Introduction
Cell proliferation and apoptosis must be properly
balanced in order to support proper development and maintain
healthy homeostasis of mature tissues (1). A highly regulated process to control
this balance involves each cell receiving survival signals from its
microenvironment and from external stimuli (1).
Cancer cells are characterized by their ability to
proliferate in an uncontrolled manner in contrast to normal cells,
in which the proliferation is tightly regulated (2). In addition, apoptosis is a continuous
physiologic process in tissue homoeostasis, and functions as a
defense against pathogens and helps with the elimination of
unwanted cells (3–5). Dysregulated apoptosis can lead to
extended aberrant cell viability or may favor the accumulation of
transforming mutations, and it is thought to contribute to
carcinogenesis (3–5). Therefore, tumor development and
progression are generally regarded as dependent on an increased
proliferation rate and an apoptosis rate too low to balance cell
growth.
Early growth response-1 (Egr-1) is a Cys2-His2-type
zinc-finger transcription factor that is rapidly induced in
response to a broad range of extracellular stimuli and plays a
critical role in controlling cell growth, proliferation,
differentiation and apoptosis (6–12).
Interestingly, Egr-1 is considered to be either a tumor-suppressor
or tumor-promoter, depending on cell type and external stimuli. The
growth suppressing activity of Egr-1 has been observed in certain
human cancers such as fibrosarcoma, glioblastoma, lung and breast
cancer (13–16). In contrast, Egr-1 has been found to
promote tumor cell growth in certain human cancer tissues such as
prostate, skin and kidney cancers, in which Egr-1 is found at
elevated levels (17–19). In particular, Egr-1 expression is
associated with higher Gleason scores and poor differentiation in
human prostatic cancer (20).
Accumulating evidence suggests that Egr-1 is involved in the
development and progression of human cancers, particularly as a
tumor promoter.
Previously, Egr-1 was found to promote tumor cell
growth and inhibit apoptosis in human colon cancer cells (21–23).
Therefore, Egr-1 expression is believed to be involved in tumor
development and progression by affecting cell proliferation and
apoptosis in human colorectal cancer. However, there are no data
concerning the impact of Egr-1 on tumor cell proliferation and
apoptosis in human colorectal cancer tissues.
The aim of the present study was to evaluate the
expression of Egr-1 in human colorectal cancer and its correlation
with tumor cell proliferation, apoptosis and clinicopathological
features including patient survival.
Materials and methods
Patient and sample selection
The mRNA and protein expression of Egr-1 was
evaluated in human colorectal cancer tissues, paired normal
colorectal mucosa, metastatic or non-metastatic lymph node tissues
of the same patients from colonoscopic biopsies and surgical
specimens. For immunohistochemical staining, formalin-fixed and
paraffin-embedded tumor specimens from 158 randomly chosen patients
who had undergone surgery for colorectal cancer at Chonnam National
University, Hwasun Hospital (Jeonnnam, Korea) between July 2004 and
June 2005 were studied. None of the patients had received
preoperative radiotherapy or chemotherapy. Pathological reports and
clinical histories at the time of surgery were reviewed through the
medical records. Tumor staging was in accordance with the American
Joint Committee on Cancer (AJCC) staging system (24). Survival was measured from the time
of surgery until follow-up in December 2010. The study group
comprised 94 males and 64 females. The median age was 66.3±12.3
(means ± SD) with a range of 26–90 years. The mean size of the
tumors was 5.1±2.3 cm (means ± SD) with a range of 1.5–14.0 cm. The
mean follow-up period was 43.1 months with a range of 0.7–56.0
months. This study was approved by the Institutional Review Board
of Chonnam National University, Hwasun Hospital. Written informed
consent was obtained from each participant prior to tissue
acquisition. All participants provided written consent for their
information to be stored in the hospital database and used for
research.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from cells in 1 ml of TRIzol
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according
to the manufacturer’s protocol and subjected to reverse
transcriptase polymerase-chain reaction (PCR). For cDNA synthesis,
1 μg of RNA was reverse transcribed with Molony-murine leukemia
virus (MMLV) transcription reagents (Invitrogen Life Technologies).
PCR amplification of cDNA was performed using gene-specific primers
and Go Taq® DNA polymerase (Promega Corporation,
Madison, WI, USA). The following primers were used; Egr-1,
5′-CAGTGGCCTAGTGAGCATGA-3′ and 5′-CCGCAAGTGGATCTTGGTAT-3′; GAPDH,
5′-ACC ACA GTC CAT GCC ATC AC-3′/5′-TCC ACC ACC CTG TTG CTG
TA-3′.
Western blotting
Total cell extracts were prepared in RIPA buffer
using the Halt™ protease and phosphatase inhibitor cocktail (both
from Thermo Scientific Rockford, IL, USA). Cells were disrupted by
sonication and centrifuged at 4°C. Equal amounts of protein were
separated on polyacrylamide gels, and transferred to PVDF membranes
(Millipore, Billerica, MA, USA). Blots were blocked with 5% milk
and incubated with primary antibodies against Egr-1 (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). Immunoreactive proteins
were visualized by the enhanced chemiluminescence detection system
HRP substrate (Millipore). Immunoreactive protein bands were
quantified using the luminescent image analyzer LAS-4000 and
MultiGauge V3.2 image analyzer software (Fujifilm, Tokyo,
Japan).
Immunohistochemistry
Formalin-fixed and paraffin-embedded tissue sections
(4 μm) with mounted probe on slides, were immunostained with
anti-rabbit polyclonal antibody for the Egr-1 antigen (R&D
Systems, Inc.) using the avidin-biotin peroxidase complex method.
Sections were deparaffinized and heated in a microwave oven for 7
min to retrieve the antigens. They were immersed in 0.6% hydrogen
peroxide for 10 min to block the endogenous peroxidase activity.
The primary antibody, at a concentration of 1:100, was diluted in
phosphate-buffered saline supplemented with 5% normal horse serum
and 1% bovine serum albumin and then incubated with tissues
overnight at room temperature. Anti-mouse immunoglobulin G (Sigma,
St. Louis, MO, USA) labeled with biotin was used as a secondary
antibody for the detection of primary antibodies and was incubated
for 10 min at 45°C. After multiple rinses with universal buffer,
the streptavidin-alkaline phosphatase detection system (Biomeda,
Foster City, CA, USA) was applied for 8 min. As the final step, the
slides were developed for 10 min with the enzyme substrate,
3,3-diaminobenzidine (Sigma). The slides were counterstained with
hematoxylin solution for 3 min (Research Genetics, Huntsville, AL,
USA). After dehydration, the tissue was sealed with a universal
mount (Research Genetics). For negative controls, the primary
antibody was omitted and replaced with phosphate-buffered
saline.
Assessment of Egr-1 expression
The immunoreactivity was evaluated independently by
2 observers without knowledge of the clinical outcomes, through
analysis of intensity, area and pattern of immunostaining. Staining
intensity was classified from 0 (no staining) to 3 (strong
staining), and the percentage of the staining area was classified
as 0 for no positive staining of tumor cells, 1 for positive
staining in <10% of the tumor cells, 2 for positive staining in
10–50% of the tumor cells, or 3 for positive staining in >50% of
the tumor cells. The staining index was calculated as the product
of staining intensity and staining area. Assessment of the staining
was evaluated by 2 independent pathologists without knowledge of
the clinical outcomes such as tumor stage, grade and survival.
Consensus scores were assigned for each case by reviewing the
slides with discrepancies in scoring. All sections for which there
was disagreement between the 2 observers were re-evaluated and
discussed. There was total agreement on the classification. The
tumors were categorized as having positive expression (staining
index ≥4) or negative expression (staining index <4).
Assessment of tumor cell
proliferation
Immunohistochemical staining with the polyclonal
Ki-67 antibody (MIB-1; diluted 1:150; Dakopatts, Glostrup, Denmark)
using the avidin-biotin peroxidase complex method and
3,3-diaminobenzidine (Sigma) as chromogen was performed. A distinct
brown staining of the nuclei with strong intratumoral heterogeneity
was considered Ki-67-positive. The Ki-67-positive tumor cells were
evaluated with a light microscope holding a ×100 oil immersion
objective by scoring a minimum of 1,000 tumor cells in randomly
selected fields. For each case, 3 different counts were performed,
and the highest score was chosen as the corresponding index. The
Ki-67 labeling index (KI) was presented as the number of
Ki-67-positive nuclei/1,000 tumor cell nuclei.
Detection of apoptotic cells and
bodies
For detection of apoptotic cells, sections of
formalin-fixed and paraffin-embedded tissue were processed for
in situ immunohistochemical localization of nuclei
exhibiting DNA fragmentation, by the terminal deoxynucleotidyl
transferase (TdT)-mediated deoxyuridine triphosphate (dUTP) nick
end labeling (TUNEL) method, using the ApopTag™ Plus In situ
Apoptosis Detection kit (Intergen, Purchase, NY, USA). Sections
were treated according to the manufacturer’s instructions as
previously described (25).
Briefly, sections were deparaffinized and rehydrated with xylene
and ethanol, and permeabilized with 20 μg/ml proteinase K (Sigma)
for 15 min at room temperature. Endogenous peroxidase was
inactivated by coating the samples with 2% hydrogen peroxide
(H2O2). Sections were rinsed with
phosphate-buffered saline, and then immersed for 120 min in TdT
buffer at 37°C. Afterwards, they were incubated for 60 min with the
anti-digoxygenin peroxidase-conjugate, followed by the peroxidase
substrate diaminobenzidine. Finally, sections were counterstained
with Mayer’s hematoxylin. The positive control sections were
treated with 0.7 g/ml DNase I (Sigma) in potassium cacodylate
buffer (pH 7.2) for 10 min before treatment with TdT buffer. As a
negative control, a number of tissue samples were subjected to
treatment without TdT. The percentage of apoptotic cells was
determined by counting labeled cells at a ×400 magnification in
randomly selected and homogeneous fields. Apoptotic cells were also
identified by their characteristic morphological features in
hematoxylin and eosin-stained sections such as cell shrinkage and
chromatin margination or chromatin condensation with formation of
apoptotic bodies (25). The
apoptotic index (AI) was expressed as the number of positive nuclei
including apoptotic bodies among 1,000 tumor cell nuclei.
Statistical analysis
All statistical analyses were carried out using the
Statistical Package for the Social Sciences (SPSS/PC Plus
Professional Statistics 15.0; SPSS, Inc., Chicago, IL, USA). The
correlation between Egr-1 expression and the clinicopathological
parameters was examined by χ2 test and Fisher’s exact
test. The relationship between Egr-1 expression and KI or AI was
evaluated by the Student’s t-test. Survival curves were calculated
according to the Kaplan-Meier method, and the differences were
tested with a log-rank test. A p-value of <0.05 was considered
to indicate a statistically significant result.
Results
Expression of Egr-1 in human colorectal
cancer and metastatic lymph node tissues
We evaluated the expression of Egr-1 at the mRNA and
protein levels by RT-PCR, western blotting and immunohistochemistry
in human colorectal cancer tissues, paired normal colorectal
mucosa, and metastatic or non-metastatic lymph node tissues of the
same patients taken by colonoscopic biopsy and as surgical
specimens. In the colonoscopic biopsy specimens, we confirmed
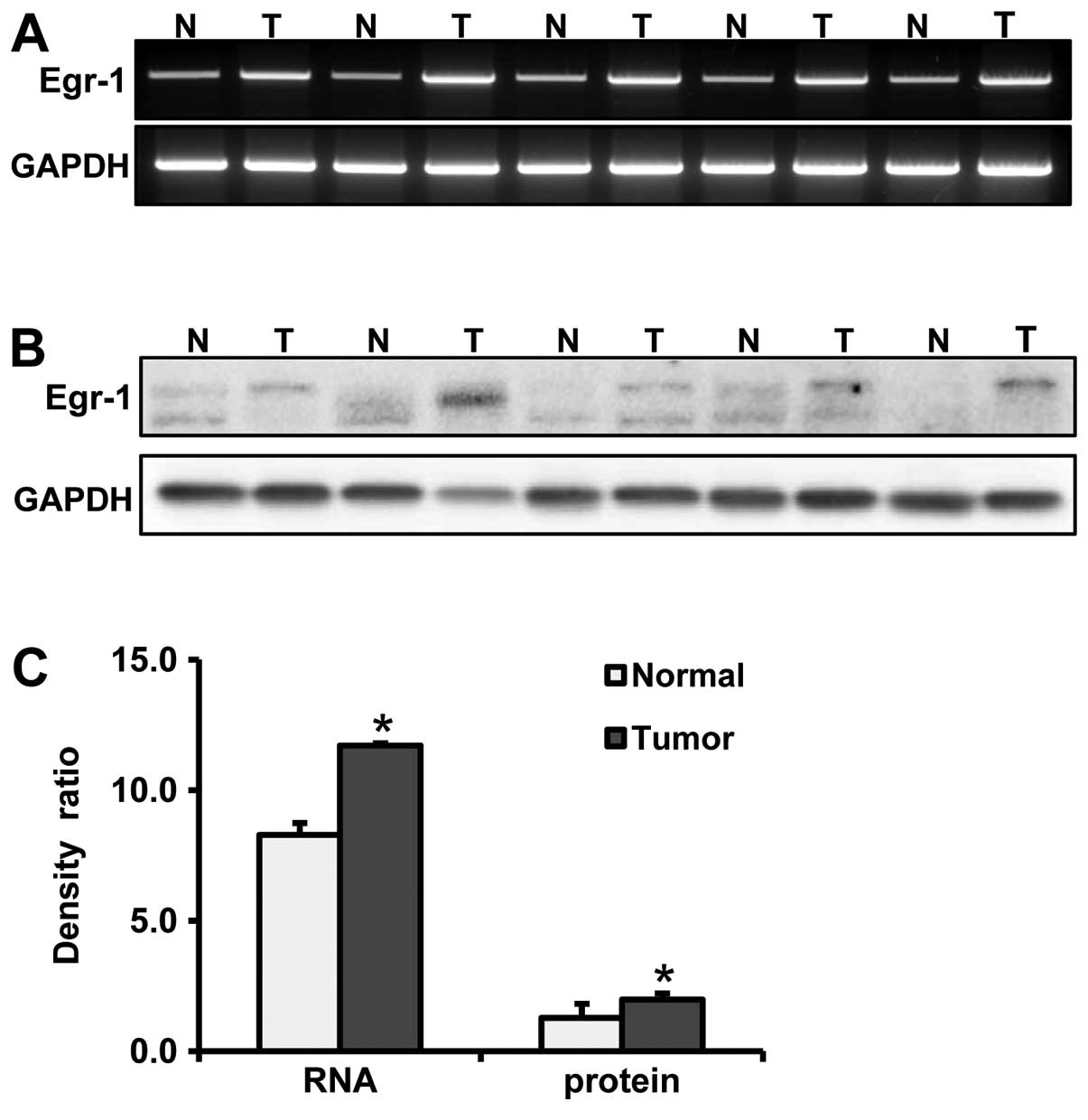
upregulation of Egr-1 expression at both the mRNA and protein
levels in cancer tissues when compared to levels in the paired
normal mucosa (p=0.002 and p=0.046, respectively) (Fig. 1). In the paraffin tissue sections,
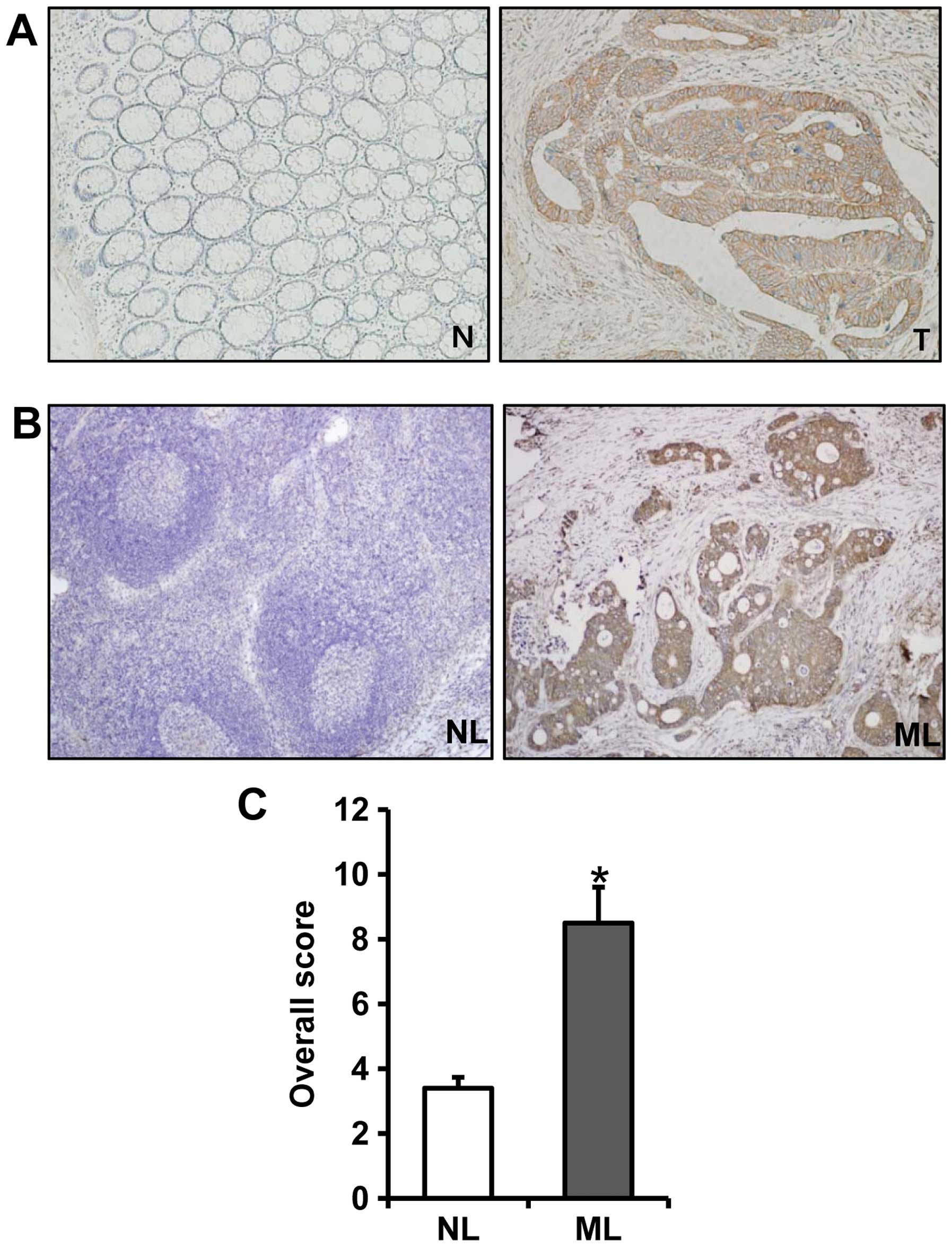
immunostaining of Egr-1 protein in the normal colorectal mucosa was
absent or weak (Fig. 2A). The
immunostaining of Egr-1 protein was predominantly identified in the
cytoplasm of cancer cells and was not detectable in the tumor
stromal (Fig. 2A). The
immunostaining of Egr-1 in metastatic lymph node tissues was
significantly stronger than that in the non-metastatic lymph node
tissues (Fig. 2B). The overall
score for immunostaining of Egr-1 in the metastatic lymph node
tissues was significantly higher than that in the non-metastatic
lymph node tissues (p<0.001) (Fig.
2C).
Expression of Ki-67 protein and detection
of apoptosis in tissue specimens
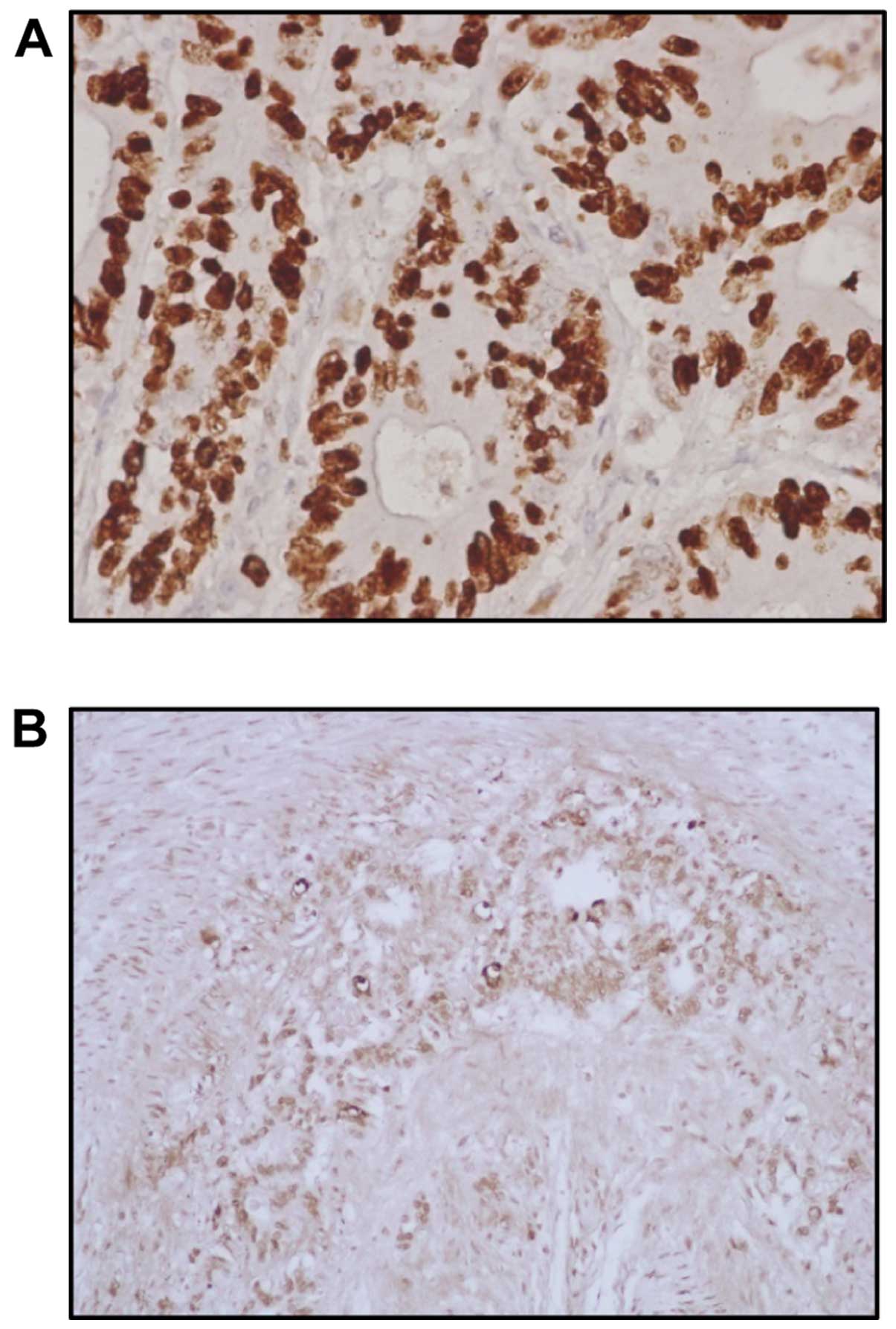
Ki-67 immunoreactivity was found in the nuclei of
cancer cells (Fig. 3A). Positive
cells were commonly observed in the advancing margin of the tumors.
The standard morphologic criteria of apoptotic cells using the
TUNEL method include cell shrinkage and chromatin margination or
chromatin condensation with formation of apoptotic bodies. Almost
all of the positively stained cells and bodies were considered to
be apoptotic cells as they corresponded morphologically to the
standard criteria of apoptotic cells (Fig. 3B). Nonspecific staining in necrotic
foci showed a faint and diffuse staining and it was distinguished
from apoptotic nuclei by simple morphological examination.
Correlation between the expression of
Egr-1 protein and clinicopathological features
The correlation between Egr-1 protein expression and
clinicopathological parameters is shown in Table I. Positive expression of Egr-1 was
significantly associated with age, lymphovascular invasion, lymph
node and distant metastasis and tumor stage (p=0.023, p=0.024,
p<0.001, p<0.001 and p<0.001, respectively). Furthermore,
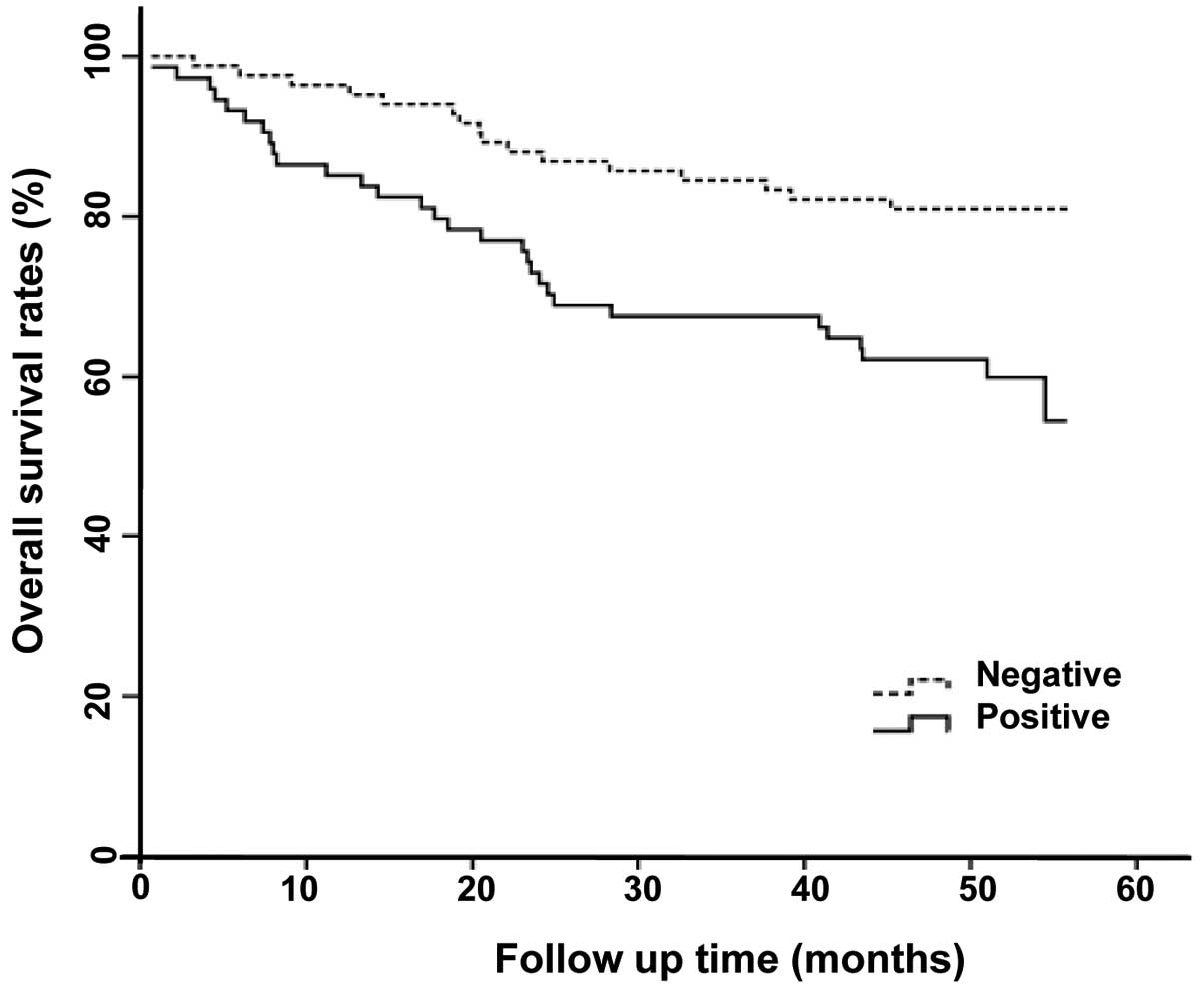
positive expression of Egr-1 was associated with poor survival
(p=0.007) (Fig. 4).
 | Table ICorrelation between Egr-1 protein
expression and clinicopathological parameters of the human
colorectal cancer cases. |
Table I
Correlation between Egr-1 protein
expression and clinicopathological parameters of the human
colorectal cancer cases.
| | Egr-1 | |
|---|
| |
| |
|---|
| Parameters | Total (n=158) | Negative
(n=84) | Positive
(n=74) | P-value |
|---|
| Age (years) | | | | 0.023 |
| <67 | 62 | 26 | 36 | |
| ≥67 | 96 | 58 | 38 | |
| Gender | | | | 0.334 |
| Male | 94 | 47 | 47 | |
| Female | 64 | 37 | 27 | |
| Tumor size
(cm) | | | | 0.186 |
| <5.0 | 92 | 53 | 39 | |
| ≥5.0 | 66 | 31 | 35 | |
| Stage | | | | <0.001 |
| I/II | 78 | 62 | 16 | |
| III/IV | 80 | 22 | 58 | |
| Lymphovascular
invasion | | | | 0.024 |
| Negative | 100 | 60 | 40 | |
| Positive | 58 | 24 | 34 | |
| Histological
type | | | | 0.811 |
| Well
differentiated | 93 | 51 | 42 | |
| Moderately
differentiated | 45 | 24 | 21 | |
| Poorly
differentiated | 7 | 3 | 4 | |
| Mucinous | 12 | 6 | 6 | |
| Signet | 1 | 0 | 1 | |
| Depth of invasion
(T) | | | | 0.324 |
| T1/T2 | 26 | 17 | 9 | |
| T3/T4 | 132 | 67 | 65 | |
| Lymph node
metastasis (N) | | | | <0.001 |
| N0 | 80 | 62 | 18 | |
| N1–3 | 78 | 22 | 56 | |
| Distant metastasis
(M) | | | | <0.001 |
| M0 | 148 | 84 | 64 | |
| M1 | 10 | 0 | 10 | |
Correlation between the expression of
Egr-1 protein and tumor cell proliferation or apoptosis
The Ki-67 labeling index (KI) for 158 tumors ranged
from 21.9 to 85.7 with a mean KI of 53.6±15.4. The mean KI value of
Egr-1-positive tumors was 60.1±14.9 which was significantly higher
than that of the Egr-1-negative tumors (p=0.031) (Table II). The apoptotic index (AI) for
158 tumors ranged from 0.9 to 30.0 with a mean AI of 9.0±1.0.
However, there was no significant difference between Egr-1
expression and AI (p=0.357) (Table
III).
 | Table IICorrelation between KI and the status
of Egr-1 protein expression in human colorectal cancer. |
Table II
Correlation between KI and the status
of Egr-1 protein expression in human colorectal cancer.
| Egr-1 protein
expression | Total (n=158) | KI (means ±
SD) | P-value |
|---|
| Negative | 84 | 49.1±14.7 | 0.031 |
| Positive | 74 | 60.1±14.9 | |
 | Table IIICorrelation between AI and the status
of Egr-1 protein expression in human colorectal cancer. |
Table III
Correlation between AI and the status
of Egr-1 protein expression in human colorectal cancer.
| Egr-1 protein
expression | Total (n=158) | AI (means ±
SD) | P-value |
|---|
| Negative | 84 | 10.2±6.3 | 0.357 |
| Positive | 74 | 8.4±4.6 | |
Correlation between KI or AI and
clinicopathological features
The correlation between KI or AI and
clinicopathological parameters associated with Egr-1 expression is
shown in Table IV. The mean KI
value of the positive distant metastatic tumors was 64.2±13.7 which
was significantly higher than that of the negative tumors
(p=0.042). The mean AI value of the negative lymph node metastatic
tumors was 11.5±7.1 and was significantly higher than that of the
positive tumors (p=0.024). However, there was no association
between KI or AI and age, tumor stage or lymphovascular invasion.
In addition, KI or AI was not associated with patient survival
(data not shown).
 | Table IVCorrelation between KI or AI and
clinicopathological parameters of the human colorectal cancer
cases. |
Table IV
Correlation between KI or AI and
clinicopathological parameters of the human colorectal cancer
cases.
| Parameters | Total (n=158) | KI (means ±
SD) | P-value | AI (means ±
SD) | P-value |
|---|
| Age (years) | | | 0.586 | | 0.431 |
| <67 | 62 | 51.7±18.30 | | 8.1±4.27 | |
| ≥67 | 96 | 55.0±13.85 | | 9.7±6.68 | |
| Stage | | | 0.138 | | 0.134 |
| I/II | 78 | 55.7±14.73 | | 12.5±6.3 | |
| III/IV | 80 | 56.7±15.31 | | 7.6±3.60 | |
| Lymphovascular
invasion | | | 0.256 | | 0.332 |
| Negative | 100 | 49.1±19.00 | | 9.2±5.44 | |
| Positive | 58 | 55.8±14.33 | | 7.4±6.53 | |
| Lymph node
metastasis (N) | | | 0.715 | | 0.024 |
| N0 | 80 | 54.5±13.59 | | 11.5±7.09 | |
| N1–3 | 78 | 52.6±17.15 | | 7.3±4.01 | |
| Distant metastasis
(M) | | | 0.042 | | 0.753 |
| M0 | 148 | 49.9±13.96 | | 9.3±6.20 | |
| M1 | 10 | 64.2±13.73 | | 8.4±2.90 | |
Discussion
Egr-1 is a member of the zinc-finger transcription
factor family that is rapidly and transiently induced by a number
of external stimuli including growth factors, cytokines and
radiation injury. It regulates a wide spectrum of biological
processes including cell growth, proliferation, apoptosis, cell
cycle arrest, senescence, differentiation and cancer progression
(6–12). Notably, Egr-1 expression has been
found to be elevated in human prostatic (20) and gastric cancer tissues (26,27)
and it has been suggested that Egr-1 plays an important role in
carcinogenesis and cancer progression in the prostate and stomach
(20,26,27).
Our study showed that expression of Egr-1 mRNA and protein was
increased in colorectal cancer tissue when compared with the barely
detectable levels that were present in the matched normal
colorectal mucosa. This result suggests that Egr-1 expression is
implicated in colorectal carcinogenesis.
Although Egr-1 is considered to be a
tumor-suppressor gene in several types of cancers (13–16),
Egr-1 expression has been reported to be associated with cancer
progression in prostatic and gastric cancers (20,26,27).
In our study, increased expression of Egr-1 was significantly
associated with age, lymphovascular invasion, lymph node and
distant metastasis, tumor stage and poor survival. Moreover, Egr-1
expression at the protein level was significantly increased in
metastatic lymph node tissues, when compared to that in the
non-metastatic lymph node tissues. These results strongly suggest
that Egr-1 is involved in colorectal cancer progression and may
have prognostic significance for colorectal cancer patients.
The continuous balance between cell proliferation
and apoptosis is essential for the optimal function of nearly all
tissues and organ systems, and must be properly coordinated.
However, if cell proliferation exceeds apoptosis, neoplastic
diseases and cancer may occur. Previous studies have shown that
Egr-1 is involved in tumor development and progression by promotion
of tumor cell growth and inhibition of apoptosis in human colon
cancer cells (21–23). Therefore, we investigated the impact
of Egr-1 on cell proliferation and apoptosis in colorectal cancer
tissues.
Ki-67 is a nuclear antigen that is expressed in all
stages of the cell cycle except for G0 and early G1, and it is an
established proliferation marker and has been extensively used to
estimate the growth fraction of tumors (28,29).
Previous reports showed that Ki-67 expression was associated with
tumor progression and patient survival in various human cancers
including colorectal cancer (30–32).
Recently, Egr-1 was found to promote tumor cell proliferation in
gastric cancer cells (33). In the
present study, higher KI values were significantly associated with
distant metastasis, and the mean KI value of Egr-1-positive tumors
was significantly higher than that of Egr-1-negative tumors. These
results suggest that Egr-1 may be involved in tumor development and
progression of colorectal cancer by affecting tumor cell
proliferation.
The TUNEL method has been designed to detect
apoptotic cells that undergo extensive DNA degradation during the
late stages of apoptosis (25,34,35).
Previously, apoptosis-related genes have been identified as
independent prognostic factor, and they have proven to be useful
therapeutic targets in cancer therapy (36–38).
Egr-1 did exert an effect as an inhibitor of the apoptotic pathway
in colorectal cancer cells (23).
In our study, a lower AI value was significantly associated with
lymph node metastasis, but the AI value did not correlate with
patient survival. Furthermore, no significant difference was noted
between Egr-1 expression and AI in colorectal cancer tissues. These
results suggest that the steps in apoptosis are not dependent on
Egr-1 alone, and are controlled by numerous regulators including
pro-apoptotic and anti-apoptotic genes (3–5).
In summary, Egr-1 expression was significantly
elevated in colorectal cancer tissues, when compared to that in
paired normal mucosa. In addition, Egr-1 expression was
significantly increased in metastatic lymph node tissues, when
compared to that in non-metastatic lymph node tissues. Increased
expression of Egr-1 was significantly associated with age,
lymphovascular invasion, lymph node and distant metastasis, tumor
stage and poor survival. KI was significantly associated with
distant metastasis. The mean KI value of Egr-1-positive tumors was
significantly higher than that of Egr-1-negative tumors. However,
there was no significant difference between Egr-1 expression and
the AI value. These results indicate that Egr-1 may be associated
with colorectal cancer progression via tumor cell
proliferation.
Acknowledgements
This study was supported by a grant (0720570) from
the National R&D Program for Cancer Control, Ministry of Health
and Welfare, Republic of Korea, and partly by a research funds for
the Research Institute of Clinical Medicine, Chonnam National
University Hospital in 2012 (CRI 12035-1), Republic of Korea.
Abbreviations:
|
Egr-1
|
early growth response-1
|
|
AJCC
|
American Joint Committee on Cancer
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
|
MMLV
|
molony-murine leukemia virus
|
|
KI
|
Ki-67 labeling index
|
|
TUNEL
|
terminal deoxynucleotidyl transferase
(TdT)-mediated deoxyuridine triphosphate (dUTP) nick end
labeling
|
|
AI
|
apoptotic index
|
References
|
1
|
Raff MC: Social controls on cell survival
and cell death. Nature. 356:397–400. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeBerardinis RJ, Lum JJ, Hatzivassiliou G
and Thompson CB: The biology of cancer: metabolic reprogramming
fuels cell growth and proliferation. Cell Metab. 7:11– 20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schultz DR and Harrington WJ Jr:
Apoptosis: programmed cell death at a molecular level. Semin
Arthritis Rheum. 32:345–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kiechle FL and Zhang X: Apoptosis:
biochemical aspects and clinical implications. Clin Chim Acta.
326:27–45. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Milbrandt J: A nerve growth factor-induced
gene encodes a possible transcriptional regulatory factor. Science.
238:797–799. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gashler A and Sukhatme VP: Early growth
response protein 1 (Egr-1): prototype of a zinc-finger family of
transcription factors. Prog Nucleic Acid Res Mol Biol. 50:191–224.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fahmy RG and Khachigian LM: Antisense
Egr-1 RNA driven by the CMV promoter is an inhibitor of vascular
smooth muscle cell proliferation and regrowth after injury. J Cell
Biochem. 84:575–582. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rupprecht HD, Hoffer G, de Heer E, Sterzel
RB, Faller G and Schoecklmann HO: Expression of the transcriptional
regulator Egr-1 in experimental glomerulonephritis: requirement for
mesangial cell proliferation. Kidney Int. 51:694–702. 1997.
View Article : Google Scholar
|
|
10
|
Yan SF, Fujita T, Lu J, et al: Egr-1, a
master switch coordinating upregulation of divergent gene families
underlying ischemic stress. Nat Med. 6:1355–1361. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O’Donovan KJ, Tourtellotte WG, Millbrandt
J and Baraban JM: The EGR family of transcription-regulatory
factors: progress at the interface of molecular and systems
neuroscience. Trends Neurosci. 22:167–173. 1999.PubMed/NCBI
|
|
12
|
Lemaire P, Revelant O, Bravo R and Charnay
P: Two mouse genes encoding potential transcription factors with
identical DNA-binding domains are activated by growth factors in
cultured cells. Proc Natl Acad Sci USA. 85:4691–4695. 1988.
View Article : Google Scholar
|
|
13
|
Liu C, Yao J, de Belle I, Huang RP,
Adamson E and Mercola D: The transcription factor EGR-1 suppresses
transformation of human fibrosarcoma HT1080 cells by coordinated
induction of transforming growth factor-β1, fibronectin, and
plasminogen activator inhibitor-1. J Biol Chem. 274:4400–4411.
1999.PubMed/NCBI
|
|
14
|
Liu C, Yao J, Mercola D and Adamson E: The
transcription factor EGR-1 directly transactivates the fibronectin
gene and enhances attachment of human glioblastoma cell line U251.
J Biol Chem. 275:20315–20323. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shareef MM, Cui N, Burikhanov R, et al:
Role of tumor necrosis factor-α and TRAIL in high-dose
radiation-induced bystander signaling in lung adenocarcinoma.
Cancer Res. 67:11811–11820. 2007.
|
|
16
|
Huang RP, Fan Y, de Belle I, et al:
Decreased Egr-1 expression in human, mouse and rat mammary cells
and tissues correlates with tumor formation. Int J Cancer.
72:102–109. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gitenay D and Baron VT: Is EGR1 a
potential target for prostate cancer therapy? Future Oncol.
5:993–1003. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Riggs PK, Rho O and DiGiovanni J:
Alteration of Egr-1 mRNA during multistage carcinogenesis in mouse
skin. Mol Carcinog. 27:247–251. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scharnhorst V, Menke AL, Attema J, et al:
EGR-1 enhances tumor growth and modulates the effect of the Wilms’
tumor 1 gene products on tumorigenicity. Oncogene. 19:791–800.
2000.PubMed/NCBI
|
|
20
|
Eid MA, Kumar MV, Iczkowski KA, Bostwick
DG and Tindall DJ: Expression of early growth response genes in
human prostate cancer. Cancer Res. 58:2461–2468. 1998.PubMed/NCBI
|
|
21
|
Arimochi H, Morita K, Kataoka K, Nakanishi
S, Kuwahara T and Ohnishi Y: Suppressive effect of Clostridium
perfringens-produced heat-stable substance(s) on proliferation
of human colon adenocarcinoma HT29 cells in culture. Cancer Lett.
241:228–234. 2006.
|
|
22
|
Chen A, Xu J and Johnson AC: Curcumin
inhibits human colon cancer cell growth by suppressing gene
expression of epidermal growth factor receptor through reducing the
activity of the transcription factor Egr-1. Oncogene. 25:278–287.
2006.
|
|
23
|
Mahalingam D, Natoni A, Keane M, Samali A
and Szegezdi E: Early growth response-1 is a regulator of
DR5-induced apoptosis in colon cancer cells. Br J Cancer.
102:754–764. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
Springer; New York: pp. 143–163. 2010
|
|
25
|
Gavrieli Y, Sherman Y and Ben-Sasson SA:
Identification of programmed cell death in situ via specific
labeling of nuclear DNA fragmentation. J Cell Biol. 119:493–501.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kobayashi D, Yamada M, Kamagata C, et al:
Overexpression of early growth response-1 as a
metastasis-regulatory factor in gastric cancer. Anticancer Res.
22:3963–3970. 2002.PubMed/NCBI
|
|
27
|
Zheng L, Pu J, Jiang G, et al: Abnormal
expression of early growth response 1 in gastric cancer:
association with tumor invasion, metastasis and heparanase
transcription. Pathol Int. 60:268–277. 2010. View Article : Google Scholar
|
|
28
|
McCormick D, Chong H, Hobbs C, Datta C and
Hall PA: Detection of the Ki-67 antigen in fixed and wax-embedded
sections with the monoclonal antibody MIB1. Histopathology.
22:355–360. 1993. View Article : Google Scholar
|
|
29
|
Weidner N, Moore DH II and Vartanian R:
Correlation of Ki-67 antigen expression with mitotic figure index
and tumor grade in breast carcinomas using the novel
‘paraffin’-reactive MIB1 antibody. Hum Pathol. 25:337–342.
1994.PubMed/NCBI
|
|
30
|
Oka S, Uramoto H, Shimokawa H, Iwanami T
and Tanaka F: The expression of Ki-67, but not proliferating cell
nuclear antigen, predicts poor disease free survival in patients
with adenocarcinoma of the lung. Anticancer Res. 31:4277–4282.
2011.PubMed/NCBI
|
|
31
|
He WL, Li YH, Yang DJ, et al: Combined
evaluation of centromere protein H and Ki-67 as prognostic
biomarker for patients with gastric carcinoma. Eur J Surg Oncol.
39:141–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hilska M, Collan YU, O Laine VJ, et al:
The significance of tumor markers for proliferation and apoptosis
in predicting survival in colorectal cancer. Dis Colon Rectum.
48:2197–2208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun T, Tian H, Feng YG, Zhu YQ and Zhang
WQ: Egr-1 promotes cell proliferation and invasion by increasing
β-catenin expression in gastric cancer. Dig Dis Sci. 58:423–430.
2013.PubMed/NCBI
|
|
34
|
Kyrylkova K, Kyryachenko S, Leid M and
Kioussi C: Detection of apoptosis by TUNEL assay. Methods Mol Biol.
887:41–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lifshitz S, Lamprecht SA, Benharroch D,
Prinsloo I, Polak-Charcon S and Schwartz B: Apoptosis (programmed
cell death) in colonic cells: from normal to transformed stage.
Cancer Lett. 163:229–238. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sezer C, Yildirim M, Yildiz M, et al:
Prognostic significance of biological apoptosis factors in gastric
cancer. J BUON. 18:138–146. 2013.PubMed/NCBI
|
|
37
|
Hernandez JM, Farma JM, Coppola D, et al:
Expression of the antiapoptotic protein survivin in colon cancer.
Clin Colorectal Cancer. 10:188–193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Theodoropoulos GE, Gazouli M, Vaiopoulou
A, et al: Polymorphisms of caspase 8 and caspase 9
gene and colorectal cancer susceptibility and prognosis. Int J
Colorectal Dis. 26:1113–1118. 2011.
|