Introduction
The most common type of thyroid carcinoma, an
endocrine-related malignancy, is papillary thyroid cancer (PTC)
that accounts for 80–90% of total cases (1). The incidence of thyroid cancer has
been steadily increasing over the past 3 decades in many countries
worldwide. Thyroid cancer has become the fifth leading type of
cancer in women in the United States (2) and commonly occurs in younger
individuals. However, the incidence rate of thyroid cancer has been
increasing in all age groups since 1990s. It is estimated that
60,220 individuals will be newly diagnosed with thyroid cancer and
1,850 deaths will result from thyroid cancer in the US in 2013
(2). Although the 5-year survival
rate for patients with PTC, particularly in younger patients under
40 years of age, is quite favorable, the prognosis declines with
each subsequent decade of life, such as in aged patients older than
60 years to a large degree. Despite the outright success of current
therapies, 10–30% of patients with PTC develop recurrence and/or
metastasis and eventually ~6% of patients die from distant
metastasis (3,4). Currently, prognostic evaluation of PTC
is based mainly on age, tumor size and clinical stage. However, the
mechanisms underlying the association between clinicopathological
features and molecular biology are not well understood. Recent
studies have shown that a number of molecular markers of the
primary tumor may serve as potent parameters with which to predict
the clinical outcome of patients (5,6).
Human stomatin-like protein (SLP) is a member of the
superfamily of stomatin proteins, including SLP-1, SLP-2 and SLP-3.
SLP-2, also known as HSPC108, EPB72-like protein 2, was first
cloned in 2000 and was identified as a novel plasma membrane-linked
protein in erythrocytes (7); it is
at present mainly considered as a mitochondrial inner membrane
protein (8). The gene for human
SLP-2, STOML2, is localized on chromosome 9p13 (9). Phylogenic analysis shows that SLP-2 is
acquired through mitochondrial endosymbiosis and is widely
distributed in most major kingdoms (10). Although it is now known to be
expressed in a range of mammalian tissues and involved in the
regulation of ion channel permeability, mechanoreception and lipid
domain organization (7), the exact
function and mechanisms are unclear. Recent studies have revealed
that SLP-2 is required for stress-induced mitochondrial hyperfusion
(11) and may be implicated in the
interaction with prohibitins in the mitochondrial inner membrane,
thereby contributing to mitochondrial stability and regulating
their biogenesis and functions (12,13).
SLP-2 was first identified as a novel cancer-related
gene in 2006 and was found to be overexpressed in several types of
human tumors, including breast cancer, gastric cancer, colorectal
cancer, glioma, laryngeal squamous cell carcinoma (LSCC),
esophageal squamous cell carcinoma (ESCC), lung cancer and
endometrial adenocarcinoma (14–19).
Inhibition of SLP-2 was found to decrease cell proliferation,
adhesion and tumor cell motility (20), suggesting that SLP-2 may play an
important role in tumorigenesis. A high level of SLP-2 has been
associated with decreased survival of patients with breast cancer
(17). A recent case study suggests
that SLP-2 may be used as a biomarker for the early detection of
colorectal cancer (18). However,
expression of SLP-2 in thyroid cancer has not yet been explored.
Furthermore, whether SLP-2 expression is regulated by cytokines
that play important roles in PTC tumorigenesis is unknown.
Transforming growth factor-β (TGF-β) is one of the important
cytokines involved in PTC development (21,22).
Changes in the expression of TGF-β have been noted in the
multistage development of thyroid cancer (23). Immunohistochemical (IHC) analysis
detected TGF-β1 in epithelial cells in 58% of malignant thyroid
tumors (including follicular, papillary and anaplastic variants),
but was not noted in any of 7 benign tumors nor in any normal
thyroid epithelium (23).
Therefore, we speculated that an alteration in the expression of
TGF-β1 occurring specifically in PTC may regulate SLP-2
expression.
In the present study, we report for the first time
that the expression of SLP-2 at the mRNA and protein levels is
associated with the clinicopathological features of human PTC.
Whether expression of SLP-2 is regulated by TGF-β1 in thyroid
cancer cells was also examined. Furthermore, we evaluated the
correlation between SLP-2 expression and the proliferation index
Ki-67 and explored the diagnostic value of SLP-2 in patients with
PTC.
Materials and methods
Patients and tissue preparation
A total of 107 patients diagnosed with PTC at
Jinshan Hospital, Fudan University from August 2009 to September
2012, was examined. The study on human subjects was approved by the
Ethics Committee of Jinshan Hospital, Fudan University. Among these
cases confirmed by surgery and histopathology, 99 patients were
diagnosed with classical PTC and 8 patients were diagnosed with
follicular variant of PTC. The mean age of the patients was 46
years (range, 13–73 years) with a median age of 47 years. None of
the patients had received radiotherapy or chemotherapy prior to
surgery.
The 10% formalin-fixed paraffin-embedded samples
were used for histological examination and were classified based on
the tumor, node, metastasis (TNM) classification method from the
American Joint Committee on Cancer (AJCC). In addition, freshly
isolated primary tumor and adjacent normal thyroid tissues from the
same cases were subjected to quantitative real-time PCR.
Immunohistochemical staining and
scoring
Sections (4 μm) were baked at 58°C for 2 h and then
deparaffinized in xylene and hydrated with a series of graded
alcohols. Antigen recovery was performed by heating the slides
immersed in citrate buffer (pH 6.0) in a microwave oven (95–100°C)
2 times for 10 min each. Endogenous peroxidase activity was blocked
using 3% hydrogen peroxide. After an additional blocking with 10%
normal goat serum, the sections were incubated with an anti-SLP-2
rabbit polyclonal antibody (1:300 dilution; Proteintech Group,
Inc., Chicago, IL, USA) in 0.1% BSA/PBS at 4°C overnight, followed
by incubation with a biotinylated anti-rabbit secondary antibody
(Long Island Biotec., Shanghai, China) at 37°C for 15 min. The
signal was detected using a DAB kit (Maixin Bio., Fuzhou, Fujian,
China). Finally, the sections were counterstained with hematoxylin
solution. SLP-2-positive cells were counted and photographed under
a light microscope. The negative control was conducted by replacing
the primary antibody with 0.1% BSA/PBS.
SLP-2 expression was scored using an immunoreactive
scoring scale and evaluated by 2 independent pathologists without
any prior knowledge of the patient clinical information. The
proportion of positive cells was scored by the extent of
immunostaining and was assigned to one of the following categories:
0 (0%, no positive cells), 1 (≤25% positive cells), 2 (26–50%
positive cells), 3 (51–75% positive cells) and 4 (>75% positive
cells). The intensity of immunostaining was scored as 0 (no
staining), 1 (weak staining), 2 (moderate staining) and 3 (strong
staining). A final immunoreactive score, also known as the staining
index (SI), was determined by the sum of the positive extent and
staining intensity. SI was clustered into 4 groups: ‘-’, ≤ 2 sum
points; ‘+’, 3–4 sum points; ‘++’, 5–6 sum points and ‘+++’, 7 sum
points. For the present study, we defined the cases with grades
equal to ‘++’ and ‘+++’ as having a high level of SLP-2 expression,
whereas ‘−’ and ‘+’ denoted negative or a low level of SLP-2
expression. The Ki-67 (Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA) index was expressed as the percentage of positive cells
and was used to define distinct nuclear staining. At least 2,000
cells/case were analyzed. Ki-67 expression was classified as high
at ≥3.0% and low at <3.0%.
RNA extraction and quantitative real-time
PCR
Total RNA was extracted from the thyroid tissue
using TRIzol reagent (Invitrogen Life Technologies, New York, NY,
USA), according to the manufacturer’s instructions. One microgram
of total RNA was reversely transcribed using a reverse
transcription kit (Takara Bio, Inc., Dalian, Liaoning, China). The
specific gene was amplified using PCR. The primer sequences were
5′-GTGACTCTCGACAATGTAAC-3′ (forward) and 5′-TGATCTCATAACGGAGGCAG-3′
(reverse) for SLP-2; and 5′-ATGGGGAAGGTGAAGGTCGGAGTC-3′ (forward)
and 5′-GCTGATGATCTTGAGGCTGTTGTC-3′ (reverse) for GAPDH (synthesized
by Sangon Biotech Co., Ltd., Shanghai, China). PCR amplification
was performed at 94°C for 30 sec, 61°C for 20 sec and 72°C for 30
sec for 40 cycles using the SYBR-Green kit (Takara Bio, Inc.). An
initial step of denaturing RNA at 95°C for 2 min and a final
extension step at 72°C for 5 min were also performed. Assays were
conducted in triplicate and repeated 3 times. The amount of the
target (SLP-2) was normalized to an endogenous control (GAPDH)
given by 2−δδCt, in which the threshold cycle
(Ct) was obtained using the sequence detection software
v1.4 (7300 Real-Time PCR System; Applied Biosystems, Foster City,
CA, USA).
Cell culture and TGF-β1 treatment
The human papillary thyroid carcinoma K1 cell line
was originally obtained from Sigma (St. Louis, MO, USA) and was a
kind gift from Dr G.B. Yuan (Chongqing Medical University,
Chongqing, China). K1 cells were cultured in DMEM/Ham’s F12/MCDB
medium (2:1:1; HyClone, Logan, UT, USA and Sigma) supplemented with
100 IU/ml penicillin and 100 μg/ml streptomycin in the presence of
10% fetal bovine serum (Invitrogen Life Technologies, Carlsbad, CA,
USA) at 37°C in a humidified atmosphere of 5% CO2. After
24 h, cells were treated with 10 ng/ml of recombinant human TGF-β1
(R&D Systems, Minneapolis, MN, USA) in complete medium for 0,
24, 48 and 72 h.
Western blotting
Equal amounts of proteins were separated by SDS-PAGE
and transferred to PVDF membranes. After blocking with 1% skim milk
in TBS-T at room temperature for 1 h, the membranes were probed
with rabbit anti-rat SLP-2 or β-actin (1:7,000 dilution each;
Proteintech Group) and Smad2 or phospho-Smad2 (1:500 dilution each;
Cell Signaling Technology, Inc., Beverly, MA, USA) primary
antibodies, respectively, at 4°C overnight and subsequently
incubated with horseradish peroxidase-conjugated goat anti-rabbit
secondary antibody (1:8,000 dilution; Proteintech Group) at room
temperature for 1 h. Signals were detected using Pierce SuperSignal
West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific
Inc., Rockford, IL, USA) and quantified using Tanon-4500 gel
imaging system with GIS ID analysis software v4.1.5 (Tanon Science
& Technology Co., Ltd., Shanghai, China).
Statistical analysis
All analyses were performed with SPSS version 13.0
for Windows (SPSS, Inc., Chicago, IL, USA). For correlation between
SLP-2 expression and histological types or the clinicopathological
characteristics, the Chi-square test or the Fisher’s exact test was
performed as indicated. For comparison between 2 groups and
multi-groups, a Student’s t-test and ANOVA were used, respectively.
A significant difference was considered at a value of
P<0.05.
Results
Overexpression of SLP-2 in human PTC
We observed that the classical PTC cases manifested
papillary formation with nuclear characteristics. The 99 classical
PTC cases displayed the principal microscopic features of papillary
architecture and nuclear features, such as irregularity of nuclear
contours, nuclear pseudo-inclusions, enlargement, crowding,
overlapping and grooves; whereas the cases of the follicular
variants of PTC comprising 8.08% (8/107) of the total PTC cases
were defined based on diagnostic criteria as having complete lack
of well-developed papillae, but exhibited the presence of an
exclusively or predominantly follicular growth pattern with
characteristic nuclear features of papillary carcinoma.
Immunohistochemical staining analysis indicated the heterogeneous
positivity of SLP-2 expression in PTC tissues. SLP-2 expression was
mainly present in the cytoplasm with minor expression in the plasma
membrane of PTC cells with different scores of staining index. A
negative staining of SLP-2 was observed in the adjacent normal
tissues to the primary tumors (Fig. 1A
and B). However, a few cases of positive staining were also
noted in the adjacent normal tissues. Using the SI system, the
level of SLP-2 expression in the tissues was classified as high and
low. We found that a high level of SLP-2 expression was more
frequent in the tumor tissues when compared with that in the
adjacent normal tissues to the primary tumors (Table I; P<0.001), particularly in the
classical PTC cases (Fig. 1C–H);
whereas a negative or low level of SLP-2 expression was observed
more frequently in the adjacent normal tissues when compared with
that in the tumor tissues. In the follicular variant cases,
however, there was no significant difference in SLP-2 expression
between the tumor tissues and adjacent normal tissues (P>0.05).
Since the sample number was quite small (8 cases of follicular
variant), we could not make a conclusion at this point that SLP-2
is an indicator for the diagnosis of classical PTC.
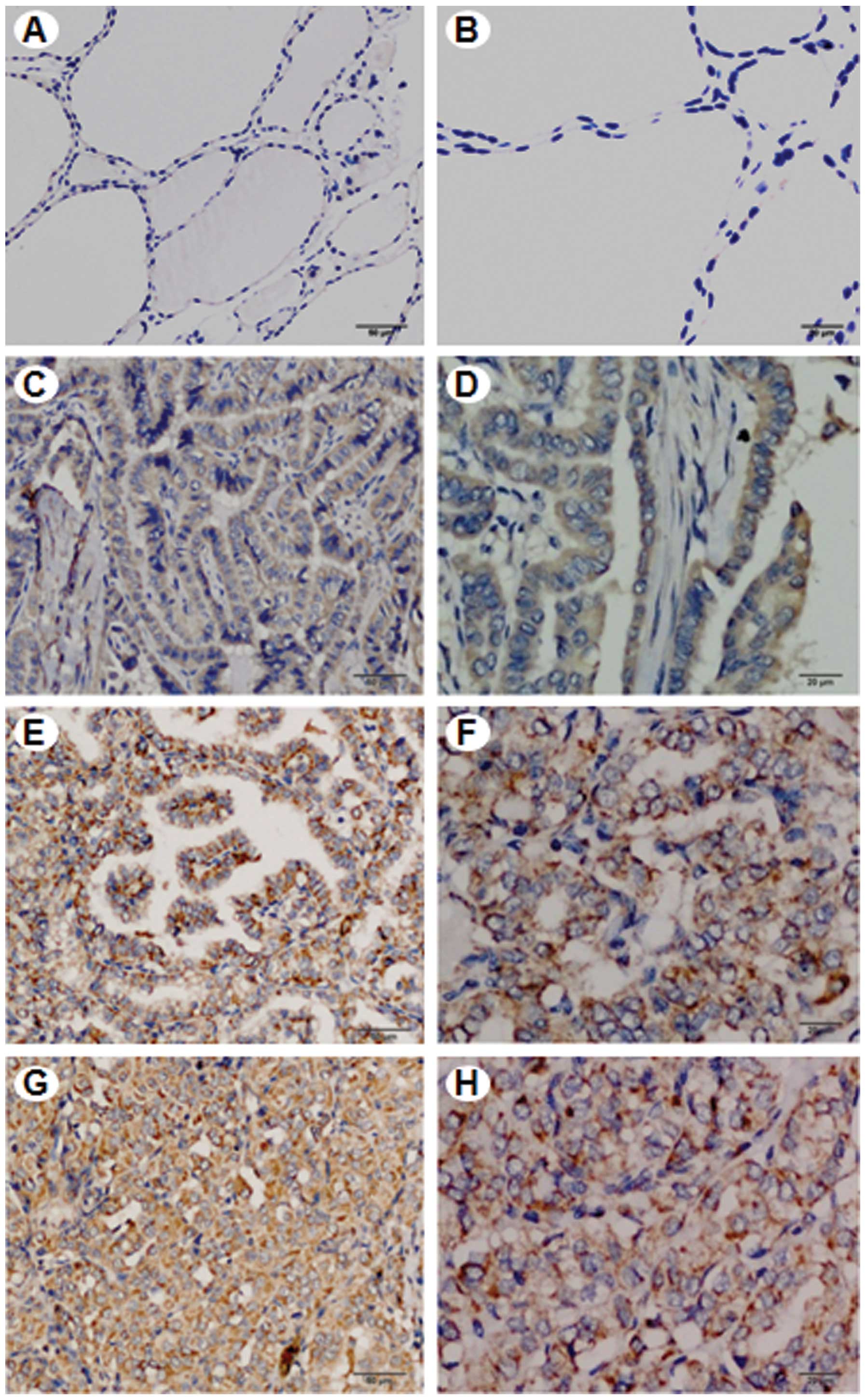 | Figure 1SLP-2 expression in human classical
PTC. Immunohistochemical staining analysis shows the heterogeneous
positivity of SLP-2 expression with the 4 different scores.
Histologically non-tumor tissue, adjacent normal tissue to the
primary tumor, was used as a control and showed negative staining
(A and B). Heterogeneous positivity of SLP-2 expression was found
in PTC tissues (C-H). A brown color shown in the cytoplasm was
considered as positive staining. Representative images are shown.
(A and B) Score of (−), negative staining. (C and D) Score of (+),
weak staining. (E and F) Score of (++), moderate staining. (G and
H) Score of (+++), strong staining. All sections were
counterstained with hematoxylin. (A,C,E,G) Original magnification,
×200 (scale bar, 50 mm). (B,D,F,H) Original magnification, ×400
(scale bar, 20 mm). SLP-2, stomatin-like protein 2; PTC, papillary
thyroid cancer. |
 | Table ISLP-2 expression in the PTC cases. |
Table I
SLP-2 expression in the PTC cases.
| Tumor n (%) | Normal n (%) | P-value |
|---|
| Total PTC | | |
<0.001a |
| Patients (n) | 107 (100.00) | 107 (100.00) | |
| High | 77 (71.96) | 15 (14.02) | |
| Low | 30 (28.04) | 92 (85.98) | |
| Classical | | |
<0.001a |
| Patients (n) | 99 (100.00) | 99 (100.00) | |
| High | 71 (71.72) | 13 (13.13) | |
| Low | 28 (28.28) | 86 (86.87) | |
| Follicular
variant | | |
0.066b |
| Patients (n) | 8 (100.00) | 8 (100.00) | |
| High | 6 (75.00) | 2 (25.00) | |
| Low | 2 (25.00) | 6 (75.00) | |
Association of SLP-2 expression with the
clinicopathological characteristics of PTC
In order to examine the correlation between SLP-2
expression and the clinicopathological features, we classified the
cases according to high and low SLP-2 expression. The clinical
information of patients was obtained from their charts and is
documented in Table II. We grouped
patients according to age at the time of diagnosis (<45 years,
young patients and ≥45 years, older patients), gender (female and
male), primary tumor size (<1 cm and ≥1 cm), presence of
multifocal tumors, lymph node involvement and tumor stage. By
comparing SLP-2 expression with the clinicopathological parameters,
we found that there was no difference in SLP-2 expression between
the young and older patients. Although PTC patients were
predominantly female (72 vs. 35 male cases), the overall expression
level of SLP-2 was not different between female and male cases, and
was not correlated with multifocal tumors. Among the 107 cases,
however, SLP-2 expression was strongly correlated with primary
tumor size (Table II; P<0.001).
In the group of tumors with size ≥1 cm, the cases having a high
level of SLP-2 expression reached 93.55%. Importantly, there was a
significant association between SLP-2 expression and lymph node
metastasis and tumor stage (P≤0.001, respectively). A high level of
SLP-2 expression was detected in 90.48% of metastatic lymph nodes
close to the tumor. The expression of SLP-2 in T1 cases (tumor
<2 cm) was significantly different than its expression in other
stages; there were no obvious differences in SLP-2 expression among
T2 (tumor 2–4 cm), T3 (tumor >4 cm limited to the thyroid) and
T4 cases (tumor extension beyond the thyroid capsule). These data
indicate that SLP-2 overexpression may be correlated with disease
progression.
 | Table IICorrelation of the
clinicopathological features of the patients with PTC subgrouped
according to low or high expression of SLP-2 as determined by
immunohistochemistry. |
Table II
Correlation of the
clinicopathological features of the patients with PTC subgrouped
according to low or high expression of SLP-2 as determined by
immunohistochemistry.
| Clinicopathological
features | n | SLP-2
expression | P-valuea |
|---|
|
|---|
| Low n (%) | High n (%) |
|---|
| Total patients | 107 | | | |
| Age (years) at
diagnosis | | | | 0.543 |
| <45 | 44 | 15 (34.09) | 29 (65.91) | |
| ≥45 | 63 | 18 (28.57) | 45 (71.43) | |
| Gender | 107 | | | 0.949 |
| Female | 72 | 21 (29.17) | 51 (70.83) | |
| Male | 35 | 10 (28.57) | 25 (71.43) | |
| Primary tumor
size | 107 | | |
<0.001 |
| <1 cm | 45 | 27 (60.00) | 18 (40.00) | |
| ≥1 cm | 62 | 4 (6.45) | 58 (93.55) | |
| Multifocal
tumors | 107 | | | 0.153 |
| Yes | 18 | 3 (16.67) | 15 (83.33) | |
| No | 89 | 30 (33.71) | 59 (66.29) | |
| LN involvement | 99 | | |
<0.001 |
| Yes | 42 | 4 (9.52) | 38 (90.48) | |
| No | 57 | 25 (43.86) | 32 (56.14) | |
| Tumor stage | 107 | | | 0.001 |
| T1 | 70 | 29 (41.43) | 41 (58.57) | |
| T2 | 16 | 0 (0.00) | 16 (100.00) | |
| T3 | 12 | 2 (16.67) | 10 (83.33) | |
| T4 | 9 | 0 (0.00) | 9 (100.00) | |
| Histological
type | 107 | | | 0.602 |
| Classical | 99 | 28 (28.28) | 71 (71.72) | |
| Follicular
variant | 8 | 2 (25.00) | 6 (75.00) | |
| Ki-67
expression | 86 | | | 0.043 |
| ≥3.0% | 21 | 2 (9.52) | 19 (90.48) | |
| <3.0% | 65 | 20 (30.77) | 45 (69.23) | |
Next, we examined whether the expression level of
SLP-2 is altered at the mRNA level and ascertained whether the
expression pattern is correlated with the clinicopathological
features of patients with PTC. Total RNA was extracted from freshly
isolated tumors (39 classical PTCs and 3 follicular variants) and
adjacent normal tissues (33 cases) and was subjected to qPCR. We
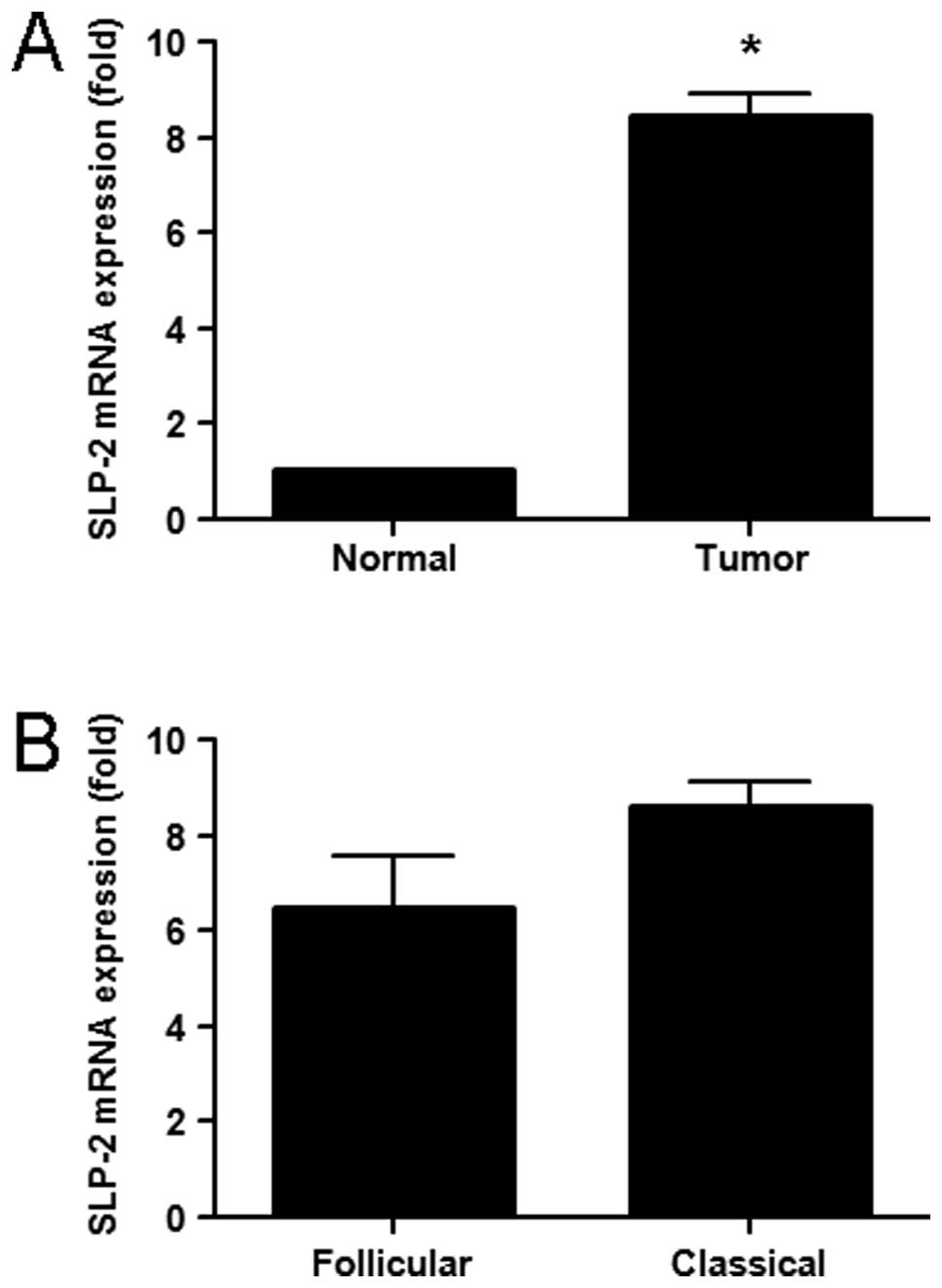
found that the expression of SLP-2 mRNA was 8-fold higher in the
primary tumor tissues than that in the normal tissue (Fig. 2A; P<0.05). Quantitative analysis
showed no difference in SLP-2 mRNA expression between classical PTC
cases and the follicular variant PTC cases (Table III; Fig. 2B). Following comparison with
clinicopathological features, we found that there was no
significant difference in SLP-2 mRNA in regards to age (<45 vs.
≥45 years), gender (female vs. male), primary tumor size (<1 cm
vs. ≥1 cm) and the presence of multifocal tumors (Table III). However, SLP-2 overexpression
was significantly correlated with lymph node metastasis and tumor
stage (P<0.005, respectively). These data were in agreement with
the IHC observation that SLP-2 may be considered as a potential
biomarker for human PTC.
 | Table IIICorrelation between expression of
SLP-2 mRNA and the clinicopathological features of the PTC
cases. |
Table III
Correlation between expression of
SLP-2 mRNA and the clinicopathological features of the PTC
cases.
| Clinicopathological
features | mRNA status of
SLP-2 (−2δδCt) | P-value |
|---|
|
|---|
| n | Mean ± SD | Median |
|---|
| Age (years) at
diagnosis | | | | 0.102a |
| <45 | 22 | 9.458±0.846 | 7.623 | |
| ≥45 | 20 | 7.507±0.543 | 7.016 | |
| Gender | | | | 0.717a |
| Female | 26 | 8.414±0.688 | 7.311 | |
| Male | 16 | 8.471±0.763 | 7.597 | |
| Primary tumor
size | | | | 0.121a |
| <1 cm | 20 | 7.430±0.545 | 6.941 | |
| ≥1 cm | 22 | 9.350±0.801 | 8.762 | |
| Multifocal
tumors | | | | 0.359a |
| Yes | 6 | 7.961±1.716 | 6.527 | |
| No | 36 | 8.515±0.533 | 7.438 | |
| LN involvement | | | |
0.005a |
| Yes | 17 | 10.370±0.868 | 9.714 | |
| No | 20 | 7.002±0.577 | 6.639 | |
| Tumor stage | | | |
0.005b |
| T1 | 26 | 7.588±0.541 | 6.941 | |
| T2 | 6 | 6.958±0.813 | 6.722 | |
| T3 | 6 | 11.490±1.180 | 11.440 | |
| T4 | 4 | 11.590±2.394 | 11.260 | |
| Histological
type | | | | 0.272a |
| Classical | 39 | 8.585±0.538 | 7.413 | |
| Follicular
variant | 3 | 6.495±1.063 | 6.364 | |
| Ki-67
expression | | | |
0.006a |
| ≥3.0% | 8 | 12.040±1.511 | 12.210 | |
| <3.0% | 33 | 7.583±0.426 | 7.013 | |
Correlation of SLP-2 expression with the
proliferation index Ki-67
Ki-67 is a nuclear protein and is highly expressed
in proliferating cells. Thus, it is a proliferation marker and has
been showed to be well correlated with tumor stage and the clinical
outcome of PTC (24). However, the
positive rate of Ki-67 is low in differentiated PTC and thus, Ki-67
is difficult to be used as a diagnostic marker alone. In the
present study, we found that a high level of SLP-2 protein
expression was positively correlated with Ki-67 expression in PTC
(Table II). There was a
significant increase in the expression of SLP-2 protein observed in
the high Ki-67-expressing group, when compared with the low
Ki-67-expressing group (P<0.05). In addition, expression of
SLP-2 mRNA was also higher in the high Ki-67-expressing group than
that in the low Ki-67-expressing group (Table III; P<0.01).
Regulation of SLP-2 expression by
TGF-β1
Since TGF-β is expressed in differentiated thyroid
cancer and plays a crucial role in thyroid tumorigenesis (25), we subsequently examined whether
TGF-β is involved in the regulation of SLP-2 expression. The effect
of TGF-β1 on SLP-2 expression was assessed in human PTC K1 cells.
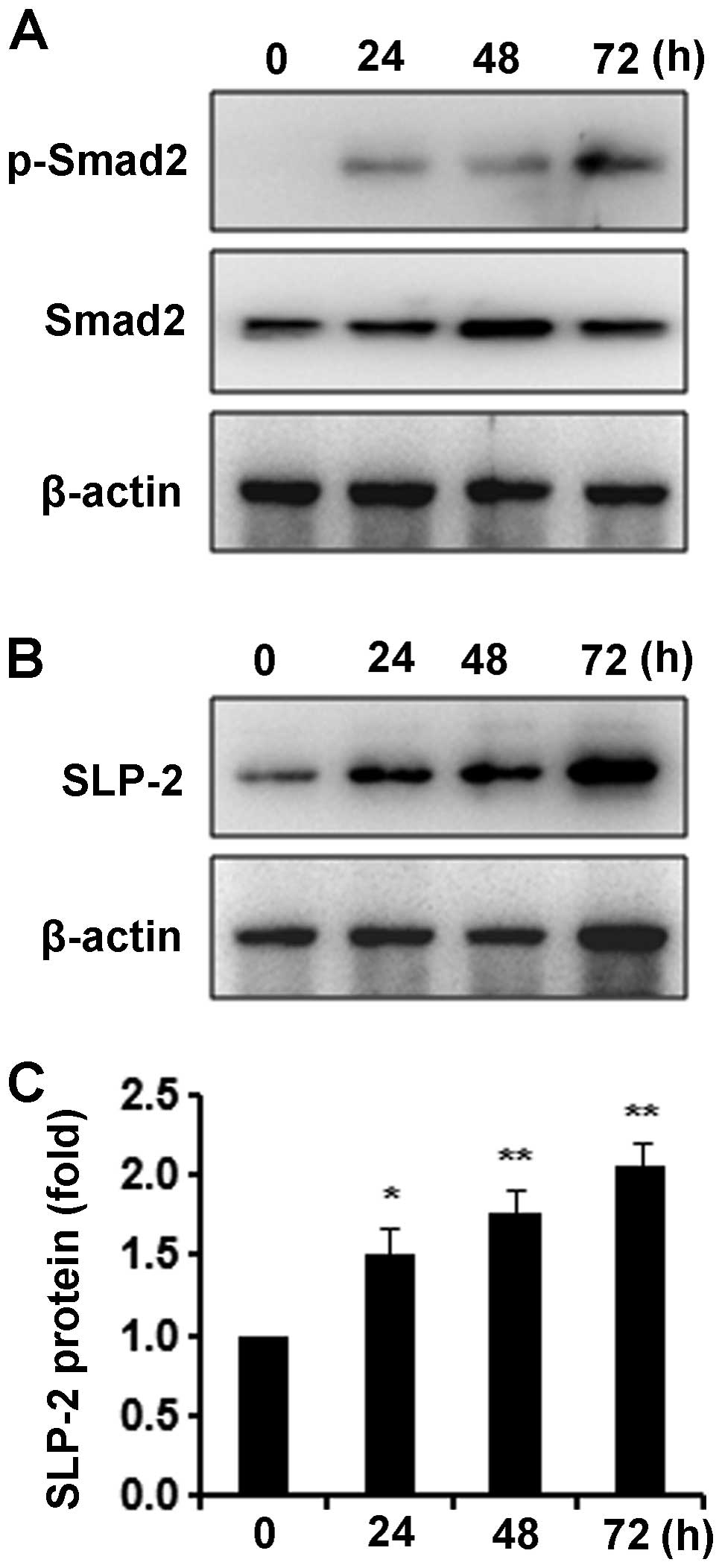
First, we found that K1 cells were responsive to TGF-β1 as an
increase in the phosphorylation of Smad2, a downstream signaling
protein of TGF-β1, was detected by western blotting after 10 ng/ml
of TGF-β1 treatment (Fig. 3A).
Next, we observed an increase in SLP-2-expression after TGF-β1
stimulation in a time-course study (Fig. 3B). Densitometric analysis showed
that TGF-β1 significantly upregulated the expression of SLP-2
protein at 24, 48 and 72 h (Fig.
3C).
Discussion
The present study showed for the first time that
SLP-2 is overexpressed in human PTC and is associated with its
clinicopathological features. SLP-2 is a novel member of the
stomatin superfamily that shares the cognate stomatin signature
sequence. However, SLP-2 is unique and is distinguished from
stomatin, SLP-1 and SLP-3 by the absence of an NH2-terminal
hydrophobic domain (7). Since 2006,
it has been determined that SLP-2 is overexpressed in several
undifferentiated human carcinomas, including ESCC, LSCC and
endometrial adenocarcinoma. Here, we identified SPL-2
overexpression in a differentiated cancer, human PTC.
In the present study, when compared with the
adjacent normal tissue, the overexpression of SLP-2 in the primary
tumors of PTC was found to be mainly located in the cytoplasm with
some distribution on the membrane as detected by IHC; these
findings were in agreement with its distribution in human
endometrial adenocarcinoma (26). A
recent study suggests that there may be a net exchange of membrane
material between mitochondria and plasma membrane (8). However, the function of SLP-2 in human
PTC is unknown. It has been showed that SLP-2 is upregulated under
conditions of mitochondrial stress and interacts with prohibitins,
chaperones in respiratory chain complexes, in the mitochondrial
inner membrane (13). SLP-2 is
required for stress-induced mitochondrial hyperfusion (SIMH)
(11). Upregulation of SLP-2
expression increases the mitochondrial membrane phospholipid
cardiolipin content and binds to cardiolipin to induce
mitochondrial biogenesis (12).
Moreover, SLP-2 deficiency is associated with impaired cardiolipin
compartmentalization in mitochondrial membranes (27). It has also been shown that SLP-2
negatively modulates mitochondrial sodium-calcium exchange
(28). These data indicate that
SLP-2 plays a role in regulating mitochondrial membrane stability
and function.
Based on clinical analysis, the present study
demonstrated that there was no significant difference in SLP-2
expression in regards to gender and age. It has been shown that the
overall outcome of women with PTC is similar to men, although
subgroup analysis showed that the first age period (<55 years)
is a period with better outcomes for women than men and the second
period (≥55 years) is a period with similar outcomes for both women
and men (29). These data suggest
that SLP-2 expression is not correlated with gender and age at the
time of diagnosis.
Tumorigenesis is complex and involves multistage
processes. IHC analysis from the present study showed that high
expression of SLP-2 was correlated with tumor size rather than
multifocal involvement. Furthermore, our data revealed expression
of SLP-2 in the cytoplasm of PTC cells that was not associated with
histological type, but significantly associated with tumor stage,
which was confirmed by qPCR after analyzing 42 cases of PTC. The
highest expression was found in stage T4 (tumor extension beyond
the thyroid capsule) tumors, which is in agreement with a previous
study that expression of SLP-2 is associated with invasiveness of
ESCC (30). SLP-2 alterations occur
early in the process of tumor formation, which may predispose to
malignant transformation. The present study suggests that SLP-2
represents an important molecular marker that is clinically
relevant to the invasion of PTC.
Following further analysis with clinical features,
we found that the expression of SLP-2 mRNA and protein was
significantly higher in the lymph nodes with metastasis than that
in the lymph nodes without metastasis. Such overexpression of SLP-2
was also found in the metastatic lymph nodes of LSCC (16). Previous evidence suggests that high
SLP-2 expression contributes to the progression and poor prognosis
of gastric cancer (19). SLP-2 may
also contribute to the promotion of cell growth and tumorigenesis.
The present study showed that SLP-2 was highly associated with
Ki-67, a cell proliferation marker, and that the expression of
SLP-2 mRNA was positively correlated with tumor stage. These data
support a previous report that Ki-67 immunoreactivity is
significantly associated with tumor stage and clinical outcome in
patients with differentiated thyroid carcinoma (24). Furthermore, downregulation of SLP-2
protein was found to inhibit tumor cell motility and proliferation
and enhance cell sensitivity to chemotherapeutic reagents (20). Transfection of SLP-2 antisense
resulted in a decrease in cell growth, adhesion and tumorigenesis
(14). These data indicate that
SLP-2 may be an important player in tumorigenesis, and the
detection of SLP-2 expression may help to characterize the
prognosis of patients with tumor growth.
The etiology of increased SLP-2 expression in human
PTC is unknown. It may be mediated by various cytokines, including
TGF-β that plays a pivotal role in many types of cancers (31). Several studies have shown that the
TGF-β/Smad signaling pathway is involved in the
epithelial-mesenchymal transformation (EMT), local invasion and
node metastasis of PTC (21,22,32).
TGF-β expression was found to be increased in the cytoplasm at the
periphery of PTC (21). Compared
with the central regions of primary PTCs, the invasive fronts
highly express TGF-β, TβRII and pSmad2 (33,34). A
correlation between TGF-β signaling and EMT was also found in human
poorly differentiated thyroid carcinoma (PDTC) (32). Previous studies have shown that
there is positive staining of TGF-β and pSmad2 in PDTC, which
provides evidence that TGF-β is involved in the transition from PTC
to PDTC (22), suggesting a role
for TGF-β in mediating this process. However, whether TGF-β
regulates SLP-2 in thyroid cancer was not previously explored. The
present study provides evidence that TGF-β1 upregulates the
expression of SLP-2. Although the TGF-β-mediated SLP-2 expression
in tumor cells is not completely understood, our study indicates
that SLP-2 induction may require the activation of TGF-β signaling
in vivo, leading to tumor formation and progression of PTC.
Further investigation is needed to identify and understand the
molecular mechanisms underlying TGF-β signaling pathway-mediated
SLP-2 expression.
In conclusion, our findings revealed for the first
time that patients with PTC exhibit SLP-2 overexpression which is
associated with clinicopathological features. The correlation
between SLP-2 expression and proliferation marker Ki-67 may be
characteristic of PTC and reflect PTC progression. Furthermore,
SLP-2 expression is upregulated by TGF-β1 in vitro in PTC
cells, indicating a possible role of SLP-2 in PTC tumorigenesis.
Thus, SLP-2 may be considered as a valuable biomarker for patients
with PTC, and the present study identifies SLP-2 as a potential
therapeutic target for the treatment of PTC.
Acknowledgements
We thank Dr G.B. Yuan (Chongqing Medical University,
Chongqing, China) for kindly providing the K1 cells. The present
study was supported by grants from the National Natural Science
Foundation of China (81272880), the Shanghai Committee of Science
and Technology (124119b1300), and the Shanghai Municipal Health
Bureau (2012-186) to G.X.
Abbreviations:
|
EMT
|
epithelial-mesenchymal
transformation
|
|
IHC
|
immunohistochemistry
|
|
PDTC
|
poorly differentiated thyroid
carcinoma
|
|
PTC
|
papillary thyroid cancer
|
|
qPCR
|
quantitative polymerase chain
reaction
|
|
SI
|
staining index
|
|
SLP-2
|
stomatin-like protein 2
|
|
TGF-β
|
transforming growth factor-β
|
References
|
1
|
Boone RT, Fan CY and Hanna EY:
Well-differentiated carcinoma of the thyroid. Otolaryngol Clin
North Am. 36:73–90. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
3
|
Mazzaferri EL and Jhiang SM: Long-term
impact of initial surgical and medical therapy on papillary and
follicular thyroid cancer. Am J Med. 97:418–428. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schlumberger MJ: Papillary and follicular
thyroid carcinoma. N Engl J Med. 338:297–306. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
French JD, Weber ZJ, Fretwell DL, Said S,
Klopper JP and Haugen BR: Tumor-associated lymphocytes and
increased FoxP3+ regulatory T cell frequency correlate
with more aggressive papillary thyroid cancer. J Clin Endocrinol
Metab. 95:2325–2333. 2010.PubMed/NCBI
|
|
6
|
Kilicarslan AB, Ogus M, Arici C, Pestereli
HE, Cakir M and Karpuzoglu G: Clinical importance of vascular
endothelial growth factor (VEGF) for papillary thyroid carcinomas.
APMIS. 111:439–443. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y and Morrow JS: Identification and
characterization of human SLP-2, a novel homologue of stomatin
(band 7.2b) present in erythrocytes and other tissues. J Biol Chem.
275:8062–8071. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Christie DA, Kirchhof MG, Vardhana S,
Dustin ML and Madrenas J: Mitochondrial and plasma membrane pools
of stomatin-like protein 2 coalesce at the immunological synapse
during T cell activation. PLoS One. 7:e371442012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Owczarek CM, Treutlein HR, Portbury KJ,
Gulluyan LM, Kola I and Hertzog PJ: A novel member of the
STOMATIN/EPB72/mec-2 family, stomatin-like 2 (STOML2), is
ubiquitously expressed and localizes to HSA chromosome 9p13.1.
Cytogenet Cell Genet. 92:196–203. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Green JB and Young JP: Slipins: ancient
origin, duplication and diversification of the stomatin protein
family. BMC Evol Biol. 8:442008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tondera D, Grandemange S, Jourdain A, et
al: SLP-2 is required for stress-induced mitochondrial hyperfusion.
EMBO J. 28:1589–1600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Christie DA, Lemke CD, Elias IM, et al:
Stomatin-like protein 2 binds cardiolipin and regulates
mitochondrial biogenesis and function. Mol Cell Biol. 31:3845–3856.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Da Cruz S, Parone PA, Gonzalo P, et al:
SLP-2 interacts with prohibitins in the mitochondrial inner
membrane and contributes to their stability. Biochim Biophys Acta.
1783:904–911. 2008.PubMed/NCBI
|
|
14
|
Zhang L, Ding F, Cao W, et al:
Stomatin-like protein 2 is overexpressed in cancer and involved in
regulating cell growth and cell adhesion in human esophageal
squamous cell carcinoma. Clin Cancer Res. 12:1639–1646. 2006.
View Article : Google Scholar
|
|
15
|
Song L, Liu L, Wu Z, et al: Knockdown of
stomatin-like protein 2 (STOML2) reduces the invasive ability of
glioma cells through inhibition of the NF-κB/MMP-9 pathway. J
Pathol. 226:534–543. 2012.PubMed/NCBI
|
|
16
|
Cao WF, Zhang LY, Liu MB, Tang PZ, Liu ZH
and Sun BC: Prognostic significance of stomatin-like protein 2
overexpression in laryngeal squamous cell carcinoma: clinical,
histologic, and immunohistochemistry analyses with tissue
microarray. Hum Pathol. 38:747–752. 2007. View Article : Google Scholar
|
|
17
|
Cao W, Zhang B, Liu Y, et al: High-level
SLP-2 expression and HER-2/neu protein expression are associated
with decreased breast cancer patient survival. Am J Clin Pathol.
128:430–436. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han CL, Chen JS, Chan EC, et al: An
informatics-assisted label-free approach for personalized tissue
membrane proteomics: case study on colorectal cancer. Mol Cell
Proteomics. 10:M110.003087. 2011.PubMed/NCBI
|
|
19
|
Liu D, Zhang L, Shen Z, et al: Increased
levels of SLP-2 correlate with poor prognosis in gastric cancer.
Gastric Cancer. Jan 31–2013.(Epub ahead of print).
|
|
20
|
Wang Y, Cao W, Yu Z and Liu Z:
Downregulation of a mitochondria associated protein SLP-2 inhibits
tumor cell motility, proliferation and enhances cell sensitivity to
chemotherapeutic reagents. Cancer Biol Ther. 8:1651–1658. 2009.
View Article : Google Scholar
|
|
21
|
Eloy C, Santos J, Cameselle-Teijeiro J,
Soares P and Sobrinho-Simões M: TGF-beta/Smad pathway and
BRAF mutation play different roles in circumscribed and
infiltrative papillary thyroid carcinoma. Virchows Arch.
460:587–600. 2012.PubMed/NCBI
|
|
22
|
Knauf JA, Sartor MA, Medvedovic M, et al:
Progression of BRAF-induced thyroid cancer is associated with
epithelial-mesenchymal transition requiring concomitant MAP kinase
and TGFβ signaling. Oncogene. 30:3153–3162. 2011.PubMed/NCBI
|
|
23
|
Jasani B, Wyllie FS, Wright PA, Lemoine
NR, Williams ED and Wynford-Thomas D: Immunocytochemically
detectable TGF-β associated with malignancy in thyroid epithelial
neoplasia. Growth Factors. 2:149–155. 1990.
|
|
24
|
Müssig K, Wehrmann T, Dittmann H, et al:
Expression of the proliferation marker Ki-67 associates with tumor
staging and clinical outcome in differentiated thyroid carcinomas.
Clin Endocrinol. 77:139–145. 2012.PubMed/NCBI
|
|
25
|
Kimura ET, Kopp P, Zbaeren J, et al:
Expression of transforming growth factor β1, β2, and β3 in
multinodular goiters and differentiated thyroid carcinomas: a
comparative study. Thyroid. 9:119–125. 1999.
|
|
26
|
Cui Z, Zhang L, Hua Z, Cao W, Feng W and
Liu Z: Stomatin-like protein 2 is overexpressed and related to cell
growth in human endometrial adenocarcinoma. Oncol Rep. 17:829–833.
2007.PubMed/NCBI
|
|
27
|
Christie DA, Mitsopoulos P, Blagih J, et
al: Stomatin-like protein 2 deficiency in T cells is associated
with altered mitochondrial respiration and defective
CD4+ T cell responses. J Immunol. 189:4349–4360. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Da Cruz S, De Marchi U, Frieden M, Parone
PA, Martinou JC and Demaurex N: SLP-2 negatively modulates
mitochondrial sodium-calcium exchange. Cell Calcium. 47:11–18.
2010.PubMed/NCBI
|
|
29
|
Jonklaas J, Nogueras-Gonzalez G, Munsell
M, et al: The impact of age and gender on papillary thyroid cancer
survival. J Clin Endocrinol Metab. 97:E878–E887. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cao W, Zhang B, Ding F, Zhang W, Sun B and
Liu Z: Expression of SLP-2 was associated with invasion of
esophageal squamous cell carcinoma. PLoS One. 8:e638902013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Massagué J: TGFβ in cancer. Cell.
134:215–230. 2008.
|
|
32
|
Montero-Conde C, Martin-Campos JM, Lerma
E, et al: Molecular profiling related to poor prognosis in thyroid
carcinoma. Combining gene expression data and biological
information. Oncogene. 27:1554–1561. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vasko V, Espinosa AV, Scouten W, et al:
Gene expression and functional evidence of
epithelial-to-mesenchymal transition in papillary thyroid carcinoma
invasion. Proc Natl Acad Sci USA. 104:2803–2808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Riesco-Eizaguirre G, Rodriguez I, De la
Vieja A, et al: The BRAFV600E oncogene induces
transforming growth factor β secretion leading to sodium iodide
symporter repression and increased malignancy in thyroid cancer.
Cancer Res. 69:8317–8325. 2009.
|