Introduction
Nasopharyngeal carcinoma (NPC) is much more common
in Southeast Asia and Southern China but is rare in most other
parts of the world (1). This
disease is managed primarily by radiotherapy with or without
chemotherapy (2). Despite
significant progress in the methods of conventional therapies such
as radiotherapy and chemotherapy, the outcome has been
disappointing. The 5-year survival rate is still approximately
between 34 and 52% (3). Both local
recurrence and lymph node metastasis are major causes of death in
NPC and are key factors affecting clinical outcome and prognosis
(4). The recurrence and metastasis
of NPC is a multi-step process that often involves many complex
biological and pathologic events. Despite extensive clinical as
well as basic research efforts, the mechanisms involved in NPC
metastasis still remain undetermined. To improve the prognosis of
NPC, it is important to identify additional reliable prognostic
factors indicating recurrence and metastasis of NPC. The search for
reliable markers to predict prognosis is important, as intensive
adjuvant treatment is critical for patients with a predictable poor
outcome.
The tropomyosin-related kinase B (TrkB) is a member
of the Trk family, and functions as a receptor tyrosine kinase.
TrkB, which has brain-derived neurotrophic factor (BDNF) as its
primary ligand, plays an essential role in nervous system
development, neuronal survival, differentiation and maintenance
(5). Recent reports indicate that
TrkB has an important function in tumor pathology. Several studies
have shown that TrkB is oncogenic not only in neurogenic original
tumors, but also in other tumors outside of the neural system.
Overexpression of TrkB has been reported in a variety of cancers,
including neuroblastoma, cancer of the lung, pancreas, stomach and
ovaries and has been associated with more aggressive malignant
behavior and a poor prognosis (6–10).
Furthermore, activation of the BDNF/TrkB pathway in some cancers is
implicated in inhibition of apoptosis, increased proliferation,
promotion of invasion, and facilitation of tumor progression by
lymphangiogenesis-associated metastasis (11). Therefore, TrkB may play an important
role in the progression and invasion of malignant tumors. However,
the role of TrkB in human NPC remains unknown. Thus, the objectives
of the present study were to determine the expression patterns of
TrkB in NPC specimens and different NPC cell lines to explore the
correlation with clinicopathologic features and prognosis in
patients with NPC.
Materials and methods
Patients and tissue samples
Specimens of tissues were obtained from 108 NPC
patients and 14 nasopharyngitis patients who underwent
nasopharyngeal biopsy at the Department of Otolaryngology, Head
Neck Surgery, The Second Xiangya Hospital of Central South
University (Changsha, China) from January 2002 to January 2004. The
NPC biopsy specimens were from patients who were diagnosed with
primary NPC, and the noncancerous nasopharyngeal biopsy samples
were from patients who were suspected of having NPC originally, but
afterwards were pathologically and clinically proven to be clear.
There were 87 male and 21 female patients, and their median age was
56 years (range, 19–86 years). The diagnoses for all patients were
confirmed by histopathologic examination. The NPC tissue specimens
were histologically confirmed and classified according to WHO
classification into keratinizing carcinoma (type 1, 8 cases),
differentiated non-keratinizing carcinoma (type 2, 30 cases) and
undifferentiated non-keratinizing carcinoma (type 3, 70 cases). All
patients were re-staged according to the 2010 American Joint
Committee on Cancer (AJCC) criteria. There were 2 cases in stage I
(T1N0M0 2 cases), 12 cases in stage II (T1N1M0 2 cases, T2N0M0 6
cases, T2N1M0 4 cases), 33 cases in stage III (T1N2M0 6 cases,
T2N2M0 12 cases, T3N0M0 6 cases, T3N1M0 1 case, T3N2M0 8 cases) and
61 cases in stage IV (T1N3M0 5 cases, T2N3M0 22 cases, T2N1M1 1
case, T2N2M1 1 case, T2N3M1 2 cases, T3N3M0 13 cases, T3N2M1 1
case, T3N3M1 2 cases, T4N0M0 2 cases, T4N1M0 2 cases, T4N2M0 1
cases, T4N3M0 4 cases, T4N1M1 1 case, T4N2M1 3 cases and T4N3M1 1
case). Demographic details and tumor characteristics are listed in
Table I.
 | Table IClinicopathological features of the
studied 108 cases of nasopharyngeal carcinoma (NPC). |
Table I
Clinicopathological features of the
studied 108 cases of nasopharyngeal carcinoma (NPC).
| Variables | No. of patients
(%) |
|---|
| Gender |
| Male | 87 (80.6) |
| Female | 21 (19.4) |
| Age (years) |
| ≤50 | 56 (51.9) |
| >50 | 52 (48.1) |
| Histological
type |
| 1
(keratinizing) | 8 (7.4) |
| 2 (differentiated
non-keratinizing) | 30 (27.8) |
| 3 (undifferentiated
non-keratinizing) | 70 (64.8) |
| T classification |
| T1 | 15 (13.9) |
| T2 | 48 (44.4) |
| T3 | 31 (28.7) |
| T4 | 14 (13.0) |
| N classification |
| N0 | 16 (14.8) |
| N1 | 11 (10.2) |
| N2 | 33 (30.6) |
| N3 | 48 (44.4) |
| M classification |
| M0 | 96 (88.9) |
| M1 | 12 (11.1) |
| Clinical stage |
| I | 2 (1.9) |
| II | 12 (11.1) |
| III | 33 (30.6) |
| IV | 61 (56.4) |
All tissue specimens were fixed in 10% neutralized
formalin and embedded in paraffin. Seventeen cases of NPC tissues
among the 108 cases and 14 cases of noncancerous nasopharyngeal
tissues were cut into 2 portions. One portion was fixed in 10%
neutralized formalin and embedded in paraffin blocks for
immunohistochemistry. The other portion was frozen immediately and
stored in liquid nitrogen for RNA and total protein extraction.
The study was approved by the Central South
University Ethics Committee. Informed consent was obtained from all
of the patients. All specimens were handled and made anonymous
according to the ethical and legal standards.
Treatment and follow-up
All patients received a complete course of
radiotherapy with or without chemotherapy. Irradiation was
administered primarily with megavoltage (6–10 MV) X-rays from a
linear accelerator. The dose was 1.8–2.0 Gy/fraction, with 5
fractions/week. All patients were evaluated by nasopharyngoscope at
10–14 weeks after completion of radiotherapy. Patients were
followed up regularly after treatments. The follow-up period was
defined as the interval between the date of treatment completion
and the date of either the patient's death or the last follow-up.
Deaths were treated as censored cases. Clinical examinations,
including an indirect mirror examination of the nasopharynx with or
without a nasopharyngoscope, were performed during subsequent
follow-ups. Chest X-ray, liver echo, whole body bone scans,
computed tomography and magnetic resonance imaging (MRI) were
performed only when clinically indicated. All disease-free patients
had a minimum follow-up of 60 months after the completion of
treatment. Locoregional or distant recurrence was confirmed by
histopathological or radiological criteria.
Immunohistochemistry
Specimens were obtained before therapy, fixed in 10%
neutral buffered formalin, and embedded in paraffin. Serial 5-μm
sections were prepared. Slides were deparaffinized in xylene,
rehydrated in graded alcohol, and incubated in 0.3% hydrogen
peroxide in methanol to quench the endogenous peroxidase activity
for 30 min. The sections were subjected to microwave heat-induced
antigen retrieval in 0.01 M citrate buffer, pH 6.0, at high power
twice for 7 min each. Nonspecific binding sites were blocked by a
20-min incubation with normal horse serum.
The sections were incubated for 2 h at room
temperature with primary rabbit polyclonal antibody detecting TrkB
(1:100) followed by the addition of HRP-labeled goat anti-rabbit
polymers. The streptavidin-biotin-peroxidase complex tertiary
system (Boster, Wuhan, China) was used according to the
manufacturer's instructions. All slides were visualized by applying
3,3′-diaminobenzidine tetrahydrochloride for 2 min and then
counterstained with hematoxylin. Negative controls were non-immune
rabbit IgG at the same dilution as for the primary antibody.
Sections were evaluated in a blinded manner and
scored by two independent experienced pathologists. Expression
levels of TrkB protein were assessed by observing the incidence and
staining intensity (i.e., color strength) of immuno-positive cells.
Staining intensity was scored as: 0, negative; 1, weak; 2, strong.
The incidence of positive cells was scored as: 1, 0–50%; 2, 51–75%;
3>75%. Tumors were categorized into three groups based on the
final staining point: low expression (scored 0–3), and high
expression (scored 4–6) (11).
Cell culture
The Epstein-Barr virus (EBV)-negative, highly
differentiated NPC cell line HNE-1 and the EBV-negative, lowly
differentiated NPC cell line HNE-2 were established by the Cancer
Research Institute, Xiangya School of Medicine, Central South
University (12). The NPC cell line
C666-1 which consistently expressed EBV was established from
undifferentiated nasopharyngeal carcinoma by the Department of
Anatomical and Cellular Pathology, Prince of Wales Hospital,
University of Hong Kong (Hong Kong, China) (13). The NPC cell lines CNE-1 and CNE-2
were established from the primary tumors of patients with
well-differentiated squamous cell NPC and poorly differentiated
squamous cell NPC, respectively, by the Chinese Academy of Medical
Sciences. The highly metastatic NPC cell line 5-8F and the
nonmetastatic NPC cell line 6-10B were established from the NPC
cell line SUNE-1 by the Cancer Research Institute of Sun Yatsen
University, Guangzhou, China (14).
The above 7 NPC cell lines were kindly provided by Cancer Research
Institute, Xiangya School of Medicine, Central South University.
The nontransformed nasopharyngeal epithelium cell line (NP-69) was
established from the nasopharynx of a human by the Department of
Anatomy, Faculty of Medicine, University of Hong Kong (15). This cell line was purchased from the
Cell Experiment Center of Shanghai, China. All cell lines were
maintained as monolayer cultures in Dulbecco's modified Eagle's
medium (DMEM)/F12 medium (1:1) supplemented with 10% fetal bovine
serum (FBS), 100 IU/ml penicillin and 100 IU/ml streptomycin at
37°C in a humidified atmosphere with 5% CO2.
Total RNA isolation and RT-PCR
Total RNA of the tissue specimens, the 7 NPC cell
lines and the nontransformed nasopharyngeal epithelium cell line
(NP-69) was isolated by Simply P Total RNA Extraction kit (Bioer
Technology, Inc., Hangzhou, China). Total RNA (1 μg) was reverse
transcribed by High-Capacity cDNA Reverse Transcription kits
(Applied Biosystems, Foster City, CA, USA) according to the
manufacturer's instructions. The primers for TrkB and GAPDH were
synthesized by Sangon Biological Engineering Technology and
Services Co., Ltd. (Shanghai, China) as follows: TrkB F,
5′-AGTCGCAGATGCTGCATATTAG-3′ and R, 5′-TAGAATGTCCAGGTAGACC-3′;
GAPDH F, 5′-CTGCAGCATC TTCTCCTTCC-3′ and R,
5′-CAAAGTTGTCATGGATGACC-3′. The reverse transcription product (1
μl) was amplified by PCR using the following conditions: 95°C for 5
min; followed by 32 cycles at 95°C for 30 sec, 58°C for 30 sec, and
72°C for 30 sec; and an extension at 72°C for 5 min. The PCR
product (8 μl) was then electrophoresed on 1.5% agarose gel. TrkB
mRNA expression levels were quantified by Quantity One software
(Bio-Rad Technical Service Department, Hercules, CA, USA) and
represented as the densitometric ratio of the targeted gene to
GAPDH. PCR experiments were repeated three times.
Western blot analysis
Total protein lysates were harvested from the tissue
specimens and cell lines by lysis buffer (20 mM
Na2PO4, 150 mM NaCl, 1% Triton X-100, 1%
aprotinin, 1 mM phenylmethylsulfonyl fluoride, 100 mM NaF and 2 mM
Na3VO4). Proteins (50 μg) were separated by
polyacrylamide gel electrophoresis on a 10% sodium dodecyl
sulfate-polyacrylamide gel and transferred to polyvinylidene
difluoride membranes. Membranes were incubated with the primary
antibody overnight at 4°C. On the next day, the membranes were
washed with phosphate-buffered saline (PBS) three times and then
the membranes were incubated with horseradish peroxidase-conjugated
goat anti-rabbit secondary antibodies (all the antibodies were
obtained from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
Finally, the proteins were detected by enhanced chemiluminescence
(ECL) procedure. TrkB protein expression levels were quantified by
Quantity One software and represented as the densitometric ratio of
the targeted protein to β-actin. Western blot experiments were
repeated three times.
Statistical analysis
Data are expressed as means ± SD. Student's
two-tailed t-test was used to compare TrkB expression between
cancer and noncancerous nasopharyngeal tissues. The protein
expression and clinicopathological parameters were compared using
the χ2 test. Survival analysis was undertaken using the
Kaplan-Meier method, and curves were compared using the log-rank
test. Multivariate analysis, using the Cox's proportional hazards
model, was applied to identify which factors were independent
indicators for prognosis. All statistical evaluations were
performed using the Statistical Package for the Social Sciences
version 10.0 software program (SPSS Inc., Chicago, IL, USA). All
tests were two-tailed, and a P-value of <0.05 was considered to
indicate a statistically significant result.
Results
Results of immunohistochemistry and
correlations between TrkB expression and clinicopathologic
variables
The 108 NPC tumor samples were initially tested for
TrkB protein expression by immunohistochemistry, and then the level
of TrkB protein expression was correlated with clinicopathological
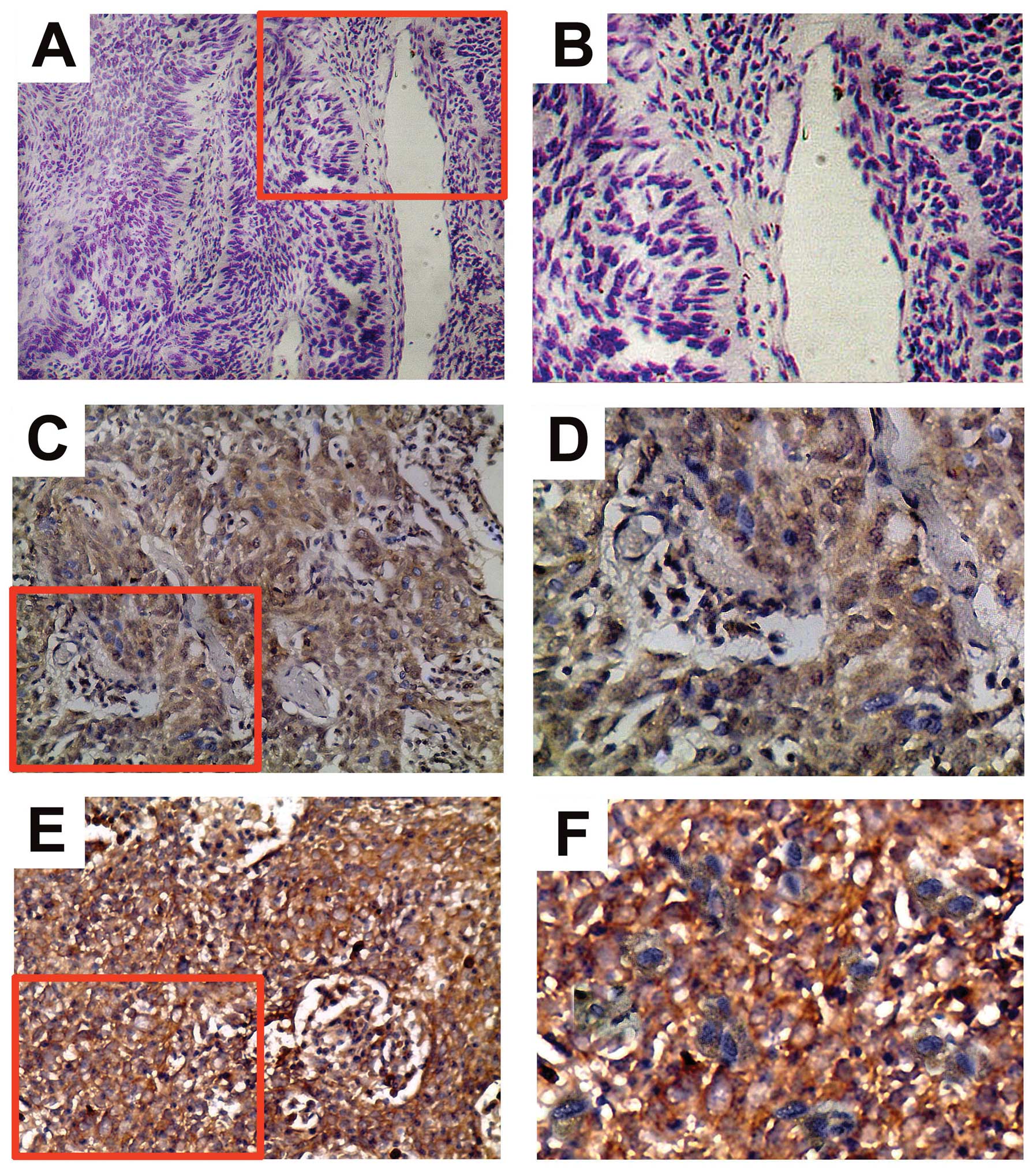
parameters. Positive TrkB immunostaining was predominantly
diffusely distributed throughout the cytoplasm of NPC cells
(Fig. 1). Of all the samples
tested, 65 (60.2%) cases displayed low TrkB protein expression
(Fig. 1C and D) and 43 (39.8%)
cases displayed high TrkB protein expression (Fig. 1E and F). The χ2 test
showed that the TrkB expression level was significantly associated
with NPC tumor size (T classification) (P=0.015), lymph node
metastasis (N classification) (P=0.009), AJCC clinical stage
(P=0.007). However, we did not find a significant association
between TrkB expression level and age, gender and distant
metastasis (M classification). The relationship between
clinicopathological characteristics and TrkB expression level in
individuals with NPC are summarized in Table II.
 | Table IICorrelation between TrkB expression
and clinicopathological variables in patients with NPC. |
Table II
Correlation between TrkB expression
and clinicopathological variables in patients with NPC.
| Variables | Total | TrkB low expression,
n (n=65) | TrkB high expression,
n (n=43) | P-value |
|---|
| Gender |
| Male | 87 | 56 | 31 | 0.071 |
| Female | 21 | 9 | 12 | |
| Age (years) |
| ≤50 | 56 | 33 | 23 | 0.782 |
| >50 | 52 | 32 | 20 | |
| Histological
type |
| 1
(keratinizing) | 8 | 4 | 4 | 0.281 |
| 2 (differentiated
non-keratinizing) | 30 | 15 | 15 | |
| 3 (undifferentiated
non-keratinizing) | 70 | 46 | 24 | |
| T
classification |
| T1+T2 | 63 | 44 | 19 | 0.015 |
| T3+T4 | 45 | 21 | 24 | |
| N
classification |
| N0+N1 | 27 | 22 | 5 | 0.009 |
| N2+N3 | 81 | 43 | 38 | |
| M
classification |
| M0 | 96 | 60 | 36 | 0.165 |
| M1 | 12 | 5 | 7 | |
| Clinical stage |
| I+II | 14 | 13 | 1 | 0.007 |
| III+IV | 94 | 52 | 42 | |
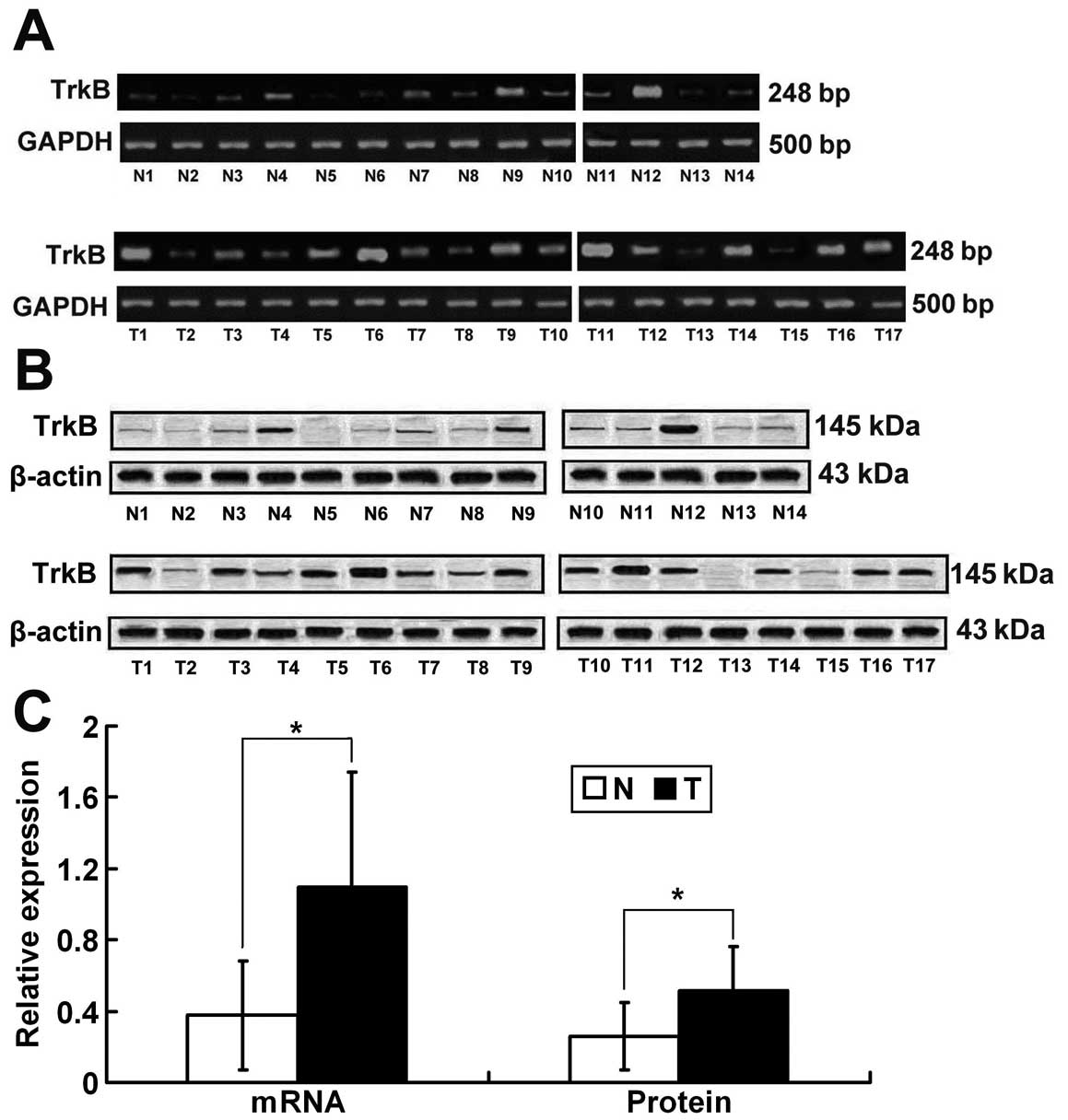
Elevated expression levels of TrkB mRNA
and protein in NPC tissues
Seventeen freshly collected clinical NPC tissues and
14 freshly collected normal nasopharyngeal mucosal tissues were
further investigated by RT-PCR and western blot analysis. The
results of RT-PCR showed that the TrkB relative mRNA expression
level was upregulated in the NPC tissues when compared to that in
the nasopharyngeal mucosal tissues (1.1±0.64 vs. 0.38±0.27,
P=0.000<0.01; Fig. 2A and C).
Consistent with the mRNA data, the TrkB relative protein expression
level in the NPC tissue was significantly higher when compared with
that in the nasopharyngeal mucosal tissues (0.52±0.24 vs.
0.26±0.18, P=0.003<0.01; Fig. 2B and
C).
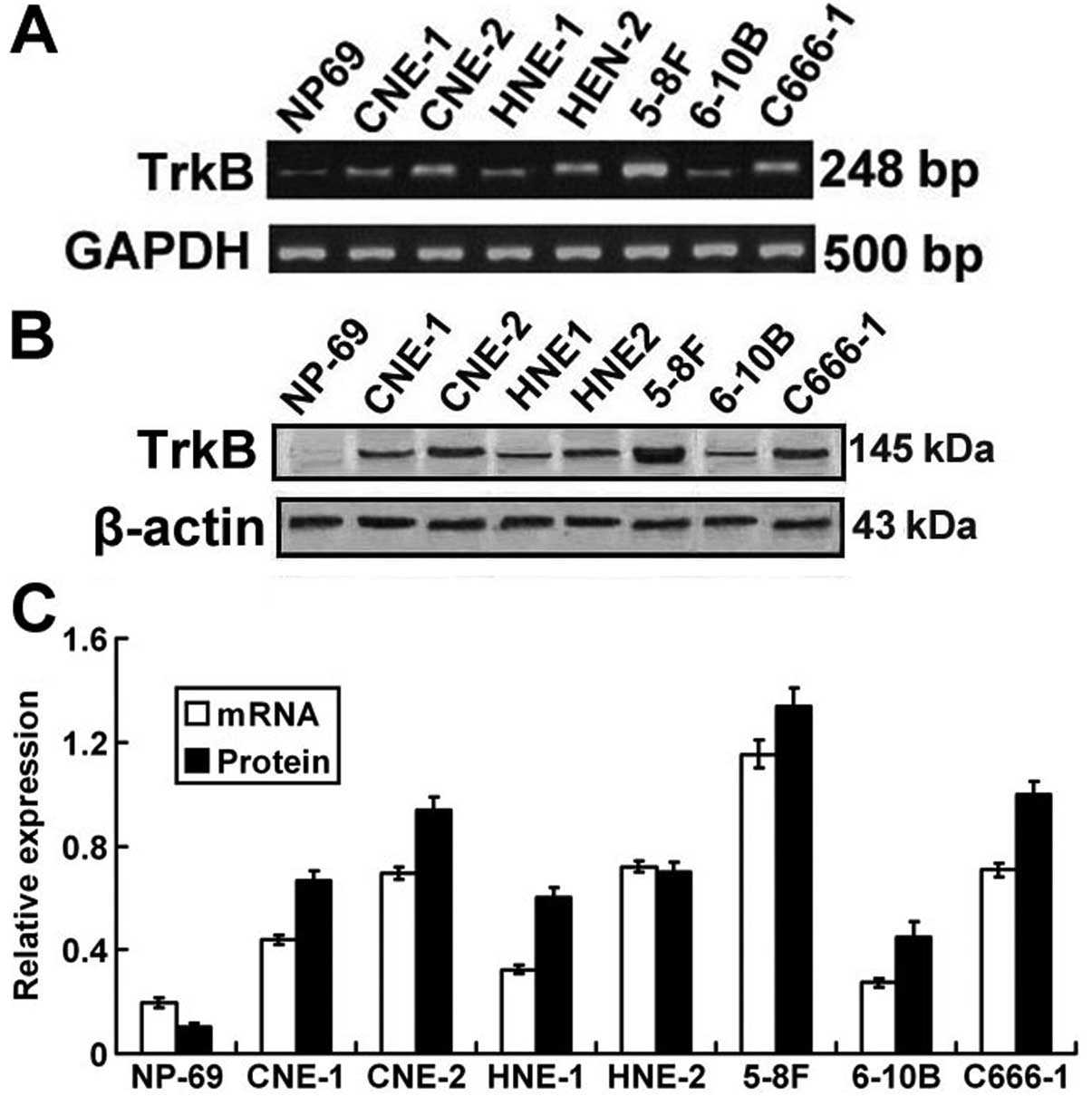
Expression of TrkB mRNA and protein in
NPC cancer cell lines
In previous experiments, we found that both TrkB
mRNA and protein expression levels were upregulated in NPC tissues
and were correlated with a number of clinicopathological
parameters. The expression pattern of TrkB in human NPC cell lines
was further investigated by RT-PCR and western blot analysis to
verify the role of TrkB in vitro. As shown in Fig. 3, both mRNA and protein expression of
TrkB in all the 7 NPC cell lines was significantly higher when
compared to that in the normal nasopharyngeal epithelium mucosal
NP-69 cell line. Moreover, compared to the other NPC cell lines,
the 5-8F cell line, which has high metastatic potential, displayed
the highest TrkB mRNA and protein expression.
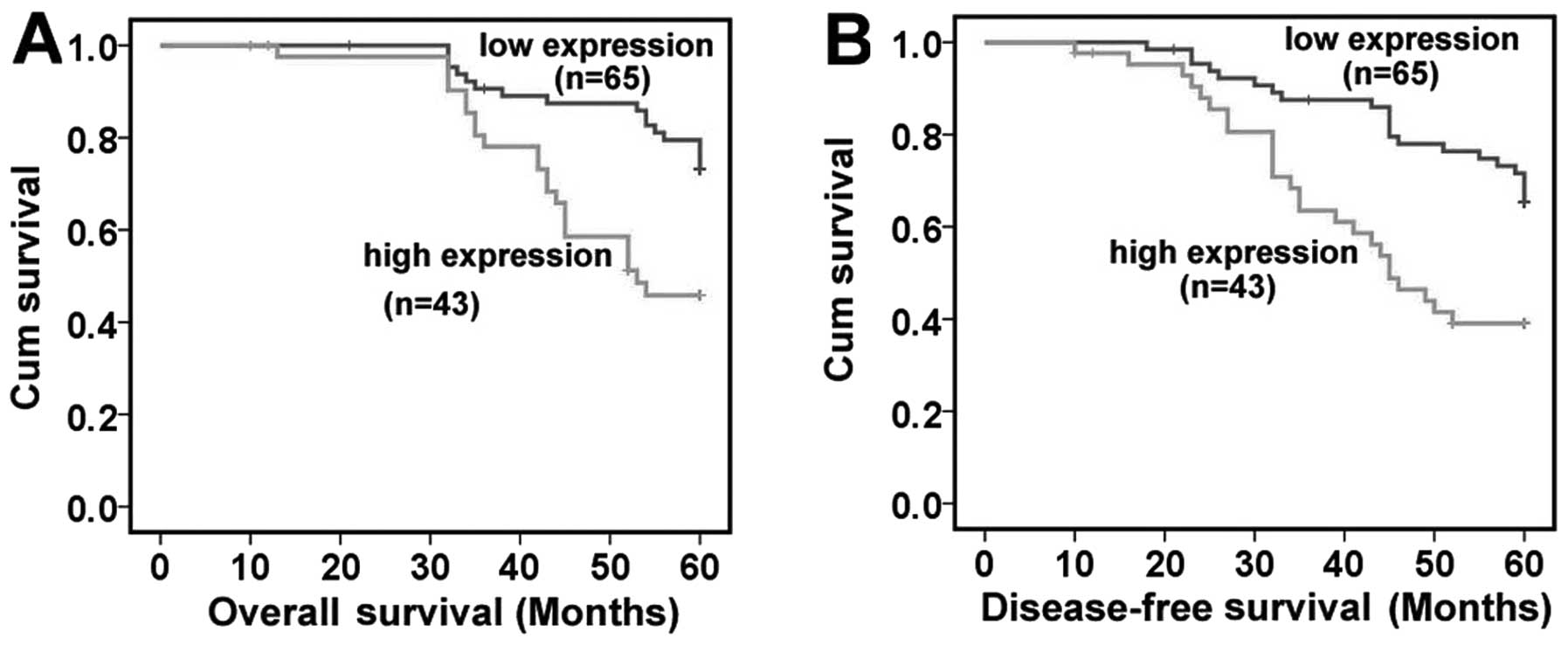
Correlation between TrkB expression and
prognosis
During the follow-up period of 5 years, 6 patients
were lost to follow-up or died from other causes. Fifty-five
patients were still alive without recurrence, and the remaining 47
patients had recurrent tumors. Thirty-nine of these 47 patients
with recurrent disease died of locoregional recurrence or distant
metastases within 5 years.
According to the TrkB protein expression levels, 108
patients were divided into 2 groups: 65 patients in the low
expression group and 43 patients in the high expression group. In
the Kaplan-Meier patient survival analysis, NPC patients in the
high expression group had both reduced overall survival and
disease-free survival when compared with patients in the low
expression group (overall survival rate, 48.8 vs. 73.8%,
P=0.001<0.05; disease-free survival rate, 41.9 vs. 66.2%,
P=0.001<0.05) (Fig. 4). In the
multivariate Cox regression analysis, high TrkB expression [risk
ratio (RR)=1.863, P=0.013], poor N classification (RR=5.216,
P=0.028) and M classification (RR=2.359, P=0.049) were identified
as independent prognostic factors for overall survival. Moreover,
these 3 clinicopathologic parameters were identified as independent
prognostic factors for disease-free survival (TrkB expression:
RR=1.600 and P=0.033; N classification: RR=6.609 and P=0.011; M
classification: RR=2.544 and P=0.025). The other
clinicopathological parameters including age, gender, histological
type, T classification and AJCC clinical stage that were examined
did not provide any independent prognostic information (Table III).
 | Table IIIMultivariate analysis of Cox model
analysis for disease-free survival and overall survival. |
Table III
Multivariate analysis of Cox model
analysis for disease-free survival and overall survival.
| Disease-free
survival | Overall
survival |
|---|
|
|
|
|---|
| Variables | Risk ratio | 95% Confidence
interval | P-value | Risk ratio | 95% Confidence
interval | P-value |
|---|
| Gender
(male/female) | 0.615 | 0.263–1.438 | 0.262 | 0.517 | 0.195–1.370 | 0.184 |
| Age (≤50/>50
years) | 1.135 | 0.631–2.040 | 0.672 | 1.295 | 0.682–2.458 | 0.430 |
| Histological type
(keratinizing/non-keratinizing) | 3.072 | 0.374–25.246 | 0.296 | 2.142 | 0.250–18.371 | 0.487 |
| T classification
(T1+T2/T3+T4) | 1.340 | 0.731–2.457 | 0.343 | 1.433 | 0.735–2.793 | 0.291 |
| N classification
(N0+N1/N2+N3) | 6.609 | 1.539–28.391 | 0.011 | 5.216 | 1.193–22.807 | 0.028 |
| M classification
(M0/M1) | 2.544 | 1.125–5.751 | 0.025 | 2.359 | 1.005–5.538 | 0.049 |
| Clinical stage
(I+II/III+IV) | 0.455 | 0.057–3.668 | 0.460 | 0.369 | 0.044–3.087 | 0.357 |
| Trkb expression
(high/low) | 1.600 | 1.040–2.461 | 0.033 | 1.863 | 1.139–3.048 | 0.013 |
Discussion
In the present study, TrkB mRNA and protein levels
were identified to be significantly upregulated in NPC tissues and
cell lines. Its expression was strongly correlated with enhanced
tumor invasion, advanced clinical stage and positive lymph node
status.
Environmental factors, genetic susceptibility and
EBV infection play roles in the development of NPC. NPC is rare in
most regions of the world; however, it is a common cancer in
Southern China, particularly in Guangdong and Guangxi and Hunan
Provinces. Radiotherapy with or without chemotherapy in NPC is the
main treatment method. However, the treatment results of late stage
NPC have been disappointing. Unfortunately, approximately 70% of
newly diagnosed NPC patients are characterized as stage II or IV
(16). The poor prognosis may be
due to lymph node and distant metastasis (16–18).
The mechanisms at the molecular level of NPC invasion and
metastasis are still not completely clear.
Receptor tyrosine kinases (RTKs) have been shown to
regulate normal cellular processes. Certain RTKs were found to be
overexpressed in malignant tissue (19). Thus, these RTKs were characterized
as oncogenes. Preclinical trials of target therapies on certain
RTKs showed promising results (20). TrkB is one of the RTKs and regulates
various cellular processes in the nervous system, including
neuronal survival, differentiation, maintenance and migration.
Support for a role of TrkB in human tumors initially came from
studies on its expression pattern in neuroblastoma. TrkB
overexpression is common in neuroblastoma, and is correlated with
poor patient prognosis (21,22).
In our present study, the mRNA and protein expression levels of
TrkB were significantly higher in NPC samples than these levels in
the normal nasopharyngeal tissues. In two independent studies on
pancreatic ductal adenocarcinomas, elevated TrkB expression was
noted in tumor cells when compared with that in the surrounding
normal tissue in 63% of 47 and 50% of 54 cases examined,
respectively (8,23). Zhang et al (9) analyzed TrkB expression in 161 gastric
carcinoma patients by immunohistochemistry and western blot
analysis, and a high level of TrkB expression was observed in
well-differentiated gastric carcinoma subtypes. In the present
study, both mRNA and protein expression of TrkB was also
upregulated in 7 NPC cell lines when compared to these levels in a
nontransformed nasopharyngeal epithelium cell line. The mechanisms
at the molecular level of the overexpression of TrkB in NPC is not
clear, but the present results that both mRNA and protein
expression of TrkB was upregulated in NPC samples and cell lines
indicate a pre-transcriptional mechanism in NPC.
TrkB has been demonstrated to be involved in the
process of invasion and metastasis in several other types of
malignancies, including lung, thyroid, breast, pancreatic and
prostate cancers, lymphoma and Wilms' tumor (24). Consistent with previous reports,
among the clinicopathologic factors examined, results from our
study revealed a significant association between TrkB expression
and tumor size, lymph node metastasis and clinical stage indicating
the close association between TrkB expression and NPC progression
and metastasis. Additionally, as compared to other NPC cell lines
(CNE-1, CNE-2, HNE-1, HEN-2, 6-10B and C666-1), the 5-8F cell line,
which has high metastatic potential (14), displayed the highest TrkB mRNA and
protein expression levels. This result demonstrated a potential
association between TrkB overexpression and a malignant phenotype
such as metastatic ability. Recently, TrkB has been reported to
increase the invasive and metastatic abilities of many types of
cancers by suppressing anoikis (25). Moreover, studies with head and neck
cancer cell lines revealed that in vitro stimulation with
BDNF, the ligand for TrkB, increased the migratory and invasive
capabilities of head and neck cancer cells by altering the
expression of molecular mediators of epithelial-to-mesenchymal
transition (EMT) (26). Our present
results and previous reports provide evidence that aberrant TrkB
signaling is sufficient to induce tumorigenesis as well as invasion
and metastasis.
The Kaplan-Meier method was used to assess the role
of TrkB expression levels to predict the prognosis of patients with
NPC. The result demonstrated that NPC patients with high TrkB
expression were significantly associated with a shorter
disease-free survival and overall survival. To determine whether
the TrkB expression level could serve as an independent prognostic
factor for patients with NPC, we subsequently constructed a Cox
regression model. In multivariate Cox regression analysis including
8 clinicopathological parameters, such as patient gender, age,
histological type, tumor size, lymph node metastasis, distant
metastasis, AJCC clinical stage and TrkB expression level, the TrkB
expression level was verified as an independent prognostic
indicator for patients with NPC. Our present results were
consistent with several other reports concerning other tumors.
Okamura et al (27)
evaluated TrkB and BDNF expression in non-small cell lung cancer
patient samples by immunohistochemistry, and found that expression
of TrkB and BDNF was associated with poor prognosis in non-small
cell lung cancer patients. A research study of 102 colorectal
cancer patients showed that patients with high TrkB mRNA expression
in clinical samples had a significantly poorer prognosis relative
to those with low TrkB levels (28).
In conclusion, our present study revealed the
possible involvement of TrkB in the induction of tumor invasion and
lymph node metastases and demonstrated that elevated TrkB
expression levels were associated with the progression and poor
prognosis of NPC. The results suggest that TrkB may be used as a
new prognostic marker for NPC. Moreover, since TrkB special
inhibitors have already been developed (29), specific drug-mediated inactivation
of TrkB may provide a novel method with which to have an effective
therapeutic impact on poor clinical stage NPC with overexpressed or
mutated TrkB.
Acknowledgements
We thank Daiqiang Li and Songqing Fang (Department
of Pathology, The Second Xiangya Hospital, Central South
University, Changsha, China) for their evaluation of the clinical
samples.
References
|
1
|
Tang L, Mao Y, Liu L, et al: The volume to
be irradiated during selective neck irradiation in nasopharyngeal
carcinoma: analysis of the spread patterns in lymph nodes by
magnetic resonance imaging. Cancer. 115:680–688. 2009.
|
|
2
|
Agulnik M and Siu LL: State-of-the-art
management of nasopharyngeal carcinoma: current and future
directions. Br J Cancer. 92:799–806. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caponigro F, Longo F, Ionna F and Perri F:
Treatment approaches to nasopharyngeal carcinoma: a review.
Anticancer Drugs. 21:471–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang ET and Adami HO: The enigmatic
epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol
Biomarkers Prev. 15:1765–1777. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brodeur GM, Minturn JE, Ho R, et al: Trk
receptor expression and inhibition in neuroblastomas. Clin Cancer
Res. 15:3244–3250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schramm A, Schulte JH, Astrahantseff K, et
al: Biological effects of TrkA and TrkB receptor signaling in
neuroblastoma. Cancer Lett. 228:143–153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ricci A, Greco S, Mariotta S, et al:
Neurotrophins and neurotrophin receptors in human lung cancer. Am J
Respir Cell Mol Biol. 25:439–446. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sclabas GM, Fujioka S, Schmidt C, et al:
Overexpression of tropomysin-related kinase B in metastatic human
pancreatic cancer cells. Clin Cancer Res. 11:440–449.
2005.PubMed/NCBI
|
|
9
|
Zhang Y, Fujiwara Y, Doki Y, et al:
Overexpression of tyrosine kinase B protein as a predictor for
distant metastases and prognosis in gastric carcinoma. Oncology.
75:17–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu X, Liu L, Cai B, He Y and Wan X:
Suppression of anoikis by the neurotrophic receptor TrkB in human
ovarian cancer. Cancer Sci. 99:543–552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu Y, Zhang S, Wang X, Yang Z and Ou G:
Overexpression of TrkB promotes the progression of colon cancer.
APMIS. 118:188–195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao KT, Zhang HY, Zhu HC, et al:
Establishment and characterization of two epithelial tumor cell
lines (HNE-1 and HONE-1) latently infected with Epstein-Barr virus
and derived from nasopharyngeal carcinomas. Int J Cancer. 45:83–89.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheung ST, Huang DP, Hui AB, et al:
Nasopharyngeal carcinoma cell line (C666-1) consistently harbouring
Epstein-Barr virus. Int J Cancer. 83:121–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Fan Y, Chen J, Yao KT and Huang ZX:
Microarray analysis of differentially expressed genes between
nasopharyngeal carcinoma cell lines 5–8F and 6–10B. Cancer Genet
Cytogenet. 196:23–30. 2010.
|
|
15
|
Tsao SW, Wang X, Liu Y, et al:
Establishment of two immortalized nasopharyngeal epithelial cell
lines using SV40 large T and HPV16E6/E7 viral oncogenes. Biochim
Biophys Acta. 1590:150–158. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chan AT, Teo PM, Ngan RK, et al:
Concurrent chemotherapy-radiotherapy compared with radiotherapy
alone in locoregionally advanced nasopharyngeal carcinoma:
progression-free survival analysis of a phase III randomized trial.
J Clin Oncol. 20:2038–2044. 2002. View Article : Google Scholar
|
|
17
|
Xu T, Hu C, Wang X and Shen C: Role of
chemoradiotherapy in intermediate prognosis nasopharyngeal
carcinoma. Oral Oncol. 47:408–413. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen MK, Yang SF, Lai JC, et al:
Expression of bcl-2 correlates with poor prognosis and modulates
migration of nasopharyngeal carcinoma cells. Clin Chim Acta.
411:400–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Casaletto JB and McClatchey AI: Spatial
regulation of receptor tyrosine kinases in development and cancer.
Nat Rev Cancer. 411:387–400. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimizu M, Adachi S, Masuda M, Kozawa O
and Moriwaki H: Cancer chemoprevention with green tea catechins by
targeting receptor tyrosine kinases. Mol Nutr Food Res. 55:832–843.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edsjö A, Lavenius E, Nilsson H, et al:
Expression of TrkB in human neuroblastoma in relation to MYCN
expression and retinoic acid treatment. Lab Invest. 83:813–823.
2003.PubMed/NCBI
|
|
22
|
Eggert A, Ikegaki N, Liu XG and Brodeur
GM: Prognostic and biological role of neurotrophin-receptor TrkA
and TrkB in neuroblastoma. Klin Padiatr. 212:200–205. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miknyoczki SJ, Lang D, Huang L,
Klein-Szanto AJ, Dionne CA and Ruggeri BA: Neurotrophins and Trk
receptors in human pancreatic ductal adenocarcinoma: expression
patterns and effects on in vitro invasive behavior. Int J Cancer.
81:417–427. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Desmet CJ and Peeper DS: The neurotrophic
receptor TrkB: a drug target in anti-cancer therapy? Cell Mol Life
Sci. 63:755–759. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Geiger TR and Peeper DS: The neurotrophic
receptor TrkB in anoikis resistance and metastasis: a perspective.
Cancer Res. 65:7033–7036. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kupferman ME, Jiffar T, El-Naggar A, et
al: TrkB induces EMT and has a key role in invasion of head and
neck squamous cell carcinoma. Oncogene. 29:2047–2059. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Okamura K, Harada T, Wang S, et al:
Expression of TrkB and BDNF is associated with poor prognosis in
non-small cell lung cancer. Lung Cancer. 78:100–106. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fujikawa H, Tanaka K, Toiyama Y, et al:
High TrkB expression levels are associated with poor prognosis and
EMT induction in colorectal cancer cells. J Gastroenterol.
47:775–784. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Perez-Pinera P, Hernandez T, García-Suárez
O, et al: The Trk tyrosine kinase inhibitor K252a regulates growth
of lung adenocarcinomas. Mol Cell Biochem. 295:19–26. 2007.
View Article : Google Scholar : PubMed/NCBI
|