the technique described by Graham et
al(20), supplemented by a
systematic internal mammary and pericardial fat node dissection.
Detailed information regarding lymph nodes was collected from the
final pathological findings. The number, location and possible
capsular rupture of lymph nodes, as described by the pathologist of
each center, were carefully assessed. Lymph nodes were classified
in different stations and chains according to the Naruke map
(21) and Riquet definitions
(22), respectively. Hilar nodes
were classified as N1, internal mammary nodes N2 and
supraclavicular nodes N3. Because of a small number, N1–N3
node-positive patients were pooled as N+ patients in further
analyses. Metastatic LNR, expressed as a percentage, was calculated
as the ratio between total positive and removed nodes.
Hyperthermic intrathoracic
chemotherapy
This technique was used as part of a tetramodal
treatment in only one center (Nice). After a double regimen of
cisplatin/pemetrexed chemotherapy, standard surgery was performed.
Upon completion of EPP resection and diaphragmatic reconstruction,
before thorax closure, the patient was given HIT chemotherapy with
cisplatin 100 mg/m2 and gemcitabine 1,250
mg/m2 for 1 h at 42°C, which was associated to a renal
protective intravenous perfusion of amifostin. A peritoneal tube
was not associated to this procedure as described by Mujoomdar
et al(23). Radiotherapy up
to 54 Gy was administered as adjuvant therapy.
Follow-up
All patients surviving operation were followed-up.
Events were defined as death before 31 May, 2012. Diagnosis of
recurrence was generally based on histological evidence. Operative
mortality included all patients who succumbed to the disease within
90 days of surgery. Survival was calculated from the date of
surgery until the death or the end of the follow-up termination
time (right censoring) including postoperative mortality. Survival
data were available for all patients.
Statistical analysis
Data are shown as absolute number and percentage,
means or median (SD/range). Correlation was expressed by the
Spearman’s rank coefficient. Continuous data were compared by
Mann-Whitney U test and ordinal data by the χ2 test or
Fisher’s exact test as appropriate. The cut-off for the total
number of lymph nodes dissected, number of positive lymph nodes,
number of metastatic nodal stations and metastatic LNR was
calculated by a data-oriented method [25th, 50th (median), 75th
percentile]. Concerning the cut-off for metastatic LNR it was
defined at the 75th percentile (value ≤13%) as at the median the
LNR value was 0. Survival was calculated by the Kaplan-Meier method
including postoperative deaths and comparisons in univariate
analysis were carried out by log-rank test. Factors identified at
univariate analysis as significantly associated with survival were
entered in a multistep Cox multivariate model to assess independent
factors. Statistics Epidémiology Medecine (SEM) software (version
3.5; Centre Jean Perrin, Clermont-Ferrand, France) was used for
statistical analysis and GraphPad Prism 5 software (Graphpad
Software Inc., La Jolla, CA, USA) was used to generate graphic
presentations of the results.
Results
Patient characteristics
Main patient characteristics are shown in Table I. The mean age at presentation was
58.7 (±7.5) years. Smoking habits in the cohort showed a mean
tobacco consumption of 16.7 (±19.5) pack years. Most patients
(84.8%) had no relevant medical history. Detailed data on
preoperative work-up were available for 62 patients. PET-CT scans
were performed in 47 (75.8%) of them and brain CT scans in 27
(43.5%) patients. In addition, 15 (24.1%) patients had undergone
mediastinoscopy to rule out a preoperative N2 status. The
neoadjuvant treatment consisted mainly of 2 or 3 rounds of
cisplatin/pemetrexed. Pathologic stages were pT1 in 10 patients
(10.1%), pT2 in 34 (34.3%), pT3 in 51 (51.5%) and pT4 in 4 (4.1%)
of them.
 | Table IPatient clinical and treatment
characteristics. |
Table I
Patient clinical and treatment
characteristics.
| Characteristic | n (%) |
|---|
| Male | 83 (83.4) |
| Center |
| 1 | 30 (30.3) |
| 2 | 29 (29.3) |
| 4 | 28 (28.3) |
| 3 | 12 (12.1) |
| Occupation |
| NA data | 45 (45.4) |
| Building worker | 18 (33.3) |
| Steelworker | 13 (24.1) |
| Others | 23 (42.6) |
| Asbestos
exposure | 75 (81.5) |
| Smokers | 58 (58.6) |
| ASA |
| NA data | 41 (41.4) |
| 1 | 18 (31) |
| 2 | 32 (55.1) |
| 3 | 8 (13.9) |
| PS |
| 0 | 72 (72.7) |
| 1 | 27 (27.3) |
| Clinical symptoms at
diagnosis |
| NA data | 7 (7) |
| Pleural
effusion | 73 (79.3) |
| Parietal pain | 7 (7.6) |
| Chronic
bronchitis | 9 (9.8) |
| Spontaneous
pneumothorax | 2 (2.1) |
| Neoadjuvant
therapy |
| None | 20 (22.9) |
| Chemotherapy | 67 (77.1) |
| Operation |
| Right sided | 56 (56.5) |
| R0 surgery | 93 (94) |
| Adjuvant therapy |
| None | 20 (20.8) |
| HIT chemotherapy
(perioperative) | 12 (12.5) |
| Chemotherapy | 1 (1.1) |
| Radiotherapy | 63 (65.6) |
| Histology |
| Epitheloid | 86 (86.8) |
| Non-epitheloid | 13 (13.2) |
| Stage |
| I | 9 (9.1) |
| II | 24 (24.2) |
| III | 61 (61.6) |
| IV | 5 (5.1) |
Lymph node dissection and LNR
The mean number of total lymph nodes in the
mediastinal dissection was 11.9 (±6.4). There was no correlation
between the total number of dissected nodes and different
clinicopathological factors assessed (weight, neoadjuvant
treatment, surgery side), however, it tended to be higher in taller
patients (P=0.08). The nodal status of patients was as follows: 65
(65.6%) N0, 3 (3.1%) N1, 30 (30.3%) N2 and 1 (1%) N3. The N3
patient was a unique case with a solitary skip subclavicular
positive lymph node. The details of the invasion of lymph node
stations are presented in Table
II. Among the 15 patients who had mediastinoscopy with negative
findings, two had pN2 at the final result, but with positive nodes
in the extra axial mediastinum, internal mammary and parietal
nodes.
 | Table IIDetailed nodal information in N2/N3
patients. |
Table II
Detailed nodal information in N2/N3
patients.
| n (% of N2/N3
patients) | n (% of all the
patients)
Total |
|---|
|
|---|
| Right | Left |
|---|
| Nodal stations |
| 2 | 1 (3.2) | - | 1 (1) |
| 3 | 1 (3.2) | - | 1 (1) |
| 4 | 7 (22.5) | 3 (9.6) | 10 (10.1) |
| 5/6 | - | 5 (16.1) | 5 (5) |
| 7 | 9 (29) | 8 (25.8) | 17 (17.1) |
| 8 | 2 (6.4) | 2 (6.4) | 4 (4) |
| 9 | 1 (3.2) | 3 (9.6) | 4 (4) |
| Parietal
nodes | 3 (9.6) | 2 (6.4) | 5 (5) |
| Internal mammary
nodes | 6 (19.3) | 6 (19.3) | 12 (12.1) |
| One-level nodal
disease | 6 (19.3) | 4 (12.9) | 10 (10.1) |
| Two-level nodal
disease | 5 (16.1) | 4 (12.9) | 9 (9.1) |
| ≥Three-level nodal
disease | 8 (25.8) | 7 (22.5) | 15 (15.1) |
| Capsular
rupture | 4 (12.9) | 5 (16.1) | 9 (9.1) |
Metastatic LNR was ≤13% in 75 (75.7%) patients. In
the whole population (including both N0 and N+ patients) mean LNR
was 11.1% (±22.03%). Among patients with N+ disease, mean LNR was
32.3% (±26.9%).
Survival analysis
The median follow-up was 84.7 months (range, 21–176)
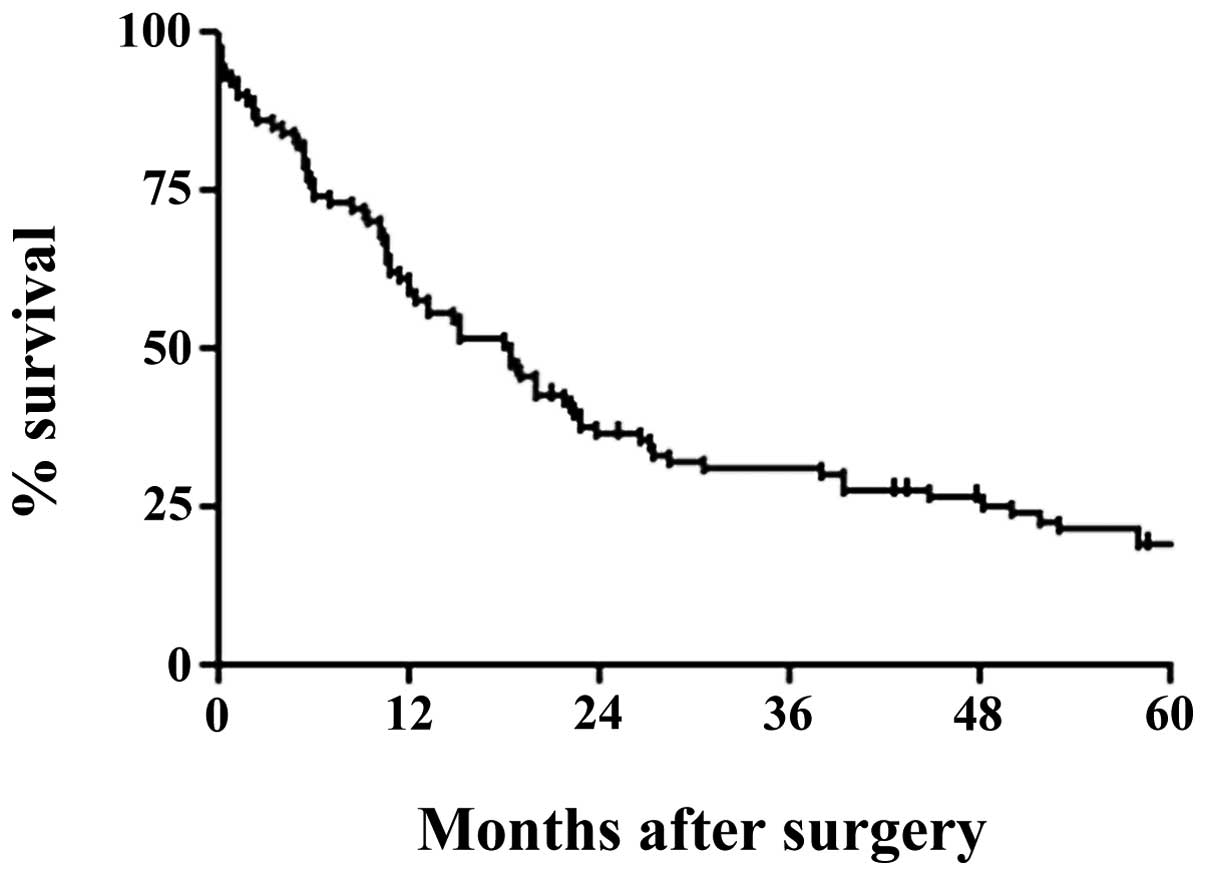
and it was complete for all patients. Median overall survival (OS)
was 18.3 months and the 5-year survival was 17.5% (Fig. 1). Table III resumes univariate and
multivariate analyses for OS. In the univariate analysis there was
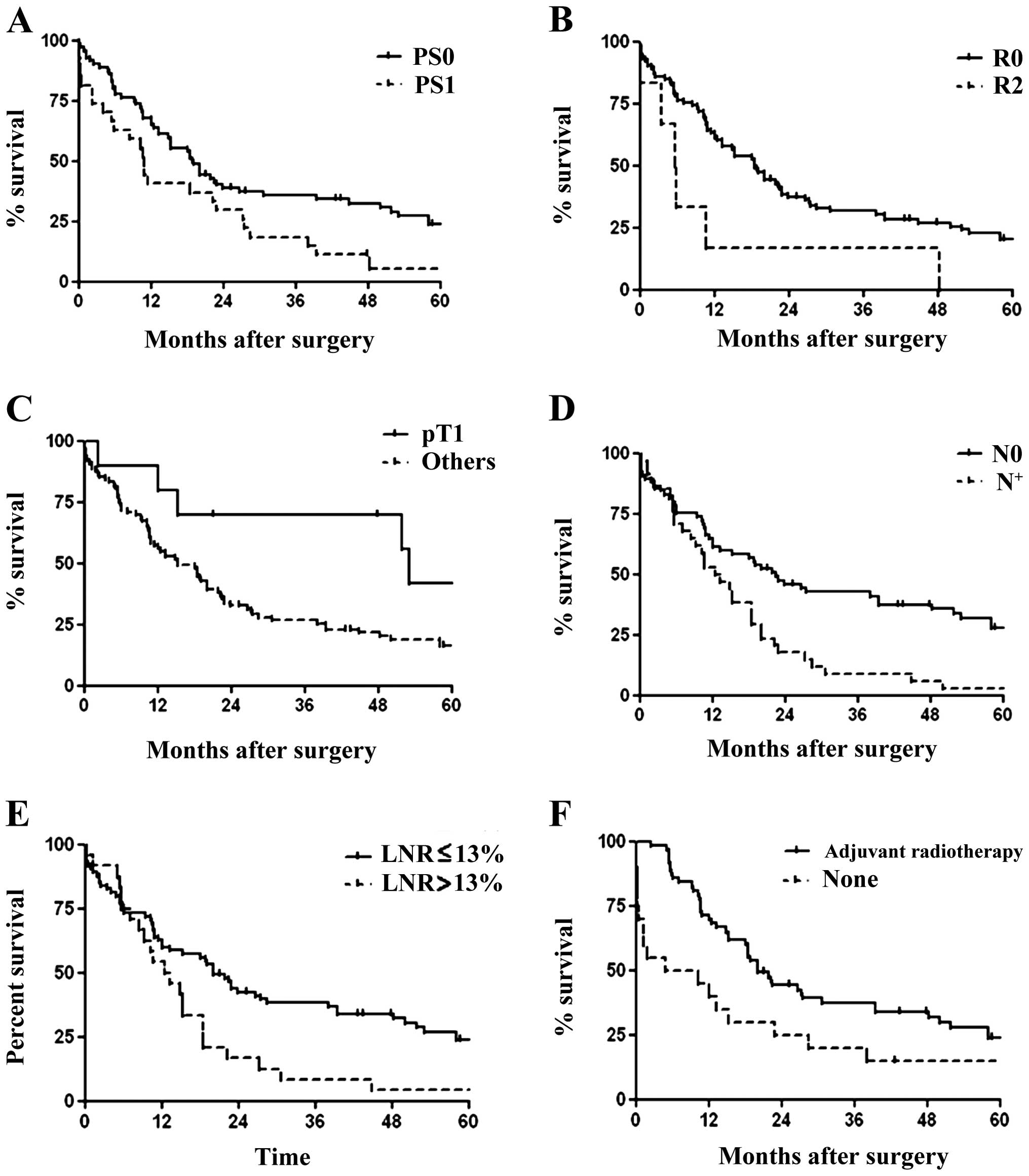
a significant difference in OS with respect to PS categories
(P=0.02; Fig. 2A). Although R0
resections were performed more frequently in patients who underwent
neoadjuvant treatment (P=0.009), neither neoadjuvant treatment
(P=0.09) nor HIT chemotherapy (P=0.38) significantly determined OS.
As for the surgical procedure, patients with R0 resection had a
better survival (P=0.02; Fig. 2B).
The pTNM staging was also an important prognostic factor. Survival
was higher in early disease (P=0.04; Fig. 2C), in N0 patients (P=0.002; Fig. 2D) and in patients with a metastatic
LNR ≤13% (P=0.01; Fig. 2E). OS was
significantly related to epitheloid histology (P=0.009) and
adjuvant radiotherapy (P=0.01; Fig.
2F). For the entire cohort, at the cut-off value of ≤13% there
was a significantly different survival with respect to LNR
(P=0.01). This survival difference disappeared in the subgroup of
N+ patients (P=0.88).
 | Table IIIUnivariate and multivariate analysis
of prognostic factors for overall survival. |
Table III
Univariate and multivariate analysis
of prognostic factors for overall survival.
| Univariate
analysis |
|---|
|
|
|---|
| Factors | Median
(months) | Log-rank
P-value |
|---|
| Gender |
| Male | 18.3 | 0.93 |
| Female | 16.9 | |
| PS |
| PS0 | 18.9 | 0.02 |
| PS1 | 10.7 | |
| Neoadjuvant
treatment |
| Administrated | 18.7 | 0.06 |
| Non
administrated | 11.9 | |
| Perioperative HIT
chemotherapy |
| Administrated | 17.9 | 0.34 |
| Non
administrated | 22 | |
| Surgical
resection |
| R0 | 18.4 | 0.02 |
| R2 | 5.6 | |
| pT |
| pT1 | 52.9 | 0.04 |
| Others | pT | 15.2 |
| pN |
| pN0 | 22.4 | 0.002 |
| pN+ | 12.7 | |
| Total no. of lymph
nodes dissected |
| ≤10 | 14.7 | 0.24 |
| >10 | 18 | |
| No. of positive
lymph nodes |
| ≤3 | 17.2 | 0.38 |
| >3 | 18.4 | |
| No. of metastatic
nodal stations |
| ≤1 | 18.7 | 0.06 |
| >1 | 12.1 | |
| LNR (%) |
| ≤13 | 19.9 | 0.01 |
| >13 | 11.7 | |
| Stage |
| I/II | 37.9 | 0.005 |
| III/IV | 12.7 | |
| Histology |
| Epitheloid | 19.4 | 0.009 |
| Non
epitheloid | 10.4 | |
| Adjuvant
radiotherapy |
| Administrated | 20 | 0.01 |
| Non
administrated | 7.4 | |
In the multivariate analysis only adjuvant
radiotherapy was a robust independent prognostic factor.
Exploratory analysis allowed the identification of a subset of
patients with better outcome: for patients with N0 status who
sustained adjuvant radiotherapy, median OS and 5-year survival were
38 months and 35.8%, respectively. By contrast, for patients with
N+ status without adjuvant radiotherapy negative status, the median
OS and 5-year survival were 8 months and 0%, respectively. The
metastatic LNR was not statistically significant in the
multivariate analysis.
Recurrence status information was available for 64
patients. Among them, 26 (40.6%) died disease-free, 17 (26.5%) had
ipsilateral recurrence, 12 (18.7%) contralateral recurrence, 7
(10.9%) peritoneal dissemination and 1 (1.5%) had pericardial
recurrence. Local recurrence was not influenced by the N+ status
(P=0.09).
Postoperative course
Thirty- and 90-day mortality were 8 and 14.1%,
respectively. The median hospitalization length of stay was 18.5
days (range, 1–391). This was longer for right side disease (38 vs.
16 days, P=0.006). Forty-one patients (41.4%) had 1 or more
postoperative morbidity (range, 1–4). Bronchopleural fistulas (BPF)
occurred in 10 patients (10.1%), all located in the right side
(P=0.005). There was no evidence of a relation between occurrence
of BPF and the total number of lymph nodes dissected in the
mediastinum (P=0.71). Eleven patients (11.1%) experienced
contralateral lung infection. Cardiac complications were observed
in 17 patients (17.1%) and included atrial arrhythmia (8), pulmonary embolism (4), acute coronary syndrome (4) and pericarditis (1). Other rarer morbidities included
hemorrhagic shock (4), recurrent
nerve paralysis (4), septic shock
(7) and ischemic colitis (1).
Discussion
Our study shows the typical demographics and
epidemiology of any MPM cohort: mostly males, history of asbestos
exposure, predominance of epithelial histology and advanced disease
at presentation. With respect to N status, almost 1/3 of our
patients had N+ disease. The latter was not related to the
neoadjuvant treatment, as shown in literature (8–10) and
despite the high proportion of this therapy in our study.
Lymphatic dissemination of pleural disease is less
known than lymphatic dissemination of lung cancer. Nevertheless,
the current staging system for MPM has the same nodal map as the
lung cancer classification. Many have questioned the validity of
this approach as the patterns of lymphatic pleural spread can
affect the prognostic value of internal mammary or parietal nodal
involvement, which could be different from the prognostic
significance of axial mediastinal nodal metastasis (14). Focusing on the involvement of
internal mammary nodes, our findings are similar to literature data
with 6.5% (9) to 11.2% (8) of the patients presenting metastatic
nodes at this level. On the other hand, only 5% had parietal nodes
involved, in our study. Edwards et al(9) observed the same rate of involvement,
but there is a lack of information on this issue in literature. One
may expect a higher level of parietal node involvement in this
primitive pleural disease but this may be underestimated by the
absence of systematic nodal research in this large anatomic region.
In addition, as the whole parietal pleura is closer to initial
tumor site and probably invaded early, parietal nodes could be the
first nodal relay and therefore be an N1 station and not an N3 one.
Similarly N1 involvement in MPM may occur only after lung invasion,
being a sign of a more aggressive disease than a ‘simple’ isolated
N2 tumor (15).
Mediastinal nodal status was explored in the
majority of our patients by the PET-CT scan couple. To better
assess preoperative nodal involvement in 15 doubtful cases we used
mediastinoscopy, which proved to be a reliable examination. All the
15 mediastinoscopy-negative patients were free of axial mediastinal
lymph node involvement in the final histological findings. Only two
of them had positive nodes in the parietal and internal mammary
stations, which were not investigated by mediastinoscopy. Given the
high specificity and the possible impact of N2 status on survival,
mediastinoscopy should be proposed every time that result from
thoracic CT and PET-CT scans are doubtful.
In this study, we aimed to clarify the potential
mismatch between different N+ status and to study the role of the
metastatic LNR in MPM survival. This simple histological tool has
shown great potential in other cancers (16,18)
but, to the best of our knowledge, LNR has not yet been evaluated
on MPM. As others before (8) we
confirm, in this study, that N+ status was an important prognostic
factor. Metastatic LNR showed a suggestive relation with OS at 13%
cut-off level. The fact that this was related to survival while
including the whole cohort (N0 and N+ patients) and not observed in
the subgroup of N+ patients, suggests that the survival of patients
with few nodes involved, tends to be similar to N0 patient
survival. This information seems more important than the location
of the involved nodes. Although it failed to reach full
conventional statistical significance in the multivariate analysis,
we believe this ratio is more reliable than the number of involved
lymph nodes >3 (9), involvement
of superior mediastinal nodes (14)
or the total number of dissected nodes, which display a wide range
of variation determined by factors not necessarily related to
disease or its prognosis, like BMI or neoadjuvant therapy.
Although neoadjuvant chemotherapy was more common in
R0 surgical resection and that R0 resection influenced survival,
neoadjuvant chemotherapy was not related to OS. By contrast,
adjuvant radiotherapy was a very important prognostic factor. This
relates to the necessity of reducing treatment morbidity thus
permitting to deliver radiotherapy as quickly as possible.
Well known predictors of poor prognosis in MPM,
described by Steele et al(24) such as poor PS and non-epitheloid
histology were confirmed by our results, while the male gender was
not significantly correlated to survival. Median OS and
perioperative mortality were in the range of previously published
systematic reviews of EPP for MPM (3).
Limitations of this study include its retrospective
nature thus, introducing some degree of heterogeneity like the
patients treated by tetramodal therapy, including HIT chemotherapy,
instead of the standard trimodal therapy. Despite the fact that
this cohort included 99 patients, the statistical power may be
insufficient. This could be explained by the relatively small
number of N+ patients (34.3%). Follow-up information was complete,
but relevant information such as recurrence status was incomplete,
not allowing a comprehensive analysis of the impact of nodal
involvement. Plus, we studied patients from a 13-year period a
factor possibly participating in the heterogeneity of this cohort.
Similarly, there is a possible bias in the uniformity of
pathological analysis which was done in four different centers.
In conclusion, except for N+ status and metastatic
LNR >13%, we did not observe any impact on OS on the number of
involved nodes >3 nor on the involvement of superior mediastinal
nodes. This follows the difficulty to unambiguously and
reproducibly identify single prognostic factors also shown by
previous studies where results were often inconsistent. We suggest
that the metastatic LNR is likely to play an important role in the
future assessment of the MPM patients, which should receive more
attention and be further confirmed by larger prospective
studies.
Acknowledgements
The authors are grateful to Claire Pinçon for her
help with statistical analysis and to Dr Fabrice Kwiatkowski who
kindly provided SEM software.
References
|
1
|
Robinson BW and Lake RA: Advances in
malignant mesothelioma. N Engl J Med. 353:1591–1603. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Treasure T, Lang-Lazdunski L, Waller D,
Bliss JM, Tan C, Entwisle J, et al: Extra-pleural pneumonectomy
versus no extra-pleural pneumonectomy for patients with malignant
pleural mesothelioma: clinical outcomes of the Mesothelioma and
Radical Surgery (MARS) randomised feasibility study. Lancet Oncol.
12:763–772. 2011. View Article : Google Scholar
|
|
3
|
Cao CQ, Yan TD, Bannon PG and McCaughan
BC: A systematic review of extrapleural pneumonectomy for malignant
pleural mesothelioma. J Thorac Oncol. 5:1692–1703. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Schil P: Malignant pleural
mesothelioma: staging systems. Lung Cancer. 49:S45–S48.
2005.PubMed/NCBI
|
|
5
|
Rusch VW: A proposed new international TNM
staging system for malignant pleural mesothelioma. From the
International Mesothelioma Interest Group. Chest. 108:1122–1128.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boutin C, Dumortier P, Rey F, Viallat JR
and De Vuyst P: Black spots concentrate oncogenic asbestos fibers
in the parietal pleura. Thoracoscopic and mineralogic study. Am J
Respir Crit Care Med. 153:444–449. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okiemy G, Foucault C, Avisse C, Hidden G
and Riquet M: Lymphatic drainage of the diaphragmatic pleura to the
peritracheobronchial lymph nodes. Surg Radiol Anat. 25:32–35. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Flores RM, Routledge T, Seshan VE, Dycoco
J, Zakowski M, Hirth Y and Rusch VW: The impact of lymph node
station on survival in 348 patients with surgically resected
malignant pleural mesothelioma: implications for revision of the
American Joint Committee on Cancer staging system. J Thorac
Cardiovasc Surg. 136:605–610. 2008. View Article : Google Scholar
|
|
9
|
Edwards JG, Stewart DJ, Martin-Ucar A,
Muller S, Richards C and Waller DA: The pattern of lymph node
involvement influences outcome after extrapleural pneumonectomy for
malignant mesothelioma. J Thorac Cardiovasc Surg. 131:981–987.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Perrot M, Uy K, Anraku M, Tsao MS,
Darling G, Waddell TK, et al: Impact of lymph node metastasis on
outcome after extrapleural pneumonectomy for malignant pleural
mesothelioma. J Thorac Cardiovasc Surg. 133:111–116.
2007.PubMed/NCBI
|
|
11
|
Sugarbaker DJ, Flores RM, Jaklitsch MT,
Richards WG, Strauss GM, Corson JM, et al: Resection margins,
extrapleural nodal status, and cell type determine postoperative
long-term survival in trimodality therapy of malignant pleural
mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg.
117:54–65. 1999. View Article : Google Scholar
|
|
12
|
Chailleux E, Dabouis G, Pioche D, de
Lajartre M, de Lajartre AY and Rembeaux Aand Germaud P: Prognostic
factors in diffuse malignant pleural mesothelioma. A study of 167
patients. Chest. 93:159–162. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aziz T, Jilaihawi A and Prakash D: The
management of malignant pleural mesothelioma; single centre
experience in 10 years. Eur J Cardiothorac Surg. 22:298–305.
2002.PubMed/NCBI
|
|
14
|
Richards WG, Godleski JJ, Yeap BY, Corson
JM, Chirieac LR, Zellos L, et al: Proposed adjustments to
pathologic staging of epithelial malignant pleural mesothelioma
based on analysis of 354 cases. Cancer. 116:1510–1517. 2010.
View Article : Google Scholar
|
|
15
|
Abdel Rahman AR, Gaafar RM, Baki HA, El
Hosieny HM, Aboulkasem F, Farahat EG, et al: Prevalence and pattern
of lymph node metastasis in malignant pleural mesothelioma. Ann
Thorac Surg. 86:391–395. 2008.PubMed/NCBI
|
|
16
|
Nwogu CE, Groman A, Fahey D, Yendamuri S,
Dexter E, Demmy TL, et al: Number of lymph nodes and metastatic
lymph node ratio are associated with survival in lung cancer. Ann
Thorac Surg. 93:1614–1620. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang CL, Li Y, Yue DS, Zhang LM, Zhang ZF
and Sun BS: Value of the metastatic lymph node ratio for predicting
the prognosis of non-small-cell lung cancer patients. World J Surg.
36:455–462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mariette C, Piessen G, Briez N and
Triboulet JP: The number of metastatic lymph nodes and the ratio
between metastatic and examined lymph nodes are independent
prognostic factors in esophageal cancer regardless of neoadjuvant
chemoradiation or lymphadenectomy extent. Ann Surg. 247:365–371.
2008. View Article : Google Scholar
|
|
19
|
Wolf AS, Daniel J and Sugarbaker DJ:
Surgical techniques for multimodality treatment of malignant
pleural mesothelioma: extrapleural pneumonectomy and
pleurectomy/decortication. Semin Thorac Cardiovasc Surg.
21:132–148. 2009. View Article : Google Scholar
|
|
20
|
Graham AN, Chan KJ, Pastorino U and
Goldstraw P: Systematic nodal dissection in the intrathoracic
staging of patients with non-small cell lung cancer. J Thorac
Cardiovasc Surg. 117:246–251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Naruke T, Suemasu K and Ishikawa S: Lymph
node mapping and curability at various levels of metastasis in
resected lung cancer. J Thorac Cardiovasc Surg. 76:832–839.
1978.PubMed/NCBI
|
|
22
|
Riquet M, Manac’h D, Dupont P, Dujon A,
Hidden G and Debesse B: Anatomic basis of lymphatic spread of lung
carcinoma to the mediastinum: anatomo-clinical correlations. Surg
Radiol Anat. 16:229–238. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mujoomdar AA and Sugarbaker DJ:
Hyperthermic chemoperfusion for the treatment of malignant pleural
mesothelioma. Semin Thorac Cardiovasc Surg. 20:298–304. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Steele JP, Klabatsa A, Fennell DA,
Palläska A, Sheaff MT, Evans MT, et al: Prognostic factors in
mesothelioma. Lung Cancer. 49:S49–S52. 2005. View Article : Google Scholar
|