Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignant tumors and ranks fifth in frequency worldwide
among all malignancies; it causes 1 million deaths annually and its
incidence is increasing steadily in various countries (1). Epidemiological studies showed that
primary liver cancer is the second cause of cancer-related
mortality in China and it accounts for 53% of all liver cancer
deaths worldwide (2). Despite
considerable developments in diagnosis and therapy, the cure rate
for patients who undergo resection is relatively low, and among
patients who are ineligible for surgical or percutaneous
procedures, only chemoembolization improves survival. Moreover, HCC
is widely regarded as a chemotherapy-resistant disease. These
drawbacks necessitate the continued search for novel HCC
therapies.
AZD8055 is an orally available, potent inhibitor of
mTORC1 and mTOR2 with superior pharmacokinetic and activity
profiles and shows inhibitory effects in a wide variety of tumor
cells by inhibiting the phosphorylation of mTORC1 substrates p70S6K
and 4E-BP1 as well as phosphorylation of the mTORC2 substrate AKT
and downstream proteins (3,4). Combination of AZD8055 and MEK
(Selumetinib) inhibitor enhanced apoptosis and tumor growth
suppression in non-small cell lung cancer (5). The promising results of AZD8055
demonstrate its potential as a therapeutic agent for solid tumors
individually or in combination, and warrant our investigation into
the therapeutic potential and molecular mechanism of AZD8055 in
liver cancer cells.
Autophagy, a process of ‘self-eating’, is a process
of self-degradation that maintains cellular viability during
periods of metabolic stress (6).
Although autophagy is considered a survival mechanism when faced
with cellular stress, extensive autophagy can also lead to cell
death. Accumulating evidence indicates the crucial role of
autophagy as a key molecular mechanism to eliminate cancer cells in
chemotherapy. AZD8055 has been shown to induce autophagy in several
cancer cell lines, although there are controversies if autophagy
induced by AZD8055 serves a pro-death or a pro-survival role
(3–5,7–9).
In the present study, we focused on the therapeutic
value and molecular mechanism of AZD8055 in HCC cells. We showed
that AZD8055 induces cell death associated with autophagic features
including an increase in the number of acidic vacuole organelles
and conversion of cytosolic LC3-I to membrane-bound LC3-II.
Furthermore, AZD8055 induced the activation of AMPK and co-treament
with AMPK inhibitor dorsomorphin attenuated AZD8055-induced cell
death. These findings provide a better understanding of
AZD8055-induced cell death and demonstrate the potential of AZD8055
as a therapeutic agent for HCC; they also provide insight into the
development of novel therapies for HCC.
Materials and methods
Chemicals and antibodies
AZD8055 was purchased from Selleck Chemicals,
dissolved in dimethyl sulfoxide (DMSO) as a stock concentration of
1 mmol/l and diluted to the indicated concentrations with culture
medium. All antibodies were obtained from Cell Signaling
Technology. Cell culture media and supplements were purchased from
Gibco/Invitrogen Corp. Other chemicals and reagents were obtained
from Sigma-Aldrich.
Cell lines and cell culture
Human HCC cell lines Huh7 and Hep3B were obtained
from the American Type Culture Collection. Cell lines were
maintained in RPMI-1640 medium with 10% FBS supplemented with 100
U/ml penicillin G and 100 μg/ml streptomycin (Sigma-Aldrich). Cells
were maintained at 37ºC in a humidified 5% CO2
incubator.
Cell viability and cell death assay
Huh7 and Hep3B cells (1×105/ml) were
plated in 96-well plates and allowed to attach overnight. Cells
were exposed to various concentrations of AZD8055 for the indicated
times. Cell viability was assessed by the reduction of tetrazolium
bromide (MTT) assay as previously described (10). For assay of cell death, cells were
treated with AZD8055 at different concentrations for the indicated
times. After treatment, cell death was determined by trypan blue
exclusion as previously described (11). Briefly, cells were stained with 0.4%
trypan blue and stained cells were counted. Data represent the mean
± SD derived from at least 3 separate experiments.
Clonogenicity assay
Clonogenicity assay was performed as previously
described (12). Briefly, Huh7 and
Hep3B cells were plated in 6-well plates in RPMI-1640 media
containing 100 nM AZD8055 and colonies were stained with crystal
violet and counted in triplicate wells after growth for a further 2
to 3 weeks. DMSO was used as a negative control. Data represent the
mean ± SD derived from at least 3 separate experiments.
Immunofluorescent staining
Cells were fixed in 4% paraformaldehyde and blocked
with normal goat serum. The mouse anti-human LC3 (Cell Signaling
Technology) primary antibody was added and incubated overnight at
4ºC. After washing 3 times with PBS, the goat anti-mouse IgG
secondary antibodies conjugated with Cy3 (Jackson ImmunoResearch
Laboratories Inc., West Grove, PA, USA) were added and incubated at
room temperature for 1 h. Cells were then counterstained with DAPI
(Sigma-Aldrich) and the images were captured using a Leica
fluorescent microscope.
Immunoblotting
Immunoblotting was performed as previously described
(10). At the end of the
treatments, Huh7 cells were harvested and lysed with ice-cold cell
lysis solution and the homogenate was centrifuged at 10,000 × g for
15 min at 4ºC. Total protein in the supernatant was quantified
using a BCA protein assay kit. Total protein (30 μg) from each
sample was separated by 12% SDS-PAGE and transferred to a PVDF
membrane; the PVDF membrane was placed in washing buffer containing
skimmed milk powder at room temperature and blocked for 2 h. After
washing 3 times, indicated primary antibodies were added
respectively and incubated at 4ºC overnight. Then, horseradish
peroxidase-conjugated secondary antibody was added to incubate for
1 h. X-ray film exposure was performed and AlphaImager HP
fluorescence/visible light gel imaging analyzer processing and
image analysis software were used to analyze gray value.
Statistical analysis
All data are presented as the mean ± SD. Differences
between groups were analyzed for statistical significance using a
two-tailed Student’s t-test. P<0.05 was considered to indicate
statistically significant differences.
Results
AZD8055-induced cell death is associated
with caspase-dependent apoptotic signaling cascade
To study the cytotoxic effect of AZD8055 on HCC
cells, Huh7 and Hep3B cells were used in the present study.
Initially, we determined the effects of AZD8055 on cell death in
both cell lines which were measured by trypan blue exclusion. DMSO
was used as control. Huh7 and Hep3B cells were treated with
different concentrations (5, 10, 20, 50 and 100 nM) of AZD8055 24 h
or with 100 nM AZD8055 for various periods and trypan blue staining
was performed. As shown in Fig. 1A,
AZD8055 increased cell death in both cell lines in a concentration-
and time-dependent manner. AZD8055 (100 nM) was used in the
following study, which was shown to significantly induce cell death
in both cell lines.
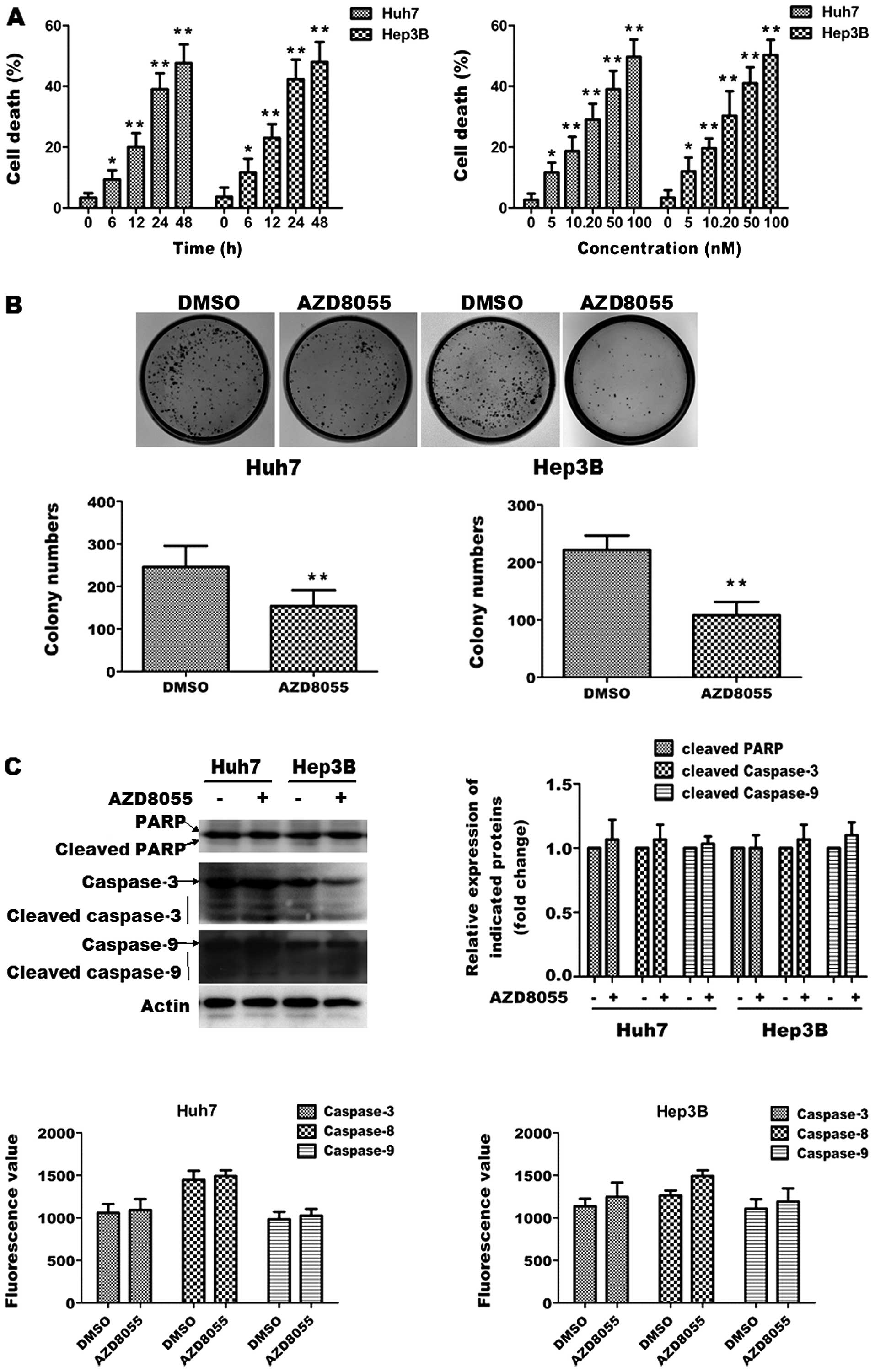 | Figure 1AZD8055-induced cell death is
associated with caspase-dependent apoptotic signaling cascade. (A)
AZD8055 induces cell death in a time- and concentration-dependent
manner. Huh7 and Hep3B cells were treated with different
concentrations (5, 10, 20, 50 and 100 nM) of AZD8055 for 24 h or
were treated with 100 nM AZD8055 for variable periods (6, 12, 24
and 48 h). Cell death was assessed by performing MTT assay and
trypan blue staining, respectively. Data represent the mean ± SD
derived from at least 3 separate experiments.
*P<0.05, **P<0.01, compared to control.
(B) AZD8055 suppresses colony formation of HCC cells. Huh7 and
Hep3B cells were plated in 6-well plates in RPMI-1640 media
containing 100 nM AZD8055 and colonies were stained with crystal
violet and counted in triplicate wells after growth for a further 2
to 3 weeks. DMSO was used as control. Data represent the mean ± SD
derived from at least 3 separate experiments.
**P<0.01. (C) AZD8055 does not cause caspase
activation. Huh7 and Hep3B cells were treated with 100 nM AZD8055
for 24 h and immunoblot analysis was performed to detect PARP
cleavage and caspase-3, caspase-9 activation. β-actin was used as a
loading control (up). Quantitative analysis of caspase-3, caspase-8
and caspase-9 activity was assessed by colorimetric assay (down).
Data represent the mean ± SD derived from at least 3 separate
experiments. |
To determine the long-term cytotoxicity of AZD8055,
colony formation assay was performed in Huh7 and Hep3B cells
treated with 100 nM AZD8055 or DMSO. As shown in Fig. 1B, AZD8055 decreased colony numbers
in both cell lines, as compared to control cells. These data
indicate that AZD8055 is cytotoxic to HCC cells.
To investigate the nature of AZD8055-induced
cytotoxicity, we first determined whether AZD8055 inhibits HCC cell
proliferation through classical apoptotic type I cell death. Huh7
and Hep3B cells were treated with DMSO or 100 nM AZD8055 for 24 h.
We explored whether AZD8055-induced cell death was associated with
caspase activation. Immunoblot analysis revealed that AZD8055
treatment did not markedly induce the formation of the active forms
of cleaved caspase-3 and caspase-9. Consistently, the cleavage of
PARP, which is a substrate of caspase-3 and a well-known apoptotic
hallmark, was not upregulated by AZD8055 in tested cell lines.
Quantitative analysis of caspase-3, caspase-8 and caspase-9
activity by colorimetric assay also showed that AZD8055 treatment
did not alter caspase-3, caspase-8 and caspase-9 activity in the
tested cell lines. These data suggest that AZD8055-induced cell
death is not associated with the caspase-dependent apoptotic
signaling cascade.
AZD8055 induces autophagy in HCC
cells
Since AZD8055-induced cell death appeared to lack
the characteristics of classical apoptotic type I cell death, we
sought to determine whether it was associated with changes in the
autophagic pathway previously described as characteristic of type
II cell death. To determine whether AZD8055 induces autophagy in
HCC cells, we investigated the effect of the drug on the
intracellular localization of microtubule-associated protein 1
light chain 3 (LC3), a specific marker of autophagosomes (13,14),
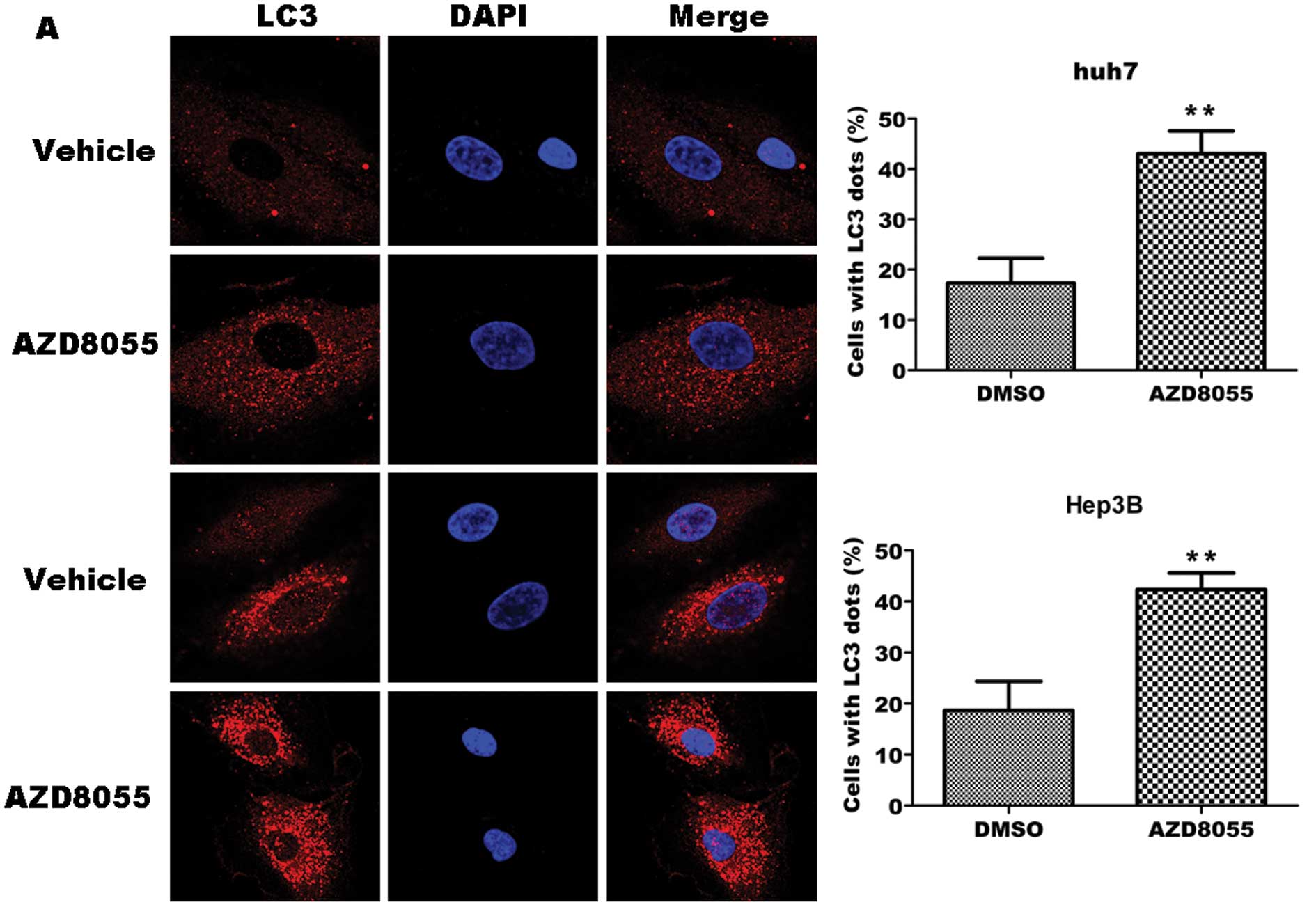
in the tested cell lines. As shown in Fig. 2A, left, representative fluorescence
micrographs show a punctate pattern of LC3 in AZD8055-treated cells
in contrast to the diffuse pattern in control cells. Quantitative
analysis revealed a significant increase in cells with LC3 punctate
pattern in AZD8055-treated cells as compared with control cells
(P<0.01), i.e., 43 vs. 17% in Huh7 cells and 41 vs. 18% in Hep3B
cells, respectively (right).
To further verify an increase in autophagic markers
induced by AZD8055, we assessed the effects of AZD8055 on the
levels of Atg-5/12, a protein complex formed by activation of
autophagy (14,15), BECN1 and on the conversion of the
cytoplasmic form of LC3-I protein (18 kDa) to the preautophagosomal
and autophagosomal membrane-bound form of LC3-II (16 kDa). As shown
in Fig. 2B, immunoblot analysis
revealed that AZD8055 treatment led to a time-dependent increase in
the levels of conjugated Atg-5, BECN1 and LC3-II proteins in both
tested cell lines. Quantitative analysis indicated that AZD8055
treatment led to an increase in Atg-5/12, BECN1 and LC3-II levels
as early as 12 h after drug exposure, gradually increasing
thereafter (Fig. 2B). Collectively,
these data suggest that AZD8055 induces autophagy in HCC cells.
Inhibition of autophagy by
3-methyladenine decreases AZD8055-induced cell death in HCC
cells
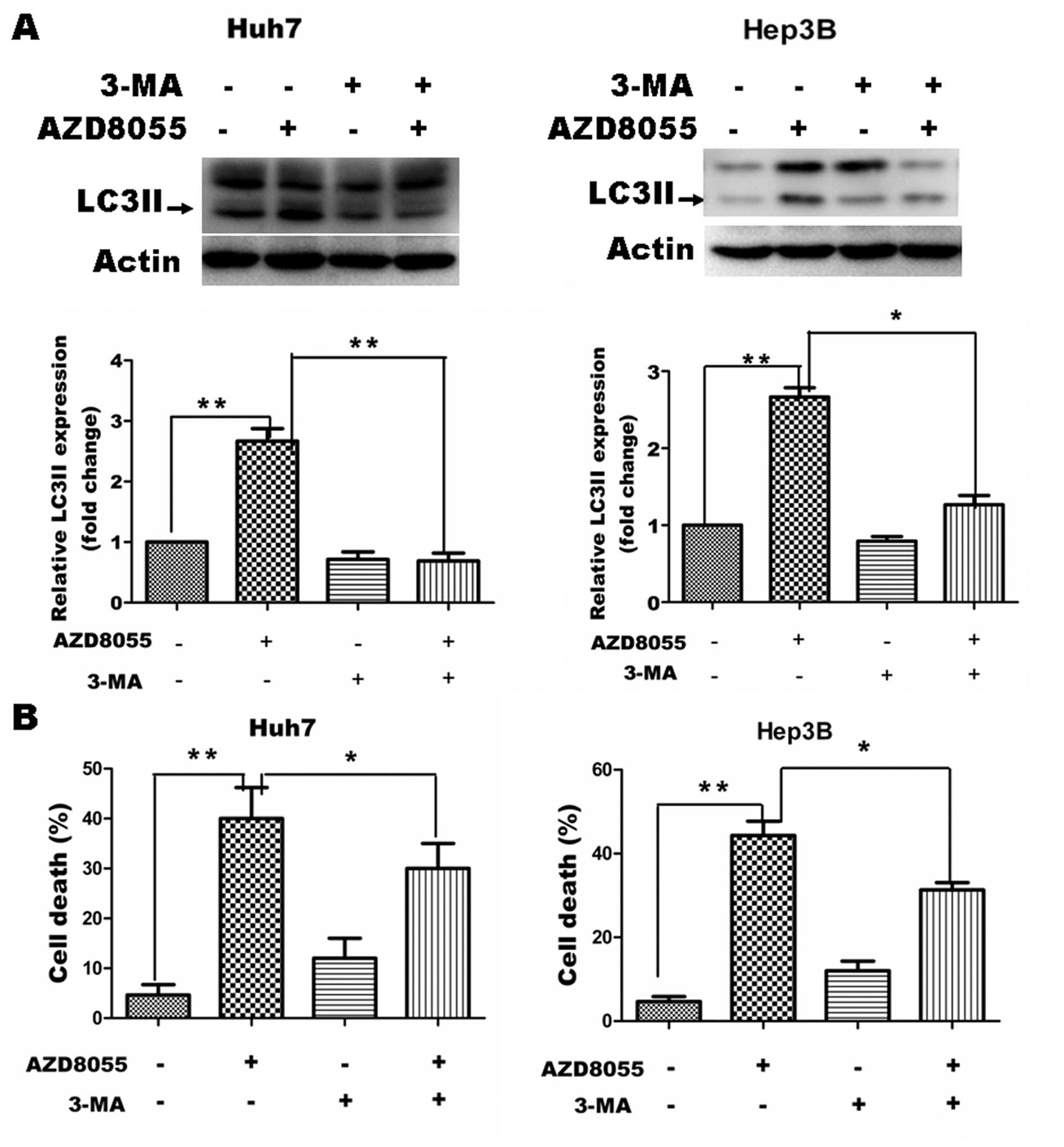
To confirm the contribution of the increase in the
autophagic compartment to AZD8055-induced cell death, we analyzed
the effect of 3-methyladenine (3-MA), a well known inhibitor of
autophagosome formation (16). Huh7
and Hep3B cells were co-treated with 2 mM 3-MA and 50 nM AZD8055
for 12 h and then cell pellets were harvested to determine the
amount of the autophagic marker LC3-II. As expected, AZD8055
treatment resulted in increased levels of LC3-II in Huh7 and Hep3B
cells. 3-MA treatment led to a significant decrease in
AZD8055-induced LC3-II expression in both tested cell lines
(Fig. 3A). In addition, we tested
whether co-treatment with 3-MA could affect AZD8055-induced cell
death. As shown in Fig. 3B,
co-treatment with 2 mM 3-MA resulted in a partial but significant
inhibition of AZD8055-induced cell death, as measured by MTT assay,
in Huh7 and Hep3B cells. The fact that chemical blockage of
autophagosome formation reduced cell death confirmed that the
observed accumulation of autophagosomes in cells treated with this
drug contributes, at least in part, to its cellular toxicity.
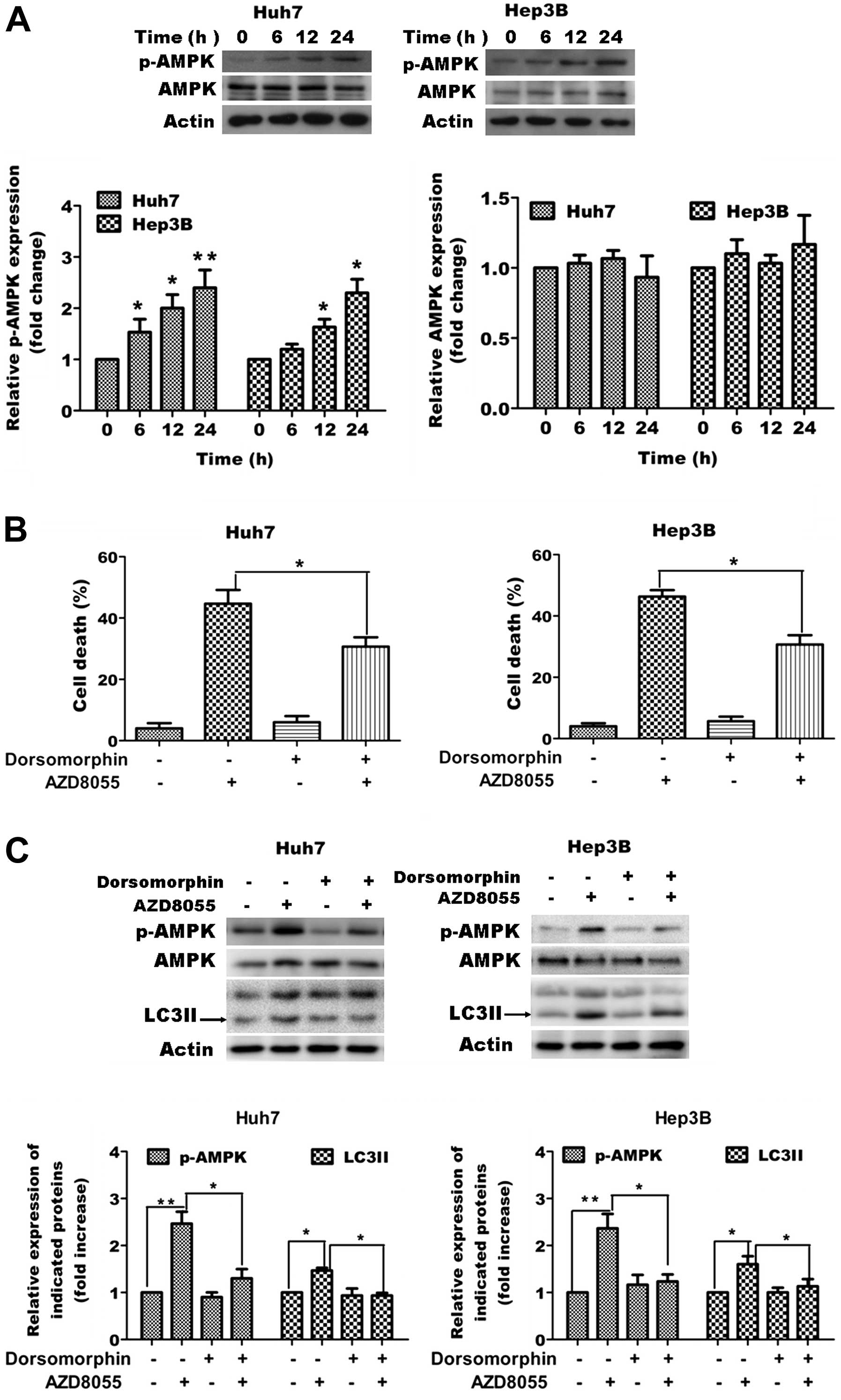
Role of AMPK activation in
AZD8055-induced autophagic cell death in HCC cells
Previous studies have demonstrated the crucial role
of AMP-dependent protein kinase (AMPK) in regulation of autophagy.
Thus, we tested whether AZD8055-induced cell death was associated
with the activation of AMPK in Huh7 cells. Initially, we tested the
effect of AZD8055 on activation of AMPK and found AZD8055 caused
increased phosphorylation of AMPK (Fig.
4A). Next we blocked activation of AMPK by using AMPK inhibitor
dorsomorphin and tested whether co-treatment with dorsomorphin
affected AZD8055-induced cell death and the amount of autophagic
marker LC3-II. As shown in Fig. 4B and
C, blocking activation of AMPK caused significant attenuation
of AZD8055-induced cell death as measured by MTT assay and
expression of LC3-II as measured by western blot analysis.
Discussion
Hepatocellular carcinoma (HCC) is one of the most
common malignant tumors with steadily increasing incidence and
mortality rates in various countries (1). HCC is widely regarded as a
chemotherapy-resistant disease. These drawbacks necessitate the
continued search for novel therapeutic strategies for HCC.
Autophagy is an evolutionarily conserved process in
which cellular organelles and macromolecules are degraded for
recycling of bioenergetic components (17). As an essential mechanism controlling
cellular homeostasis, autophagy is intimately associated with the
regulation of cell survival and cell death. Often a stress or
injury signal could activate both the cell death and autophagy
pathways, in which the role of autophagy could vary depending on
the context. In particular, the role of autophagy in liver cancer
biology/therapy remains unclear. A previous study demonstrated a
dual role of autophagy in cancer therapy (18); autophagy may suppress tumorigenesis
or it may provide cancer cells with a rescue mechanism under
unfavorable conditions.
Autophagy could serve as novel mechanism to kill
cancer cells. Autophagy is also referred to as programmed cell
death type II, as opposed to apoptosis or programmed cell death
type I (19,20). Recently, inducing autophagic cell
death became a novel treatment strategy for cancer therapy. Several
chemotherapeutic agents have been observed for inducing autophagic
cell death in various tumor cells (21,22).
In the present study, we investigated the potential of AZD8055 as a
therapeutic agent in HCC partially through the perspective of
inducing autophagic cell death.
AZD8055 is a specific mammalian target of rapamycin
kinase inhibitor with profound growth inhibitory effects in
cervical (3), lung (4), breast cancer (4), glioblastoma (4) and osteosarcoma (4) cells and antitumor activity in
vivo (4). The effects of
AZD8055 on HCC cells has not been studied. We reported that AZD8055
induced cell death associated with autophagic features in HCC
cells, as demonstrated by increased autophagy-related protein. The
induction of autophagy seems to be indispensable for inhibitory
effects of AZD8055, as demonstrated by the fact that inhibition of
autophagy by 3-MA attenuates AZD8055-induced cell death in Huh7 and
Hep3B cells.
A better understanding of the mechanism underlying
AZD8055-mediated autophagy is critical for the development of novel
therapies. We further investigated the mechanism underlying
AZD8055-mediated autophagy in HCC. AZD8055 is an ATP-competitive
mTOR kinase inhibitor (4). The
inhibitory effect of AZD8055 is closely related to ATP levels
(4). AMPK acts as a major sensor of
cellular energy status in cancer and is critically involved in the
efficiency of anticancer agents (23–27).
We observed that AZD8055 caused AMPK activation in HCC cells, which
has been proved to modulate the induction of autophagy under low
energy conditions by dual regulation of mTORC1 and ULK1 (28,29).
We found that AZD8055 triggered the activation of AMPK and
inhibition of AMPK by dorsomorphin attenuated AZD8055-induced
autophagic cell death. The data presented here indicate the key
role of AMPK activation in AZD8055-induced autophagic cell
death.
In the present study, we investigated the potential
of AZD8055 as a therapeutic agent in HCC. We reported that AZD8055
induces cell death associated with autophagic features and APMK
activation. Our findings provide a better understanding of the
molecular mechanisms underlying the inhibitory effects of AZD8055
and demonstrate the potential of AZD8055 as a therapeutic agent for
HCC.
Acknowledgements
The authors thank Dr Qitao Yan (Southern Medical
University) for his technical assistance. The present study was
supported by grants from the Science and Technology Program of
Guangdong Province (2008B030301028) and the Science and Technology
Innovation Fund of Guangdong Medical College (STIF201107).
References
|
1
|
Sherman M: Epidemiology of hepatocellular
carcinoma. Oncology. 78(Suppl 1): 7–10. 2010. View Article : Google Scholar
|
|
2
|
Tanaka M, Katayama F, Kato H, et al:
Hepatitis B and C virus infection and hepatocellular carcinoma in
China: a review of epidemiology and control measures. J Epidemiol.
21:401–416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li S, Li Y, Hu R, et al: The mTOR
inhibitor AZD8055 inhibits proliferation and glycolysis in cervical
cancer cells. Oncol Lett. 5:717–721. 2013.PubMed/NCBI
|
|
4
|
Chresta CM, Davies BR, Hickson I, et al:
AZD8055 is a potent, selective, and orally bioavailable
ATP-competitive mammalian target of rapamycin kinase inhibitor with
in vitro and in vivo antitumor activity. Cancer Res. 70:288–298.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holt SV, Logie A, Davies BR, et al:
Enhanced apoptosis and tumor growth suppression elicited by
combination of MEK (selumetinib) and mTOR kinase inhibitors
(AZD8055). Cancer Res. 72:1804–1813. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Levine B and Klionsky DJ: Development by
self-digestion: molecular mechanisms and biological functions of
autophagy. Dev Cell. 6:463–477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang S, Yang ZJ, Yu C and Sinicrope FA:
Inhibition of mTOR kinase by AZD8055 can antagonize
chemotherapy-induced cell death through autophagy induction and
down-regulation of p62/sequestosome 1. J Biol Chem.
286:40002–40012. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sini P, James D, Chresta C and Guichard S:
Simultaneous inhibition of mTORC1 and mTORC2 by mTOR kinase
inhibitor AZD8055 induces autophagy and cell death in cancer cells.
Autophagy. 6:553–554. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Willems L, Chapuis N, Puissant A, et al:
The dual mTORC1 and mTORC2 inhibitor AZD8055 has anti-tumor
activity in acute myeloid leukemia. Leukemia. 26:1195–1202. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang HL, Zheng WL, Zhao R, Zhang B and Ma
WL: FBXO31 is down-regulated and may function as a tumor suppressor
in hepatocellular carcinoma. Oncol Rep. 24:715–720. 2010.PubMed/NCBI
|
|
11
|
Strober W: Trypan blue exclusion test of
cell viability. Curr Protoc Immunol Appendix 3: Appendix 3B. 2001.
View Article : Google Scholar
|
|
12
|
Zhao R, Yan Q, Lv J, et al: CHD5, a tumor
suppressor that is epigenetically silenced in lung cancer. Lung
Cancer. 76:324–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aoki H, Kondo Y, Aldape K, et al:
Monitoring autophagy in glioblastoma with antibody against isoform
B of human microtubule-associated protein 1 light chain 3.
Autophagy. 4:467–475. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsushita M, Suzuki NN, Obara K, Fujioka
Y, Ohsumi Y and Inagaki F: Structure of Atg5. Atg16, a complex
essential for autophagy. J Biol Chem. 282:6763–6772. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pyo JO, Jang MH, Kwon YK, et al: Essential
roles of Atg5 and FADD in autophagic cell death: dissection of
autophagic cell death into vacuole formation and cell death. J Biol
Chem. 280:20722–20729. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seglen PO and Gordon PB: 3-Methyladenine:
specific inhibitor of autophagic/lysosomal protein degradation in
isolated rat hepatocytes. Proc Natl Acad Sci USA. 79:1889–1892.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang CW and Klionsky DJ: The molecular
mechanism of autophagy. Mol Med. 9:65–76. 2003.
|
|
18
|
Kondo Y, Kanzawa T, Sawaya R and Kondo S:
The role of autophagy in cancer development and response to
therapy. Nat Rev Cancer. 5:726–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bursch W, Hochegger K, Torok L, Marian B,
Ellinger A and Hermann RS: Autophagic and apoptotic types of
programmed cell death exhibit different fates of cytoskeletal
filaments. J Cell Sci. 113:1189–1198. 2000.PubMed/NCBI
|
|
20
|
Bursch W, Ellinger A, Gerner C, Frohwein U
and Schulte-Hermann R: Programmed cell death (PCD). Apoptosis,
autophagic PCD, or others? Ann NY Acad Sci. 926:1–12. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Le XF, Mao W, Lu Z, Carter BZ and Bast RC
Jr: Dasatinib induces autophagic cell death in human ovarian
cancer. Cancer. 116:4980–4990. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiong HY, Guo XL, Bu XX, et al: Autophagic
cell death induced by 5-FU in Bax or PUMA deficient human colon
cancer cell. Cancer Lett. 288:68–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fruman DA and Edinger AL: Cancer therapy:
staying current with AMPK. Biochem J. 412:e3–e5. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ji C, Yang B, Yang YL, et al: Exogenous
cell-permeable C6 ceramide sensitizes multiple cancer cell lines to
Doxorubicin-induced apoptosis by promoting AMPK activation and
mTORC1 inhibition. Oncogene. 29:6557–6568. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yun SM, Jung JH, Jeong SJ, Sohn EJ, Kim B
and Kim SH: Tanshinone IIA induces autophagic cell death via
activation of AMPK and ERK and inhibition of mTOR and p70 S6K in
KBM-5 leukemia cells. Phytother Res. Jun 27–2013.(Epub ahead of
print). View
Article : Google Scholar
|
|
26
|
Puissant A, Robert G, Fenouille N, et al:
Resveratrol promotes autophagic cell death in chronic myelogenous
leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK
activation. Cancer Res. 70:1042–1052. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chandrasekar B, Boylston WH, Venkatachalam
K, Webster NJ, Prabhu SD and Valente AJ: Adiponectin blocks
interleukin-18-mediated endothelial cell death via APPL1-dependent
AMP-activated protein kinase (AMPK) activation and IKK/NF-κB/PTEN
suppression. J Biol Chem. 283:24889–24898. 2008.PubMed/NCBI
|
|
28
|
Lee JW, Park S, Takahashi Y and Wang HG:
The association of AMPK with ULK1 regulates autophagy. PLoS One.
5:e153942010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Egan DF, Shackelford DB, Mihaylova MM, et
al: Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase
connects energy sensing to mitophagy. Science. 331:456–461. 2011.
View Article : Google Scholar : PubMed/NCBI
|