Introduction
Esophageal squamous cell carcinoma (ESCC) remains a
major worldwide gastrointestinal tract malignancy. Although the
incidence of ESCC has decreased in Western countries, it is still a
major public health problem worldwide (1,2). Since
ESCC usually occurs in the middle and upper part of the esophagus,
the risks of surgery often outweigh the benefits, making this a
fatal cancer (3,4). Due to its poor prognosis, major
efforts have been undertaken to discover new therapies for ESCC
(5).
Many ESCC patients receive chemotherapy regardless
of their TNM-stage due to a high perioperative risk (3). Consequently, chemotherapy has been
widely used in ESCC patients and has proven benefits. Paclitaxel,
platinums and fluoropyrimidines are all used for the treatment of
ESCC (1). Paclitaxel arrests the
cell cycle by interfering with the microtubular network during cell
division (1,3). It is commonly administered in patients
with breast, lung or prostate cancer and those with melanoma due to
its cytotoxic effect (1,6–9).
Although platinums and fluoropyrimidines have historically been
used to treat gastroesophageal cancer, paclitaxel alone or in
combination with other agents such as 5 FU or cisplatin is a
current therapy (1). Studies have
suggested that paclitaxel is efficacious in patients with ESCC and
its use results in improvements in clinical outcomes (1,10,11).
However, patients who are treated with paclitaxel often relapse or
do not respond to the treatment. In vitro experiments have
shown that paclitaxel induces apoptosis in ESCC cells but different
sensitivities were observed, suggesting that responses to
paclitaxel may vary (12). Higher
dosages of paclitaxel can induce toxicity in kidney and liver.
Doxorubicin is an effective cytotoxic anticancer
agent and has been used for the treatment of variety of
malignancies (13). Doxorubicin
binds to DNA-associated enzymes and intercalates between DNA base
pairs, ultimately resulting in DNA damage (13) that leads to apoptosis by inhibiting
the cell cycle and nuclear DNA polymerase (13,14).
Although doxorubicin is widely used in the treatment of several
types of cancer, its clinical use is limited by severe
dose-dependent toxicities. At higher dosages, both paclitaxel and
doxorubicin are toxic to many organs including the kidney, heart,
brain and liver. Thus, great care must be taken in the use of these
agents.
Combination chemotherapy has been reported to be
more effective than single agent therapy. However, data on
combination chemotherapy in ESCC cells are limited, especially for
paclitaxel and doxorubicin. Here, we examined a modestly toxic
combination of paclitaxel and doxorubicin to determine whether they
have more significant biological effects together than as single
agents alone in ESCC cells. We report that the combination
treatment of paclitaxel and doxorubicin enhanced the induction of
G2/M cell cycle arrest and apoptosis in human ESCC cells by
suppressing Akt activity. These findings emphasize the powerful
apoptotic effect of combination therapy with paclitaxel and
doxorubicin in ESCC cells and the potential clinical usefulness of
these two drugs in esophageal cancer.
Materials and methods
Cell culture and reagents
Human esophageal cancer cell line TE-12 was obtained
from Dr Izzo (University of Texas M.D. Anderson Cancer Center,
Houston, TX, USA). These cancer cells were maintained as a
monolayer in 100-mm dishes (BD Biosciences, Cockeysville, MD, USA)
in DMEM-F12 medium (Gibco, Grand Island, NY, USA) supplemented with
10% fetal bovine serum (FBS) (Gibco), 100 mg/ml streptomycin and
100 IU/ml penicillin (Gibco) under standard conditions at 37ºC in a
5% CO2 humidified atmosphere. Paclitaxel and doxorubicin
were obtained from Sigma-Chemical Co. (St. Louis, MO, USA)
dissolved in dimethysulphoxide (DMSO; Sigma-Chemical). Further
dilutions were performed in cell culture media and DMSO was used as
a vehicle control. Cyclin B1, p-cdc2, p-Wee1, cleaved caspase-7,
cleaved caspase-9, cleaved poly(ADP-ribose) polymerase (PARP),
p-PTEN and p-Akt antibodies were obtained from Cell Signaling
Technology (Danvers, MA, USA) and cdc2, Akt, p53 and β-actin
antibodies were obtained from Santa Cruz Biotechnology Inc.
(Dallas, TX, USA).
MTT assay
The cytotoxicity was monitored using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay as previously described (6).
Briefly, cells (1×104) were seeded in 96-well plates (BD
Biosciences, San Jose, CA, USA) containing 100 μl of DMEM-F12
medium. After 24-h incubation under standard conditions, the cells
were treated with various concentrations of paclitaxel and/or
doxorubicin. After 72 h, 50 μl of MTT (2 mg/ml PBS) was added and
the plates were incubated for an additional 3 h. The medium was
aspirated off, and 200 μl of DMSO was added. The optical density
was assessed at a wavelength of 540 nm using a scanning multiwall
spectrophotometer (SpectraMAX 340; Molecular Devices Co.,
Sunnyvale, CA, USA).
PI staining for cell cycle analysis
Cell sample preparation and PI staining were
performed according to the manufacturer’s protocol. Briefly,
1×106 cells were incubated with or without various
concentrations of paclitaxel and/or doxorubicin. Cells were washed
with PBS and the nuclei were stained with PI (Sigma Chemical) as
previously described (6). The cell
cycle distribution was measured with a FACStar flow cytometer
(Becton-Dickinson, San Jose, CA, USA) and analyzed using
Becton-Dickinson software (Lysis II and CellFit).
Immunoblot assay analysis
TE-12 human esophageal cancer cells were plated and
allowed to attach for 24 h. Paclitaxel and doxorubicin were added
to cell cultures at various concentrations for 24–72 h. Cells with
or without paclitaxel and doxorubicin were harvested and suspended
in lysis buffer (Intron Biotechnology, Inc.). Extracts were
incubated on ice for 10 min and centrifuged at 13,200 rpm for 20
min at 4ºC. After centrifuging, the supernatant was collected. The
protein concentration was determined using a BSA Protein Assay kit
(Pierce, Rockford, IL, USA). Whole lysate was resolved on an
SDS-PAGE gel and transferred to a PVDF membrane (Bio-Rad
Laboratories, Hercules, CA, USA). Membranes were probed with
specific primary antibodies and then with peroxidase-conjugated
secondary antibodies. The bands were visualized with an Enhanced
chemiluminescence kit (Amersham Health, Arlington Heights, IL,
USA). Cyclin B1, p53, cdc2, p-cdc2, p-Wee1, cleaved caspase-7,
cleaved caspase-9, cleaved PARP, p-Akt, Akt and β-actin antibodies
were used.
Statistical analysis
Statistical analysis was performed using Student’s
t-test or one-way analysis of variance (ANOVA) where appropriate.
All experiments were repeated more than three times and results are
expressed as means ± SE. P-values of <0.05 were considered to
indicate statistically significant results.
Results
Growth inhibition of TE-12 cells by
paclitaxel and doxorubicin
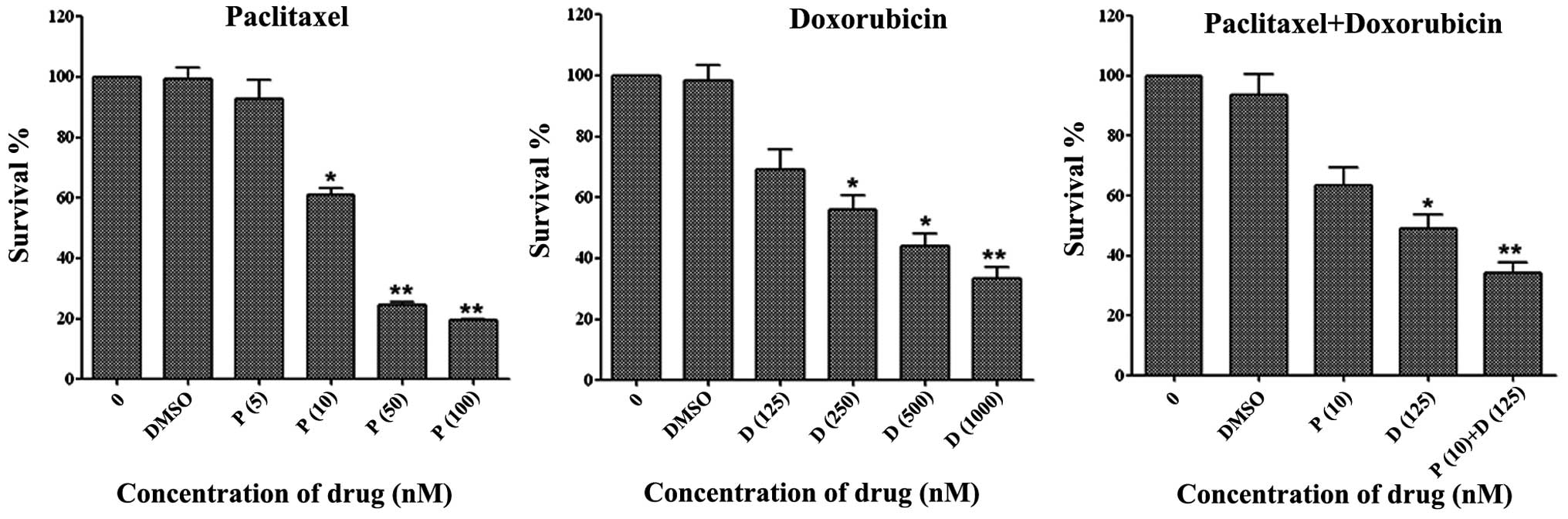
TE-12 esophageal squamous carcinoma cells were
exposed to paclitaxel and doxorubicin in varying concentrations for
72 h. As shown in Fig. 1,
paclitaxel and doxorubicin inhibited cell viability in a
dose-dependent manner. Since the IC50 of paclitaxel and
doxorubicin was ~20–25 and 250–300 nM, respectively, we performed
combination treatment experiments with 10 nM paclitaxel and 125 nM
doxorubicin. Combination treatment with paclitaxel (10 nM) and
doxorubicin (125 nM) resulted in significant growth inhibition
(60–80% at 72 h) of TE-12 cells compared with either agent alone.
These results suggest that combination treatment with paclitaxel
and doxorubicin effectively inhibits the growth of esophageal
squamous carcinoma cells.
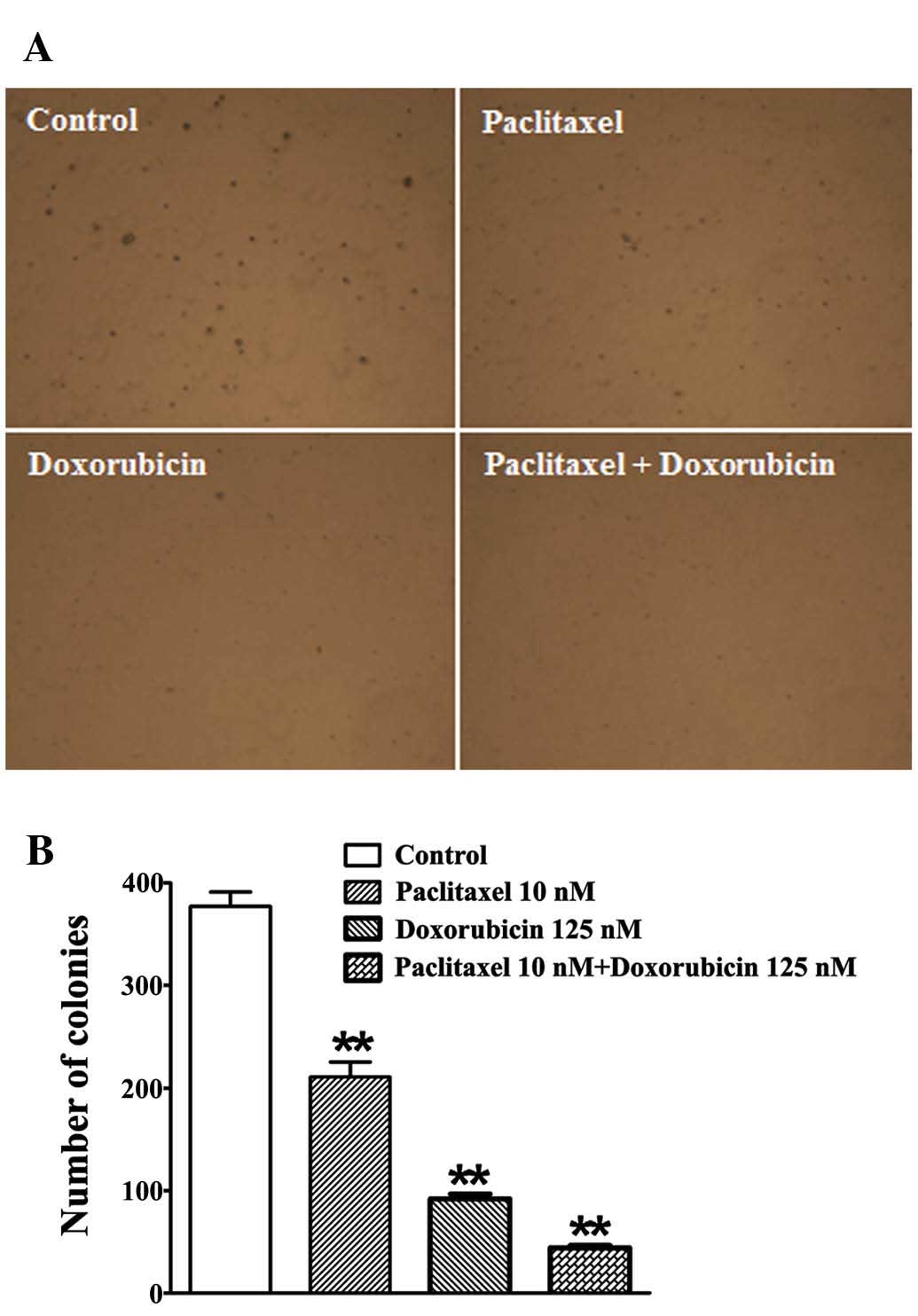
Inhibition of colony formation by
paclitaxel and doxorubicin
A soft agar cloning assay was performed to test the
effect of paclitaxel and doxorubicin on TE-12 cell in vitro
colony formation. Treatment with paclitaxel (10 nM) and doxorubicin
(150 nM) resulted in significantly greater inhibition of colony
formation in TE-12 cells when compared to treatment with a single
agent (Fig. 2).
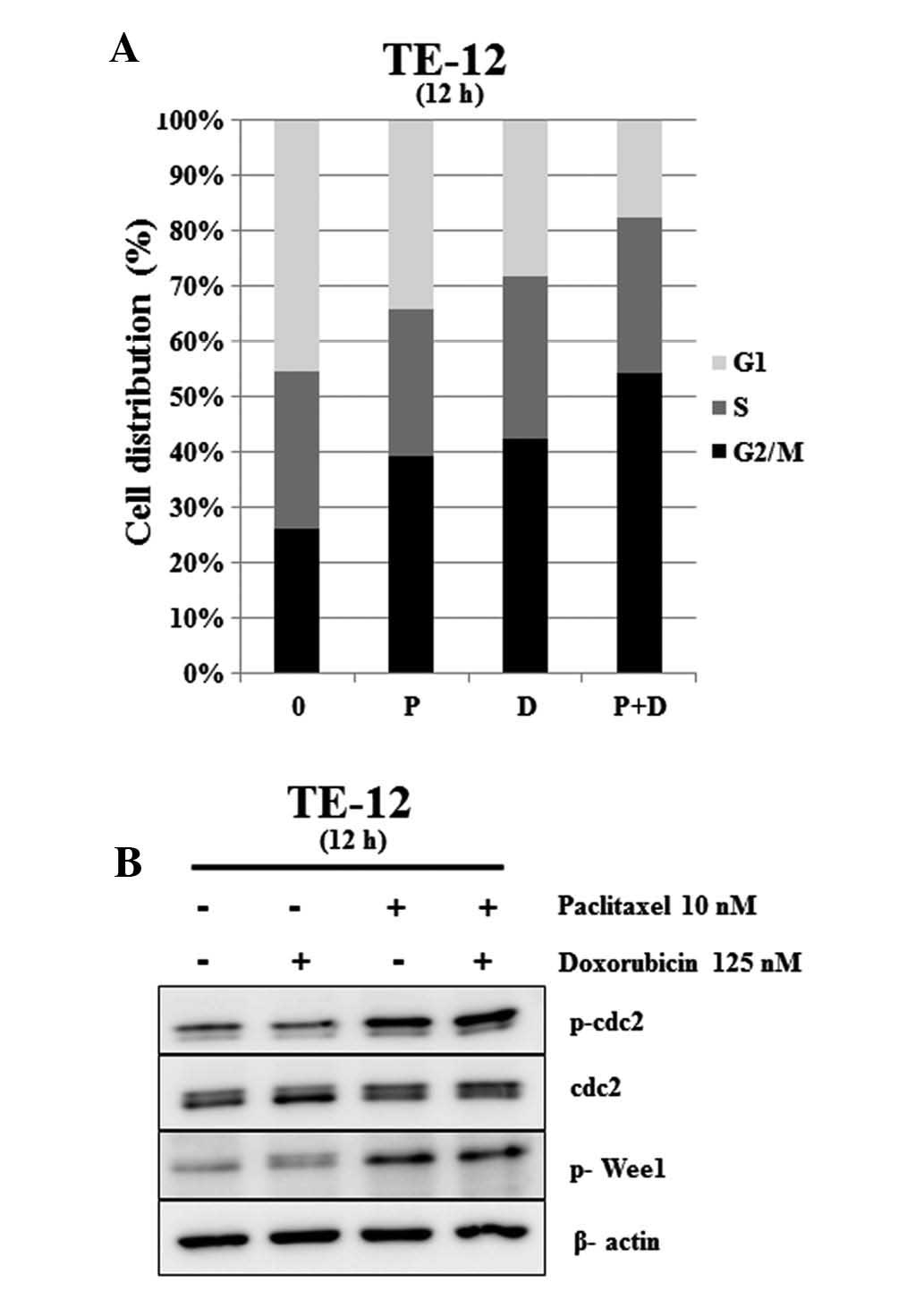
Paclitaxel and doxorubicin affect cell
cycle progression
FACS was used to investigate the effect of
paclitaxel and doxorubicin on cell cycle progression in esophageal
squamous carcinoma cells. The cell cycle distribution was measured
at 12 h after treatment with paclitaxel (10 nM) alone, doxorubicin
(150 nM) alone, and paclitaxel (10 nM) plus doxorubicin (150 nM).
As shown in Fig. 3A, significant
accumulation of cells in G2/M phase was observed after exposure to
paclitaxel and doxorubicin. Combination treatment with paclitaxel
and doxorubicin significantly increased the percentage of TE-12
cells in the G2/M (G1 percentage 54.22, P<0.05) compared to
control (G1 percentage 26.21). We further investigated alterations
in cell cycle regulatory proteins following paclitaxel and
doxorubicin treatment. p-cdc2 and p-Wee1 protein levels were
significantly increased by paclitaxel (10 nM) and combination
treatment with paclitaxel (10 nM) and doxorubicin (125 nM), while
no change was observed with doxorubicin treatment alone. Levels of
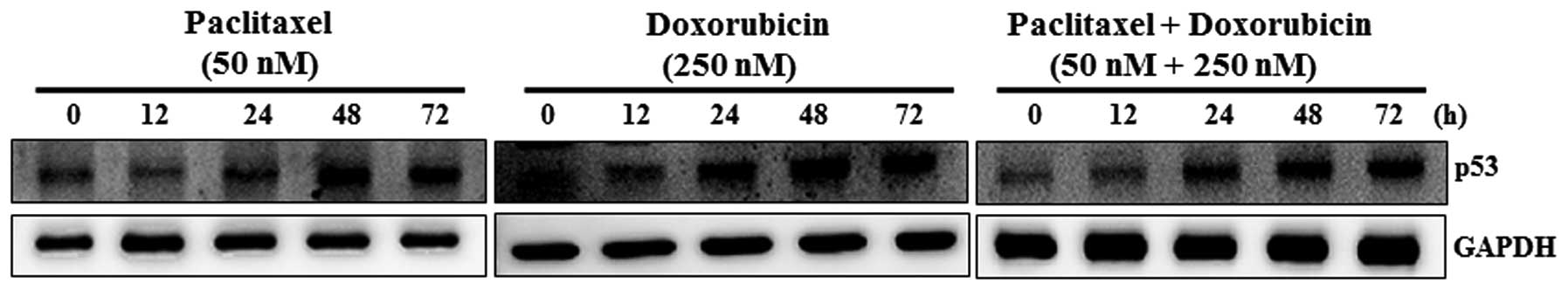
the cyclin-dependent kinase inhibitor protein p53 increased with
the concentration of paclitaxel (50 nM) and doxorubicin (250 nM) in
a time-dependent manner (Fig. 4).
Combination treatment with paclitaxel (50 nM) and doxorubicin (250
nM) also induced p53 protein expression in a time-dependent manner.
These results indicate that combination treatment with paclitaxel
and doxorubicin leads to a significant increase in the G2/M
population in esophageal squamous carcinoma cells.
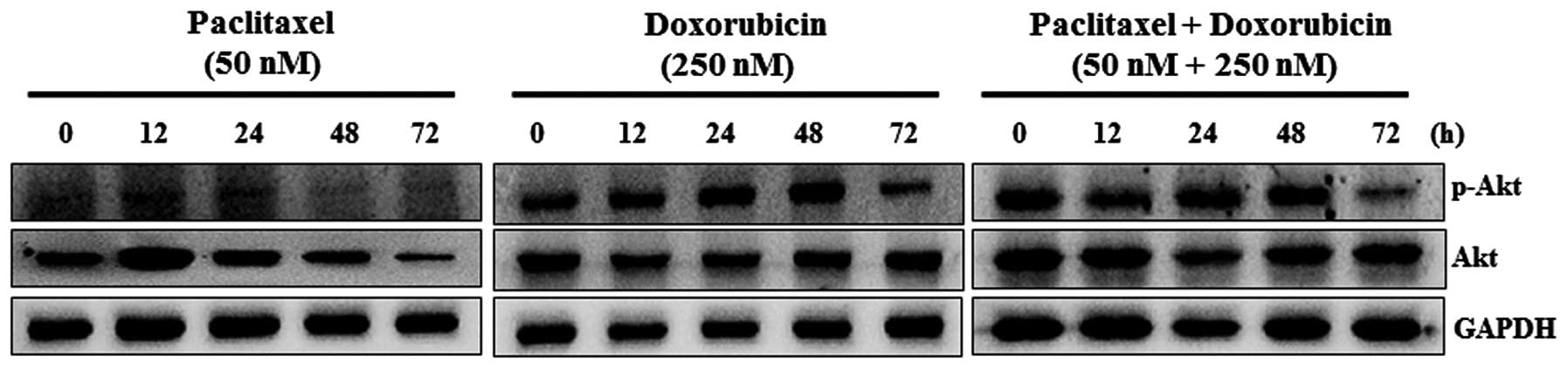
Paclitaxel and doxorubicin suppress the
phosphorylation of Akt
Cells were treated with paclitaxel and doxorubicin
and Akt protein expression was followed by western blot analysis.
As shown in Fig. 5, paclitaxel (50
nM) significantly inhibited p-Akt and Akt protein levels in a
time-dependent manner. Doxorubicin (250 nM) significantly inhibited
the expression of p-Akt at 72 h, but no change was seen at other
time-points. Doxorubicin did not affect Akt expression. Combination
treatment with paclitaxel and doxorubicin significantly suppressed
the expression of p-Akt at 72 h, but no difference was observed at
other time-points. Akt protein levels were not altered by
combination treatment with paclitaxel and doxorubicin.
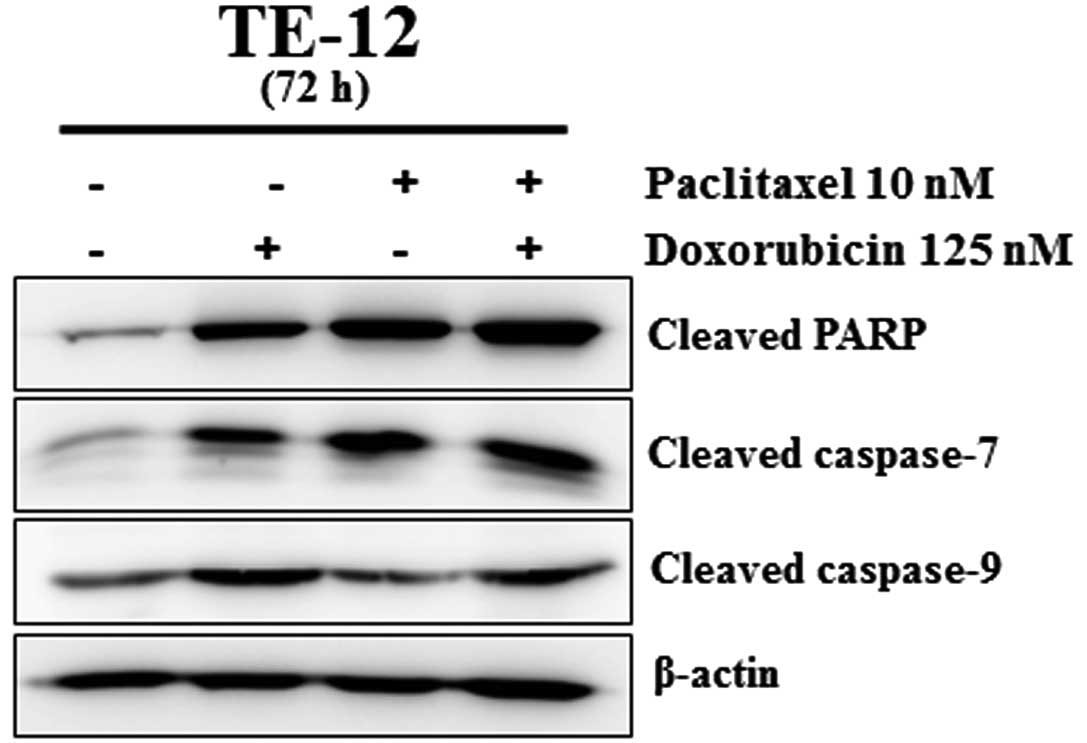
Treatment with paclitaxel and doxorubicin
induces apoptosis
We measured cleaved caspase-9, cleaved PARP and
cleaved caspase-7 protein levels in TE-12 cells. As shown in
Fig. 6, treatment with paclitaxel
or doxorubicin alone increased the expression of cleaved PARP,
cleaved caspase-7 and cleaved caspase-9 proteins. Treatment with
the combination of paclitaxel (10 nM) and doxorubicin (125 nM)
significantly increased the expression of cleaved PARP, cleaved
caspase-7 and cleaved caspase-9. These data indicate that the
combination of paclitaxel and doxorubicin induces more marked
apoptotic cell death in TE-12 esophageal carcinoma cells than a
single agent alone.
Discussion
Despite significant reductions in esophageal cancer
rates associated with lifestyle changes, ESCC remains the seventh
leading cause of mortality in the USA and the sixth leading cause
of mortality worldwide (15). It is
one of the most fatal types of cancer and chronic irritation and
inflammation appear to be the main cause of ESCC (4,15).
Since the morbidity and mortality associated with surgery for ESCC
outweigh the benefits (3,15), chemotherapy is largely employed to
reduce tumor size, lower recurrence rates and prolong survival
(3,15). Although paclitaxel and doxorubicin
are widely used as chemotherapy agents against several types of
cancer, combination therapy with these agents has not been
evaluated for ESCC. Here, we demonstrated, for the first time, the
biological effects of combination therapy with paclitaxel and
doxorubicin on ESCC cells.
In the present study, we found that paclitaxel and
doxorubicin significantly suppressed the proliferation of TE-12
cells in a dose-dependent manner. Concentrations of 10 nM
paclitaxel and 125 nM doxorubicin were chosen for their efficacy in
ESCC cells. Treatment with these two drugs in combination
suppressed proliferation of TE-12 cells significantly more
effectively than treatment with a single agent alone, suggesting
that the two agents work substantially to suppress proliferation in
ESCC cells. As expected from the cell viability assay, paclitaxel
and doxorubicin inhibited colony formation in TE-12 cells.
Combination treatment with paclitaxel and doxorubicin inhibited
colony formation in TE-12 significantly more than treatment with a
single agent alone. The antiproliferative effects of the drug
combination were more marked in the colony formation assay than in
the MTT assay, although the same dosages and cells were used.
However, the inhibition patterns caused by treatment with
paclitaxel and doxorubicin appeared similar. Therefore, combination
therapy markedly increased the antiproliferative effects of these
drugs in ESCC cells.
Several studies, including one of ours pertaining to
esophageal adenocarcinoma, reported that paclitaxel inhibits cell
replication by blocking progression beyond the late G2 and/or M
phases of the cell cycle in various types of cancer (1,12,16–18).
Zimmermann et al(19) also
demonstrated that doxorubicin induced G2/M cell cycle arrest
secondary to DNA damage. In agreement with previous reports, we
observed G2/M cell cycle arrest induced by paclitaxel and
doxorubicin in TE-12 cells. The proportion of the cell population
in G2/M was increased by paclitaxel and doxorubicin treatment.
Combination treatment with these two drugs significantly increased
the proportion of the cell population in G2/M. In addition, G2/M
phase-related proteins p-cdc2 and p-Wee1 were also significantly
induced by combination treatment with these two drugs. Expression
of the cyclin-dependent kinase inhibitor p53 was increased by
treatment with paclitaxel and doxorubicin in a time-dependent
manner. Similar to treatment with either agent alone, combination
treatment with these two drugs significantly increased expression
of p53 in a time-dependent manner. Collectively, our findings
strongly suggest that combination therapy with paclitaxel and
doxorubicin significantly induces marked G2/M cell cycle arrest in
ESCC cells.
The PI3K/Akt pathway plays an important role in
controlling cell proliferation and increased PI3K/Akt activity has
been observed in several types of cancer including ESCC (20–23). A
recent study also linked mutation in the PI3K/Akt pathway to
prognosis of patients with ESCC (24). A few studies have concluded that
paclitaxel induces apoptosis through the suppression of the Akt
pathway in ESCC (25,26). Doxorubicin induced apoptosis in
breast cancer through the suppression of Akt signaling (27). Yu et al(28) reported that doxorubicin induced
apoptosis in lung cancer via modulation of the PI3K/Akt signaling
pathway. However, Akt modulation mediated by doxorubicin has not
been investigated in ESCC cells. Therefore, we explored changes in
the levels of Akt and p-Akt induced by treatment with doxorubicin
alone and in combination with paclitaxel in ESCC cells. In the
present study, p-Akt protein levels were significantly diminished
in a time-dependent manner by single-agent treatment with
paclitaxel or doxorubicin and combination treatment further
decreased p-Akt levels. Consistent with its behavior in other types
of cancer, doxorubicin also suppressed the expression of Akt in
ESCC cells, although this effect only became significant after 72
h. Levels of the apoptosis-associated proteins cleaved PARP,
cleaved caspase-7 and cleaved caspase-9 were increased by
single-agent treatment with paclitaxel and doxorubicin. Combination
treatment with paclitaxel and doxorubicin strongly induced the
expression of these apoptosis-associated proteins. These results
indicate that apoptosis induced by combination treatment with
paclitaxel and doxorubicin may be affected by the caspase-dependent
pathway.
Our experiments suggest that paclitaxel and
doxorubicin synergistically inhibit proliferation of ESCC cells by
inducing G2/M cell cycle arrest and apoptosis. Apoptosis induced by
treatment with paclitaxel and doxorubicin may proceed secondary to
the suppression of Akt signaling, although further experiments are
required to investigate the signaling mechanisms involved. Based on
our findings, we believe that combination therapy with paclitaxel
and doxorubicin may prove to be a successful approach for the
treatment of ESCC.
Acknowledgements
The present study was supported by research funds
from the Chonbuk National University in 2012 and by the Basic
Science Research Program through the National Research Foundation
of Korea (NRF) funded by the Ministry of Education, Science and
Technology (20110014864 and 2012R1A1A2005729).
References
|
1
|
Jimenez P, Pathak A and Phan AT: The role
of taxanes in the management of gastroesophageal cancer. J
Gastrointest Oncol. 2:240–249. 2011.PubMed/NCBI
|
|
2
|
Ajani JA, Barthel JS, Bekaii-Saab T, et
al: Esophageal cancer. J Natl Compr Canc Netw. 6:818–849. 2008.
|
|
3
|
Wolf MC, Stahl M, Krause BJ, et al:
Curative treatment of oesophageal carcinoma: current options and
future developments. Radiat Oncol. 6:552011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wright JC: Update in cancer chemotherapy:
gastrointestinal cancer, cancer of the small intestines,
gallbladder, liver, and esophagus. J Natl Med Assoc. 78:753–766.
1986.
|
|
6
|
Kim SJ, Chung MJ, Kim JS, et al:
Deciphering the role of paclitaxel in the SKGT4 human esophageal
adenocarcinoma cell line. Int J Oncol. 39:1587–1591.
2011.PubMed/NCBI
|
|
7
|
Legha SS, Ring S, Papadopoulos N, Raber M
and Benjamin RS: A phase II trial of taxol in metastatic melanoma.
Cancer. 65:2478–2481. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Holmes FA, Walters RS, Theriault RL, et
al: Phase II trial of taxol, an active drug in the treatment of
metastatic breast cancer. J Natl Cancer Inst. 83:1797–1805. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Einzig AI, Wiernik PH, Sasloff J, Runowicz
CD and Goldberg GL: Phase II study and long-term follow-up of
patients treated with taxol for advanced ovarian adenocarcinoma. J
Clin Oncol. 10:1748–1753. 1992.PubMed/NCBI
|
|
10
|
van Steenbergen LN, Elferink MA, Krijnen
P, et al: Improved survival of colon cancer due to improved
treatment and detection: a nationwide population-based study in The
Netherlands 1989–2006. Annal Oncol. 21:2206–2212. 2010.PubMed/NCBI
|
|
11
|
Ajani J: Therapy of localized esophageal
cancer: it is time to reengineer our investigative strategies.
Onkologie. 31:360–361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Faried A, Faried LS, Kimura H, et al:
Differential sensitivity of paclitaxel-induced apoptosis in human
esophageal squamous cell carcinoma cell lines. Cancer Chemother
Pharmacol. 57:301–308. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tacar O, Sriamornsak P and Dass CR:
Doxorubicin: an update on anticancer molecular action, toxicity and
novel drug delivery systems. J Pharm Pharmacol. 65:157–170. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buchholz TA, Stivers DN, Stec J, et al:
Global gene expression changes during neoadjuvant chemotherapy for
human breast cancer. Cancer J. 8:461–468. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Enzinger PC and Mayer RJ: Esophageal
cancer. New Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rowinsky EK and Donehower RC: The clinical
pharmacology of paclitaxel (Taxol). Semin Oncol. 20:16–25.
1993.PubMed/NCBI
|
|
17
|
Park KW, Kang SH, Park KH, et al:
Sirolimus- vs. paclitaxel-eluting stents for the treatment of
unprotected left main coronary artery stenosis: complete 2-year
follow-up of a two-center registry. Int J Cardiol. 151:89–95.
2011.PubMed/NCBI
|
|
18
|
Toiyama Y, Tanaka K, Konishi N, et al:
Administration sequence-dependent antitumor effects of paclitaxel
and 5-fluorouracil in the human gastric cancer cell line MKN45.
Cancer Chemother Pharmacol. 57:368–375. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zimmermann M, Arachchige-Don AS, Donaldson
MS, Dallapiazza RF, Cowan CE and Horne MC: Elevated cyclin G2
expression intersects with DNA damage checkpoint signaling and is
required for a potent G2/M checkpoint arrest response to
doxorubicin. J Biol Chem. 287:22838–22853. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim AH, Khursigara G, Sun X, Franke TF and
Chao MV: Akt phosphorylates and negatively regulates apoptosis
signal-regulating kinase 1. Mol Cell Biol. 21:893–901. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang HB, Lu P, Guo QY, Zhang ZH and Meng
XY: Baicalein induces apoptosis in esophageal squamous cell
carcinoma cells through modulation of the PI3K/Akt pathway. Oncol
Lett. 5:722–728. 2013.PubMed/NCBI
|
|
22
|
Lin ML, Lu YC, Chen HY, Lee CC, Chung JG
and Chen SS: Suppressing the formation of lipid raft-associated
Rac1/PI3K/Akt signaling complexes by curcumin inhibits
SDF-1alpha-induced invasion of human esophageal carcinoma cells.
Mol Carcinog. Nov 28–2012.(Epub ahead of print). View Article : Google Scholar
|
|
23
|
Gen Y, Yasui K, Nishikawa T and Yoshikawa
T: SOX2 promotes tumor growth of esophageal squamous cell carcinoma
through the AKT/mammalian target of rapamycin complex 1 signaling
pathway. Cancer Sci. 104:810–816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shigaki H, Baba Y, Watanabe M, et al:
PIK3CA mutation is associated with a favorable prognosis among
patients with curatively resected esophageal squamous cell
carcinoma. Clin Cancer Res. 19:2451–2459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ou Y, Ma L, Ma L, et al: Overexpression of
cyclin B1 antagonizes chemotherapeutic-induced apoptosis through
PTEN/Akt pathway in human esophageal squamous cell carcinoma cells.
Cancer Biol Ther. 14:45–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nguyen DM, Chen GA, Reddy R, et al:
Potentiation of paclitaxel cytotoxicity in lung and esophageal
cancer cells by pharmacologic inhibition of the phosphoinositide
3-kinase/protein kinase B (Akt)-mediated signaling pathway. J
Thorac Cardiovasc Surg. 127:365–375. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Y, Sun Y, Chen L, et al: miRNA-200c
increases the sensitivity of breast cancer cells to doxorubicin
through the suppression of E-cadherin-mediated PTEN/Akt signaling.
Mol Med Rep. 7:1579–1584. 2013.PubMed/NCBI
|
|
28
|
Yu YH, Kuo HP, Hsieh HH, et al:
Ganoderma tsugae induces S phase arrest and apoptosis in
doxorubicin-resistant lung adenocarcinoma H23/0.3 cells via
modulation of the PI3K/Akt signaling pathway. Evid Based Complement
Alternat Med. 2012:3712862012.PubMed/NCBI
|