Introduction
Astrocytomas, among gliomas, are the most common
type of primary brain malignancies, accounting for 40–50% of
central nervous system tumors. Malignant astrocytomas constitute a
spectrum of clinicopathological entities, from low to high-grade
malignancies. The World Health Organization (WHO) classifies these
tumors into 4 grades according to their histological and anaplastic
characteristics, which include: grade I (pilocytic astrocytoma),
grade II (low grade astrocytoma), grade III (anaplastic
astrocytoma) and grade IV glioblastoma (GBM) (1). GMB is characterized by rapid cell
proliferation and a marked propensity to invade and damage the
surrounding tissues. In addition, intense angiogenesis is a
distinguishing pathological characteristic of GBM relative to
lower-grade astrocytomas (2).
Tissue factor (TF), the primary initiator of the
coagulation cascade, is a 47-kDa transmembrane protein receptor.
Upon disruption of vascular integrity, TF functions as a cofactor
for circulating blood factor VIIa, which leads to thrombin
activation, platelet aggregation and fibrin deposition (3). TF is constitutively expressed in a
cell type specific manner and upregulated in a number of
pathological processes (3,4). A strong correlation between TF
expression and malignancy grade has been reported for a number of
tumors (5). In addition, several
studies have implicated the TF-factor VIIa-initiated coagulation
pathway in several aspects of tumor biology including cancer
progression, cancer-associated thrombosis and metastasis (6,7).
The occurrence of intratumoral thrombosis in GBM has
recently been shown to be an additional distinct feature of grade
IV glioma in relation to lower-grade gliomas (8). It has been proposed that intratumoral
thrombosis is mainly driven by upregulated TF expression. This
increase in TF expression contributes to the establishment of
hypoxic areas, further stimulating the production of pro-tumor
factors such as vascular endothelial growth factor (VEGF) and
interleukin-8 (IL-8) (9,10). In vitro studies demonstrated
that hypoxia as well as loss of the PTEN tumor-suppressor
upregulate TF gene expression in human GBM cell lines (11). In addition, analysis of human GBM
specimens revealed increased TF expression in pseudopalisades,
which constitute a dense collection of neoplastic cells that
surround a central necrotic focus (11).
In addition to TF, protease-activated receptors
(PARs), a family of G protein-coupled receptors, have been
implicated in tumor biology (6,12).
PARs comprise a family of receptors (PAR1, PAR2, PAR3 and PAR4)
that are uniquely activated by proteolytic cleavage of their
extracellular domain (13). This
cleavage unmasks a new N-terminus, which serves as a tethered
ligand that binds to the second extracellular domain of the
protein, resulting in a variety of cellular responses. In
particular, PAR1 and PAR2 are overexpressed in a variety of tumor
types (14). Several studies have
demonstrated a strong correlation between PAR1 and/or PAR2
activation with a number of pro-tumor responses, including primary
growth, invasion, metastasis and angiogenesis (6,12,15).
It was recently demonstrated that TF-mediated signaling through
PAR2 modulates proliferation, migration and invasion of malignant
glioma cell lines (16).
In the present study, we analyzed the expression of
TF signaling pathway elements (TF, PAR1 and PAR2) as well as
downstream products (VEGF and IL-8) in samples from human
astrocytoma patients. Our data suggest a role for TF signaling
pathway elements in astrocytoma progression, particularly in GBM.
Collectively, our results suggest that blood clotting proteins may
offer additional strategies for new therapies against aggressive
glioma.
Materials and methods
Reagents
Anti-PAR1 (clone ATAP2), anti-PAR2 (clone SAM11),
and anti-actin antibodies were obtained from Santa Cruz
Biotechnology, Inc., (Santa Cruz, CA, USA). Secondary antibodies
conjugated with biotin and peroxidase-conjugated streptavidin were
obtained from Zymed Invitrogen Corp. The PAR1 agonist peptide
(PAR1-AP, TFLLR-NH2) and the PAR2 agonist peptide (PAR2-AP,
SLIGKL-NH2) were synthesized by Bio-Synthesis Inc.
Tissue samples
Human astrocytomas of different malignant grades
were obtained during therapeutical surgical management, as
previously described (17). The
specimens were immediately snap-frozen in liquid nitrogen upon
surgical removal. The astrocytoma specimens were categorized
according to the WHO classification system. Then, the tissue
samples were analyzed and graded independently by histopathological
analysis into the following groups: non-tumor (n=14); pilocytic
astrocytoma, grade I (n=15); low-grade astrocytoma, grade II
(n=15); anaplastic astrocytoma, grade III (n=15); or GBM, grade IV
(n=30). Non-tumor tissue samples, surgically resected from the
anterior temporal lobe tissue, were obtained from patients selected
for surgical treatment of temporal-lobe epilepsy associated with
hippocampus sclerosis (TLE-HS). The present study was performed
according to the ethics guidelines approved by the Department of
Neurology, School of Medicine, at the University of Sao Paulo and
by the Brazilian Health Ministry.
RNA isolation and cDNA synthesis
Total RNA from the astrocytomas and non-tumor
samples was isolated with RNeasy Mini kit (Qiagen). Total RNA from
the cell cultures was isolated and the mRNA expression levels were
determined as previously described (18). The sequence-specific primers were
designed using Primer Express (Applied Biosystems) and validated
using BLAST and BLAT. Primers used were: TF (F,
5′-CAGGCACTACAAATACTGTGG-3′ and R, 5′-TGTAGA CTTGATTGACGGGTT-3′);
PTEN (F, 5′-CGGTGTCAT AATGTCTTTCAGC-3′ and R, 5′-TGAAGGCGTATACAGG
AACAAT-3′); PAR1 (F, 5′-GCAGGCCAGAATCAAAAG CAA-3′ and R,
5′-CATTTTTCTCCTCATCCTCCC-3′); PAR2 (F, 5′-GCACCATCCAAGGAACCAAT-3′
and R, 5′-TGTGC CATCAACCTTACCAATAA-3′); VEGF (F, 5′-AGTGGT
GAAGTTCATGGATGT-3′ and R, 5′-GCACACAGGAT GGCTTGAAGA-3′); IL-8 (F,
5′-CTGGACCCCAAGGAAA ACTG-3′ and R, 5′-TGTGCCATCAACCTTACCAATAA-3′);
GAPDH (F, 5′-ACCCACTCCTCCACCTTTGA-3′ and R,
5′-ACCGAGCCCATTTCATTTCTG-3′) and HBMS (F,
5′-TGGACCTGGTTGTTCACTCCTT-3′ and R, 5′-CAACAG CATCATGAGGGTTTTC-3′).
A normalization value was generated employing the geNorm program,
which selected GAPDH and HBMS as housekeeping genes. The relative
expression levels were estimated utilizing a previously described
method, using the mean of control non-tumor samples as calibrator
(19).
Immunohistochemistry
Tissue staining was performed on paraffin-embedded
sections (4 μm thick), which were incubated overnight following
heat antigen retrieval at 122°C for 3 min using an electric
pressure cooker (BioCase Medical, USA) with the primary antibodies
anti-PAR1 or anti-PAR2 at 1:400 dilution. The sections were further
examined using a NovoLink kit (Newcastle upon Tyne, UK) with
3,3′-diaminobenzidine as the chromogen and counterstained with
Harris hematoxylin. Optimization using positive controls suggested
by the manufacturer of each antibody was performed in order to
obtain optimal dilution. The negative control sections were
obtained by omitting the primary antibody in each staining batch.
The slides were independently analyzed by 2 observers (SKNM and
SMOS). The immunoreactivities of PAR1 and PAR2 were determined
semi-quantitatively in 5 cases of each grade of malignancy and
non-tumor brain tissue, considering both intensity of staining and
percentage of cells. Intensity of staining was applied as follows:
0 (negative); 1 (weak), 2 (moderate) and 3 (strong). The percentage
of stained cells was determined according to the following scale:
negative (no cells stained), low (1–25%), moderate (26–50%), high
(51–75%), and strong intensity (>75%). The total score for each
case was calculated as the percentage vs. intensity.
Cell culture
The human GBM cell line U87-MG and oligodendroglioma
cell line HOG were cultured as previously described (18). Adult primary human astrocytes were
isolated from non-tumor tissues collected as described above and
further cultured as previously described (20). Primary cultures were used until the
third passage in the present study. For the experiments, the cells
were maintained in DMEM-F12 (Gibco-BRL) supplemented with 10% FBS
(Cultilab, Brazil), 60 mg/l penicillin, 100 mg/l streptomycin and
1.2 g/l sodium bicarbonate in culture flasks. The cells were
incubated in 5% CO2 at 37°C. The subconfluent cultures
were washed twice with PBS, and the cells were detached with Hank’s
solution containing 10 mM HEPES and 0.2 mM EDTA.
Western blotting
Adult primary human astrocytes, U87-MG and HOG cells
were seeded at 5×105 cells/well in 6-well plates for the
quantitative analysis of PAR1 and PAR2, as previously described
(21) the cells were washed with
phosphate-buffered saline and lysed in cold buffer containing a
phosphatase inhibitor cocktail. The cell lysates (20 μl) were
separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE,
10%). The proteins were transferred onto polyvinylidene fluoride
(PVDF) membranes (Millipore) and further blocked with Tris-buffered
saline (TBS) containing 5% BSA and 0.1% Tween-20 for 1 h at room
temperature. Then, the membranes were probed with the primary
antibodies overnight at 4°C. The membranes were washed 3 times with
TBS/Tween before addition of the secondary antibody for 1 h at room
temperature, and further washed and probed with
peroxidase-conjugated streptavidin for 1 h at room temperature.
Immunodetection was carried out with a chemiluminescent method
using a Western Lightning ECL kit (Amersham Pharmacia Biotech).
Immunoassay for IL-8 and VEGF
U87-MG and HOG cells were seeded at 5×106
cells/well in 6-well plates. The cells were serum-starved for 30
min prior to stimulation with PAR1-AP or PAR2-AP for 8 h at 37°C.
IL-8 and VEGF protein secretion into the cell supernatants was
measured using human ELISA kits from PeproTech, Inc. (Rocky Hill,
NJ, USA), according to the manufacturer’s instructions.
Statistical analysis
All statistical analyses were performed using the
GraphPad Prism program. The gene expression assays were analyzed
using the Mann-Whitney test (Student’s t- and non-parametric test).
Immunohistochemistry was analyzed by one-way analysis of variance
(ANOVA) complemented by the Bonferroni post-hoc test. The
Spearman’s correlation test was used for correlation analysis.
Immunoassay for IL-8 and VEGF were analyzed by unpaired t-test. The
differences were considered to be statistically significant at
p<0.05.
Results
Expression of TF and PTEN are inversely
correlated in human astrocytoma patients
Previous studies demonstrated that TF expression
correlates with the histological grade of malignancy in glioma
patients (22,23). In the present study, we analyzed TF
expression in 89 samples which included non-tumor tissues and
astrocytoma patient specimens classified into different grades.
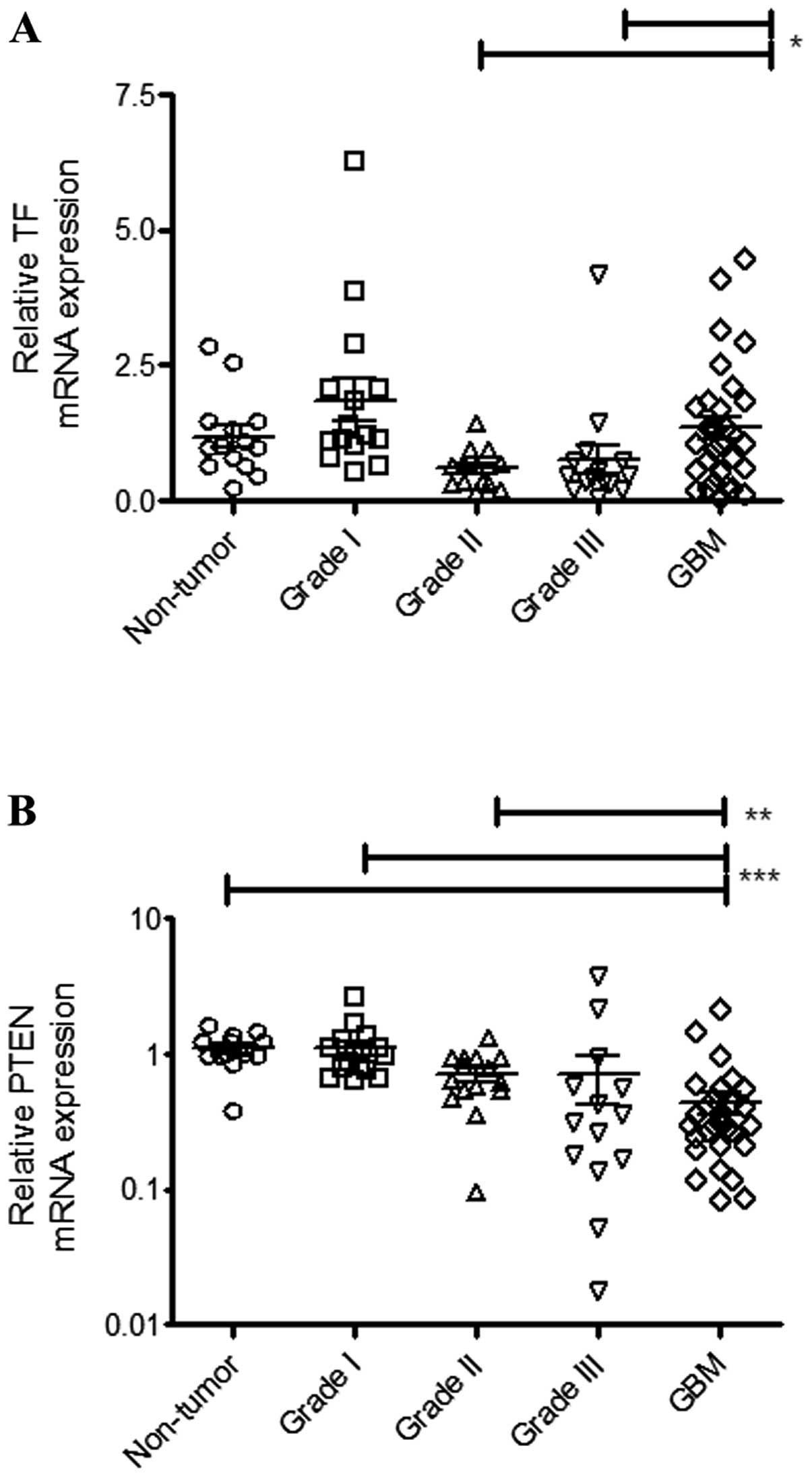
Analysis by qPCR demonstrated that TF expression was positive in
all the samples (Fig. 1A). However,
as shown in Fig. 1A, a
significantly elevated TF expression was only observed in the GBM
samples relative to grade II (p=0.0356) and grade III (p=0.0312)
astrocytoma patients.
In vitro studies suggested that upregulation
of TF in high-grade gliomas is associated with loss of the
tumor-suppressor PTEN (11).
Consistent with this hypothesis, Fig.
1B shows that PTEN expression levels were inversely correlated
with the malignancy in astrocytoma patients. We found a significant
correlation between PTEN and TF mRNA expression levels in GBM
samples (Table I).
 | Table IRelationship between the expression
levels of TF, PAR1, PAR2 and analyzed genes in GBM. |
Table I
Relationship between the expression
levels of TF, PAR1, PAR2 and analyzed genes in GBM.
| TF | PAR1 | PAR2 |
|---|
|
|
|
|
|---|
| P | r | P | r | P | r |
|---|
| PTEN | 0.0004 | 0.3979 | NS | −0.1748 | 0.0002 | 0.4246 |
| VEGF | 0.0018 | 0.3550 | <0.0001 | 0.5939 | 0.0115 | −0.3003 |
| IL-8 | <0.0001 | 0.9997 | NS | 0.1572 | 0.1563 | 0.1688 |
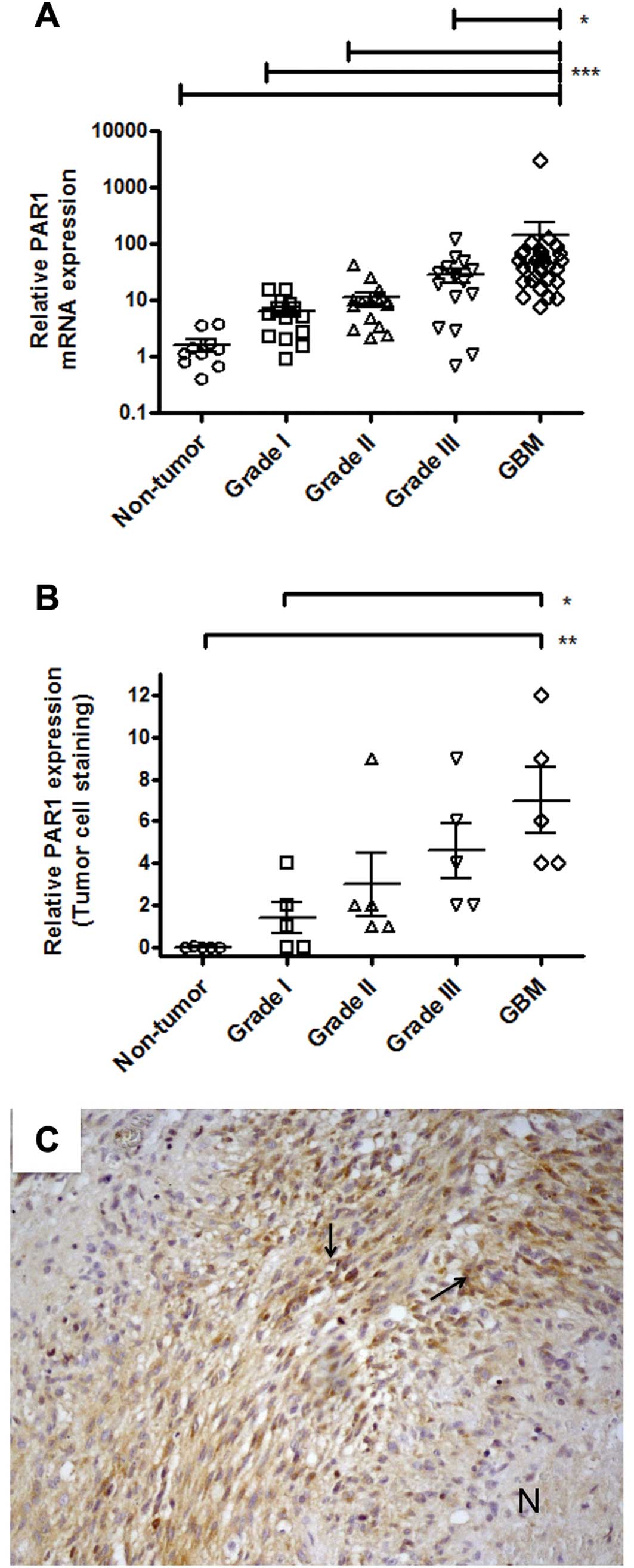
Expression of PAR1, but not PAR2, is
correlated with the malignancy in human astrocytoma patients
The pro-tumor role of TF has been correlated with
its ability to generate thrombin and indirectly promote the
activation of the G protein-coupled receptor PAR1 (5,6).
Furthermore, elevated expression levels of PAR1 have been
demonstrated in studies employing tumor cell lines and patient
specimens (14).
Analysis by qPCR showed that PAR1 expression levels
were positively correlated with malignancy in astrocytoma patients,
as shown in Fig. 2A. PAR1 mRNA
expression levels were significantly more abundant in GBM when
compared to the non-tumor tissue (p<0.0001) and to lower grade
astrocytoma samples (p<0.0001, relative to grade I; p<0.0001,
relative to grade II; p=0.0146 relative to grade III). These
results were further confirmed by immunohistochemistry analyses, as
shown in Fig. 2B. PAR1 staining in
the tumor-associated endothelium was significantly elevated in GBM
when compared to non-tumor tissue (p=0.001) and samples from
lower-grade astrocytoma (data not shown).
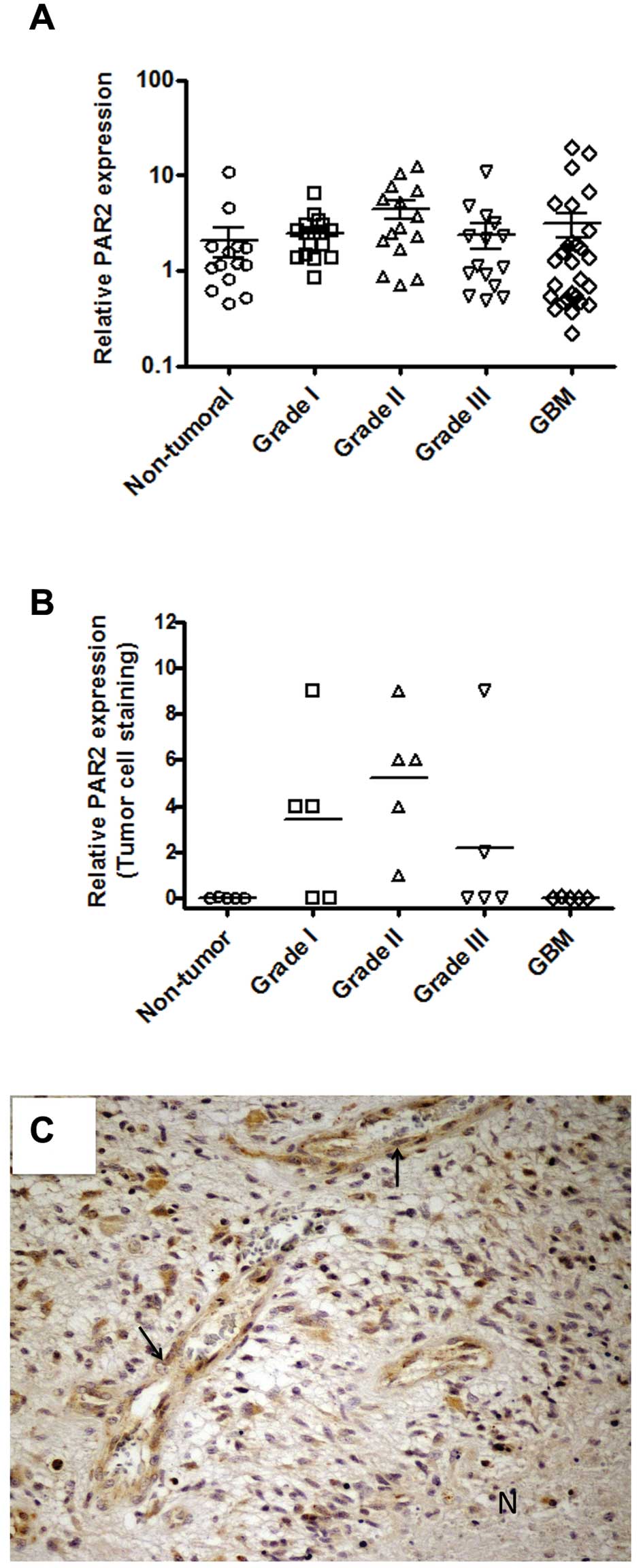
In addition to PAR1, PAR2 has been shown to be
overexpressed in several types of cancer (14). Therefore, we further employed qPCR
and immunohistochemistry for analysis of PAR2 expression in tissue
samples. Fig. 3A shows that mRNA
encoding PAR2 was detected in all the samples analyzed, but no
significant difference was observed between the non-tumor tissue
and astrocytoma samples. These observations were further confirmed
by immunohistochemistry, as shown in Fig. 3B. The immunohistochemistry results
were somewhat heterogeneous and some astrocytoma samples
demonstrated high antigen reactivity either in tumor cells
(Fig. 3B) or in tumor-associated
endothelium (data not shown).
A stronger positive staining was observed around the
perinecrotic area mainly in pseudopalisading cells for either PAR1
(Fig. 2C) or PAR2 (Fig. 3C), although PAR2 staining was more
intense than that observed in PAR1.
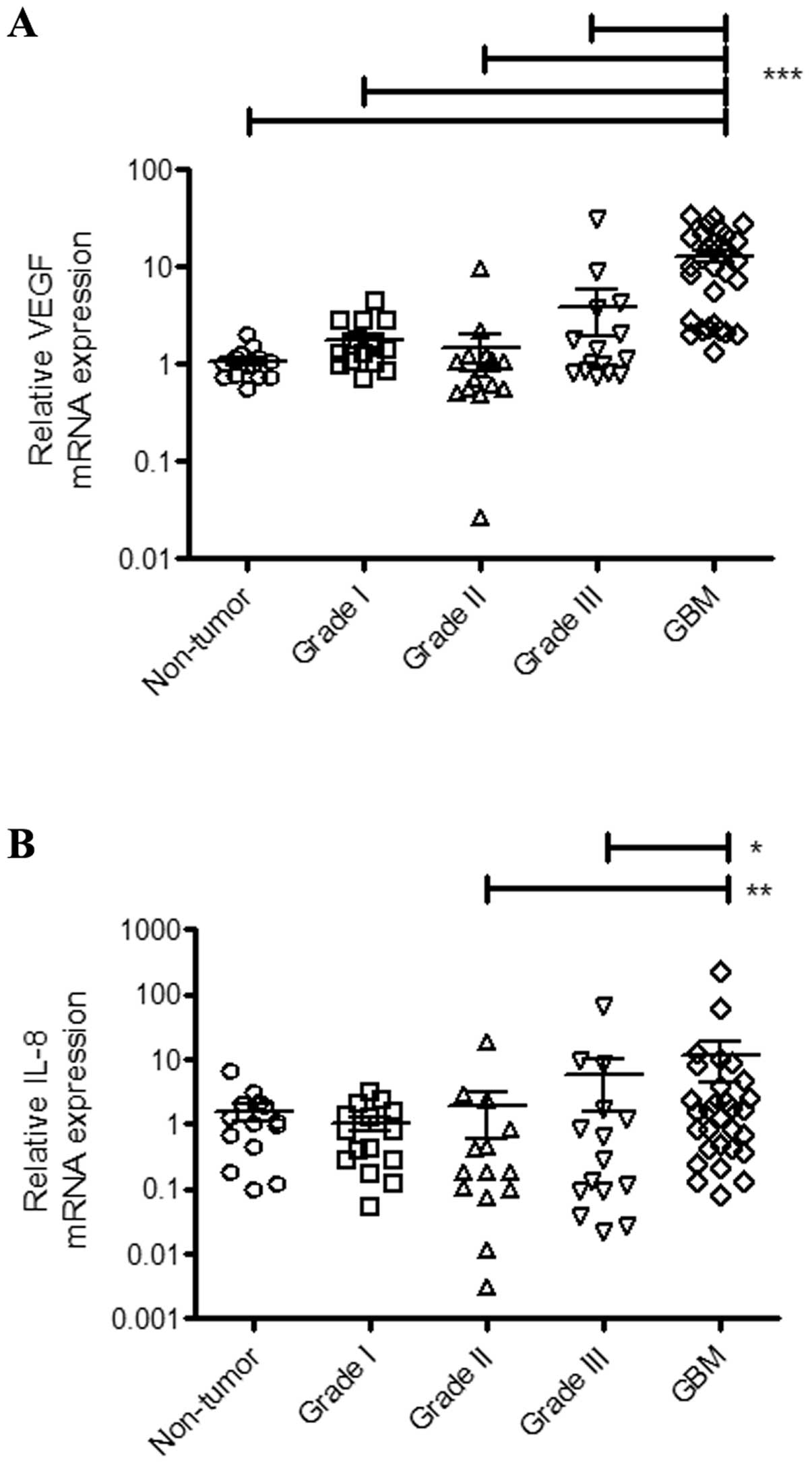
Expression of VEGF and IL-8 correlates
with TF signaling pathway elements
A key event that accompanies astrocytoma progression
is the upregulation of VEGF levels. Intense angiogenesis is a
pathological hallmark of GBM relative to lower-grade gliomas
(2). GBM is a highly vascularized
malignant tumor and there is strong evidence that VEGF plays a key
role in this process (24). In this
context, Fig. 4A shows that VEGF
expression is significantly upregulated in GBM relative to
non-tumor tissues (p<0.0001) and grade I (p<0.0001), grade II
(p<0.0001) and grade III (p<0.0001) astrocytomas.
In addition to VEGF, the chemokine IL-8 has been
recognized as an important factor in astrocytoma progression and
GBM angiogenesis (25,26). Accordingly, our data demonstrate
that GBM samples exhibit increased expression of IL-8 when compared
to grade II (p=0.0098) and grade III (p=0.0420) astrocytomas
(Fig. 4B). Furthermore, we
evaluated the correlation between the TF mRNA expression levels of
the angiogenic factors. We found a significant correlation between
TF and VEGF expression, as well as between TF and IL-8 expression
(Table I). Analysis of the
correlation between the mRNA expression levels of PAR1 or PAR2 and
the angiogenic factors in GBM samples showed a significant
correlation between PAR1 and VEGF expression (Table I). There was no significant
correlation between PAR1 and IL-8 expression. Furthermore, PAR2
expression was correlated with VEGF, but not with IL-8 (Table I).
A PAR2 agonist stimulates VEGF and IL-8
production in vitro
Several studies demonstrated that PAR1 and PAR2 are
constitutively expressed in a variety of tumor cell lines.
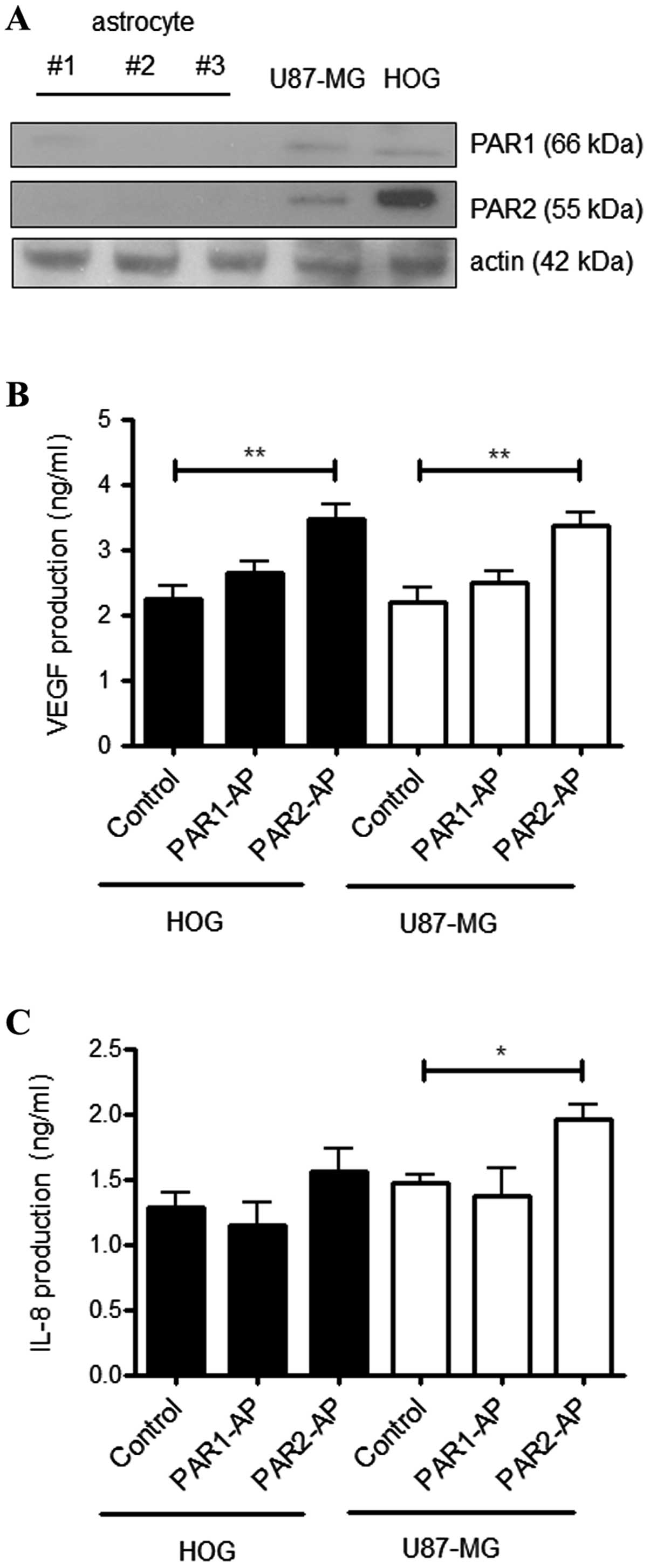
Accordingly, Fig. 5A compares the
expression of PAR1 and PAR2 in primary astrocytes to the human GBM
cell line U87-MG and the human oligodendroglioma cell line HOG.
Western blot analysis showed no detectable levels of PAR1 or PAR2
in the astrocytes, while both the tumor cell lines constitutively
expressed PAR1 and PAR2. Both receptors have been implicated in
VEGF and IL-8 production by tumor cells (15,21,27).
To determine the effect of PAR1 and PAR2 activation on VEGF and
IL-8 production in the U87-MG and HOG cell lines, we employed
synthetic agonist peptides and further quantified VEGF and IL-8 in
the conditioned medium. Fig. 5B
shows that activation of PAR2, but not PAR1, increased VEGF
production in both cell lines. In addition, the PAR2 agonist
peptide induced IL-8 production in U87-MG, but not in HOG,
cells.
Discussion
A marked shift in biological behavior occurs
following the transition from low- to high-grade astrocytoma. These
changes include the acquisition of proliferative and invasive
phenotypes, which account for the extensive damage in normal brain
tissue. Intense angiogenesis is another pathological hallmark of
high-grade glioma. Indeed, GBM is one of the most highly
vascularized malignant tumors. Despite significant advances in
diagnostics and characterization of molecular markers associated
with aggressive astrocytoma, the search for new targets remains a
challenge (28). The present study
provides evidence that the signaling pathways triggered by the
clotting initiator protein TF may play a role in GBM progression.
Expression of TF, as well as PAR1 and PAR2, correlated with
increased levels of VEGF and IL-8, which are well recognized
factors in astrocytoma progression and GBM aggressiveness.
Intratumoral thrombosis has been documented as an
additional distinguishing pathological feature in GBM relative to
lower grade astrocytoma (8).
According to our results, this may be explained by the significant
elevation of TF expression in GBM. Different mechanisms have been
proposed for the increased expression of TF in high-grade glioma
including hypoxia and oncogenic events (10,11,27).
In vitro studies employing tumor cell lines have
demonstrated that loss of the tumor-suppressor PTEN is directly
correlated with upregulation of TF (11). In accordance with these
observations, we demonstrated that decreased PTEN mRNA levels
correlate with increased TF expression in astrocytoma patients.
Studies employing tumor cell lines demonstrated that
simultaneous TF expression and phosphatidylserine exposure
contribute to massive generation of clotting enzymes (29,30),
which may account for the activation of signaling pathways elicited
by cleavage of PARs. PAR1 and PAR2 activation have been associated
with transcriptional programs regulating increased expression of
angiogenic molecules (6,15). Our results demonstrate that PAR1
expression is correlated with malignancy and is highly expressed in
high-grade astrocytoma. This is in agreement with the
immunohistochemistry data from a study performed by Zhang et
al (31), which also
demonstrated that PAR1 expression levels are an independent
prognostic factor in GBM patients. Notably, the authors reported
the significant upregulation of metalloprotease-1 (MMP1) in
high-grade glioma. MMP-1 has been described as a possible PAR1
activator and may possibly elicit PAR1 pro-tumor effects through a
TF-independent route (32). PAR1
supports oncogenic transformation and angiogenesis by upregulating
VEGF in vitro and in vivo (33). In this context, elevated levels of
PAR1 in GBM may directly contribute to the angiogenic switch during
astrocytoma progression. This hypothesis is reinforced by the
observation that thrombin, which activates PAR1, upregulates VEGF
production in vitro and co-localizes with VEGF in
vivo in a rat glioma model (34).
TF-dependent activation of PAR2 has recently been
implicated in increased proliferation, migration and invasion of
GBM cell lines (16). Antibodies
that specifically target TF-dependent signaling without affecting
TF procoagulant activity diminish these pro-tumor properties.
Inhibition of proliferation, migration and invasion of GBM cell
lines has also been attained by independently silencing TF or PAR2.
Studies employing a spontaneous mammary tumor model in mice
demonstrated that the TF/PAR2 signaling axis is coupled to
angiogenesis in breast cancer (35,36).
Thus, PAR2-deficient mice have delayed mammary tumor progression,
as well as decreased angiogenesis in the initial phase of tumor
development. Svensson et al demonstrated that PAR2
activation in endothelial cells may be triggered by TF-bearing
microvesicles derived from GBM cells (37). This phenomenon is observed
particularly under hypoxic conditions and is consistent with the
observation that TF is more highly expressed proximal to the
necrotic areas in GBM samples (11). Recently, Harter et al
demonstrated that PAR2 is heterogeneously overexpressed in GBM
(38). These findings may explain
our observation that analyses of PAR2 expression by qPCR or
immunohistochemistry show no differences between GBM and
lower-grade gliomas as well as between GBM and non-tumor
samples.
Targeting the blood clotting cascade may be a
feasible therapeutic approach for treatment of GBM (39). In this regard, argatroban, a
specific thrombin inhibitor, has been shown to reduce the in
vivo growth of rat GBM. However, argatroban treatment in this
model displayed only a modest improvement in survival (40). It was demonstrated that Ixolaris, a
TF inhibitor that blocks PAR2 signaling in vitro (41), blocks the in vivo growth of
human GBM (U87-MG) cells in a xenograft model (18). This phenomenon was accompanied by a
significant decrease in VEGF expression as well as diminished tumor
angiogenesis. Additionally, Harter et al demonstrated that a
monoclonal anti-TF antibody reduces tumor cell invasiveness in a
xenograft model (38).
Collectively, our results strongly suggest that
signaling pathway elements comprising TF, PAR1 and PAR2 are
connected to the upregulation of VEGF and IL-8 expression in
high-grade glioma. Therefore, these proteins may offer additional
targets for the development of new therapies to treat aggressive
glioma.
Acknowledgements
The authors thank Zizi de Mendonça for the technical
assistance. The present study was supported by the Brazilian
National Council for Scientific and Technological Development
(CNPq), the State of Rio de Janeiro Research Foundation (FAPERJ),
the State of São Paulo Research Foundation (FAPESP), and the
Brazilian Cancer Foundation.
References
|
1
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Williams JC and Mackman N: Tissue factor
in health and disease. Front Biosci. 1:358–372. 2012. View Article : Google Scholar
|
|
4
|
Francischetti IM, Seydel KB and Monteiro
RQ: Blood coagulation, inflammation, and malaria. Microcirculation.
15:81–107. 2008. View Article : Google Scholar
|
|
5
|
Rak J, Milsom C, Magnus N and Yu J: Tissue
factor in tumour progression. Best Pract Res Clin Haematol.
22:71–83. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruf W, Disse J, Carneiro-Lobo TC, Yokota N
and Schaffner F: Tissue factor and cell signalling in cancer
progression and thrombosis. J Thromb Haemost. 9(Suppl 1): 306–315.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lima LG and Monteiro RQ: Activation of
blood coagulation in cancer: implications for tumor progression.
Biosci Rep. 33:e000642013.PubMed/NCBI
|
|
8
|
Tehrani M, Friedman TM, Olson JJ and Brat
DJ: Intravascular thrombosis in central nervous system
malignancies: a potential role in astrocytoma progression to
glioblastoma. Brain Pathol. 18:164–171. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brat DJ and Van Meir EG: Vaso-occlusive
and prothrombotic mechanisms associated with tumor hypoxia,
necrosis, and accelerated growth in glioblastoma. Lab Invest.
84:397–405. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Anand M and Brat DJ: Oncogenic regulation
of tissue factor and thrombosis in cancer. Thromb Res. 129(Suppl
1): S46–S49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rong Y, Post DE, Pieper RO, Durden DL, Van
Meir EG and Brat DJ: PTEN and hypoxia regulate tissue factor
expression and plasma coagulation by glioblastoma. Cancer Res.
65:1406–1413. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kasthuri RS, Taubman MB and Mackman N:
Role of tissue factor in cancer. J Clin Oncol. 27:4834–4838. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coughlin SR: Protease-activated receptors
in hemostasis, thrombosis and vascular biology. J Thromb Haemos.
3:1800–1814. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elste AP and Petersen I: Expression of
proteinase-activated receptor 1–4 (PAR 1–4) in human cancer. J Mol
Histol. 41:89–99. 2010.
|
|
15
|
Albrektsen T, Sørensen BB, Hjortø GM,
Fleckner J, Rao LV and Petersen LC: Transcriptional program induced
by factor VIIa-tissue factor, PAR1 and PAR2 in MDA-MB-231 cells. J
Thromb Haemost. 5:1588–1597. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gessler F, Voss V, Dützmann S, Seifert V,
Gerlach R and Kögel D: Inhibition of tissue
factor/protease-activated receptor-2 signaling limits
proliferation, migration and invasion of malignant glioma cells.
Neuroscience. 165:1312–1322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oba-Shinjo SM, Bengtson MH, Winnischofer
SM, Colin C, Vedoy CG, de Mendonça Z, Marie SK and Sogayar MC:
Identification of novel differentially expressed genes in human
astrocytomas by cDNA representational difference analysis. Brain
Res Mol Brain Res. 140:25–33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carneiro-Lobo TC, Konig S, Machado DE,
Nasciutti LE, Forni MF, Francischetti IM, Sogayar MC and Monteiro
RQ: Ixolaris, a tissue factor inhibitor, blocks primary tumor
growth and angiogenesis in a glioblastoma model. J Thromb Haemost.
7:1855–1864. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De Groot CJ, Langeveld CH, Jongenelen CA,
Montagne L, Van Der Valk P and Dijkstra CD: Establishment of human
adult astrocyte cultures derived from postmortem multiple sclerosis
and control brain and spinal cord regions: immunophenotypical and
functional characterization. J Neurosci Res. 49:342–354. 1997.
|
|
21
|
Dutra-Oliveira A, Monteiro RQ and
Mariano-Oliveira A: Protease-activated receptor-2 (PAR2) mediates
VEGF production through the ERK1/2 pathway in human glioblastoma
cell lines. Biochem Biophys Res Commun. 421:221–227. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hamada K, Kuratsu J, Saitoh Y, Takeshima
H, Nishi T and Ushio Y: Expression of tissue factor correlates with
grade of malignancy in human glioma. Cancer. 77:1877–1883. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guan M, Jin J, Su B, Liu WW and Lu Y:
Tissue factor expression and angiogenesis in human glioma. Clin
Biochem. 35:321–325. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Plate KH, Breier G, Weich HA and Risau W:
Vascular endothelial growth factor is a potential tumour
angiogenesis factor in human gliomas in vivo. Nature. 359:845–848.
1992. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koch AE, Polverini PJ, Kunkel SL, Harlow
LA, DiPietro LA, Elner VM, Elner SG and Strieter RM: Interleukin-8
as a macrophage-derived mediator of angiogenesis. Science.
258:1798–1801. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brat DJ, Bellail AV and Van Meir EG: The
role of interleukin-8 and its receptors in gliomagenesis and
tumoral angiogenesis. Neuro Oncol. 7:122–133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Magnus N, Garnier D and Rak J: Oncogenic
epidermal growth factor receptor up-regulates multiple elements of
the tissue factor signaling pathway in human glioma cells. Blood.
116:815–818. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lima FR, Kahn SA, Soletti RC, Biasoli D,
Alves T, da Fonseca AC, Garcia C, Romão J, Brito L, Holanda-Afonso
R, Faria J, Borges H and Moura-Neto V: Glioblastoma: therapeutic
challenges, what lies ahead. Biochim Biophys Acta. 1826:338–349.
2012.PubMed/NCBI
|
|
29
|
Fernandes RS, Kirszberg C, Rumjanek VM and
Monteiro RQ: On the molecular mechanisms for the highly
procoagulant pattern of C6 glioma cells. J Thromb Haemost.
4:1546–1552. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kirszberg C, Lima LG, Da Silva de Oliveira
A, Pickering W, Gray E, Barrowcliffe TW, Rumjanek VM and Monteiro
RQ: Simultaneous tissue factor expression and phosphatidylserine
exposure account for the highly procoagulant pattern of melanoma
cell lines. Melanoma Res. 19:301–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Y, Zhan H, Xu W, Yuan Z, Lu P, Zhan
L and Li Q: Upregulation of matrix metalloproteinase-1 and
proteinase-activated receptor-1 promotes the progression of human
gliomas. Pathol Res Pract. 207:24–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boire A, Covic L, Agarwal A, Jacques S,
Sherifi S and Kuliopulos A: PAR1 is a matrix metalloprotease-1
receptor that promotes invasion and tumorigenesis of breast cancer
cells. Cell. 120:303–313. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yin YJ, Salah Z, Maoz M, Even Ram SC,
Ochayon S, Neufeld G, Katzav S and Bar-Shavit R: Oncogenic
transformation induces tumor angiogenesis: a role for PAR1
activation. FASEB J. 17:163–174. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu Y, Gu Y, Keep RF, Heth J, Muraszko KM,
Xi G and Hua Y: Thrombin up-regulates vascular endothelial growth
factor in experimental gliomas. Neurol Res. 31:759–765. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Versteeg HH, Schaffner F, Kerver M, Ellies
LG, Andrade-Gordon P, Mueller BM and Ruf W: Protease-activated
receptor (PAR) 2, but not PAR1, signaling promotes the development
of mammary adenocarcinoma in polyoma middle T mice. Cancer Res.
68:7219–7227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schaffner F, Versteeg HH, Schillert A,
Yokota N, Petersen LC, Mueller BM and Ruf W: Cooperation of tissue
factor cytoplasmic domain and PAR2 signaling in breast cancer
development. Blood. 116:6106–6113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Svensson KJ, Kucharzewska P, Christianson
HC, Sköld S, Löfstedt T, Johansson MC, Mörgelin M, Bengzon J, Ruf W
and Belting M: Hypoxia triggers a proangiogenic pathway involving
cancer cell microvesicles and PAR-2-mediated heparin-binding EGF
signaling in endothelial cells. Proc Natl Acad Sci USA.
108:13147–13152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Harter PN, Dützmann S, Drott U, Zachskorn
C, Hattingen E, Capper D, Gessler F, Senft C, Seifert V, Plate KH,
Kögel D and Mittelbronn M: Anti-tissue factor (TF9–10H10) treatment
reduces tumor cell invasiveness in a novel migratory glioma model.
Neuropathology. 33:515–525. 2013.
|
|
39
|
Ornstein DL, Meehan KR and Zacharski LR:
The coagulation system as a target for the treatment of human
gliomas. Semin Thromb Hemost. 28:19–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hua Y, Tang L, Keep RF, Schallert T, Fewel
ME, Muraszko KM, Hoff JT and Xi G: The role of thrombin in gliomas.
J Thromb Haemost. 3:1917–1923. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Carneiro-Lobo TC, Schaffner F, Disse J,
Ostergaard H, Francischetti IM, Monteiro RQ and Ruf W: The
tick-derived inhibitor Ixolaris prevents tissue factor signaling on
tumor cells. J Thromb Haemost. 10:1849–1858. 2012. View Article : Google Scholar : PubMed/NCBI
|