Introduction
BCL6 is a transcriptional repressor which has
emerged as a critical regulator of germinal centers (GCs) of lymph
nodes. BCL6 is also a frequently activated oncogene in the
pathogenesis of human B cell lymphomas, most of which derive from
the GC B cells (1). Especially in
the last 10 years, knowledge concerning this gene and its
expression has improved considerably. Identification of factors
acting together with the BCL6 protein as well as delineation
of several target genes have allowed a determination of its
biological function. This set of BCL6 targets points to a
number of cellular functions which are likely to be directly
controlled by BCL6 during GC development, including
activation, survival, DNA-damage response, cell cycle arrest,
cytokine-, toll-like receptor-, TGFβ-, WNT-signaling and
differentiation (2).
BCL6 protein is a 92- to 98-kDa nuclear
phosphoprotein that is produced at low levels in several tissues
and is expressed at high levels exclusively in GC B cells (3,4). High
levels of BCL6 gene expression in non-Hodgkin’s lymphomas
have a favorable prognostic value (5,6).
BCL6 contains an N-terminal BTB/POZ domain and 6
Kruppel-type (C2H2) zinc-finger (ZnF) motifs in the C terminus
(7). The BTB/POZ domain displays a
conserved protein-protein interaction motif that is required for
the repressive activity of the protein (8). The BTB domain interacts with several
other proteins which are members of the BTB/POZ-zinc finger family
(BAZF, LRF, PLZF) (9–11). Although BCL6 contributes to
lymphomagenesis by allowing conditions favorable to lymphoma
pathogenesis, its precise role in the malignant transformation
remains to be fully elucidated.
Logarajah et al(12) and Bos et al(13) reported that BCL6 has an
important function in breast cancer. Walters et al(14) evaluated the BCL6 protein
expression in a subset of mesenchymal tumors. However, to our
knowledge, there are no data on BCL6 expression/role in the
adenoma-carcinoma sequence of colorectal carcinogenesis.
Colorectal cancer remains the second leading cause
of cancer-related mortality in Western countries, and it develops
mainly from adenomatous polyps and shows a morphological and
genetic progression through an adenoma-carcinoma sequence, both in
hereditary and in sporadic colorectal cancer (15). Microadenomas (MAs) have been
generally accepted as precancerous lesions on the basis of
histopathological characteristics, biochemical and
immunohistochemical features, as well as genetic and epigenetic
alterations (16–19). The purpose of the present study was
two-fold: (i) to determine whether BCL6 is expressed during
malignant transformation of the large bowel and (ii) to assess
whether, and to what extent, BCL6 immunoreactivity is
related to the different stages of neoplastic progression.
Therefore, samples of human normal mucosa, MA and
cancer were evaluated with different methods, i.e.,
immunohistochemistry, immunofluorescence and western blot
analysis.
The results obtained showed a significant expression
of BCL6 protein in preneoplastic and neoplastic lesions and
suggest a possible involvement of BCL6 during the malignant
transformation of colorectal mucosa.
Materials and methods
Study population
Twenty-two samples of normal colorectal mucosa (NM)
were collected from 10 patients during colonoscopy, at least 2
samples from each patient. One sample from each patient was fixed
in formalin and embedded in paraffin for immunohistochemistry; the
others were frozen at −80°C. All patients had normal colonoscopy.
Twenty-two MAs were also identified in 6 patients, and removed
after operation for colorectal cancer on surgical specimens; the
precise localization of the lesion was made staining the resected
mucosa with a 0.1% methylene-blue solution in saline, and observing
it under a dissecting microscope in order to take and process only
the fields of the lesion, as previously described (16,19).
All MAs, defined as dysplastic aberrant crypt foci at histology
were referred to as MAs. Ten MAs were fixed in formalin and
embedded in paraffin, the others were frozen at −80°C. Finally, 22
samples of colorectal cancer (K) were collected from 11 patients
operated on for cancer, fixed in formalin or frozen at −80°C. All
cases of cancer were Dukes’ C adenocarcinomas.
The patients enrolled in the present study, who
underwent colonoscopy or surgical resection for colorectal cancer
at the University Hospital of Modena, provided written informed
consent to the study protocol, which was approved by the local
Ethics Committee.
Immunohistochemistry
Ten samples of NM, 10 of MA, and 10 of K were fixed
in a 10% formalin solution for 1 h. They were then dehydrated,
embedded in paraffin wax, and sectioned (3-μm thick); the sections
were placed on Super Frost Plus microscope slides (Menzel). Prior
to immunohistochemistry, routine histology of all tissue samples
was carried out after hematoxylin and eosin (H&E) staining of
the sections. Slides were dried overnight at 37°C, dewaxed in 2
changes of fresh xylene, and rehydrated in a descending alcohol
series. Antigen retrieval involved treatment with a protease
(Pronase 1:20; DakoCytomation) for 7 min at 37°C. Prior to
immunohistochemical staining, the sections were washed with PBS and
blocked with 20% Swine Serum (Normal; DakoCytomation) in PBS for 30
min to reduce nonspecific antibody binding. Primary antibody was
then applied at appropriate dilutions for 30 min at room
temperature. Polyclonal rabbit anti-mouse BCL6 antibody
(DakoCytomation) was used as primary antibody at a dilution of
1:200 in PBS. Endogenous peroxidase activity was blocked by
treating the slides with 0.3% hydrogen peroxide in methanol for 12
min. After washing with PBS, biotinylated anti-mouse secondary
antibody (1:20; DakoCytomation) was incubated for 30 min, and the
slides were incubated with pre-diluted streptavidin-HRP conjugates
for 30 min according to the manufacturer’s instructions. In order
to reveal the color of the antibody staining, DAB substrate
solution (freshly made immediately prior to use: 0.05% DAB-0.015%
H2O2 in PBS) was used. Slices were
counterstained with Mayer’s hematoxylin, dehydrated in ascending
alcohol series and covered with a coverslip (Histovitrex mounting
medium; Carlo Erba). For all these procedures, the negative control
was obtained by staining samples without the primary antibody.
Human B cell lymphomas were used as positive control.
Scoring system
One or 2 slices for each sample were scored as
described below for immunofluorescence analysis. For each group of
colorectal tissue and lesions, about 25 ×100 microscopic fields
were scored. Moreover slices for each group of normal mucosa and
lesions were scored by 2 independent observers and there was <5%
variance between the results of the 2 counts. To quantify the
staining area, the slices were processed with an ImageJ software.
The images were converted into 16-bit images and then thresholded
(the same value was used in all analyzed images). The resulting
thresholded images were binary and they showed only the staining
area. The total staining area was calculated by the software.
Immunofluorescence confocal
microscopy
Immunofluorescence analysis was carried out to
evaluate the expression of BCL6 protein. Six samples of NM,
6 of MA and 6 of K, were fixed in 4% paraformaldehyde in PBS,
cryoprotected in 15% sucrose in PBS, and frozen in isopentane
cooled in liquid nitrogen. Horizontal cryosections of the samples
were cut (10-μm thick), and H&E staining was performed on
sections to control tissue integrity. After a treatment with 3% BSA
in PBS for 30 min at room temperature, the cryostatic sections were
incubated with the primary antibodies (mouse anti-BCL6;
DakoCytomation) diluted 1:25 in PBS containing 3% BSA for 1 h at
room temperature. After washing in PBS, the samples were incubated
for 1 h at room temperature with the secondary antibodies diluted
1:20 in PBS containing 3% BSA (sheep anti-mouse FITC-conjugated;
Sigma). After washing in PBS and in H2O, the samples
were counterstained with 1 μg/ml DAPI in H2O and then
mounted with anti-fading medium (0.21 M DABCO and 90% glycerol in
0.02 M Tris, pH 8.0). Negative control samples were not incubated
with the primary antibody. The confocal imaging was performed on a
Leica TCS SP2 AOBS confocal laser scanning microscope. For DAPI and
FITC double detection, samples were sequentially excited with the
405-nm/25-mW lines of a blue diode laser and the 488-nm/20-mW lines
of the Argon laser. The emission signals from DAPI and FITC were
detected by 2 photomultiplier tubes. Excitation and detection of
the samples were carried out in sequential mode to avoid
overlapping of signals. Sections were scanned with laser intensity,
confocal aperture, gain and black-level setting kept constant for
all samples. Optical sections were obtained at increments of 0.3-μm
in the z-axis and digitized with a scanning mode format of 512×512
or 1,024×1,024 pixels and 256 grey levels. The confocal serial
sections were processed with Leica LCS software to obtain
3-dimensional projections. Image rendering was performed by adobe
Photoshop software.
Evaluation of BCL6
immunofluorescence
The original green fluorescent confocal images were
converted to grey-scale and median filtering was performed. An
intensity value ranging from 0 (black) to 255 (white) was assigned
to each pixel. Background fluorescence was subtracted and the
immunofluorescence intensity was calculated as the average for each
selected area. To quantify BCL6 expression, all blocks were
completely sectioned and 3–4 slices were examined at a
magnification of ×40 for each patient. Starting randomly, 3
microscopic fields for each section were used for sampling all
sections within the unbiased sampling frame. The fluorescence
intensity at the selected areas, linearly correlated with the
number of pixels, was quantitatively analyzed using the standard
imaging analysis software of the NIS-Elements system. To each
sample was assigned a code number and the score, referred to as
immunofluorescence intensity score, determined by an observer who
was blind to tissue groups during analysis (20).
Western blot analysis
Whole cell lysates were obtained from 6 samples of
NM, 6 of MA and 6 of K, extracted with hypotonic buffer (50 mM
Tris-Cl, pH 7.8, containing 1% Nonidet P-40, 140 mM NaCl, 0.1% SDS,
0.1% Na deoxycholate, 1 mM Na3VO4 and freshly
added protease inhibitor cocktail). Lysates were then cleared by
centrifugation for 15 min in a refrigerated centrifuge (1,500 rpm)
and immediately boiled in SDS sample buffer. Protein extract (40
μg) from each sample (NM, MA and K) was electrophoresed on SDS-PAGE
and transferred to nitrocellulose membranes. The protocols of the
western blotting were performed as previously described (20). The membranes were blocked with 3%
dry milk and 2% BSA in PBS-T and were then incubated with mouse
anti-BCL6 (DakoCytomation) antibody, diluted 1:1,000
overnight at 4°C under agitation. After washing, the membranes were
incubated with secondary HPR-conjugated goat anti-mouse IgG
antibody (1:10,000) for 30 min at room temperature. Immunoreactive
proteins were detected with ECL (Amersham). Furthermore, the
membranes were stripped and incubated with anti-mouse β-tubulin
(Sigma) to control and correct for loading error. Densitometry
analysis was performed using a Kodak Image Station 440CF system
(Rochester, NY, USA).
Statistical analysis
All quantitative data for NM, MA and K are reported
as means ± SD. The difference in BCL6 average expression in
the different groups of colorectal lesions was tested for
statistical significance using ANOVA one-way analysis, followed by
Student-Newman-Keuls tests. P<0.05 was considered to indicate a
statistically significant difference between groups.
Results
Morphological evaluation
The quality of staining obtained by the
immunohistochemical method was generally very good; BCL6
staining was evident as brown spots both in the cytoplasm and in
the nucleus of stromal cells, easily allowing their identification
even at low magnification. Examples of BCL6 staining are
shown in Fig. 1, in which panels A
and B show low levels of BCL6-positive cells
(fibroblast-like cells and leukocytes) in NM, especially in the
stroma adjacent to the crypts. In all samples of normal mucosa,
appreciable BCL6 staining of epithelial cells was not found.
In MAs, BCL6 expression was higher compared to normal mucosa
and it was very evident in the stromal compartment (Fig. 1C and D). Moreover, a consistent
epithelial positivity was observed in MA and K: the protein
aggregated in small clumps appears distributed in the cytoplasm at
the lateral and basal portions of the epithelial cells and also
partially in their nucleus (Fig.
1G). Concerning the stromal compartment, the samples of K were
characterized by a higher number of BCL6-positive cells with
respect to MA (Fig. 1E and F); in
carcinoma, positive cells were not evenly distributed in the
tissue, but tended to gather in some areas, usually at the edge of
the neoplastic tissue (Fig. 1E),
not only in the stroma inside the tumor (Fig. 1F), but also among the neoplastic
cells at the margins of the lesions (Fig. 1H). Inside the epithelial-positive
cells, BCL6 protein accumulated predominantly at the apical
region of cells towards the lumen of colonic crypts, as well as at
the nuclear level (Fig. 1H). The
semi-quantitative evaluation of immunostaining intensity reported
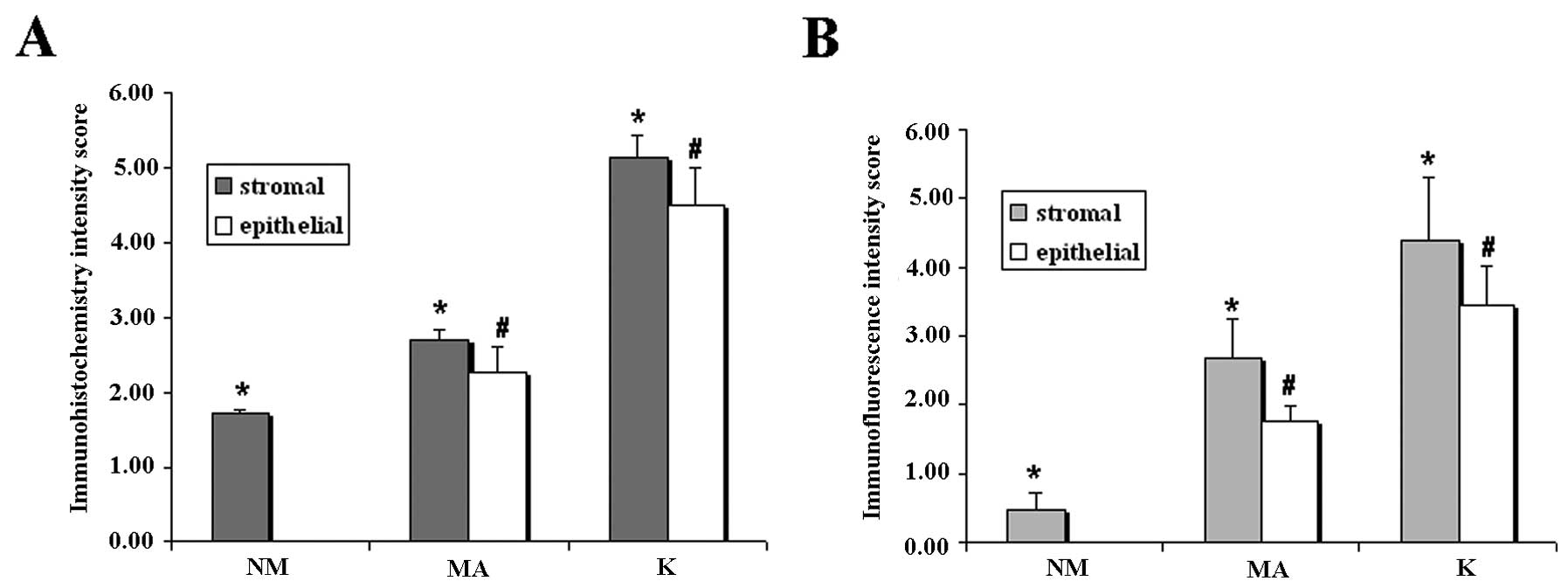
as immunohistochemistry intensity score in Fig 3A, showed that the level of
BCL6 protein in both the epithelial and the stromal
compartment had an increasing trend from NM to K, with statistical
significance of the different expression among the groups. In order
to obtain a better resolution of subcellular structures in very
thick samples, the confocal analysis of BCL6 was performed
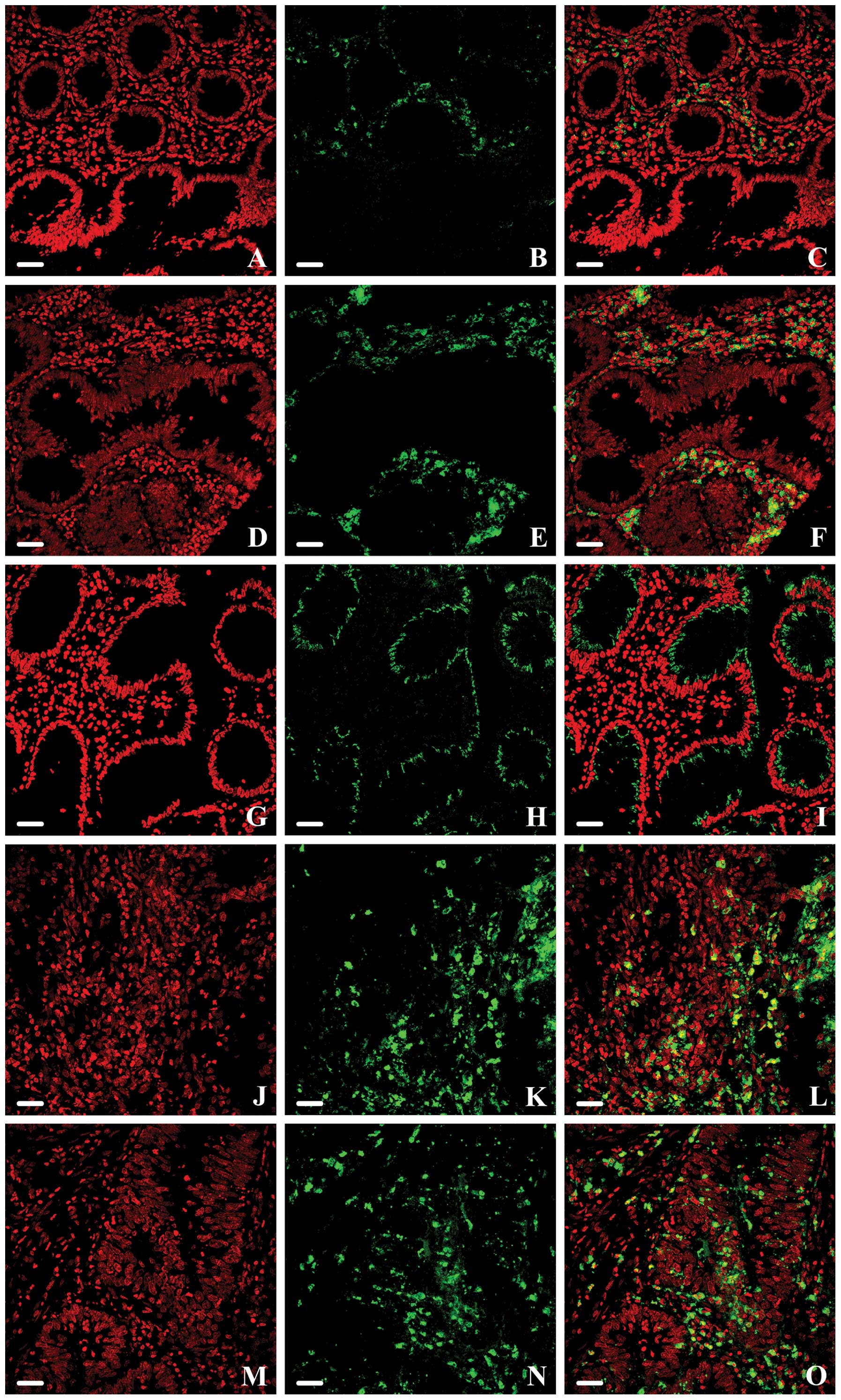
in a subset of NM, MA and K samples (Fig. 2). Moreover, this technique allowed
to define the precise distribution pattern and the quantification
of BCL6 protein. The staining patterns of BCL6 varied
from a diffuse mode to a granular one, both in the cytoplasm and in
the nucleus of stromal cells, appearing consistent and unchanged in
the same samples, without appreciable variations in intensity and
localization among the cells. In all NM samples, a few scattered
areas in the stroma compartment exhibited a moderate
BCL6-reactivity, although the epithelial cells were not
marked (Fig. 2A–C). The results of
immunofluorescence analysis were in line with those of
immunohistochemistry: intense staining was evident in MA, in
stromal cells surrounding colonic crypts (Fig. 2D–F); moreover, a moderate expression
appeared in epithelial cells, but only at the cytoplasmic level
(Fig. 2G–I). BCL6-positive
cells were observed throughout the resected tissue in all colon
cancer lesions and they were particularly numerous within the
central tumor stroma (Fig. 2J–L).
Some cells (both the epithelial and stromal ones) showed a strong
BCL6 immunoreactivity in the cytoplasm as well as in the
nucleus inside some tumor areas (Fig.
2M–O); in particular, they appeared located deeply, inside the
tumor lesion. These features were confirmed by the measurement of
the BCL6 expression levels, both in the epithelial and in
the stromal compartment, by means of immunohistochemistry and
immunofluorescence, that showed statistically significant
differences in the 3 different types of samples (Fig. 3A and B). These results were in
agreement with those obtained by immunohistochemical analysis.
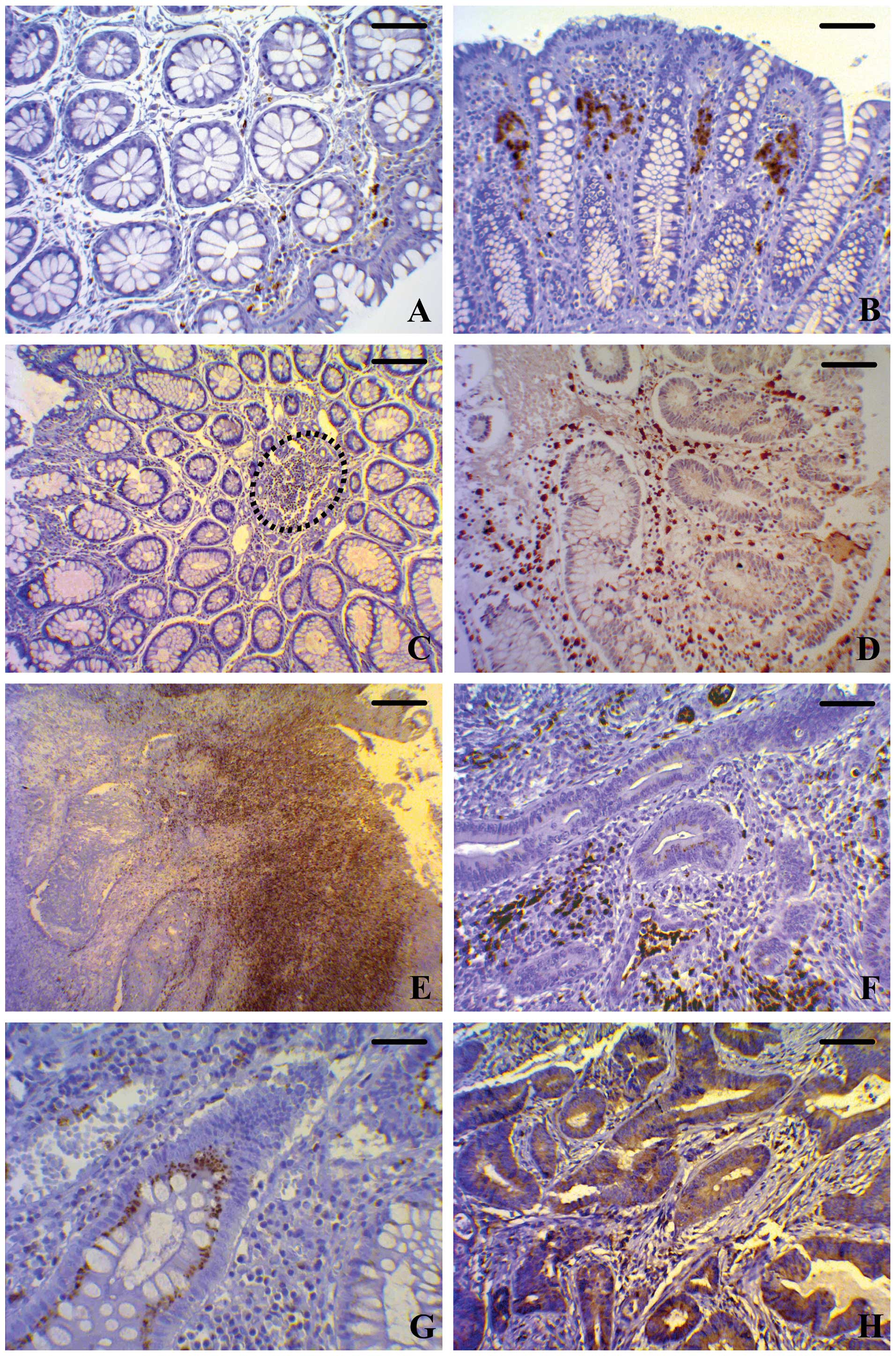 | Figure 1Immunohistochemical staining of
BCL6 in different human colonic tissues. (A and B) Normal
mucosa at different magnifications. (C and D) MA at different
magnifications. (E and F) Colorectal cancer at different
magnifications. (G and H) High magnifications of MAs and colorectal
cancer, respectively. The expression of BCL6 in samples of
normal mucosa is low and restricted to the stromal compartment, as
is well evident in both (A) transverse and in (B) longitudinal
sections. The BCL6-positive cells show nuclear and
cytoplasmic localization of the protein. MA samples are
characterized by high expression at both the cytoplasmic and the
nuclear level of fibroblast-like cells and leukocytes that fill the
stromal compartment (dotted line). (C) Transverse section; (D)
longitudinal section. In MA, the epithelial cells of the crypt show
well evident BCL6 immunostaining, particularly in the (G)
cytoplasm. In the lamina propria of colorectal cancer, the
number of BCL6-positive fibroblast-like cells and leukocytes
is significantly higher than in MA. (E and F) The protein is
localized at both the cytoplasmic and the nuclear level. In
colorectal cancer samples, diffuse high cytoplasmic and nuclear
BCL6 expression is present in tumor cells (H). (A and D:
bar, 50 μm; B: bar, 70 μm; C: bar, 100 μm; E: bar, 200 μm; F and H:
bar, 50 μm; G: bar, 25 μm). MAs, microadenomas. |
Western blot quantification
To confirm the findings of the morphological
evaluation previously described, cell lysates of NM, MA and K were
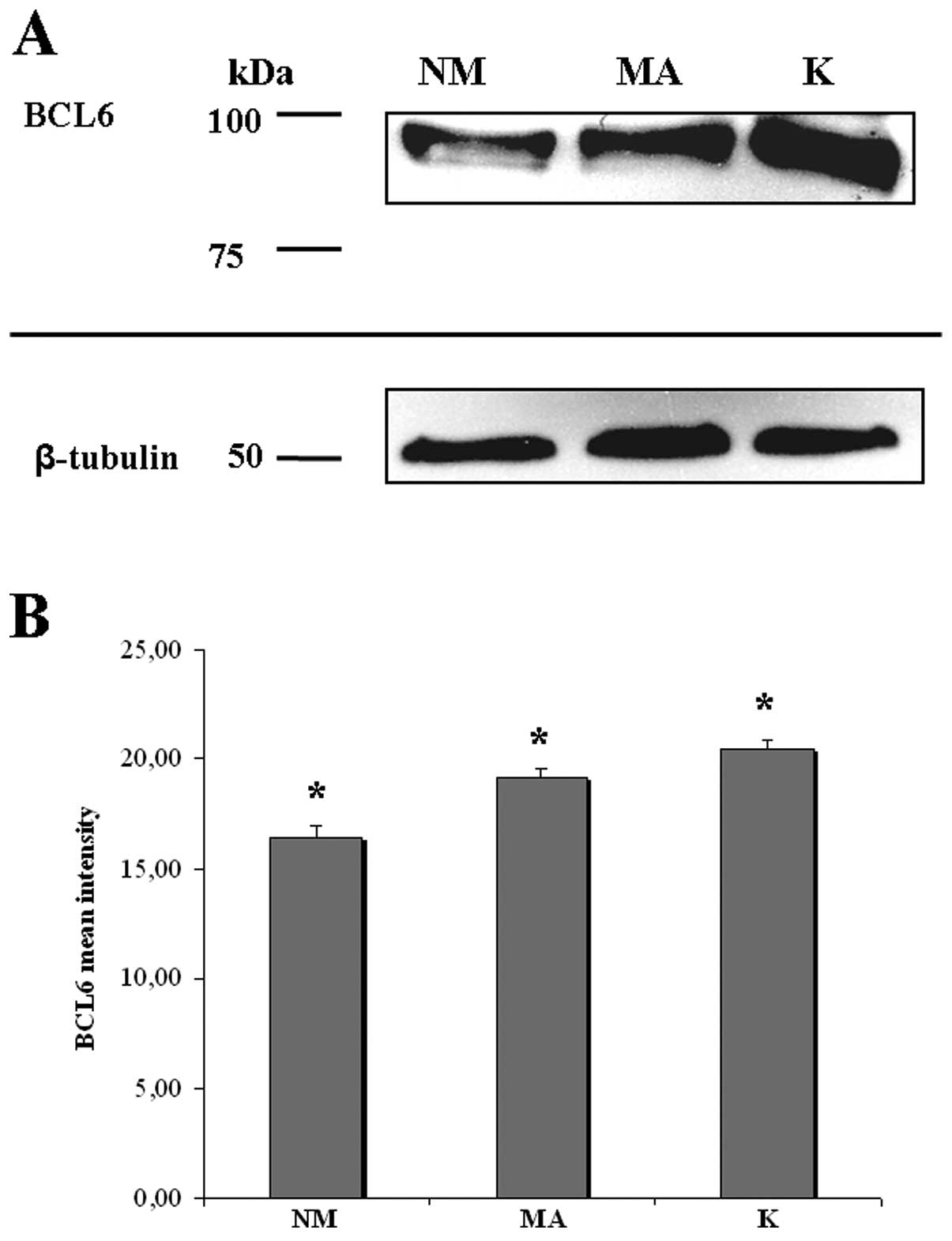
analyzed by western blotting. The protein profile of BCL6
clearly showed 1 single weak band at the expected molecular weight
(98-kDa) in NM, an intense band in MA and a very intense band in K
(Fig. 4A). Densitometric analysis
and normalization (with equal amounts of protein loading) of the
immunoreactivity signal from protein extracts of NM, MA and K
showed that the level of BCL6 protein has an increasing
trend from NM to K, with statistical significance of the different
expressions (Fig. 4B).
Discussion
The aim of the present study was to evaluate
BCL6 expression during the neoplastic progression of human
intestinal mucosa. BCL6 is a transcriptional repressor
required in mature B cells during the germinal center (GC) reaction
(3). The critical functions exerted
by BCL6 during normal B-cell development can be hijacked by
the malignant transformation process, thus indicating its critical
involvement in colorectal cancer. Indeed, BCL6 is targeted
by genetic aberrations and acts as an oncogene in GC-derived
lymphomas (2); despite this, its
precise role in the malignant transformation process remains to be
fully elucidated. In non-hematolymphoid neoplasms, BCL6
expression has been demonstrated in subsets of high grade ductal
human carcinomas of the breast, both at the gene and the protein
level (12,13).
To our knowledge, this is the first comprehensive
report from morphological and quantitative analyses of BCL6
in human colorectal carcinogenesis, both in the epithelial and the
stromal compartment. The results clearly outline that there is a
marked increase in the intensity and density of staining for
BCL6 protein in the normal mucosa-microadenoma-carcinoma
sequence. Immunohistochemistry and immunofluorescence analyses
showed that BCL6 is expressed in normal mucosa, although at
low levels, whereas it significantly increases in microadenoma (MA)
and in cancer with statistical significance. These results were
confirmed by western blotting data, with no difference according to
the various techniques used. In BCL6-positive cells, both
the nucleus and the cytoplasm appeared stained and this finding is
in accordance with previous data on cellular localization of
BCL6 in gastric cancer (21). Moreover, our observations
consistently showing that the BCL6 protein is expressed only
in the stromal compartment of the normal mucosa support some
observations of other authors; BCL6 was considered to be
produced at low levels in various tissues, as reported in EST
libraries obtained from several epithelial tissues, although Hirata
et al(21) reported that
BCL6 is expressed in normal gastric epithelial cells and
that it is not expressed in normal mucosa of colon and oesophagus.
However, in their study, the authors did not examine the stromal
compartment of the colon and did not show quantitative data.
In addition, our data showed that the BCL6
protein is highly expressed in epithelial cells, in premalignant
lesions as well as in carcinomatous tissues. For this reason, we
hypothesized that the increased expression of the BCL6
protein is due to the transformation of the epithelium, that
represents an important source of this protein.
Another notable consideration is that the
BCL6 expression/production by the epithelium appears to be
linked to the early stages of carcinogenesis, since the positivity
is strongly detectable in MAs. Although MAs are not the only
microscopic lesions in the large bowel, they represent the most
common precursors of cancer in this organ (22,23).
In these neoplasms, the transformation process begins in the crypts
and seems to result from qualitative, quantitative and spatial
reorganization of some pathways that represent the physiologic
regulator of epithelial homeostasis (24–26).
Although the key role played by MAs in colorectal tumorigenesis is
widely acknowledged, the modifications of gene expression that
trigger or accompany their development have never been
comprehensively studied. This investigation identifies, for the
first time, BCL6 as a putative marker of colorectal
tumorigenesis and may also provide new insight for future molecular
characterization of colorectal precancerous lesions.
Recently, Walters et al(14) examined a series of mesenchymal
tumors with immunohistochemical and fluorescence in situ
hybridization techniques. They reported that BCL6 expression
is relatively common in solitary fibrous tumor (SFT) and that,
markedly, BCL6 protein seems to be significantly more
expressed in malignant SFT, by the histological point of view, as
compared to benign SFT. This finding is significant, inasmuch as
our results suggest that the BCL6 protein may play some
roles in the malignant transformation occurring in the large bowel.
Our data should be validated with further investigations on
premalignant and malignant lesions and/or different subtypes of
colorectal cancer.
As is well known, microarray data serve as a
springboard and reference point for further studies on the
molecular basis of colorectal transformation along the
adenocarcinoma pathway; in this regard, Saadi et al(27) recently carried out a gene set
enrichment analysis (GSEA) to demonstrate that genes of the stromal
cellular compartment discriminate pre-invasive from invasive
disease and suggest outcome prediction in digestive tumors,
including colorectal cancer. The authors identified several genes,
including BCL6, and underlined that the upregulation of this
protein shows a trend for significantly poorer outcome in
esophageal adenocarcinoma. Although the genes identified are
relevant to Barrett’s carcinogenesis, it is likely that these genes
are involved in a dysregulated process common to other
gastrointestinal tumors rather than being isolated critical
oncogenes.
Furthermore, we consider immunohistochemistry
critical in performing the identification of proteins which are
current as well as new putative therapeutic targets.
On the basis of our excellent results obtained by
immunohistochemical analysis, fully confirmed by immunofluorescence
and western blotting methods, it would be useful to determine
whether the BCL6 protein can be considered a histological
marker for colorectal cancer.
Considering the well documented accuracy of the
prognostic relevance of BCL6 in large B-cell lymphomas
(28–30), it is possible that quantification of
BCL6 expression may have a potential role in risk evaluation
of large bowel tumors.
In conclusion, the findings of the present study
clearly show the qualitative and quantitative expression of
BCL6 in human colorectal cancer development (in particular,
the localization of BCL6 expression in precancerous as well
as tumor epithelia), and demonstrate that BCL6 appears to be
involved in tumor progression, from the earliest stage of
carcinogenesis. The improved understanding of the biological
function of BCL6, the key role of which was already
demonstrated in lymphomagenesis, should lead to a re-evaluation of
the importance of this protein in other tissues and may highlight
the relevance of performing further studies in order to identify
novel therapeutic targets for colorectal cancer.
Acknowledgements
The present study was supported by funds of the
‘Fondazione di Vignola’ (Vignola-MO, Italy), the ‘Banca Popolare
dell’Emilia Romagna’ (Modena, Italy) and the Cremonini Group
(Castelvetro, MO, Italy). The authors thank the Centro
Interdipartimentale Grandi Strumenti (C.I.G.S.) of the University
of Modena and Reggio Emilia for software, instrument availability
and assistance, the Fondazione ALTEG (Associazione per la Lotta ai
Tumori nell’ Età Giovanile), the Fondazione Umberto Veronesi, and
the Associazione per la Ricerca sui Tumori Intestinali (A.R.T.I),
Modena, Italy.
References
|
1
|
Dent AL, Shaffer AL, Yu X, Allman D and
Staudt LM: Control of inflammation, cytokine expression, and
germinal center formation by BCL-6. Science. 276:589–592. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Basso K and Dalla-Favera R: Roles of
BCL6 in normal and transformed germinal center B cells.
Immunol Rev. 247:172–183. 2012.
|
|
3
|
Cattoretti G, Chang CC, Cechova K, Zhang
J, Ye BH, Falini B, Louie DC, Offit K, Chaganti RS and Dalla-Favera
R: BCL-6 protein is expressed in germinal-center B cells. Blood.
86:45–53. 1995.PubMed/NCBI
|
|
4
|
Onizuka T, Moriyama M, Yamochi T, Kuroda
T, Kazama A, Kanazawa N, Sato K, Kato T, Ota H and Mori S: BCL-6
gene product, a 92- to 98-kD nuclear phosphoprotein, is highly
expressed in germinal center B cells and their neoplastic
counterparts. Blood. 86:28–37. 1995.
|
|
5
|
Alizadeh AA, Eisen MB, Davis RE, Ma C,
Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell
JI, Yang L, Marti GE, Moore T, Hudson J Jr, Lu L, Lewis DB,
Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD,
Armitage JO, Warnke R, Levy R, Wilson W, Grever MR, Byrd JC,
Botstein D, Brown PO and Staudt LM: Distinct types of diffuse large
B-cell lymphoma identified by gene expression profiling. Nature.
403:503–511. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lossos IS, Jones CD, Warnke R, Natkunam Y,
Kaizer H, Zehnder JL, Tibshirani R and Levy R: Expression of a
single gene, BCL-6, strongly predicts survival in patients
with diffuse large B-cell lymphoma. Blood. 98:945–951.
2001.PubMed/NCBI
|
|
7
|
Dhordain P, Albagli O, Ansieau S, Koken
MH, Deweindt C, Quief S, Lantoine D, Leutz A, Kerckaert JP and
Leprince D: The BTB/POZ domain targets the LAZ3/BCL6
oncoprotein to nuclear dots and mediates homomerisation in vivo.
Oncogene. 11:2689–2697. 1995.PubMed/NCBI
|
|
8
|
Jardin F, Ruminy P, Bastard C and Tilly H:
The BCL6 proto-oncogene: a leading role during germinal
center development and lymphomagenesis. Pathol Biol. 55:73–83.
2007.
|
|
9
|
Okabe S, Fukuda T, Ishibashi K, Kojima S,
Okada S, Hatano M, Ebara M, Saisho H and Tokuhisa T: BAZF, a novel
Bcl6 homolog, functions as a transcriptional repressor. Mol
Cell Biol. 18:4235–4244. 1998.
|
|
10
|
Davies JM, Hawe N, Kabarowski J, Brand NJ,
Leprince D, Dhordain P, Cook M, Morriss-Kay G and Zelent A: Novel
BTB/POZ domain zinc-finger protein, LRF, is a potential target of
the LAZ-3/BCL-6 oncogene. Oncogene. 18:365–375. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dhordain P, Albagli O, Honore N, Guidez F,
Lantoine D, Schmid M, The HD, Zelent A and Koken MH: Colocalization
and heteromerization between the two human oncogene POZ/zinc finger
proteins, LAZ3 (BCL6) and PLZF. Oncogene. 19:6240–6250.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Logarajah S, Hunter P, Kraman M, Steele D,
Lakhani S, Bobrow L, Venkitaraman A and Wagner S: BCL-6 is
expressed in breast cancer and prevents mammary epithelial
differentiation. Oncogene. 22:5572–5578. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bos R, van Diest PJ, van der Groep P,
Greijer AE, Hermsen MA, Heijnen I, Meijer GA, Baak JP, Pinedo HM,
van der Wall E and Shvarts A: Protein expression of B-cell lymphoma
gene 6 (BCL-6) in invasive breast cancer is associated with cyclin
D1 and hypoxia-inducible factor-1α (HIF-1α). Oncogene.
22:8948–8951. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Walters MP, McPhail ED, Law ME and Folpe
AL: BCL-6 expression in mesenchymal tumours: an immunohistochemical
and fluorescence in situ hybridisation study. J Clin Pathol.
64:866–869. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suehiro Y and Hinoda Y: Genetic and
epigenetic changes in aberrant crypt foci and serrated polyps.
Cancer Sci. 99:1071–1076. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di Gregorio C, Losi L, Fante R, Modica S,
Ghidoni M, Pedroni M, Tamassia MG, Gafà L, Ponz de Leon M and
Roncucci L: Histology of aberrant crypt foci in the human colon.
Histopathology. 30:328–334. 1997.
|
|
17
|
Roncucci L, Pedroni M, Vaccina F, Benatti
P, Marzona L and De Pol A: Aberrant crypt foci in colorectal
carcinogenesis. Cell and crypt dynamics. Cell Prolif. 33:1–18.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takayama T, Miyanishi K, Hayashi T,
Kukitsu T, Takanashi K, Ishiwatari H, Kogawa T, Abe T and Niitsu Y:
Aberrant crypt foci: detection, gene abnormalities, and clinical
usefulness. Clin Gastroenterol Hepatol. 3(Suppl 1): S42–S45. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roncucci L, Mora E, Mariani F, Bursi S,
Pezzi A, Rossi G, Pedroni M, Luppi D, Santoro L, Monni S, Manenti
A, Bertani A, Merighi A, Benatti P, Di Gregorio C and de Leon PM:
Myeloperoxidase-positive cell infiltration in colorectal
carcinogenesis as indicator of colorectal cancer risk. Cancer
Epidemiol Biomarkers Prev. 17:2291–2297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sena P, Roncucci L, Marzona L, Mariani F,
Maffei S, Manenti A and De Pol A: Altered expression of apoptosis
biomarkers in human colorectal microadenomas. Cancer Epidemiol
Biomarkers Prev. 19:351–357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hirata Y, Ogasawara N, Sasaki M, Mizushima
T, Shimura T, Mizoshita T, Mori Y, Kubota E, Wada T, Tanida S,
Kataoka H, Kamiya T, Higashiyama S and Joh T: BCL6
degradation caused by the interaction with the C-terminus of
pro-HB-EGF induces cyclin D2 expression in gastric cancers. Br J
Cancer. 100:1320–1329. 2009. View Article : Google Scholar
|
|
22
|
Roncucci L, Medline A and Bruce WR:
Classification of aberrant crypt foci and microadenomas in human
colon. Cancer Epidemiol Biomarkers Prev. 1:57–60. 1991.PubMed/NCBI
|
|
23
|
Takayama T, Katsuki S, Takahashi Y, Ohi M,
Nojiri S, Sakamaki S, Kato J, Kogawa K, Miyake H and Niitsu Y:
Aberrant crypt foci of the colon as precursors of adenoma and
cancer. N Engl J Med. 339:1277–1284. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Korinek V, Barker N, Morin PJ, van Wichen
D, de Weger R, Kinzler KW, Vogelstein B and Clevers H: Constitutive
transcriptional activation by a β-catenin-Tcf complex in
APC−/−colon carcinoma. Science. 275:1784–1787. 1997.
|
|
25
|
Morin PJ, Sparks AB, Korinek V, Barker N,
Clevers H, Vogelstein B and Kinzler KW: Activation of β-catenin-Tcf
signalling in colon cancer by mutations in β-catenin or APC.
Science. 275:1787–1790. 1997.
|
|
26
|
Pinto D and Clevers H: Wnt, stem cells and
cancer in the intestine. Biol Cell. 97:185–196. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saadi A, Shannon NB, Lao-Sirieix P,
O’Donovan M, Walker E, Clemons NJ, Hardwick JS, Zhang C, Das M,
Save V, Novelli M, Balkwill F and Fitzgerald RC: Stromal genes
discriminate preinvasive from invasive disease, predict outcome,
and highlight inflammatory pathways in digestive cancers. Proc Natl
Acad Sci USA. 107:2177–2182. 2010. View Article : Google Scholar
|
|
28
|
Bilalovic N, Blystad AK, Golouh R, Nesland
JM, Selak I, Trinh D and Torlakovic E: Expression of bcl-6 and CD10
protein is associated with longer overall survival and time to
treatment failure in follicular lymphoma. Am J Clin Pathol.
121:34–42. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Berglund M, Thunberg U, Amini RM, Book M,
Roos G, Erlanson M, Linderoth J, Dictor M, Jerkeman M,
Cavallin-Ståhl E, Sundström C, Rehn-Eriksson S, Backlin C, Hagberg
H, Rosenquist R and Enblad G: Evaluation of immunophenotype in
diffuse large B-cell lymphoma and its impact on prognosis. Mod
Pathol. 18:1113–1120. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Levy O, Deangelis LM, Filippa DA, Panageas
KS and Abrey LE: Bcl-6 predicts improved prognosis in primary
central nervous system lymphoma. Cancer. 112:151–156. 2008.
View Article : Google Scholar : PubMed/NCBI
|