Introduction
Lung cancer is one of the leading causes of cancer
death worldwide. In the United States, the annual mortality rate of
lung cancer (estimated 180,000 deaths, male: 73.5/105
person-year; female: 41.5/105 person-year) is
approximately 30% of total cancer-related deaths (1), and nearly 85–90% of lung cancer deaths
are attributed to tobacco smoking (2). In Taiwan, the annual mortality rate of
lung cancer is approximately 20% (estimated 6,000 deaths, male:
21/105 person-year; female: 10.3/105
person-year) (3). Lung carcinoma is
categorized into small cell lung cancer (SCLC) or non-small cell
lung cancer (NSCLC) with neuroendocrine features of the cancer
cells. Based on the histopathological characteristics, NSCLC can be
subcategorized into adenocarcinoma (ADC), squamous cell carcinoma
and large cell carcinoma (4). Of
note, among these, ADC, which is associated with a higher frequency
of drug resistance and mortality than the other types, is most
commonly found in women and smokers (5).
Previous studies have indicated that tobacco smoking
is a key risk factor for lung cancer (1,2,5,6).
In patients with stage I NSCLC, we demonstrated that tobacco
smoking and tumor size, but not visceral pleural invasion, are
major factors influencing overall and disease-free survival
(6). Moreover, accumulated evidence
showed that NSCLC patients who continued to smoke were more
resistant to chemotherapy and irradiation, and had poorer prognosis
(6–9). Although nicotine per se is not
directly associated with tumorigenesis, catalyzed nicotine is
carcinogenic (10,11). In addition, nicotine induces NSCLC
growth, and increases angiogenesis in tumors probably via
activating nicotinic acetylcholine receptor (nAchR), epidermal
growth factor receptor (EGFR) and Akt (12–19).
Using differential display alone or in combination
with microarray, we previously identified a spectrum of
NSCLC-specific tumor markers, such as dihydrodiol dehydrogenase
(DDH), c-MET, matrix metalloproteinase (MMP) and short oncostatin M
receptor (OSMRs), which were closely associated with resistance to
chemotherapy, tumor recurrence, metastasis and poor prognosis
(20–24). Although we expected that DDH would
correlate with drug resistance (20–22)
and MMP with tumor metastasis (23), DDH was not directly involved in
cisplatin deactivation. However, since DDH overexpression is
closely related to tobacco smoking, and tobacco smoking is the key
risk factor for carcinogenesis and disease progression of lung
cancer, we used the same methods to examine gene expression
profiles in biopsy specimens from smokers and non-smokers, and we
detected that hepatocyte growth factor (HGF) was frequently
overexpressed in smokers with NSCLC. HGF was correlated with tumor
stages and poor prognosis (25). Of
note, HGF not only increased resistance to cisplatin, but also
reduced levels of apoptosis inducing factor (AIF), a vital factor
of caspase-independent cell death (CICD), in NSCLC (25,26).
Reduced effect of EGFR tyrosine kinase inhibitor (TKI), gefitinib,
in patients with smoking habits or amplified c-MET gene suggested
that in addition to cisplatin HGF might be involved in resistance
to TKI as well (27). However, the
prognostic value of HGF downstream effector AIF and its association
with metastasis-related genes in NSCLC have not been reported.
In this study, we investigated AIF expression, and
evaluated the statistical relationship between AIF expression and
the clinicopathological factors as well as the prognostic
significance of AIF expression in patients with NSCLC. We also
studied the biological correlation between AIF and positive tumor
marker genes in NSCLC.
Materials and methods
Tissue specimens
From August 1986 to November 2003, pathology
specimens from 452 patients with NSCLC were reviewed. Pathology
samples from all patients, for whom at least one follow-up
examination or death was documented, were pathologically confirmed
NSCLC. Of the 452 patients, 219 were diagnosed as having lung ADC.
The stage of the disease was classified (patients after 1999) or
re-classified (patients before 1999) according to the new
international staging system for lung cancer (28). The Medical Ethics Committee approved
the protocol in 2001, and written informed consent of donating
biopsy specimens had been obtained from every patient before
surgery since 2001. All patients had undergone surgical resection
and radical N2 lymph node dissection. Tumor size, lymph node
number, differentiation, vascular invasion and mitotic number were
documented. Patients with lymph node involvement or locoregional
recurrence received irradiation at the afflicted areas. Those with
distant metastasis were treated with chemotherapy. After treatment,
patients were routinely followed every 3 to 6 months in the
outpatients department. Tumor recurrence and metastasis were
diagnosed when biochemical studies, chest radiography, whole body
bone scan and computerized tomography scans of chest showed any
evidence of the disease. Immunohistochemical staining was carried
out using a single-blinded procedure.
Immunoblotting analysis
Total cell lysate was prepared by mixing
5×107 cells/100 μl phosphate-buffered saline with equal
volume of 2X loading buffer (50 mM Tris, pH 6.8, 150 mM NaCl, 1 mM
disodium EDTA, 1 mM PMSF, 10% glycerol, 5% β-mercaptoethanol, 0.01%
bromophenol blue and 1% SDS). Eletrophoresis was carried out in two
10% polyacrylamide gels with 4.5% stacking. One gel was processed
for immunoblotting (20–26), and the other gel was stained with
Coomassie blue. After electrophoresis, proteins on the first gel
were transferred to a nitrocellulose membrane for immunoblotting.
The membrane was probed with specific antibodies. The signal was
amplified by biotin-labeled goat anti-mouse IgG, and
peroxidase-conjugated streptavidin. The protein was visualized by
exposing the membrane to an X-Omat film (Eastman Kodak, Rochester,
NY, USA) with enhanced chemiluminescent reagent (NEN, Boston, MA,
USA). After getting unsatisfactory results from several batches of
commercially obtained antibodies for Her2/neu, we decided to raise
our own antibodies.
Preparation of mouse antibodies to
HER2/neu
DNA sequence corresponding to C-terminal amino acids
807-1183 of HER2/neu was amplified by primer sequences containing
SalI (sense) and NotI (antisense) restriction sites
respectively. The primer sequences were 5′-TCCGTCGACAAATGGACCAT
GTCCGGGAAAAC-3′ (SalI site is underlined) and
5′-AGCGGCCGCAGTCTTTGACGACCCCATTCTT-3′
(NotI site is underlined).
The 1131-bp cDNA of HER2/neu was cloned into an
expression vector pET-32a+ (Promega KK, Tokyo, Japan).
Bacterial colony containing the pET32+-HER2/neu was
selected, and induced by isopropyl-β-D-thiogalactopyranoside (IPTG)
to mass-produce HER2/neu. The recombinant protein was purified by a
nickel-affinity column, and protein identity was determined by
MALDI-TOF. Affinity-purified HER2/neu was used to immunize BALB/c
mice, and sensitivity of antiserum (OD405 >0.3 at
1:6,000 dilutions) was measured by enzyme-linked immunosorbent
assay (ELISA). Specificity of antibodies was determined by showing
distinct bands with molecular weight of 185 kDa in the
immunoblotting of breast cancer cell extract. Monoclonal antibodies
were produced by a hybridoma technique, and HER2/neu-specific
antibodies were screened by the above-mentioned methods.
Immunohistochemistry
Immunohistochemical staining was performed according
to the immunoperoxidase method previously reported (20–26).
Slide evaluation
In each pathological section, non-tumor lung tissue
(NTLT) served as the internal negative control. Slides were
evaluated by two independent pathologists blinded to the
clinicopathological knowledge. The ImmunoReactive Scoring system
was adapted for this study (29).
Briefly, a specimen was considered having strong signals when
>50% of cancer cells were positively stained; intermediate, if
25–50% of the cells stained positive; weak, if <25% or >10%
of the cells were positively stained; and negative, if <10% of
the cells were positively stained. Cases with strong and
intermediate AIF signals were classified as AIF+, and
those with weak or negative AIF signals were classified as
AIF−. Those with AIF detecting in the nuclei were
classified as cases with nuclear AIF index (NAI).
Statistical analysis
The relationship between AIF expression and
clinicopathological parameters was analyzed by Chi-square test.
Survival curves were plotted using the Kaplan-Meier estimator
(30). Statistical difference in
survival among different groups was compared by the log-rank test
(between AIF+ and AIF− groups) and log-rank
test for trend [among NAI, cAIF+ (cytoplasmic AIF,
AIF+ patients minus NAI patients) and AIF−
groups] (31). Statistical analysis
was performed using GraphPad Prism5 statistical software (San
Diego, CA, USA). Statistical significance was set at p-value
<0.05.
Results
Expression of AIF in NSCLC and
correlation with patient survival
Using AIF-specific monoclonal antibodies, we
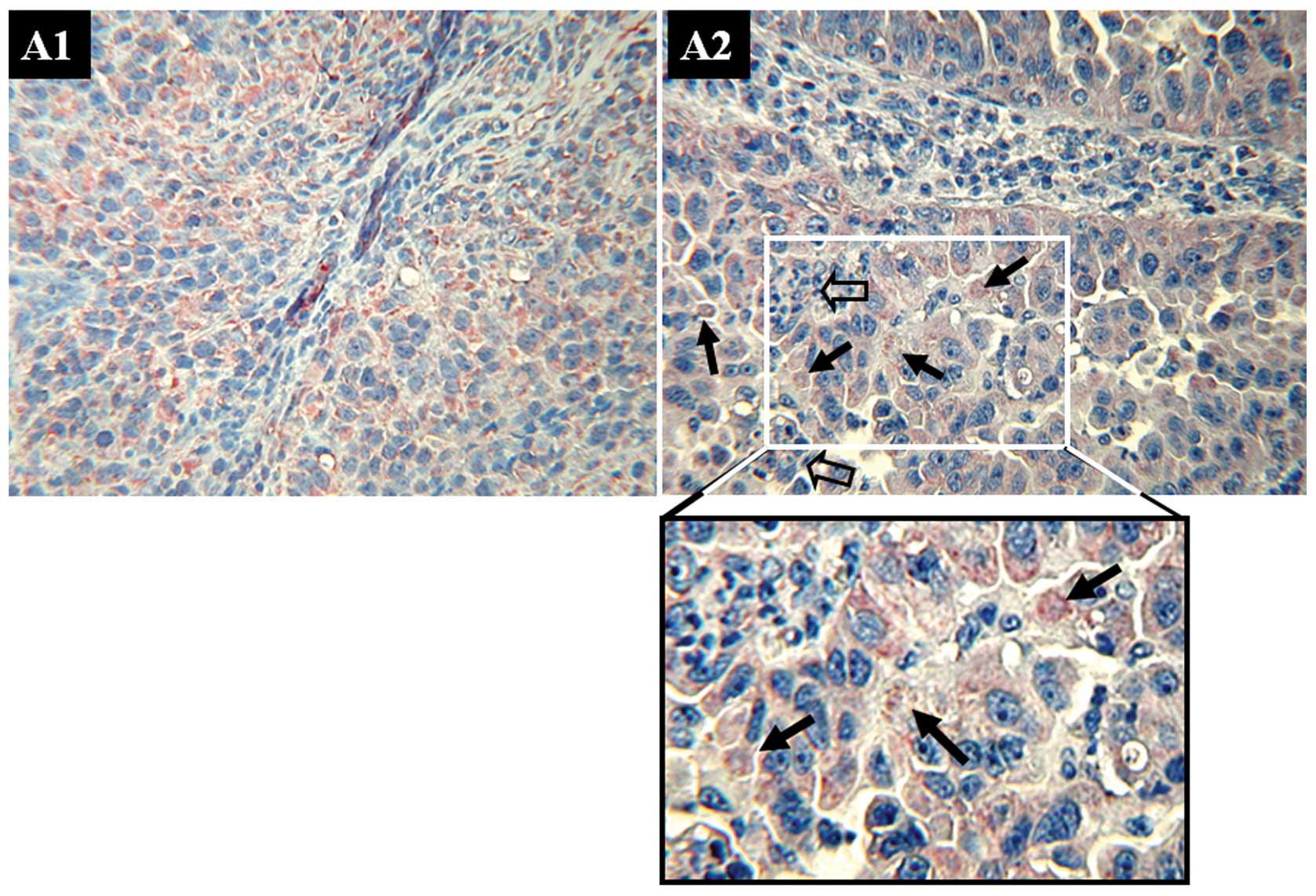
detected AIF expression (Fig. 1A1 and
A2) in tumor cells in 109 NSCLC patients (24.1%). In 14
(12.84%) of the 109 patients, some AIF signals were identified in
the nuclei of tumor cells (Fig.
1A2). Nuclear AIF indicated that these tumor cells are under
apoptosis. Judging from vicinity of the tumor, apoptotic cancer
cells could be associated with the infiltrating immune cells. AIF
was also detected in 40.45% (36/89) of metastatic lymph nodes.
However, no nuclear AIF was identified in the metastatic lymph
nodes. AIF expression in was verified by immunoblotting (Fig. 1B). Expression of AIF decreased
following advances of tumor stage. From stage 1b, AIF level reduced
markedly.
Among the 109 patients whose tissue samples had high
AIF expression, 31 (28.44%) patients had tumor recurrence. Among
the 343 patients whose tissue samples had low AIF expression, 168
(48.98%) patients had tumor recurrence during follow-up
examination. All 199 patients who had recurrence developed new
tumors within 18 months after operation and cisplatin-based
chemotherapy. Recurrence rate of patients with low AIF expression
was 1.72-fold higher than that of patients with high AIF
expression. The difference was significant (p<0.01). Moreover,
survival of patients with high AIF levels was significantly better
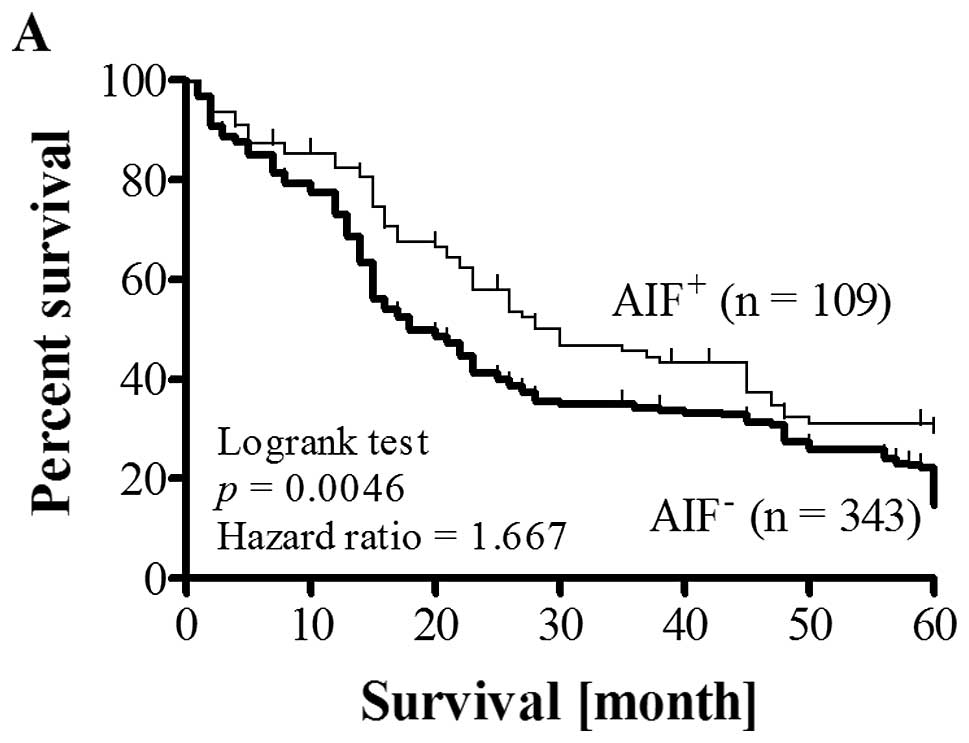
than that of patients with low AIF levels (Fig. 2A). Survival rate of patients with
high nuclear AIF index (NAI) was the best among patient groups
(Fig. 2B). The differences in
cumulative survival between groups were significant (p=0.0046 and
p=0.009).
Functional characterization of monoclonal
antibodies to HER2/neu
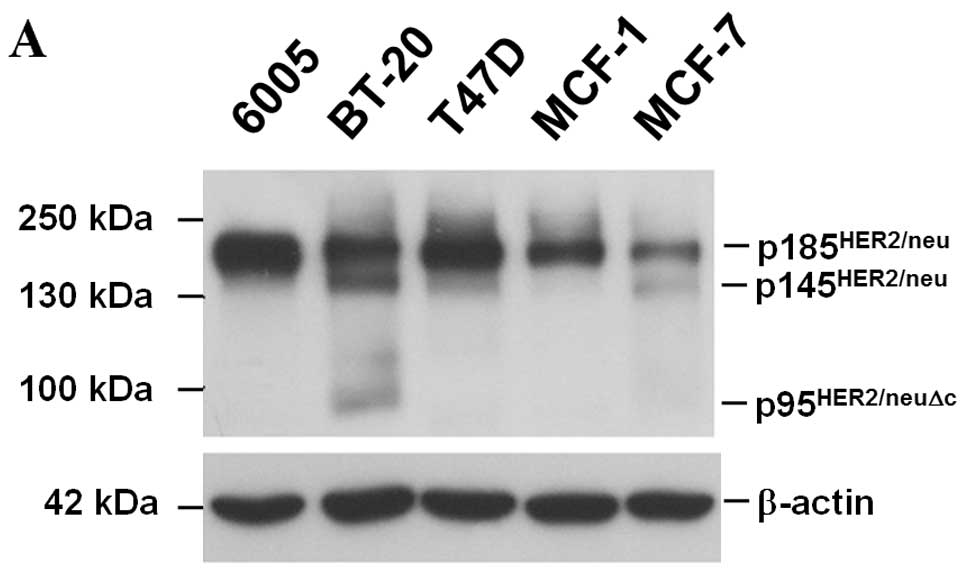
Specificity of the monoclonal antibodies was
determined by an immunoblotting analysis of the whole cell lysate.
The unique 185-kDa protein was detected by monoclonal antibodies
(Fig. 3A). Immunohistochemical
staining showed that HER2/neu was present on plasma membranes of
breast cancer cells (Fig. 3B, left
panels). Infection with lentivirus expressing siRNA to HER2/neu
reduced HER2/neu expression validated that our monoclonal
antibodies uniquely recognized HER2/neu (Fig. 3B, right panel).
Levels of AIF inversely correlates with
expression of positive tumor markers in NSCLC
Our previous studies showed that overexpression of
DDH, c-MET, MMP1 and OSMRs in NSCLC was associated with drug
resistance, tumor recurrence, metastasis and poor prognosis
(20–26). Moreover, our recent data suggested
that expression of HGF, which was frequently detected in smokers
with NSCLC, reduced levels of AIF (25). In this study, we investigated the
correlation between levels of AIF and expression of positive tumor
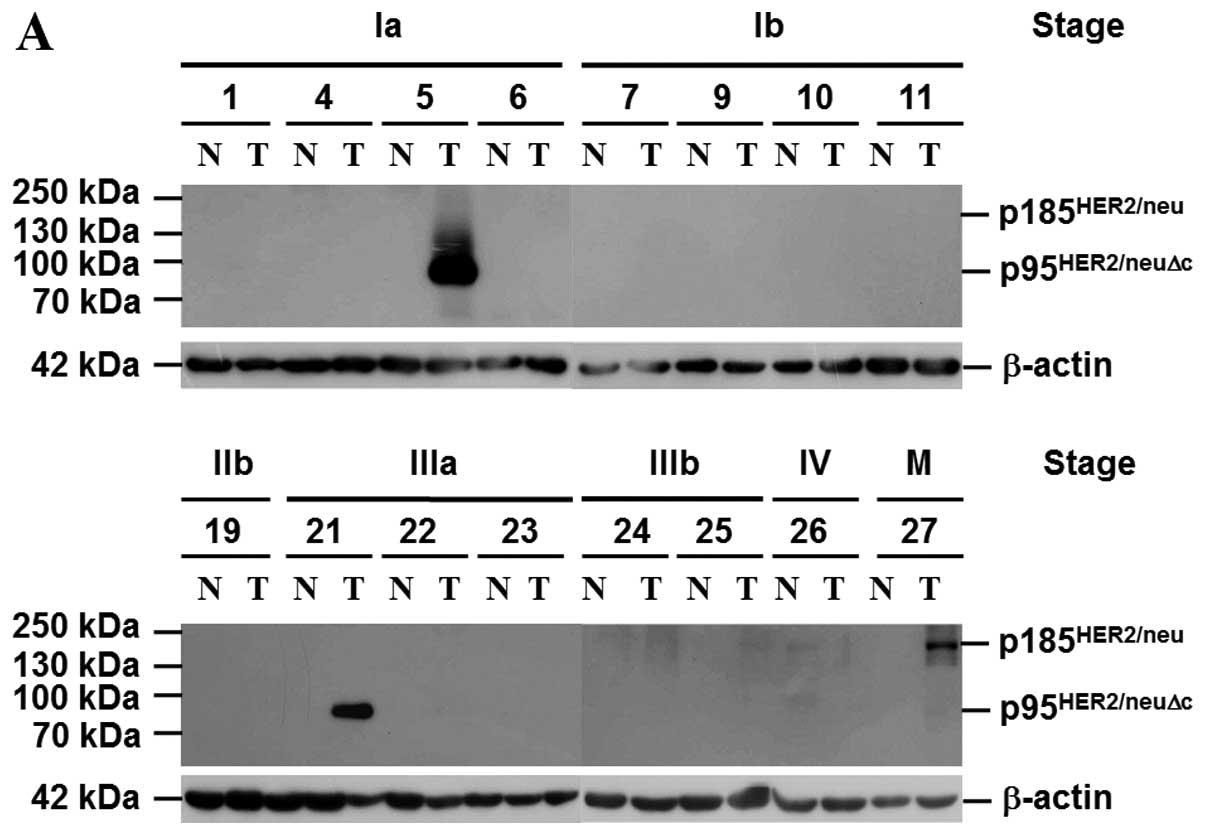
markers, e.g., DDH, c-MET, MMP1, HGF, OSMRs and HER2/neu (Fig. 4A), in NSCLC. HER2/neu in patients
with the early stages was mainly p95HER2/neuΔc (HER2/neu
with deletion of extracellular domain) (32); in advanced stages it was the
p185HER2/neu. As shown in Fig. 4B, higher AIF expression ratio was
detected in 37 (43.5%) of 85 tumor specimens. When gene expression,
tumor staging and smoking habit were used to categorize patient
groups, our data showed that AIF expression, as determined by
immunoblotting, was inversely correlated with positive tumor
markers, tumor staging and cigarette smoking. Noteworthy, cancer
samples that had higher level of HER2/neu expressed less AIF.
Levels of checkpoint kinase 1 (CHK1) and Nijmegen breakage syndrome
1 (NBS1, nibrin) protein, which are essential for mediating cell
cycle arrest and maintaining genome stability during DNA
replication, on the other hand, were proportional to those of AIF.
The results are consistent with our in vitro data that AIF
level is comparative to that of NSB1 (22) and HGF inhibits AIF expression
(26).
In vivo, expression level of AIF is
comparatively low in the center of cigarette smoking (CS)-induced
lung adenocarcinoma in BALB/c3 mice
To study the in vivo effect of cigarette
smoking on AIF expression, male BALB/c mice (National Animal
Center, National Science Council, Taipei, Taiwan) at six weeks of
age were treated with passive cigarette smoking daily (5 min a day
in a 40 cm × 40 cm ×60 cm chamber, and each cigarette contained 0.9
mg of nicotine, Marlboro, USA) for 25 weeks before
histopathological and biochemical examinations (Animal exposure
protocols were approved by the Institutional Animal Care and Use
Committee of National Chung-Hsing University). Among 100 male mice,
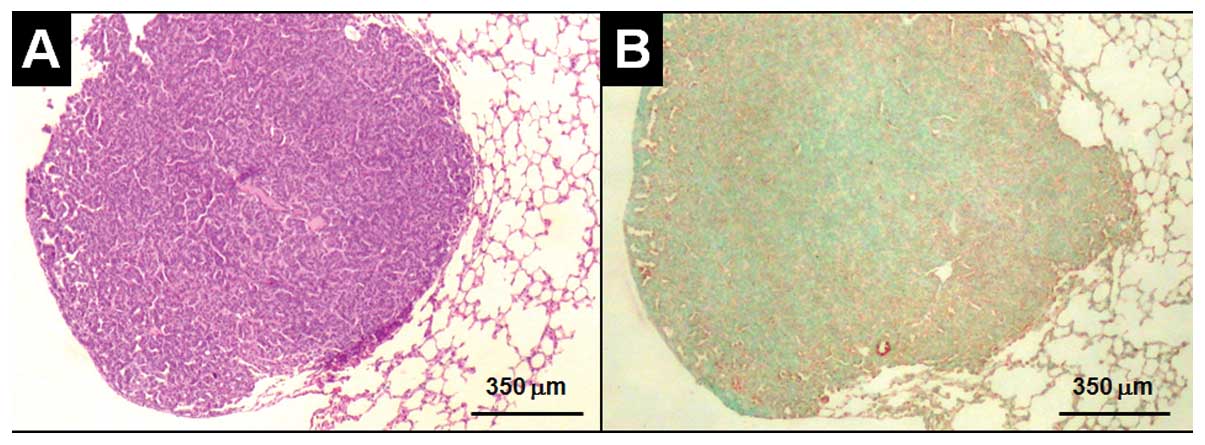
lung cancer was detected in seven mice. Compared to alveolar type
II (ATII) pneumocytes and cancer cells in the periphery (Fig. 5A), which were highly expressing AIF,
AIF level decreased toward the center of the tumor (Fig. 5B). The results are consistent with
our in vitro data that nicotine reduced AIF expression
(26,33).
Effect of HER2/neu on AIF expression and
cell survival following cisplatin challenge in NSCLC cells
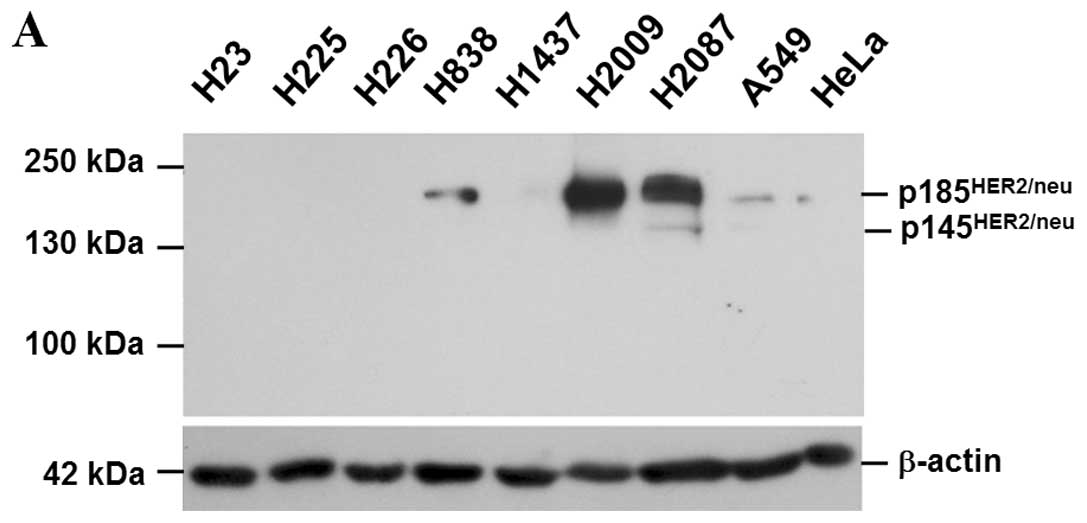
Eight NSCLC cells, H23, H225, H226, H838, H1437,
H2009, H2087 and A549, examined by immunoblotting, expressed
various levels of HER2/neu: high in H2009 and H2087; and low in
H838 and A549 (Fig. 6A). HER2/neu
was not detected in H23, H225, H226 and H1437. Of note, silence of
HER2/neu expression by siRNA increased AIF expression; however, it
did not affect the HGF effect on downregulation of AIF (Fig. 6B). The data indicated that HER2/neu
also influences AIF expression in NSCLC cells. Since c-MET and
HER2/neu share FAK as a common signal transducer, we examined the
effect of p60src and p23ras on FAK
expression. As shown in Fig. 6C,
overexpression of p60src protected FAK protein; however,
p23ras had no effect. The presence of HGF saved FAK
protein from proteolytic degradation and facilitated cell moving
out of the agarose trap (Fig.
6D).
Discussion
The results show that AIF expression in NSCLC is
inversely correlated with tumor stage and patient cigarette smoking
history. By demonstrating that in patients with more advanced lung
cancer, especially, in those who smoked more than 20 pack-years,
AIF expression was low in tumor cells (~67.6% of specimens), our
data suggest that, besides being involved in lung carcinogenesis,
cigarette smoking increases drug resistance by downregulating
expression of AIF in lung tumor cells. Reduced AIF expression
correlated with poor prognosis and was inversely associated with
expressions of positive tumor marker genes.
The results corresponded well with our previous
study that HGF was upregulated by tobacco smoke in ATII and NSCLC
cells (25). Increase of HGF in
turn reduces AIF expression and cisplatin sensitivity (26), which could be mediated via c-MET,
FAK, phosphoinositide kinase 3 (PI3K) and protein kinase B (PKB,
also called AKT) pathway in NSCLC cells (26,34).
This study showed that HER2/neu was also involved in downregulation
of AIF expression. Since signals from HER2/neu and c-MET converge
onto a common transducer FAK protein, our results suggested that
these two receptors might in part play a role in activation of PI3K
and AKT (26), and activated AKT
decreased expression levels of AIF, a vital factor of
caspase-independent cell death, to induce drug resistance in NSCLC
(25,26). Expression level of FAK alone,
however, was not associated with that of AIF or drug resistance
(data not shown).
In addition, AIF level correlated with expression of
critical sensor proteins of DNA damage and replication stress, NBS1
and CHK1, which are essential for mediating cell-cycle arrest and
DNA-repair mechanisms (35).
Noteworthy, FERM (band four point one, Ezrin/Radxin/Moesin) region
of FAK protein interacts with the N-terminal transactivation domain
of p53, which binds mouse double minute 2 (MDM-2), a ubiquitin
ligase (36). Selective binding of
p53 to MDM-2 or FAK facilitates cell transformation and
epithelial-mesenchymal transition (37). By showing that overexpression of
v-src protein and addition of HGF or fetal calf serum (FCS)
protected FAK from integrin-related protein degradation, our data
suggested that integrin, v-src and H-ras might have different
effects on FAK function. ATR, a cell stress-responsive kinase that
is essential for sensing DNA replication error, contains a stretch
of focal adhesion targeting (FAT) and PI3K motifs (38). Myers et al demonstrated that
ATR and Chk1 alleviate replication-related stress by suppressing a
caspase-3-dependent apoptotic response (39). Our data supported their findings and
showed that in combination with reduced AIF expression,
downregulation of NBS1 and CHK1, two mediators of ATR, might elude
the inhibitory effect of p53 on cell cycle progression, and
increase genomic heterogeneity as well as resistance to
doxorubicin, etoposide, cisplatin and gemcitabine in NSCLC cells,
which overexpressed HER2/neu (40).
By showing that levels of HER2/neu increased following addition of
FCS, our data suggested that HER2/neu expression might not be
constitutive (41), but could be
upregulated by yet to be determined serum factors.
Moreover, our previous studies showed that drug
resistance, tumor recurrence, metastasis and poor prognosis of
NSCLC correlated with overexpression of DDH, c-MET, MMP1, HGF and
OSMRs (20–25). Recently, by confocal
immunofluorescence microscopy we found that HGF, which was
frequently detected in smoker patients, was expressed on surface of
cancer cells (33). Therefore, the
overexpressed HGF could only interact with nearby cells. Of note,
HGF overexpression was induced by prostaglandin F2α
(PGF2α), which was synthesized by DDH when cells were
under hypoxic condition (33). As
shown in cigarette smoking-induced murine lung ADC, AIF expression
was much lower in the center of tumor mass when tumor size was
larger than one millimeter. However, we are less certain whether
this phenomenon was caused by tumor size-related hypoxia or the
effect of tobacco smoke could not reach the interior of tumor mass.
In addition to reducing levels of AIF, HGF upregulates expression
of interleukin (IL)-1α, -1β, -6, -8 and -24 (a member of IL-10
family), as well as that of tumor necrosis factor (TNF) superfamily
member 10 (TNFSF10), MMP1 and transforming growth factor α (TGF-α),
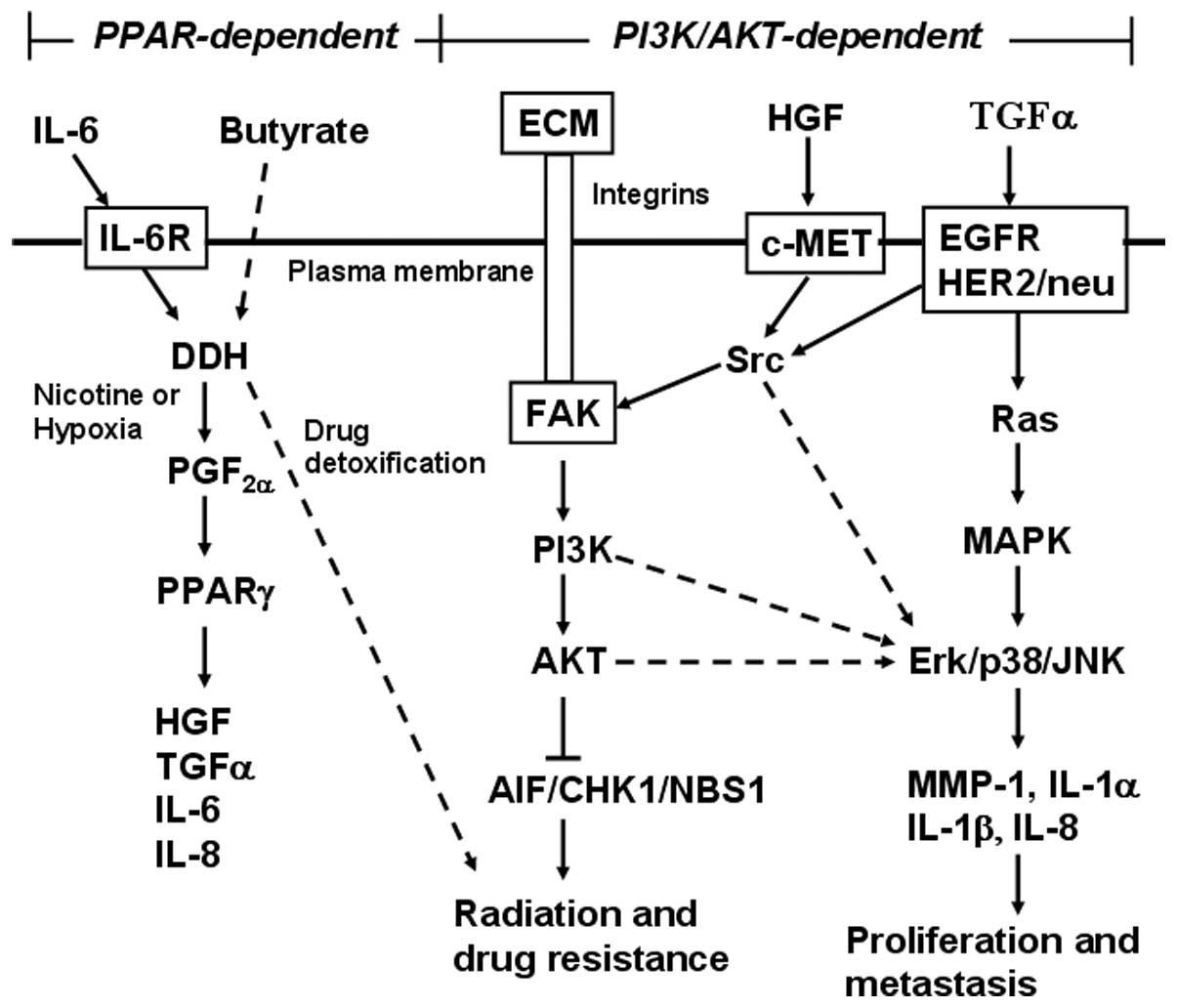
an alternative ligand of EGFR (33). TGF-α activates EGFR and IL-6 induces
overexpression of DDH (22), which
constitute a vicious cycle to maintain cancer cell survival
(Fig. 7); in particular, activated
EGFR is vital for cell proliferation and DDH is essential for
detoxification of anticancer drugs, including cisplatin (21), doxorubicin, etoposide, mitoxantrone,
gefitinib and erlotinib, of which chemical structures are highly
similar to polycyclic aromatic hydrocarbons (PAH) (20).
These results considered together with the current
data provided in vitro explanations to support our previous
findings that tobacco smoking and tumor size are the two major
factors influencing overall and disease-free survival in patients
with stage I NSCLC (6); in
particular in patients with stage Ib disease AIF levels were
markedly reduced. Patients who continued to smoke after proper
resections were more resistant to chemotherapy and irradiation, and
had poorer prognosis (6–8,20–25).
Cigarette smoking and hypoxia respectively induce expression of
HGF, which decreases AIF levels and drug sensitivity in NSCLC cells
(25,26). In conclusion, our data show that AIF
expression was frequently downregulated in patients with NSCLC,
especially in those with a previous or current smoking history.
Statistical analysis showed that decreased AIF level in NSCLC was
closely associated with patient survival. These results suggest
that cigarette smoking plays an important role in drug resistance
and cancer cell survival, which were probably mediated via HGF,
HER2/neu and FAK activation-induced downregulation of AIF, CHK1 and
NBS1 expression in patients with NSCLC (42).
Abbreviations:
|
AIF
|
apoptosis inducing factor
|
|
ATII
|
alveolar type II epithelial cells
|
|
ATM
|
ataxia-telangiectasia mutated
kinase
|
|
ATR
|
ATM and Rad 3-related kinase
|
|
HGF
|
hepatocyte growth factor
|
|
HGFR
|
HGF receptor/c-MET
|
|
MMP-1
|
matrix metalloproteinase-1
|
|
NSCLC
|
non-small cell lung cancer
|
|
PI3K
|
phosphoinositide 3-kinase
|
References
|
1
|
Schiller JH, Harrington D, Belani CP, et
al; The Eastern Cooperative Oncology Group. Comparison of four
chemotherapy regimens for advanced non-small-cell lung cancer. N
Engl J Med. 346:92–98. 2002. View Article : Google Scholar
|
|
2
|
Phillips DH: Smoking-related DNA and
protein adducts in human tissues. Carcinogenesis. 23:1979–2004.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Annual reports of the Department of
Health, the Executive Yuan, Taiwan, Republic of China, 2012.
|
|
4
|
Moran CA and Suster S: Tumours of the lung
and pleura. Diagnostic Histopathology of Tumours. Fletcher CDM:
Churchill Livingstone; London: pp. 171–208. 2000
|
|
5
|
Ko YC, Lee CH, Chen MJ, et al: Risk
factors for primary lung cancer among non-smoking women in Taiwan.
Int J Epidemiol. 26:24–31. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hung JJ, Wang CY, Huang MH, Huang BS, Hsu
WH and Wu YC: Prognostic factors in resected stage I non-small cell
lung cancer with a diameter of 3 cm or less: visceral pleural
invasion did not influence overall and disease-free survival. J
Thorac Cardiovasc Surg. 134:638–643. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Browman GP, Wong G, Hodson I, et al:
Influence of cigarette smoking on the efficacy of radiation therapy
in head and neck cancer. N Engl J Med. 328:159–163. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johnston-Early A, Cohen MH, Minna JD, et
al: Smoking abstinence and small cell lung cancer survival. An
association. JAMA. 244:2175–2179. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Videtic GM, Stitt LW, Dar AR, et al:
Continued cigarette smoking by patients receiving concurrent
chemoradiotherapy for limited-stage small-cell lung cancer is
associated with decreased survival. J Clin Oncol. 21:1544–1549.
2003. View Article : Google Scholar
|
|
10
|
Hoffmann D, Lavoie EJ and Hecht SS:
Nicotine: a precursor for carcinogens. Cancer Lett. 26:67–75. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Minna JD: Nicotine exposure and bronchial
epithelial cell nicotinic acetylcholine receptor expression in the
pathogenesis of lung cancer. J Clin Invest. 111:31–33. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garnier M, Lamacz M, Tonon MC and Vaudry
H: Functional characterization of a nonclassical nicotine receptor
associated with inositolphospholipid breakdown and mobilization of
intracellular calcium pools. Proc Natl Acad Sci USA.
91:11743–11747. 1994. View Article : Google Scholar
|
|
13
|
Jull BA, Plummer HK III and Schuller HM:
Nicotinic receptor-mediated activation by the tobacco-specific
nitrosamine NNK of a Raf-1/MAP kinase pathway, resulting in
phosphorylation of c-myc in human small cell lung carcinoma cells
and pulmonary neuroendocrine cells. J Cancer Res Clin Oncol.
127:707–717. 2001.
|
|
14
|
Masaki T, Igarashi K, Tokuda M, et al:
pp60c-src activation in lung adenocarcinoma. Eur J Cancer.
39:1447–1455. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park PG, Merryman J, Orloff M and Schuller
HM: Beta-adrenergic mitogenic signal transduction in peripheral
lung adenocarcinoma: implications for individuals with preexisting
chronic lung disease. Cancer Res. 55:3504–3508. 1995.PubMed/NCBI
|
|
16
|
Schuller HM and Cekanova M: NNK-induced
hamster lung adenocarcinomas over-express beta2-adrenergic and EGFR
signaling pathways. Lung Cancer. 49:35–45. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsurutani J, Castillo SS, Brognard J, et
al: Tobacco components stimulate Akt-dependent proliferation and
NFkappaB-dependent survival in lung cancer cells. Carcinogenesis.
26:1182–1195. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
West KA, Brognard J, Clark AS, et al:
Rapid Akt activation by nicotine and a tobacco carcinogen modulates
the phenotype of normal human airway epithelial cells. J Clin
Invest. 111:81–90. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
West KA, Linnoila IR, Belinsky SA, Harris
CC and Dennis PA: Tobacco carcinogen-induced cellular
transformation increases activation of the phosphatidylinositol
3′-kinase/Akt pathway in vitro and in vivo. Cancer Res. 64:446–451.
2004.PubMed/NCBI
|
|
20
|
Hsu NY, Ho HC, Chow KC, et al:
Overexpression of dihydrodiol dehydrogenase as a prognostic marker
of non-small cell lung cancer. Cancer Res. 61:2727–2731.
2001.PubMed/NCBI
|
|
21
|
Deng HB, Parekh HK, Chow KC and Simpkins
H: Increased expression of dihydrodiol dehydrogenase induces
resistance to cisplatin in human ovarian carcinoma cells. J Biol
Chem. 277:15035–15043. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang HW, Lin CP, Chiu JH, et al: Reversal
of inflammation-associated dihydrodiol dehydrogenases (AKR1C1 and
AKR1C2) overexpression and drug resistance in nonsmall cell lung
cancer cells by wogonin and chrysin. Int J Cancer. 120:2019–2027.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin TS, Chiou SH, Wang LS, et al:
Expression spectra of matrix metalloproteinases in metastatic
non-small cell lung cancer. Oncol Rep. 12:717–723. 2004.PubMed/NCBI
|
|
24
|
Chen DR, Chu CY, Chen CY, et al:
Expression of short form oncostatin M receptor as a decoy receptor
in lung adenocarcinomas. J Pathol. 215:290–299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen JT, Lin TS, Chow KC, et al: Cigarette
smoking induces overexpression of HGF in type II pneumocytes and
lung cancer cells. Am J Repir Cell Mol Biol. 34:264–273. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen JT, Huang CY, Chiang YY, et al: HGF
increases cisplatin resistance via down-regulation of AIF in lung
cancer cells. Am J Respir Cell Mol Biol. 38:559–565. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yano S, Wang W, Li Q, et al: Hepatocyte
growth factor induces gefitinib resistance of lung adenocarcinoma
with epidermal growth factor receptor-activating mutations. Cancer
Res. 68:9479–9487. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mountain CF: Revisions in the
international system for staging lung cancer. Chest. 111:1710–1717.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Remmele W and Schicketanz KH:
Immunohistochemical determination of estrogen and progesterone
receptor content in human breast cancer. Computer-assisted image
analysis (QIC score) vs subjective grading (IRS). Pathol Res Pract.
189:862–866. 1993. View Article : Google Scholar
|
|
30
|
Kaplan EL and Meier P: Nonparametric
estimation from incomplete observations. J Am Stat Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
31
|
Mantel N: Evaluation of survival data and
two new rank order statistics arising in its consideration. Cancer
Chemother Rep. 50:163–170. 1966.PubMed/NCBI
|
|
32
|
Swanton C, Futreal A and Eisen T:
Her2-targeted therapies in non-small cell lung cancer. Clin Cancer
Res. 12:4377s–4383s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chiang YY: Hepatocyte growth factor
induces hypoxia-related interleukin-8 expression in lung
adenocarcinoma cells. Mol Carcinog. 48:662–670. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma PC, Jagadeeswaran R, Jagadeesh S, et
al: Functional expression and mutations of c-Met and its
therapeutic inhibition with SU11274 and small interfering RNA in
non-small cell lung cancer. Cancer Res. 65:1479–1488. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kastan MB and Bartek J: Cell-cycle
checkpoints and cancer. Nature. 432:316–323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lim ST, Chen XL, Lim Y, et al: Nuclear FAK
promotes cell proliferation and survival through FERM-enhanced p53
degradation. Mol Cell. 29:9–22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mitra SK and Schlaepfer DD:
Integrin-regulated FAK-Src signaling in normal and cancer cells.
Curr Opin Cell Biol. 18:516–523. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shiloh Y: ATM and related protein kinases:
safeguarding genome integrity. Nat Rev Cancer. 3:155–168. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Myers K, Gagou ME, Zuazua-Villar P,
Rodriguez R and Meuth M: ATR and Chk1 suppress a
caspase-3-dependent apoptotic response following DNA replication
stress. PLoS Genet. 5:e10003242009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tsai CM, Chang KT, Wu LH, et al:
Correlations between intrinsic chemoresistance and HER-2/neu gene
expression, p53 gene mutations, and cell proliferation
characteristics in non-small cell lung cancer cell lines. Cancer
Res. 56:206–209. 1996.PubMed/NCBI
|
|
41
|
Noro R, Gemma A, Kosaihira S, et al:
Gefitinib (IRESSA) sensitive lung cancer cell lines show
phosphorylation of Akt without ligand stimulation. BMC Cancer.
6:2772006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
King FW, Skeen J, Hay N and Shtivelman E:
Inhibition of Chk1 by activated PKB/Akt. Cell Cycle. 3:634–637.
2004.PubMed/NCBI
|