Introduction
Genistein (4′,5,7-trihydroxyisoflavone), one of the
isoflavones derived from soybeans, exhibits many therapeutic
effects, particularly in anticancer treatment. Several
epidemiological studies have reported that Asian women who consume
more soy products when compared to Western women have a lower risk
of breast cancer. This is based on the fact that breast cancer
incidence in Asian women who have immigrated to Western countries
is similar to that of Western women (1). It has also been shown that genistein
inhibits cancer growth in many types of tumors in vitro and
in vivo. Genistein pre-treatment was found to lead to
increased cancer cell death following cancer chemotherapy by
inhibiting the activity of nuclear factor κB. Genistein was also
found to regulate DNA methylation and histone modification
(2,3). The therapeutic action of genistein in
gastric cancer is through suppression of the cancer cell growth via
the apoptosis cascade (4). It was
also found that genistein inhibits cell proliferation through cell
cycle arrest in a dose-dependent manner (5).
Cancer stem cells are a subpopulation of cancer
cells that have similar characteristics to normal stem cells.
Cancer stem cells initiate new tumors, have self-renewing capacity
and are pluripotent (6). The
identification and management of cancer stem cells are important
issues for targeted therapy in cancer treatment. In gastric cancer,
cancer stem cells have not been well identified, but several
studies have supported the existence of gastric cancer stem cells
(7). CD44, a cell surface
glycoprotein, is commonly used to isolate cancer stem cells in
various types of tumors including gastric cancer (8–11).
Hedgehog (Hh) signaling is essential for embryonic
development and is responsible for maintaining many tissues and
organs. Hh proteins [Sonic Hh (Shh), Indian Hh (Ihh) and Desert Hh
(Dhh)] activate the transmembrane receptor Patched (Ptch). Ptch
inhibits another transmembrane protein, Smoothened (Smo), and in
the absence of Hh ligands, Hh signaling is blocked. Hh inhibits
Ptch by binding to it, and as a result, the transcription factor
Gli is activated (12,13). There are three Gli proteins: Gli1,
Gli2 and Gli3. Gli1 is a strong activator of targets, whereas Gli2
and Gli3 can be repressors or activators depending on the
circumstance (14). Shh signaling
is one of the important signaling pathways that have been
implicated in human carcinogenesis. In addition, the Shh signaling
pathway is reported to be crucial for maintaining the
characteristics of cancer stem cells in gastric cancer (15).
In the present study, we investigated CD44(+)
gastric cancer stem-like cells through the expression of stem
cell-related genes and Shh signaling molecules. To determine the
effect of genistein, we treated CD44(+) gastric cancer stem-like
cells with genistein and analyzed gene expression changes and
sphere-forming ability. Taken together, the present study evaluated
the specific molecular targets by genistein in the Shh signaling
pathway by attenuating gastric cancer stem cell
characteristics.
Materials and methods
Cell culture
AGS (ATCC CRL 1739) and MKN45 (KCLB 80103) gastric
cancer cells were maintained in RPMI-1640 medium (Thermo Fisher
Scientific, Rockford, IL, USA) supplemented with 10% fetal bovine
serum (FBS) (Thermo Fisher Scientific) and 1%
penicillin-streptomycin sulfate (Thermo Fisher Scientific). All
cultures were maintained in a 37°C incubator supplemented with 5%
CO2.
Flow cytometric analysis and
fluorescence-activated cell sorting (FACS)
For cell surface marker analysis by flow cytometry,
~80% confluent cells in a 100-mm cell plate were washed with PBS,
and then cells were detached from the plates using Trypsin-EDTA and
centrifuged at 4°C. Cell pellets were resuspended in HBSS (Gibco,
Grand Island, NY, USA) supplemented with 1 mM HEPES (Gibco) and 2%
FBS and filtered with a 40-μm mesh filter (BD Biosciences, San
Jose, CA, USA). The cells were stained with a 400-fold dilution of
anti-CD44-FITC (BD Biosciences) and incubated for 30 min in a 37°C
incubator supplemented with 5% CO2. The cells were then
washed with HBSS and resuspended in HBSS supplemented with 1 mM
HEPES, 2% FBS and 1% penicillin-streptomycin sulfate. The cells
were analyzed and sorted immediately with FACS Aria III (BD
Biosciences).
Spheroid colony formation assay
FACS-sorted MKN45 cells were plated in each well of
ultra-low-attachment 24-well plates (Corning Life Sciences,
Corning, NY, USA) with DMEM/F12 medium (Thermo Fisher Scientific)
supplemented with 20 ng/ml EGF (R&D Systems, Minneapolis, MN,
USA), 10 ng/ml basic FGF (R&D Systems), 1%
insulin-transferrin-selenium (Gibco) and 1% penicillin-streptomycin
sulfate. To investigate the effect of genistein, we added genistein
to the assay media (10 μg/ml) and DMSO was used as the control.
Every 3 days, each well was examined using a light microscope.
RT-PCR and real-time PCR
Total RNA was extracted from the cultured cells
using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to
the manufacturer’s instructions. First strand cDNA was synthesized
using oligo(dT) primers and Superscript™ II reverse transcriptase
(Invitrogen). PCR was carried out with a PCR Maxi kit (Intron,
Sungnam, Korea) according to the manufacturer’s instructions.
Amplification conditions included denaturation at 95°C for 15 min,
followed by 30 cycles of 30 sec each at 95, 55 and 72°C. PCR
products were separated on 2% agarose gels. Real-time PCR was
carried out with a PCR mixture containing 1 μmol/l of each primer
and SYBR-Green Master Mix (Applied Biosystems, Foster City, CA,
USA). The amplifications were conducted at 95°C for 10 sec and 60°C
for 60 sec using a StepOnePlus™ real-time PCR system (Applied
Biosystems). Each sample was examined in triplicate, and the amount
of PCR product was normalized with respect to β-actin as an
internal control. PCR primers are shown in Table I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Direction | Sequence (5′-3′) |
|---|
| CD44 | F |
GCTATTGAAAGCCTTGCAGAG |
| R |
CGCAGATCGATTTGAATATAACC |
| Oct4 | F |
GACAACAATGAAAATCTTCAGGAGA |
| R |
TTCTGGCGCCGGTTACAGAACCA |
| Bmi | F |
ATGTGTGTGCTTTGTGGAG |
| R |
AGTGGTCTGGTCTTGTGAAC |
| Nestin | F |
AACAGCGACGGAGGTCTCTA |
| R |
TTCTCTTGTCCCGCAGACTT |
| ABCG2 | F |
CTGAGATCCTGAGCCTTTGG |
| R |
TGCCCATCACAACATCATCT |
| Shh | F |
GCAGAGTAGCCCTAACCGCT |
| R |
CAGGAGCCAGGTGCCTATTT |
| Ptch | F |
CTCCCTACAGCAGCCACAGC |
| R |
AGGTCCCTTGTGGAGCRGGT |
| Gli1 | F |
ACCCAGCCACCCCCTGATTA |
| R |
CCCCACCCTCCCACAGTAGC |
| β-actin | F |
TTGCCGACAGGATGCAGAAG |
| R |
AGGTGGACAGCGAGGCCAGG |
Western blot analysis
Prepared cells were harvested after washing with
PBS. Collected cells were lysed with buffer [50 mM Tris-Cl (pH
7.5), 150 mM NaCl, 1 mM EDTA (pH 8.0), 1% Triton X-100, 1 mM PMSF,
1 mM Na3VO4 and protease inhibitor cocktail
(Roche Molecular Biochemicals, Indianapolis, IN, USA)]. The same
amount of protein was boiled at 95°C after adding SDS sample buffer
[62.5 mM Tris-Cl (pH 6.8), 2% SDS, 10% glycerol, β-mercaptoethanol,
and 0.002% bromophenol blue]. Samples were loaded on 12% SDS-PAGE
gels for Shh, Bmi and Oct4; 8% SDS-PAGE gels for Ptch, Gli1, CD44,
Nestin and ABCG2. Samples were transferred to PVDF membranes
(Amersham Bioscience, Piscataway, NJ, USA).
Antibodies used included: Shh (1:1,000; Santa Cruz
Biotechnology), Ptch1 (1:1,000; Abcam), Gli1 (1:1,000; Abcam), CD44
(1:1,000; Abcam), Oct4 (1:1,000; Santa Cruz Biotechnology), Bmi
(1:1,000; Abcam), Nestin (1:1,000; Abcam), ABCG2 (1:1,000; Santa
Cruz Biotechnology) and β-actin (1:2,000; Santa Cruz
Biotechnology).
Immunofluorescence assay
Cells were grown on glass coverslips in a 6-well
plate. After overnight incubation, the cells were washed with PBS
three times, fixed in 10% formaldehyde for 10 min and permeabilized
in 0.1% Triton X-100 in PBS for 3 min. The cells were washed three
times with PBS and blocked with 1% BSA in PBS buffer. The cells
were incubated with the primary antibodies overnight at 4°C. The
slides were washed with PBS and incubated with FITC-conjugated
secondary antibody for 1 h at room temperature. The slides were
mounted with DAPI. All samples were photographed using a Zeiss LSM
700 confocal microscope (Carl Zeiss, Oberkochen, Germany).
siRNA transfection
MKN45 cells were seeded onto 6-well plates and
incubated overnight. The cells were transfected with siGli and
scramble siRNA using Lipofectamine 2000 (Invitrogen) according to
the manufacturer’s instructions. After 48 h, the cells were
harvested and RNA and protein were isolated. siGli1 and scramble
siRNA sequences are shown in Table
II.
 | Table IIGli siRNA sequence. |
Table II
Gli siRNA sequence.
| Gene | Sequence |
|---|
| Scramble siRNA |
CGUACGCGGAAUACAACGA |
| Gli1siRNA |
CUCCACAGGCAUACAGGAU |
Invasion assay
Transwells (24-well, 8.0 μm) (BD Biosciences) were
coated with diluted Matrigel (BD Biosciences) in PBS and dried for
6 h. The cells were resuspended in serum-free media, and then
4×105 cells were seeded in each top chamber. The lower
chambers were filled with 10% FBS complete media for
chemoattraction. To identify the effect of genistein, we added 10
μg/ml genistein and control DMSO to the top chambers. Cells were
also transfected with siGli1 and scramble siRNA for Gli1 knockdown.
After 24 h, the cells were seeded into the inserts. Cell invasion
chambers were incubated for 48 h in a 37°C incubator supplemented
with 5% CO2. Non-invaded cells on the upper surface were
removed by a cotton swap, and the cells on the lower surface were
fixed and stained using the Diff-Quik kit according to the
manufacturer’s instructions. The number of migrated cells on each
insert was counted under a light microscope. Each sample was
examined in triplicate.
Results
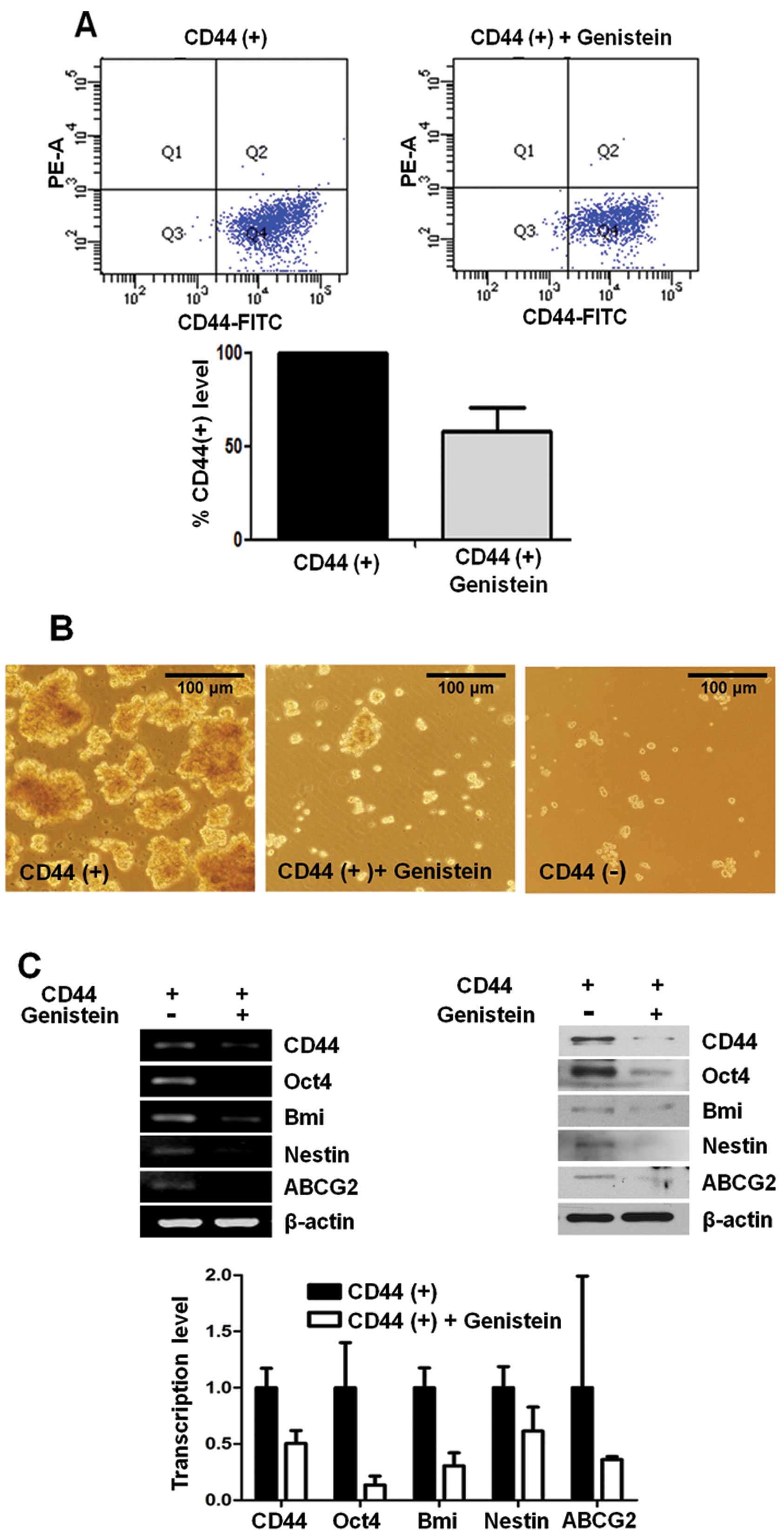
Genistein inhibits CD44 expression and
other cancer stem cell markers
CD44 has been used as a cancer stem cell marker in
many types of tumor cells including gastric cancer. To investigate
the effect of genistein on the CD44(+) stem-like cell population,
we incubated CD44(+) cells with 10 μg/ml genistein or DMSO as a
control. After 72 h, CD44 expression was analyzed by flow
cytometry. CD44(+) cells decreased ~40% in the genistein-treated
CD44(+) cells when compared with the DMSO-treated CD44(+) control
cells (Fig. 1A).
Formation of sphere colonies in serum-free media is
one of the hallmarks of cancer stem cells. CD44(+) cells formed
more spheres than CD44(−) cells in a time-dependent manner, and
genistein significantly reduced the ability of sphere formation by
CD44(+) gastric cancer cells (Fig.
1B).
Expression of cancer stem cell-related genes, Oct4,
Bmi, Nestin and ABCG2, was determined at the mRNA level by RT-PCR
and real-time PCR. We also examined the protein expression levels
of several stemness-related genes, Oct4, Bmi, Nestin and ABCG2,
using western blot analysis in CD44(+) and CD44(−) cells. We found
that the expression of these genes was significantly decreased in
the genistein-treated CD44(+) cells when compared to these levels
in the control CD44(+) cells at the mRNA and protein levels
(Fig. 1C).
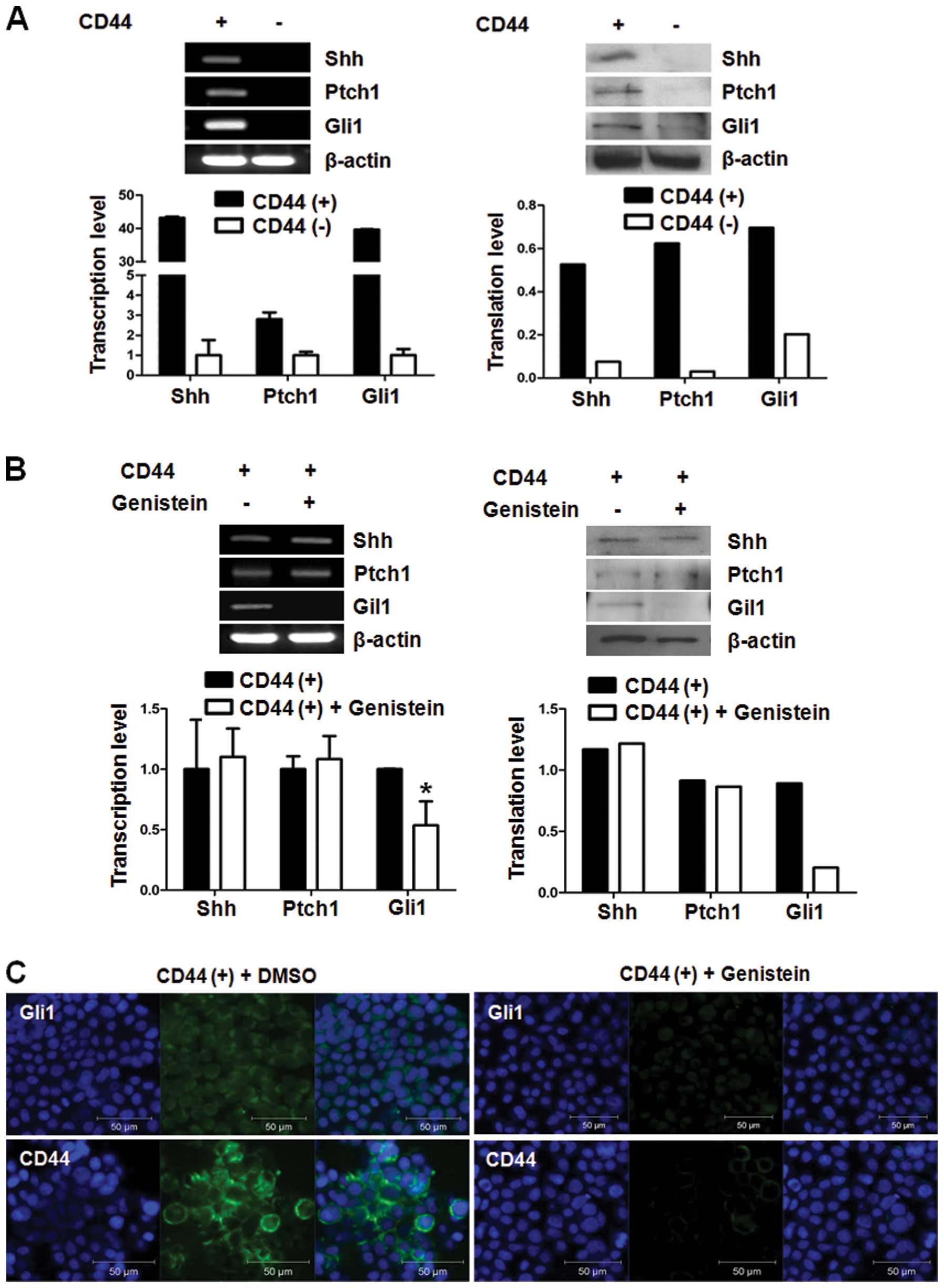
Genistein suppresses the Shh signaling
molecule, Gli1
The Shh signaling pathway has been reported to be
involved in cancer stem cell properties. Therefore, we performed
RT-PCR and real-time PCR to identify the change in Shh signaling
pathways and to validate the effect of Shh signaling on cancer
stemness of CD44(+) cells at the mRNA level. CD44(+) cells showed
upregulated expression of Shh, Ptch and Gli1 mRNA when compared to
the expression in the CD44(−) cells. In addition, the protein
levels of the Shh signaling genes were also overexpressed in the
CD44(+) cells when compared to the protein levels in the CD44(−)
cells (Fig. 2A).
We treated CD44(+) cells with genistein to confirm
its inhibitory action on the Shh-Gli1 signaling pathway. We found
that genistein downregulated only Gli1 at the mRNA level. Gli1 was
also downregulated at the protein level (Fig. 2B). Based on these results, genistein
affects the Shh signaling pathway through the inhibition of Gli1
expression in CD44(+) cells.
Gli1 and CD44 overexpression was confirmed by
immunofluorescence assay. The results showed that the number of
Gli1 and CD44 overexpressing cells in the CD44(+) cells were
reduced in the genistein-treated CD44(+) cells (Fig. 2C).
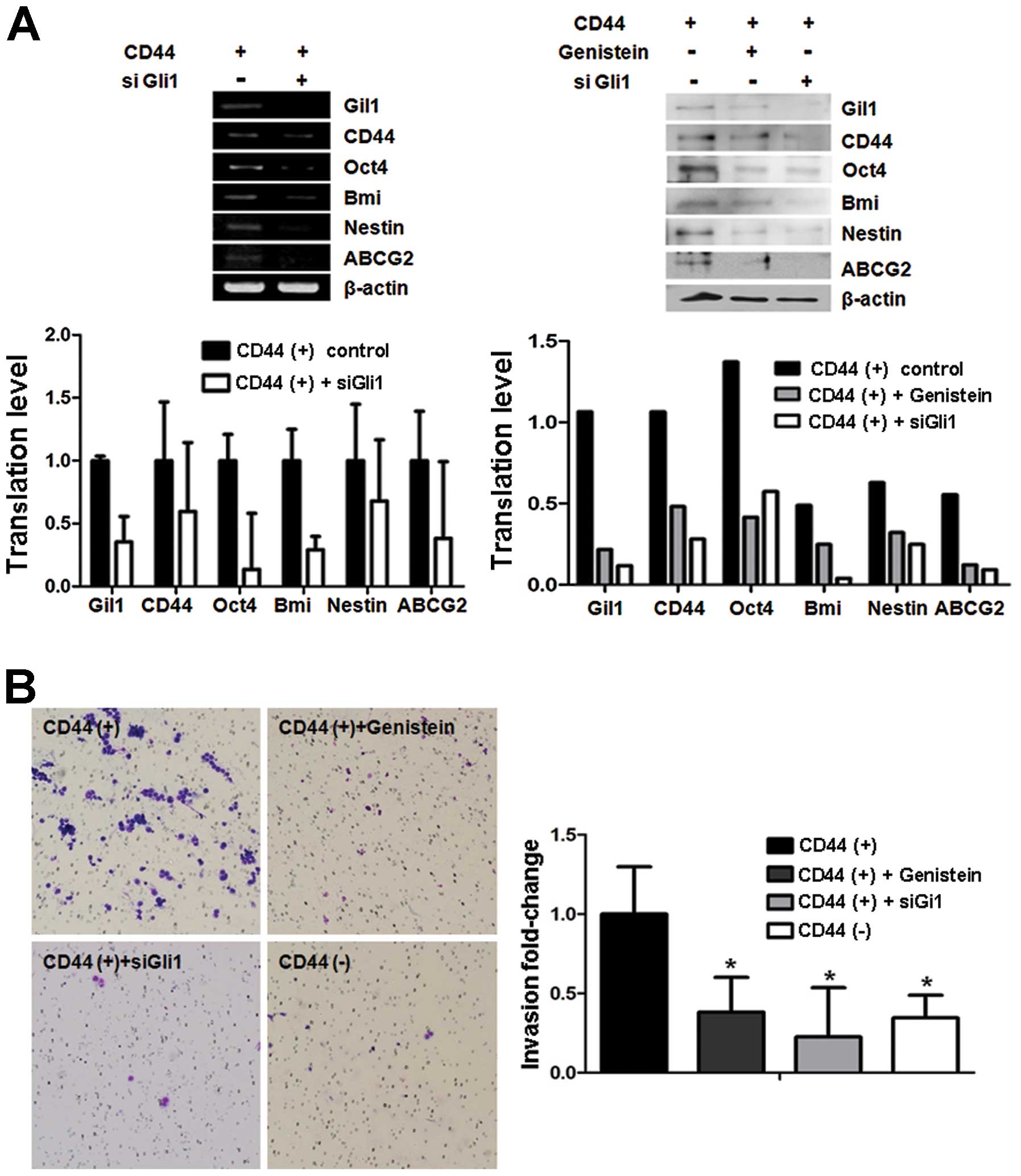
Gli1-knockdown cells exhibit similar
characteristics as genistein-treated cells and genistein inhibits
the migration capacity of CD44(+) stem-like gastric cancer cells in
vitro
We transfected Gli1 siRNA into CD44(+) cells to
confirm the inhibitory effect of genistein on Gli1 expression in
gastric cancer cells. CD44(+) cells were transfected with either
scramble siRNA or Gli1 siRNA, and RNA and protein were isolated
from cancer cells after 48 h. The expression of cancer stem cell
markers including CD44, Oct4, Bmi, Nestin and ABCG2 was assessed
using real-time PCR and western blot analysis. The results showed
that mRNA of cancer stem cell markers was decreased in the
Gli1-downregulated CD44(+) cells by Gli1 siRNA pre-treatment.
Following Gli1 knockdown, the changes in the protein expression
levels of these markers in CD44(+) cells were validated by western
blot analysis (Fig. 3A).
Migration and invasion properties are important
characteristics of cancer stem cells responsible for tumor
metastasis and growth. Cancer stem cells are assumed to have higher
migration capacity than normal cancer cells. In addition, genistein
at dietary concentrations can inhibit cancer invasion in
vitro and in vivo (16).
Genistein treatment reduced the invasive capacity of CD44(+) cells,
and siGli1 treatment showed a comparable effect in CD44(+) cells.
The number of CD44(+) cells treated with siGli1 that had migrated
was nearly 50% of the number of migratory CD44(+) cells that were
untreated, and the results were similar following genistein
pre-treatment (Fig. 3B).
Discussion
In the present study, we investigated the
feasibility of genistein as an anticancer molecule in gastric
cancer, particularly for targeting cancer stem cells. The present
study was based on previous research that genistein induces
suppression of the Hh signaling pathway (17,18).
We focused on the Shh-Gli1 pathway among many targets of genistein
since Shh signaling is related to many of the characteristics noted
in cancer stem cells. Cancer stem cells are considered to be a new
target of cancer therapy as they have the potential to form new
tumors and are difficult to kill due to their multidrug resistance
potential (9). Therefore,
inhibition of Gli1 to suppress cancer stemness would eliminate
tumor cells completely. A previous report indicated that regulation
of Hh-Gli1 signaling is a possible candidate for cancer therapy
(19).
In the present study, cancer stem-like cells were
identified in gastric cancer cell lines, AGS and MKN45, using CD44.
CD44(+) cells exhibited upregulation of several cancer stem cell
markers (data not shown) as well as the Shh-Gli1 signaling pathway
and displayed high sphere colony forming ability. We found that
cancer stem-like cells were enriched in the CD44(+) fraction when
compared to the CD44(−) counterpart.
Next, we chose the appropriate concentration of
genistein based on a cell proliferation assay and cell cytotoxicity
assay to exclude effects through cell death and apoptotic damage
(data not shown). After genistein treatment, Gli1 and CD44
expression was significantly decreased, and genistein affected the
expression of cancer stem cell-related genes at both the
transcriptional and translational levels. Moreover, our
observations demonstrated that the Shh-Gli1 pathway is implicated
in cancer stem cell properties. Gli1 knockdown in CD44(+) cells led
to a reduction in the expression of stem cell-related genes,
similar to that observed following genistein treatment. Cell
invasion ability is highly maintained in cancer stem cells and this
is strongly implicated in cancer growth and metastasis (20). Another important finding of our
study was the effect of genistein on cell mobility. It has been
demonstrated that genistein affects epithelial-mesenchymal
transition (EMT) and cell invasion (21). We confirmed that genistein is an
inhibitory agent for cancer invasion and found that Gli1
downregulation also affects the migratory capacity of cancer stem
cells.
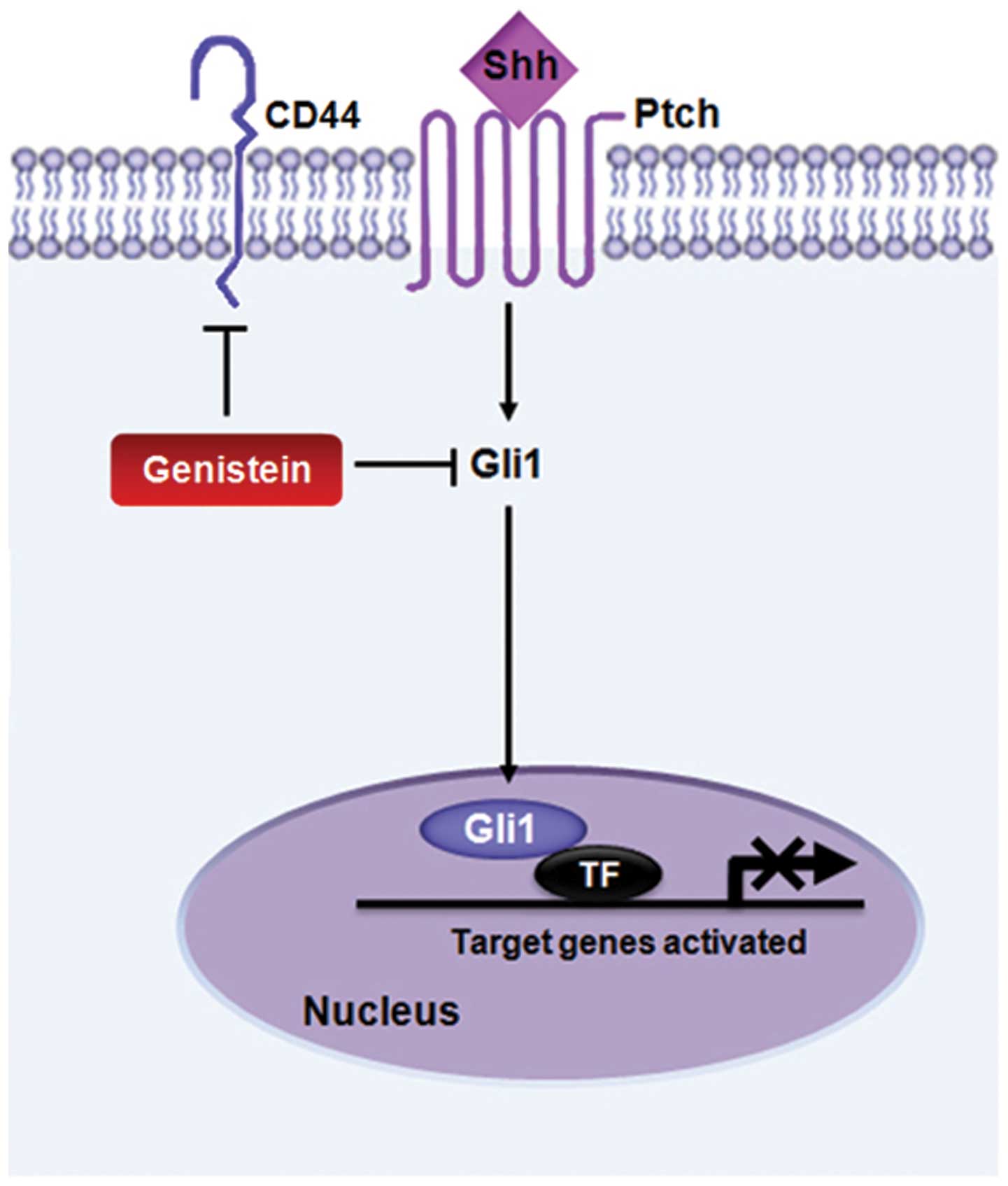
In summary, these data indicate that the soy
isoflavone genistein controls Shh-Gli1 signaling and CD44
expression, and that it attenuates cancer stem cell properties
(Fig. 4). We propose that genistein
treatment may not only lead to cancer prevention, but that
genistein itself could be used as an effective cancer therapy by
modulating cancer stem cell characteristics.
Acknowledgements
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education, Science and Technology
(grant no. NRF-2013R1A1A2009707 and NRF-2010-0024601).
References
|
1
|
Taylor CK, Levy RM, Elliott JC and Burnett
BP: The effect of genistein aglycone on cancer and cancer risk: a
review of in vitro, preclinical, and clinical studies. Nutr Rev.
67:398–415. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y, Li Q and Chen H: DNA methylation
and histone modifications of Wnt genes by genistein during colon
cancer development. Carcinogenesis. 34:1756–1763. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Y, Ahmed F, Ali S, Philip PA, Kucuk O
and Sarkar FH: Inactivation of nuclear factor κB by soy isoflavone
genistein contributes to increased apoptosis induced by
chemotherapeutic agents in human cancer cells. Cancer Res.
65:6934–6942. 2005.
|
|
4
|
Yanagihara K, Ito A, Toge T and Numoto M:
Antiproliferative effects of isoflavones on human cancer cell lines
established from the gastrointestinal tract. Cancer Res.
53:5815–5821. 1993.PubMed/NCBI
|
|
5
|
Matsukawa Y, Marui N, Sakai T, et al:
Genistein arrests cell cycle progression at G2-M. Cancer Res.
53:1328–1331. 1993.PubMed/NCBI
|
|
6
|
Guo W, Lasky JL and Wu H: Cancer stem
cells. Pediatr Res. 59:59R–64R. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takaishi S, Okumura T and Wang TC: Gastric
cancer stem cells. J Clin Oncol. 26:2876–2882. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rocco A, Compare D and Nardone G: Cancer
stem cell hypothesis and gastric carcinogenesis: experimental
evidence and unsolved questions. World J Gastrointest Oncol.
4:54–59. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Du L, Wang H, He L, et al: CD44 is of
functional importance for colorectal cancer stem cells. Clin Cancer
Res. 14:6751–6760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang C, Li C, He F, Cai Y and Yang H:
Identification of CD44+ CD24+ gastric cancer
stem cells. J Cancer Res Clin Oncol. 137:1679–1686. 2011.PubMed/NCBI
|
|
11
|
Takaishi S, Okumura T, Tu S, et al:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Akiyoshi T, Nakamura M, Koga K, et al:
Gli1, downregulated in colorectal cancers, inhibits proliferation
of colon cancer cells involving Wnt signalling activation. Gut.
55:991–999. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Douard R, Moutereau S, Pernet P, et al:
Sonic Hedgehog-dependent proliferation in a series of patients with
colorectal cancer. Surgery. 139:665–670. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yanai K, Nagai S, Wada J, et al: Hedgehog
signaling pathway is a possible therapeutic target for gastric
cancer. J Surg Oncol. 95:55–62. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song Z, Yue W, Wei B, et al: Sonic
hedgehog pathway is essential for maintenance of cancer stem-like
cells in human gastric cancer. PLoS One. 6:e176872011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pavese JM, Farmer RL and Bergan RC:
Inhibition of cancer cell invasion and metastasis by genistein.
Cancer Metastasis Rev. 29:465–482. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Œlusarz A, Shenouda NS, Sakla MS, et al:
Common botanical compounds inhibit the hedgehog signaling pathway
in prostate cancer. Cancer Res. 70:3382–3390. 2010.PubMed/NCBI
|
|
18
|
Zhang L, Li L, Jiao M, et al: Genistein
inhibits the stemness properties of prostate cancer cells through
targeting Hedgehog-Gli1 pathway. Cancer Lett. 23:48–57. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Clement V, Sanchez P, de Tribolet N,
Radovanovic I and Ruiz i Altaba A: HEDGEHOG-GLI1 signaling
regulates human glioma growth, cancer stem cell self-renewal and
tumorigenicity. Curr Biol. 17:165–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: an emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang LL, Li L, Wu DP, et al: A novel
anti-cancer effect of genistein: reversal of epithelial mesenchymal
transition in prostate cancer cells. Acta Pharmacol Sin.
29:1060–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|