Introduction
Vault particles are composed of multiple copies of
three proteins, a major vault protein (MVP), also known as lung
resistance-related protein (LRP), a telomerase-associated protein,
and a vault-poly(ADP-ribose) polymerase, as well as untranslated
RNA molecules (1–3). The distribution of MVP in normal human
tissues is similar to other drug resistance-related proteins. MVP
is expressed in tissues chronically exposed to xenobiotics. MVP
expression is high in certain multidrug-resistant tumors (4) and increased MVP expression represents
an early event in colorectal carcinogenesis (5). We previously reported that the
expression of the MVP protein, MVP mRNA and the promoter
activity of MVP were increased by osmostress (7). The downregulation of MVP
expression by MVP interfering RNA (RNAi) in SW620 cells increased
the sensitivity of cells to hyperosmotic stress. These results
suggest that the exposure of cells to hyperosmotic stress may
induce MVP, which may play an important role in protecting cells
from the adverse effects of osmotic stress. Although the expression
of MVP was also associated with an adverse clinical outcome and
shorter overall survival (6),
little is known about the regulation of MVP expression. The
aim of the present study was to investigate the molecular basis for
MVP gene expression in human SW620 colon cancer cells.
Materials and methods
Materials
SW620, a human colon carcinoma cell line, was
provided by Dr A.T. Fojo (National Cancer Institute, Bethesda, MD,
USA). RPMI-1640 medium was purchased from Nissui Seiyaku Co.
(Tokyo, Japan), and fetal calf serum (FCS) was from JRH Biosciences
(Lenexa, KS, USA). A rabbit anti-ERK polyclonal antibody was
obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). A
rabbit anti-MVP polyclonal antibody was prepared using a
glutathione S-transferase (GST)-MVP (aa 694–794) fusion protein as
an antigen (4). Multiple tissue
cDNA panels were from Clontech Laboratories Inc. (Mountain View,
CA, USA). Anti-α-tubulin antibody was from Calbiochem (Darmstadt,
Germany).
Cell culture
SW620 and ACHN cells, a human renal adenocarcinoma
cell line, were grown in RPMI-1640 containing 10% FCS, 2 mM
glutamine and 100 U/ml of penicillin at 37ºC in a 5% CO2
humidified atmosphere. Cell cultures were maintained in exponential
growth by media replacement every 2–3 days. Cells were provided
with fresh media the day before use for experimentation.
Fluorescence and laser scanning confocal
microscopy (LSCM)
Imaging was conducted using a confocal laser
scanning microscope (FV500; Olympus Corp., Tokyo, Japan). SW620
cells (1×106) were incubated for 24 h at 37ºC. After
being carefully washed twice with PBS, SW620 cells were fixed with
3% formaldehyde for 30 min at room temperature. They were then
washed twice with PBS and permeabilized with 100% methanol for 20
min at room temperature. After blocking for 30 min with 3% skimmed
milk in PBS, cells were incubated for 1 h with a polyclonal
antibody against MVP diluted in PBS containing 3% skimmed milk.
After washing three times in PBS, cells were incubated for 20 min
with FITC-conjugated goat anti-rabbit IgG diluted 1:100 in PBS
containing 3% skimmed milk. Cells were then washed three times in
PBS and samples were examined by confocal microscopy. Fluorescence
was imaged at an excitation wavelength (λex) = 488 and
with an emission bandpass filter set for FITC.
Immunoblot analysis
Samples were subjected to 6 or 12.5% sodium dodecyl
sulfate polyacrylamide gel electrophoresis (SDS-PAGE) according to
the method of Laemmli. Gel proteins were electrophoretically
transferred onto polyvinylidene difluoride membranes (Immobilon-P
transfer membrane; Millipore, Bedford, MA, USA) using the Bio-Rad
Trans-Blot SD apparatus. The membrane was treated with a buffer
[350 mM NaCl, 10 mM Tris-HCl (pH 8.0), 0.05% Tween-20] containing
3% skimmed milk for 1 h and incubated with the indicated antibody
(1:1,000) in the buffer containing 3% skimmed milk for 1 h.
Following four washes with the buffer (10 min each), the membrane
was incubated with a peroxidase-conjugated horse anti-rabbit IgG
diluted 1:1,000 in the buffer containing 3% skimmed milk for 1 h.
The membrane was washed with buffer, and developed using the
enhanced chemiluminescence western blotting detection system
(Amersham Pharmacia, Buckinghamshire, UK).
Dual-luciferase reporter assay
The human MVP promoter ranging from −407 and
−78 to +66 was amplified from the genomic DNA of SW620 cells as
previously described (22). The PCR
product was cloned into the pT7 Blue-2 vector (Merck Biosciences,
San Diego, CA, USA) and sequenced. Following digestion with
KpnI and SacI, the MVP promoter fragment was
gel-purified and ligated into the KpnI and
SacI-linearized pGL3-Basic vector (Promega, Madison, WI,
USA). The resulting constructs were designated pMVP407 and pMVP78
(22). SW620 cells were grown in
RPMI-1640 medium containing 10% FCS, 2 mM glutamine, and 100 U/ml
of penicillin at 37ºC in a 5% CO2 humidified atmosphere.
Cells (3.0×105/well in 6-well plates) were transfected
with 1 μg of luciferase reporter plasmid (pGL3, Promega) or 100 ng
of control plasmid (pRL-TK; Promega). Following incubation for 24 h
with the transfected cells, the luciferase assay was performed
using the Dual-Luciferase Reporter Assay System following the
manufacturer’s protocol (Promega). Luminescence assays were
performed using a luminometer (TD-20/20 Luminometer; Turner
Designs, Sunnyvale, CA, USA). All experiments were performed in
triplicate and the results were normalized to pRL-TK activity.
RNA interference
Small interfering RNA (siRNA) duplexes were
synthesized using the Silencer® siRNA construction kit
(Ambion Inc.). A target site within the gene was chosen from the
mRNA sequence of USF1. Following selection, the target site
was searched with the National Center for Biotechnology Information
Blast to confirm its specificity. The siRNA used in the present
study consisted of a 21-nucleotide sense strand and a 21-nucleotide
antisense strand with a two-nucleotide overhang at the end.
Sequences were as follows: USF1-siRNA sense,
5′-GGAAGGUGCAGUGGCUACUGG-3′, starting at nucleotide 54 from the AUG
start codon of the human USF1 coding sequence;
USF1-siRNA antisense, 5′-GGCCAGUAG CCACTGCACCUU-3′; siRNA
was transfected to cells using Lipofectamine 2000™ according to the
manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA).
RT-PCR method
Total cellular RNA was extracted using TRIzol
reagent according to the manufacturer’s instructions (Invitrogen).
RT-PCR was performed with the SuperScript One-Step RT-PCR system
and gene-specific primers according to the manufacturer’s
instructions (Invitrogen). Reaction mixtures containing total RNA
(500 ng of each), 0.2 μM of each primer, and an enzyme mixture
composed of SuperScript II RT, Platinum Taq DNA polymerase, were
maintained at 50ºC for 20 min, then at 94ºC for 2 min, and PCR was
performed as follows: 30 cycles at 94ºC for 15 sec, 55ºC for 30
sec, and 70ºC for 30 sec. Primers for RT-PCRs were designed based
on human sequences in GenBank. These sequences used the following
primers: MVP (GenBank accession no. NM_017458),
5′-CAGGATGTGTATGTGCTGT CGG-3′ and 5′-GCTGGAGGCTCTTAGCTGTGTC-3′;
USF1 (GenBank accession no. NM_ 007122), 5′-ATGAAGGGG
CAGCAGAAAACA-3′ and 5′-TTAGTTGCTGTCATTCT TGA-3′ and GAPDH
(GenBank accession no. NM_002046), 5′-AGAACATCATCCCTGCCTCTACTGG-3′
and 5′-AAAG GTGGAGGAGTGGGTGTCGCTG-3′.
Real-time reverse-transcription PCR
quantification
Total cellular RNA was extracted using TRIzol
reagent according to the manufacturer’s instructions (Invitrogen).
One microgram of RNA was reverse transcribed using a first-strand
cDNA synthesis kit (ReverTra Ace®; Toyobo, Osaka,
Japan). Human MVP and GAPDH gene expression levels
were assayed by real-time reverse PCR (PRISM 7900HT; Applied
Biosystems, Foster City, CA, USA) according to the technical
specifications. Human GAPDH was used for normalization. The
quantification of target gene expression was obtained with the
comparative cycle threshold method according to the instructions of
the manufacturer.
Chromatin immunoprecipitation assay
Cells were fixed with 1% formaldehyde for 10 min at
37ºC to cross-link protein to DNA. A chromatin immunoprecipitation
(ChIP) assay was carried out using a ChIP assay kit (Upstate
Biotechnology) according to the manufacturer’s instructions. The
soluble DNA fraction was mixed with an anti-USF-1 antibody (Santa
Cruz Biotechnology) or non-immunized mouse IgG (Santa Cruz
Biotechnology) and the precipitated DNA was amplified with primers
for the MVP promoter, 5′-GCCAGCTGGCTCCAAG GTAG-3′ (sense)
and 5′-GGCAGGGCAAGGCAGGCCAA-3′ (antisense).
Results
Tissue expression profiles of MVP
mRNA
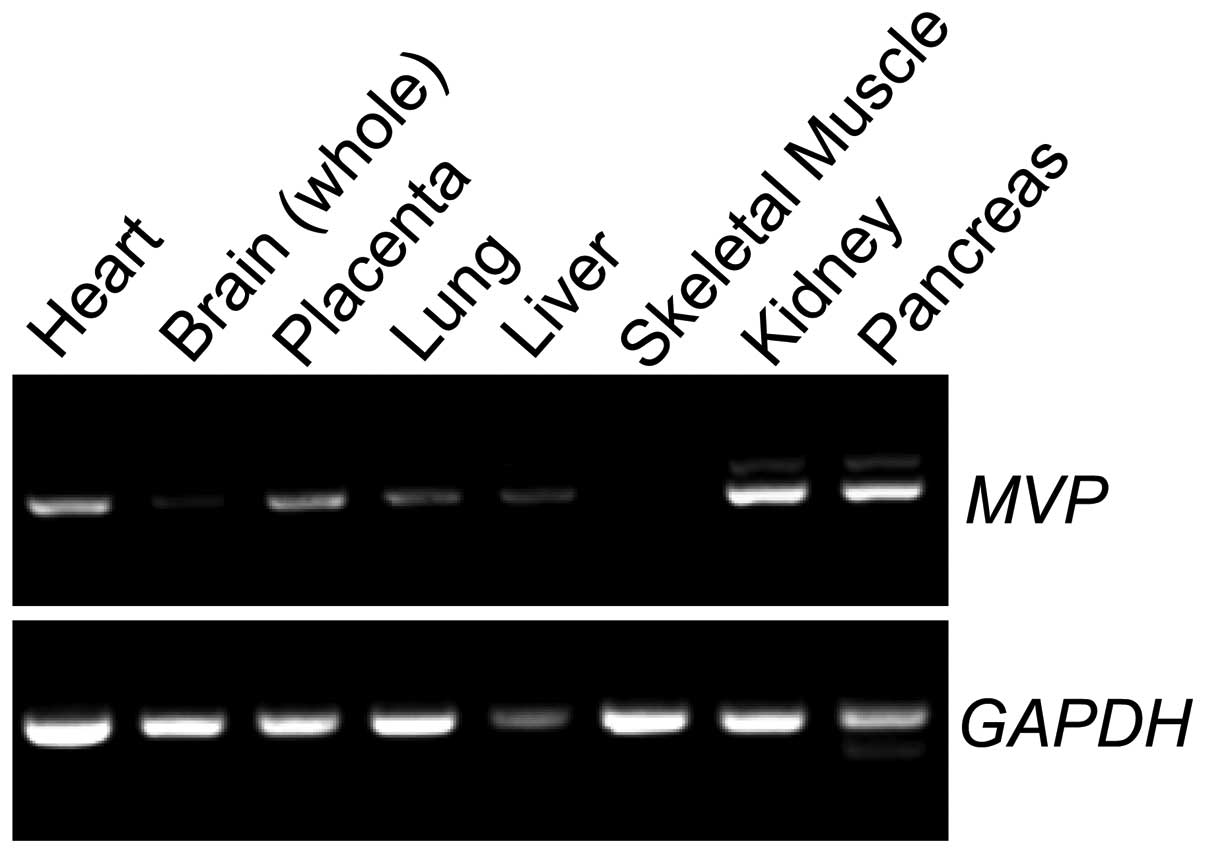
At the start of the present study, we examined the
tissue expression profile of MVP mRNA by performing PCR. MVP
was found to be expressed in the heart, placenta, lung, liver,
kidney and pancreas in normal tissues (Fig. 1).
Expression of MVP in cancer cell lines
and intracellular localization of MVP
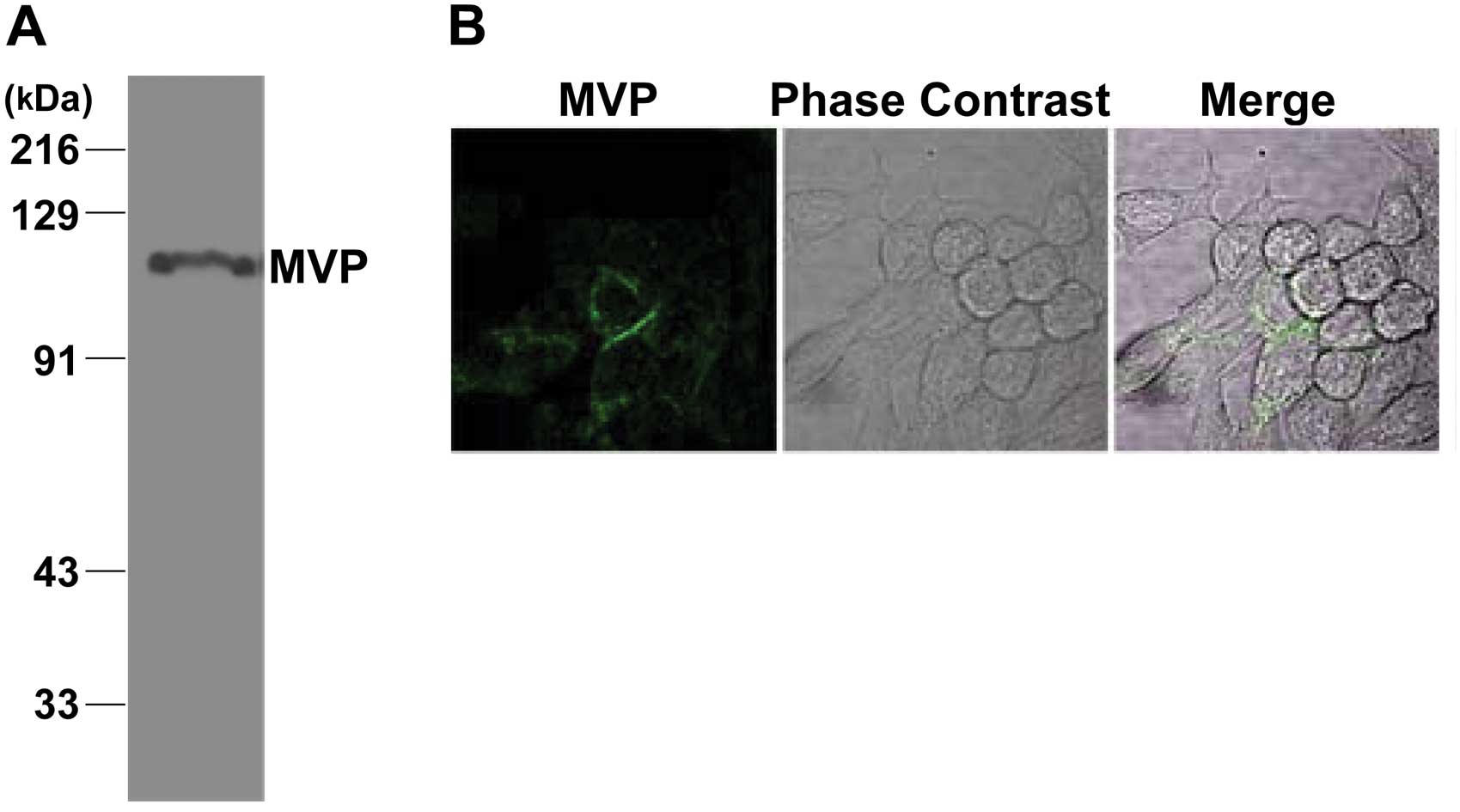
To examine if the MVP protein was expressed in
cancer cell lines, we cultured human colon adenocarcinoma SW620
cells. As shown in Fig. 2A, the MVP
protein was expressed in SW620 cells. It is known that MVP can
shuttle between the nucleus and the cytoplasm. We examined the
localization of MVP in SW620 cells by immunofluorescence. MVP was
mainly localized in the cytoplasm of SW620 cells (Fig. 2B).
Identification of MVP cis-elements
responsible for the transcription activity of the 5′-flanking
region of the MVP gene
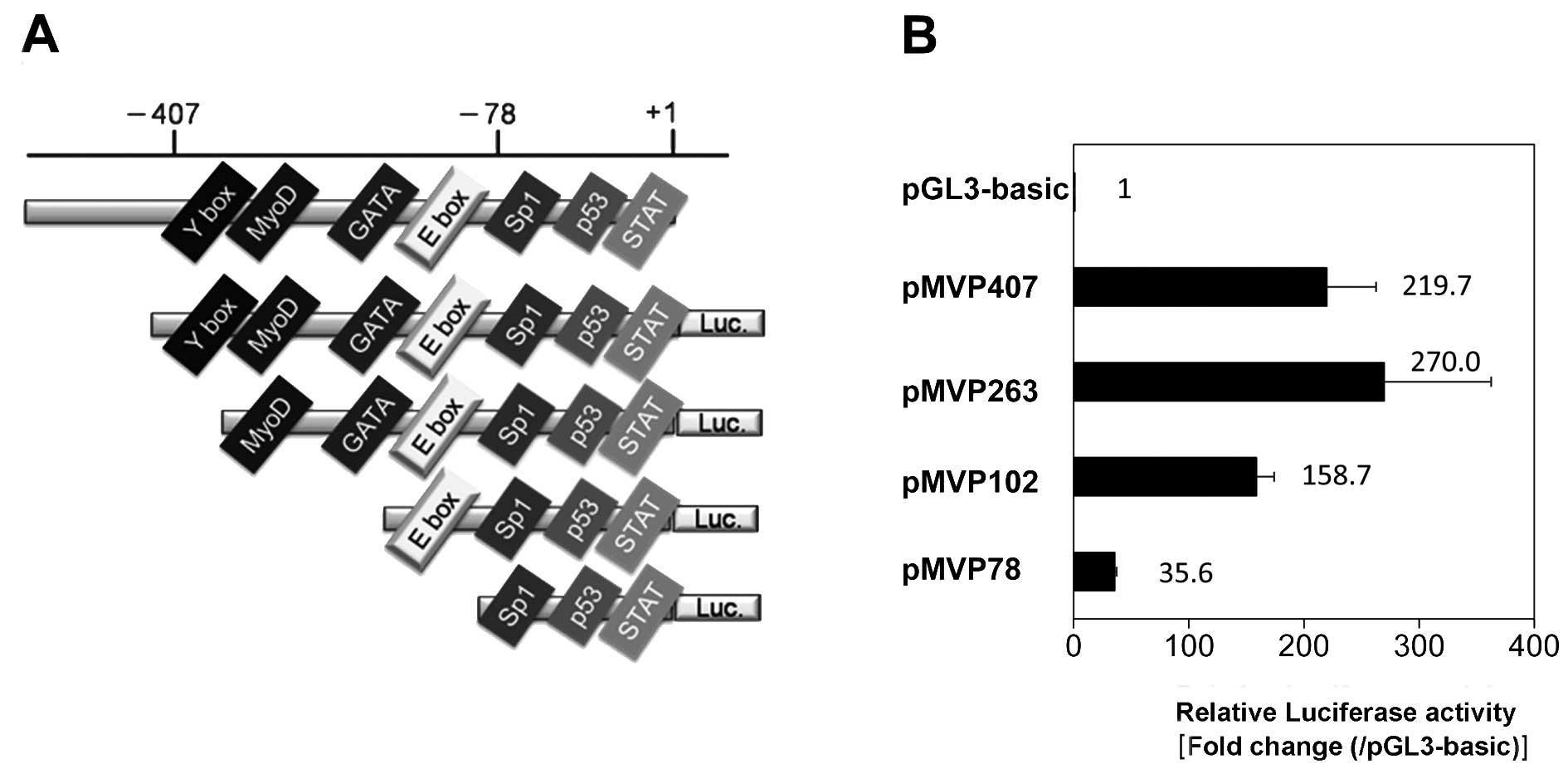
To confirm basal MVP gene transcription, we
cloned human MVP promoter fragments and inserted them into a
luciferase reporter vector. One construct, pMVP407, contained all
the known conserved promoter elements, Y-box, Myo D, GATA, E-box,
Sp1, p53 and STAT of the MVP promoter (22). Following transfection of the
individual plasmids into SW620 cells, the cells and MVP
promoter activity was monitored by a luciferase assay (Fig. 3). Luciferase activity in pMVP407 and
pMVP263-transfected cells was 200-fold higher than that of cells
transfected with the control luciferase vector. The level of
luciferase activity was gradually reduced as the fragment became
shorter. Basal luciferase activity in pMVP78-transfected cells was
significantly lower than that in pMVP102-transfected cells. The
construct pMVP78 lacks the E-box binding site of the construct
pMVP102. These findings suggest that the E-box-binding motif may
play a crucial role in maintaining basal MVP promoter activity.
The effect of USF1 on the expression of
MVP in SW620 cells
USF1 and USF2 are members of the eukaryotic
evolutionary conserved Basic-Helix-Loop-Helix Leucine Zipper
transcription factor. They interact with high affinity to cognate
E-box regulatory elements and are ubiquitously expressed. To
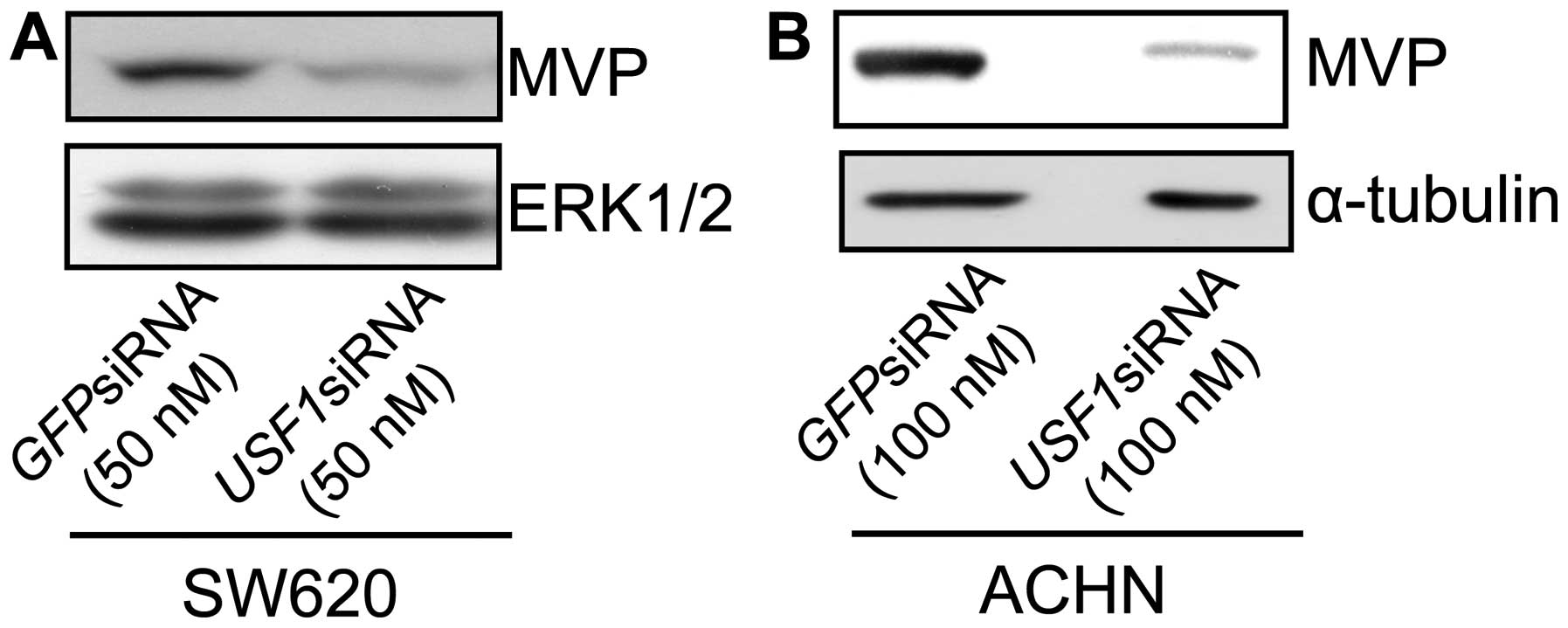
examine whether the expression of USF1 regulates the expression of
MVP, SW620 cells transfected with siRNA duplexes targeting
USF1 or green fluorescent protein (GFP) as a control
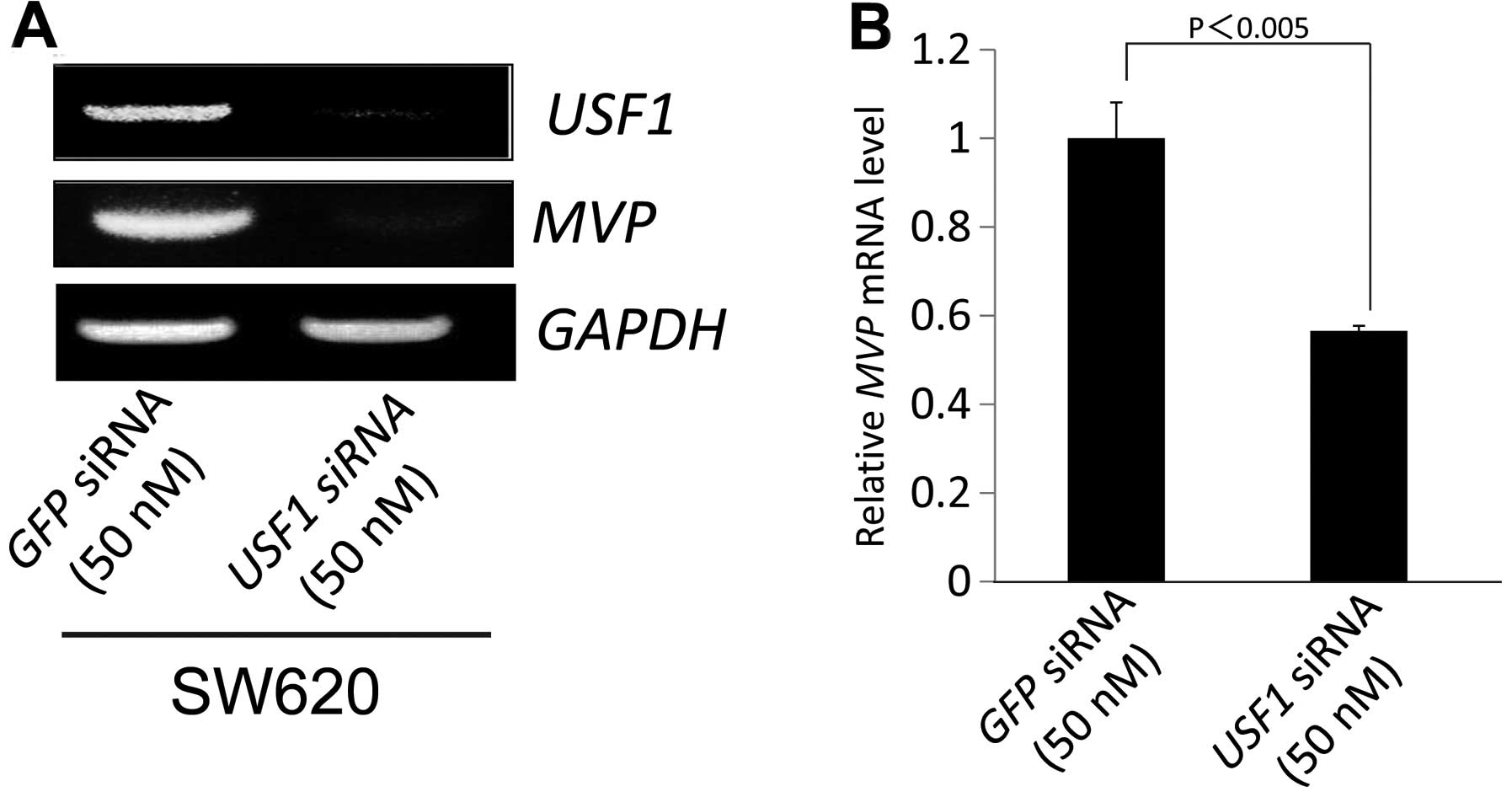
were cultured. As shown in Fig. 4A,
the introduction of siRNA against USF1 resulted in a
downregulation in MVP expression in SW620 cells. To examine the
effects in ACHN cells, ACHN cells were transfected with siRNA
duplexes targeting USF1 or green fluorescent protein
(GFP). The introduction of siRNA against USF1 also resulted
in a downregulation in MVP expression in ACHN cells. These results
indicate that the expression of MVP is affected by the
expression of USF1 in SW620 cells.
The effect of USF1 on MVP mRNA levels in
SW620 cells
To determine if MVP mRNA expression was
altered by the introduction of siRNA against USF1, we
examined the siRNA against USF1 on the expression levels of
MVP in SW620 cells by RT-PCR and real-time RT-PCR. RT-PCR and
real-time RT-PCR analyses confirmed that the introduction of siRNA
against USF1 in SW620 cells downregulated the expression of
USF1 and consequently suppressed MVP mRNA levels in
SW620 cells (Fig. 5A and B). These
results suggest that USF1 modulates the expression of MVP.
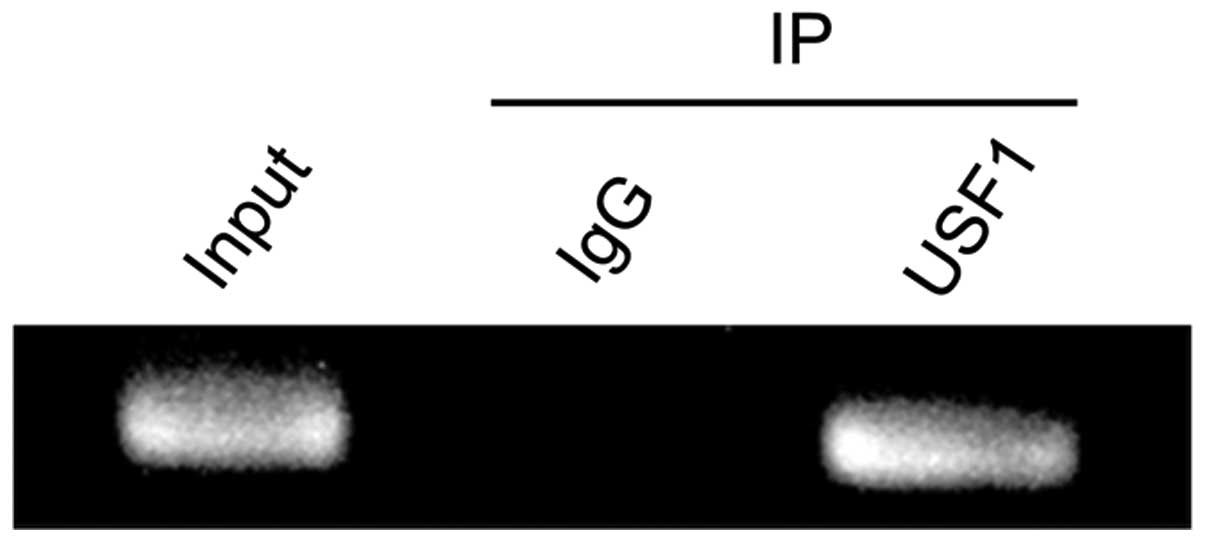
Binding of USF1 to the MVP promoter in
SW620 cells
To test for in vivo USF1 binding to the
MVP promoter, we performed a ChIP assay using the antibody
against USF1. The chromatin immunoprecipitated by the antibody was
then amplified by PCR with primers specific for MVP. As
shown in Fig. 6, USF1 bound the
MVP promoter in SW620 cells.
Discussion
The high evolutionary conservation and wide species
distribution of vaults suggest an important role of vaults in
cellular function. The human vault particle is a 13-MDa
ribonucleoprotein complex comprised of the ~100-kDa major vault
protein (MVP), 193-kDa vault-associated poly(ADP-ribose) polymerase
(vPARP), and the 280-kDa vault protein that has been demonstrated
to be identical to a component of telomerase, TEP1, and four small
untranslated vault RNAs (vRNAs) (1–3). MVP
is expressed in the kidney, adrenal glands, heart, lung, muscle,
thyroid, prostate, liver, pancreas, placenta, bone marrow and
testis (8) (Fig. 1). These findings suggest that MVP
plays distinct physiological roles in these organs. However, the
physiological role of vaults remains unknown.
The MVP promoter contains conserved promoter
elements, Y-box, Myo D, GATA, E-box, Sp1, p53 and STAT of the
MVP promoter (9). YB-1 is a
transcription factor that binds to the Y-box and responds to
environmental stresses such as anticancer agents, UV light and
hyperthermia (10–12). Treatment with IFN-γ resulted in a
significant upregulation of MVP promoter activity as well as mRNA
and protein levels. The activation of MVP expression by IFN-γ
involved transcriptional upregulation through the JAK/STAT pathway
based on the interaction of STAT1 within the proximal MVP
promoter (13). Sp-transcription
factors are essential for the basal MVP promoter via binding to the
GC-box element with the MVP promoter sequence, and
stimulation of the MVP promoter was increased by HDAC inhibitors
(14). As shown in Fig. 3B, deletion of the region from −102
to −79, which contained the E-box element, resulted in a decrease
in the level of transcriptional activity by 22.4% in SW620 cells.
This result suggests that the E-box-binding motif may play a
crucial role in maintaining basal MVP promoter activity in SW620
cells.
USF is a ubiquitous transcription factor in
mammalian cells. Two different genes, defined as USF1 and USF2,
have been isolated from HeLa cells, and these genes encode 43 and
44-kDa polypeptides, respectively (15–18).
These two peptides have identical DNA-binding specificities and
transcriptional activities (15–18).
Although USF1 and USF2 polypeptides are highly divergent in their
N-terminal sequences, they share a highly conserved C-terminal
basic/helix-loop-helix/leucine zipper (B-HLH-LZ) sequence that is
important for the homo- and heterodimerization and DNA-binding of
USF (18). Proteins of the B-HLHLZ
gene family bind to the DNA sequences of the general type CANNTG,
which is referred to as the E-box (19,20).
In mammalian cells, two ubiquitously expressed genes, USF1 and
USF2, have been characterized and have pleiotropic effects in cells
and tissues (18,21). The patterns of USF1 and
MVP genes were expressed in similar tissues; therefore, we
investigated the effect of USF1 on the expression of MVP in SW620
cells (Fig. 1) (18). The introduction of siRNA against
USF1 decreased the expression of MVP in SW620 cells (Fig. 4). These results indicated that the
expression of MVP was regulated by USF1 in SW620 cells.
Our findings showed that a conserved proximal E-box
binding site is important for basal human MVP promoter
transactivation by deletion analysis of the MVP promoter
assay and the introduction of siRNA against USF1 in SW620 cells
also decreased the expression of MVP. These results suggest that
USF1 binding to the E-box element may be critical for basal
MVP promoter activation. However, further study is required
to confirm whether USF1 and other transcription factors regulate
the tissue-specific MVP expression involved in cell development and
differentiation and malignant transformation.
References
|
1
|
Scheffer GL, Wijngaard PL, Flens MJ,
Izquierdo MA, Slovak ML, Pinedo HM, Meijer CJ, Clevers HC and
Scheper RJ: The drug resistance-related protein LRP is the human
major vault protein. Nat Med. 1:578–582. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scheper RJ, Broxterman HJ, Scheffer GL,
Kaaijk P, Dalton WS, van Heijningen TH, van Kalken CK, Slovak ML,
de Vries EG, van der Valk P, Meijer CJ and Pinedo HM:
Overexpression of a M(r) 110,000 vesicular protein in
non-P-glycoprotein-mediated multidrug resistance. Cancer Res.
53:1475–1479. 1993.PubMed/NCBI
|
|
3
|
Scheffer GL, Schroeijers AB, Izquierdo MA,
Wiemer EA and Scheper RJ: Lung resistance-related protein/major
vault protein and vaults in multidrug-resistant cancer. Curr Opin
Oncol. 12:550–556. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kitazono M, Sumizawa T, Takebayashi Y,
Chen ZS, Furukawa T, Nagayama S, Tani A, Takao S, Aikou T and
Akiyama S: Multidrug resistance and the lung resistance-related
protein in human colon carcinoma SW-620 cells. J Natl Cancer Inst.
91:1647–1653. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pohl G, Suchomel RW, Stranzl T, Depisch D,
Stiglbauer W, Filipits M and Pirker R: Expression of the lung
resistance protein in primary colorectal carcinomas. Anticancer
Res. 21:201–204. 2001.PubMed/NCBI
|
|
6
|
Meijer GA, Schroeijers AB, Flens MJ,
Meuwissen SG, van der Valk P, Baak JP and Scheper RJ: Increased
expression of multidrug resistance related proteins Pgp, MRP1, and
LRP/MVP occurs early in colorectal carcinogenesis. J Clin Pathol.
52:450–454. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ikeda R, Iwashita K, Sumizawa T, Beppu S,
Tabata S, Tajitsu Y, Shimamoto Y, Yoshida K, Furukawa T, Che XF,
Yamaguchi T, Ushiyama M, Miyawaki A, Takeda Y, Yamamoto M, Zhao HY,
Shibayama Y, Yamada K and Akiyama S: Hyperosmotic stress
up-regulates the expression of major vault protein in SW620 human
colon cancer cells. Exp Cell Res. 314:3017–3026. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sugawara I, Akiyama S, Scheper RJ and
Itoyama S: Lung resistance protein (LRP) expression in human normal
tissues in comparison with that of MDR1 and MRP. Cancer Lett.
112:23–31. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lange C, Walther W, Schwabe H and Stein U:
Cloning and initial analysis of the human multidrug
resistance-related MVP/LRP gene promoter. Biochem Biophys Res
Commun. 278:125–133. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Asakuno K, Kohno K, Uchiumi T, Kubo T,
Sato S, Isono M and Kuwano M: Involvement of a DNA binding protein,
MDR-NF1/YB-1, in human MDR1 gene expression by actinomycin D.
Biochem Biophys Res Commun. 199:1428–1435. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ohga T, Uchiumi T, Makino Y, Koike K, Wada
M, Kuwano M and Kohno K: Direct involvement of the Y-box binding
protein YB-1 in genotoxic stress-induced activation of the human
multidrug resistance 1 gene. J Biol Chem. 273:5997–6000. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stein U, Jürchott K, Walther W, Bergmann
S, Schla PM and Royer HD: Hyperthermia-induced nuclear
translocation of transcription factor YB-1 leads to enhanced
expression of multidrug resistance-related ABC transporters. J Biol
Chem. 276:28562–28569. 2001. View Article : Google Scholar
|
|
13
|
Steiner E, Holzmann K, Pirker C, Elbling
L, Micksche M, Sutterlüty H and Berger W: The major vault protein
is responsive to and interferes with interferon-γ-mediated STAT1
signals. J Cell Sci. 119:459–469. 2006.
|
|
14
|
Steiner E, Holzmann K, Pirker C, Elbling
L, Micksche M and Berger W: SP-transcription factors are involved
in basal MVP promoter activity and its stimulation by HDAC
inhibitors. Biochem Biophys Res Commun. 317:235–243. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sawadogo M, Van Dyke MW, Gregor PD and
Roeder RG: Multiple forms of the human gene-specific transcription
factor USF. I Complete purification and identification of USF from
HeLa cell nuclei. J Biol Chem. 263:11985–11993. 1988.
|
|
16
|
Sawadogo M: Multiple forms of the human
gene-specific transcription factor USF. II DNA binding properties
and transcriptional activity of the purified HeLa USF. J Biol Chem.
263:11994–12001. 1988.PubMed/NCBI
|
|
17
|
Gregor PD, Sawadogo M and Roeder RG: The
adenovirus major late transcription factor USF is a member of the
helix-loop-helix group of regulatory proteins and binds to DNA as a
dimer. Genes Dev. 4:1730–1740. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sirito M, Walker S, Lin Q, Kozlowski MT,
Klein WH and Sawadogo M: Members of the USF family of
helix-loop-helix proteins bind DNA as homo- as well as
heterodimers. Gene Expr. 2:231–240. 1992.PubMed/NCBI
|
|
19
|
Miyamoto NG, Moncollin V, Wintzerith M,
Hen R, Egly JM and Chambon P: Stimulation of in vitro transcription
by the upstream element of the adenovirus-2 major late promoter
involves a specific factor. Nucleic Acids Res. 12:8779–8799. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu YT and Manley JL: Generation and
functional analyses for base substitution mutants of the adenovirus
2 major late promoter. Nucleic Acids Res. 12:9309–9321. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sirito M, Lin Q, Maity T and Sawadogo M:
Ubiquitous expression of the 43- and 44-kDa forms of transcription
factor USF in mammalian cells. Nucleic Acids Res. 22:427–433. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shimamoto Y, Sumizawa T, Haraguchi M,
Gotanda T, Jueng HC, Furukawa T, Sakata R and Akiyama S: Direct
activation of the human major vault protein gene by DNA-damaging
agents. Oncol Rep. 15:645–652. 2006.PubMed/NCBI
|