Introduction
The sterile alpha motif domain and HD
domain-containing protein 1 (SAMHD1) spans 59,523 bp of genomic
sequence in 16 exons and encodes a 626-amino acid protein. It is
related to type 1 interferon and mediates a TNF-α-related
proinflammatory response in a series of autoimmune diseases, such
as Aicardi-Goutières syndrome (AGS) (1–3) and
systemic lupus erythematosus (SLE) (4). Recent reports have shown that SAMHD1
might function as a Vpx-sensitive retroviral restriction factor
that inhibits HIV-1 infection in macrophages and dendritic cells
through its intrinsic dGTP-dependent deoxynucleoside triphosphate
(dNTP) hydrolase activity (5–7).
Therefore, SAMHD1 might mediate its restriction activity by
reducing the pool of dNTPs in virus-infected cells. This
characteristic feature of SAMHD1 indicates that it might operate
similarly in other diseases related to viral infections, such as
nasopharyngeal carcinoma caused by Epstein-Barr virus (EB),
cervical cancer caused by human papilloma virus (HPV) and liver
cancer caused by hepatitis B/C virus (HBV/HCV).
HBV infection is a major etiologic factor in the
pathogenesis of hepatocellular carcinoma (HCC) (8,9).
Approximately 170 million individuals are chronically infected by
HCV, and some of them develop progressive liver disease leading to
cirrhosis and HCC (10–12). Despite identification of the main
factors resulting in virus-mediated HCC, the roles of SAMHD1 in
this disease are still unclear, and therefore we undertook a study
of SAMHD1 in 44 patients with HCC and 10 healthy controls (HC).
Although it has been reported that a number of SNPs in SAMHD1 are
related to AGS syndrome or SLE, these mutations were found to occur
at low frequencies in both groups (data not shown). In contrast,
several splice variants of SAMHD1 exhibited high frequencies,
including a novel insertion of exon4, deletions of exon8-9, 13, and
14 (13,14). In the present study, we investigated
the frequency of SAMHD1 splice variants, and compared their
different activities in reversing sensitivity to chemotherapeutic
drugs. Our results suggest that the exon4 insertion might act as an
indicator of hepatocarcinogenesis.
Materials and methods
Patients
Forty-four patients diagnosed with HCC by both
surgical procedures and pathology participated in the present study
in The First Hospital of Jilin University from 2009 to 2011. Since
SAMHD1 is closely associated with the immune system, patients were
selected with no previous history of autoimmune diseases to exclude
from our results confounding contributions from other factors. The
patients were divided into three groups: the HBV-infected group,
the HCV-infected group, and the non-virus-infected group. Each
patient in the virus-induced groups had been infected with the
corresponding type of virus for more than seven years.
The 10 healthy controls (HC) were also recruited at
The First Hospital of Jilin University. Each individual had
undergone a medical examination during the period 2010–2011, and
all were free from any autoimmune diseases or cancer. All
participants in this study provided a written informed consent.
Tissues and blood treatment
Forty-four liver tissue samples from the
corresponding cancer patients were resected from the center of the
tumor block (20–30 mg in weight and 3–8 mm in size). Following
ice-cold PBS washes, the tumor tissues were split into small pieces
for RNA isolation and protein extraction for further immunoblot
analyses. All RNA and protein samples were kept in a refrigerator
at −80°C.
Five ml of venous blood from each patient was
collected and used to extract peripheral blood mononuclear cells
(PBMCs). The same procedure was applied to the 10 HC.
Cell cultures and transfections
The human liver carcinoma cell line HepG2, obtained
from the American Type Culture Collection (ATCC), was cultured in
RPMI-1640 containing 10% v/v fetal bovine serum (FBS), and
cultivated at 37°C in a 5% CO2 incubator. One day before
plasmid transfections, cells (3×106) were seeded onto
6-well plates (Iwaki Cell Biology, Tokyo, Japan) at 80% confluency.
Following incubation for 24 h, cells were prepared for transient
transfection with 2 μg of plasmid DNA/plate, using
Lipofectamine® 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA), according to the manufacturer's instructions.
After this step, cells were incubated in serum- and antibiotic-free
culture medium for 6 h, and then cultured with complete medium.
Cells were collected at 48 h following transfection.
Plasmid construction
Human SAMHD1 cDNA (Genbank: NM_015474) was amplified
from tissues of the HCC patients by PCR using the WT primer, as
shown in Table I. PCR products were
then purified and cloned into a pGEM-T vector (Promega Corporation,
Madison, WI, USA), and the target monoclones were selected through
blue-white colony screenings. The following splice variants of
SAMHD1 were identified with sequencing: wild-type (WT), exon4
insertion (ins4), exon8-9 deletion (del8-9), exon13 deletion
(del13) and exon14 deletion (del14). The sequence of each variant
was excised from the pGEM-T vector by ApaI and NotI
(Takara Bio Inc., Shiga, Japan), and inserted into the plasmid
pcDNA3.1(−)/Myc-His version A (Invitrogen Life Technologies) to
construct pcDNA-His-SAMHD1 WT, ins4, del8-9, del13 and del14,
respectively. Stop codons were excluded from the reverse primer of
WT SAMHD1 to allow using the His-tag of pcDNA. To exclude the
influence of endogenous SAMHD1 on the results, the Vpx gene was
amplified from SIVmac239 (using primers listed in Table I) to induce intracellular SAMHD1
degradation. The Vpx start/stop codons were included in the
primers, and XbaI and ApaI restriction sites were
also created for subcloning into pCMV-N-His (Beyotime Institute of
Biotechnology Co., Ltd., Shanghai, China). The resulting vector is
referred to as pCMV-His-Vpx.
 | Table IPrimers designed for vector
construction and validation of mutations. |
Table I
Primers designed for vector
construction and validation of mutations.
| Amplicon | Primer sequence | Length (bp) |
|---|
| WT | F:
5′-ATGCAGCGAGCCGAT-3′
R: 5′-CATTGGGTCATCTTTAA-3′ | 1,869 |
| Vpx | F:
5′-TCTAGAGCATGTCAGATCCCAGGGAGAGAAT-3′
R: 5′-GGGCCCGCTTATGCTAGTCCTGGAGGGGG-3′ | 339 |
| ins4 | F:
5′-GATACAATGAAGGAAGAAGTAAAAT-3′
R: 5′-CGGGCGAGCAAGTGG-3′ | 408 |
| del8-9 | F:
5′-ACCACTTGAATCACCTGTCG-3′
R: 5′-TAACCTGCGGCTTGGTG-3′ | 719 |
| del13 | F:
5′-GGGTTATCAACATGGATTATGG-3′
R: 5′-GAGTTGGATTTTGGACTGAAG-3′ | 315 |
| del14 | F:
5′-CCAGGGACTGCCATCATC-3′
R: 5′-GCAGAAGTTGTGAAACATCCA-3′ | 570 |
| ins4 genomic | F:
5′-CACTTGAGGTCAGGAGTTCGC-3′
R: 5′-CGATTGTGTGAAGCTCCTGGA-3′ | 763 |
| del8-9 genomic | F:
5′-TTGGTGCCTATCCTAAAACTTCC-3′
R: 5′-AAACCCCAAAACCAGTGTCTAAC-3′ | 598 |
| del13 genomic | F:
5′-TCAGGCTGGTCTCGAACTCC-3′
R 5′-ACTTTTTCCTCTGTGCTTGTATGC-3′ | 672 |
| del14 genomic | F:
5′-GTTCCATTTCTTGTTATGCTCCTAC-3′
R: 5′-GCTGATCTTAAGCTCTTCGTCTC-3′ | 390 |
RNA/DNA extractions and RT-PCR
The HepG2 cells were collected after transfection,
and total RNA was isolated using TRIzol (Invitrogen Life
Technologies) according to the manufacturer's instructions. RNA
pellets were ethanol-precipitated, washed, and resuspended in
sterile ribonuclease-free water. Reverse transcription was carried
out using the Superscript II® enzyme (Gibco-BRL,
Carlsbad, CA, USA). The presence of SAMHD1 mutants was checked
using the primers shown in Table
I.
In order to verify whether the mutations occurred at
the level of the genome, we prepared DNA samples using a Universal
Genomic DNA Extraction kit (Takara Bio Inc.) and designed primers
(Table I) based on the
SAMHD1 genomic sequence (Genbank: NG_017059). PrimeSTAR HS
DNA Polymerase (Takara Bio Inc.) was used in the subsequent PCR
reactions, performed following the manufacturer's instructions.
Western blot analysis
Cells were harvested 48 h after transfection, and
lysed in RIPA buffer with protease inhibitors (Roche Diagnostics
KK, Basel, Switzerland, USA). Following incubation for 15 min on
ice, cells were centrifuged for 10 min at 12,000 × g at 4°C. The
proteins were incubated for 3 min at 100°C, and then separated on a
10% SDS-PAGE gel before transferring onto a polyvinylidene
difluoride (PVDF) membrane (GE Healthcare). Anti-His monoclonal
antibody (BioVision Inc., Mountain View, CA, USA) and anti-GAPDH
monoclonal antibody (LifeTein LLC, South Plainfield, NJ, USA) were
used for western blot analysis. The secondary antibody used was
horseradish peroxidase (Santa Cruz Biotechnology Inc., Santa Cruz,
CA, USA) and it was visualized using enhanced chemiluminescence
(ECL) (Thermo Fisher Scientific, Jan Jose, CA, USA).
Cell cycle analysis
Following transfections, cells were collected by
trypsinization, washed twice with PBS before fixation in 75%
pre-cooled ethanol, and kept at 4°C overnight. Following RNaseA (1
μg/ml) treatment for 30 min at 37°C, cells were re-suspended with
propidium iodide (50 μg/ml), and incubated on ice in the dark for
30 min. The cell cycle distribution was determined by FACSCalibur
flow cytometry (BD Biosciences, San Jose, CA, USA) and analyzed
using Multicycle software.
Statistical analyses
All the experiments in this study were independently
repeated for at least three times. Statistical significance of
comparisons between treatments was evaluated using a one-way ANOVA
followed by a Tukey's test. A p-value (P) <0.05 was considered
to indicate a statistically significant difference.
Results
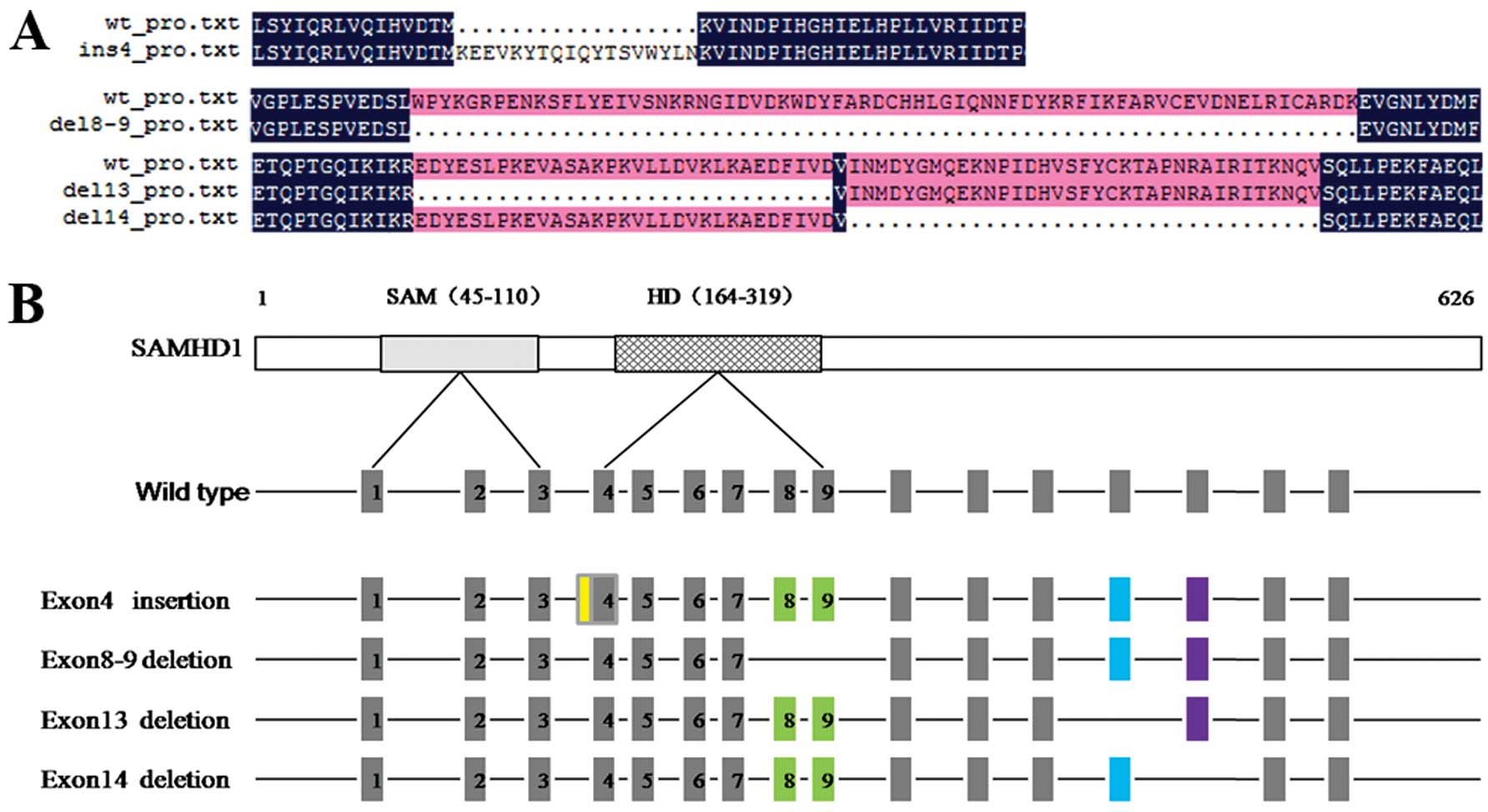
Natural SAMHD1 splice variants in
HCC
Deletions of exon8-9 and 14 have been identified in
a variety of cell types, including 293T, THP-1 and primary human
CD4+ T cells. It is very likely that the deleted
variants are naturally expressed along with the full-length SAMHD1
(13). In order to determine
whether these variants also occurr in liver cancer, 44 tissues from
patients with HCC were tested. We identified, by sequencing, a
natural insertion in exon4 and deletions in exons8-9, 13 and 14
(Fig. 1).
Frequency of splice variants in patients
and controls
Among the identified splice variants, deletions of
exon8-9, 13 and 14 existed naturally in both the HCC and the
controls, but the insertion of exon4, which contained 62 bp,
occurred more frequently in the HCC and was seldom found in the HC.
As shown in Table II, the
frequency of exon4 insertion was 36.4% and 30% in the
HBV/HCV-infected groups respectively, while it was 25% in the
non-virus-infected group and only 10% in the HC. The high frequency
of the insertion in the virus-infected groups compared to the
non-virus-infected and HC groups was statistically significant
(P<0.01), while the frequencies of the deletions showed no
difference in these groups (data not shown). These results suggest
that the exon4 insertion might correlate with the occurrence of
virus-infected HCC.
 | Table IIClinical data of liver cancer patients
and healthy controls. |
Table II
Clinical data of liver cancer patients
and healthy controls.
| Parameters | HBV-infected patients
(n=22) | HCV-infected patients
(n=10) | Non-virus-infected
patients (n=12) | HC (n=10) |
|---|
| Mean age (range),
years | 54 (37–68) | 60 (55–66) | 62 (44–66) | 35 (28–42) |
| Gender
(male/female) | 16/6 | 7/3 | 8/4 | 7/3 |
| Mean AFP (range),
ng/ml | 70.64
(1.3–3,430) | 21.57
(11.14–325.4) | 8.29
(1.74–121.3) | 8.21 (1.8–10.21) |
| Mean AST (range),
U/l | 40 (13–75) | 53.8 (21–68) | 32 (15–38) | 18 (15–23) |
| Mean ALT (range),
U/l | 39 (12–65) | 41 (24–94) | 21 (9–50) | 20 (14–28) |
| ins4 frequency | 36.4% (8/22)a,b | 30% (3/10)a,b | 25% (3/12)a | 10% (1/10) |
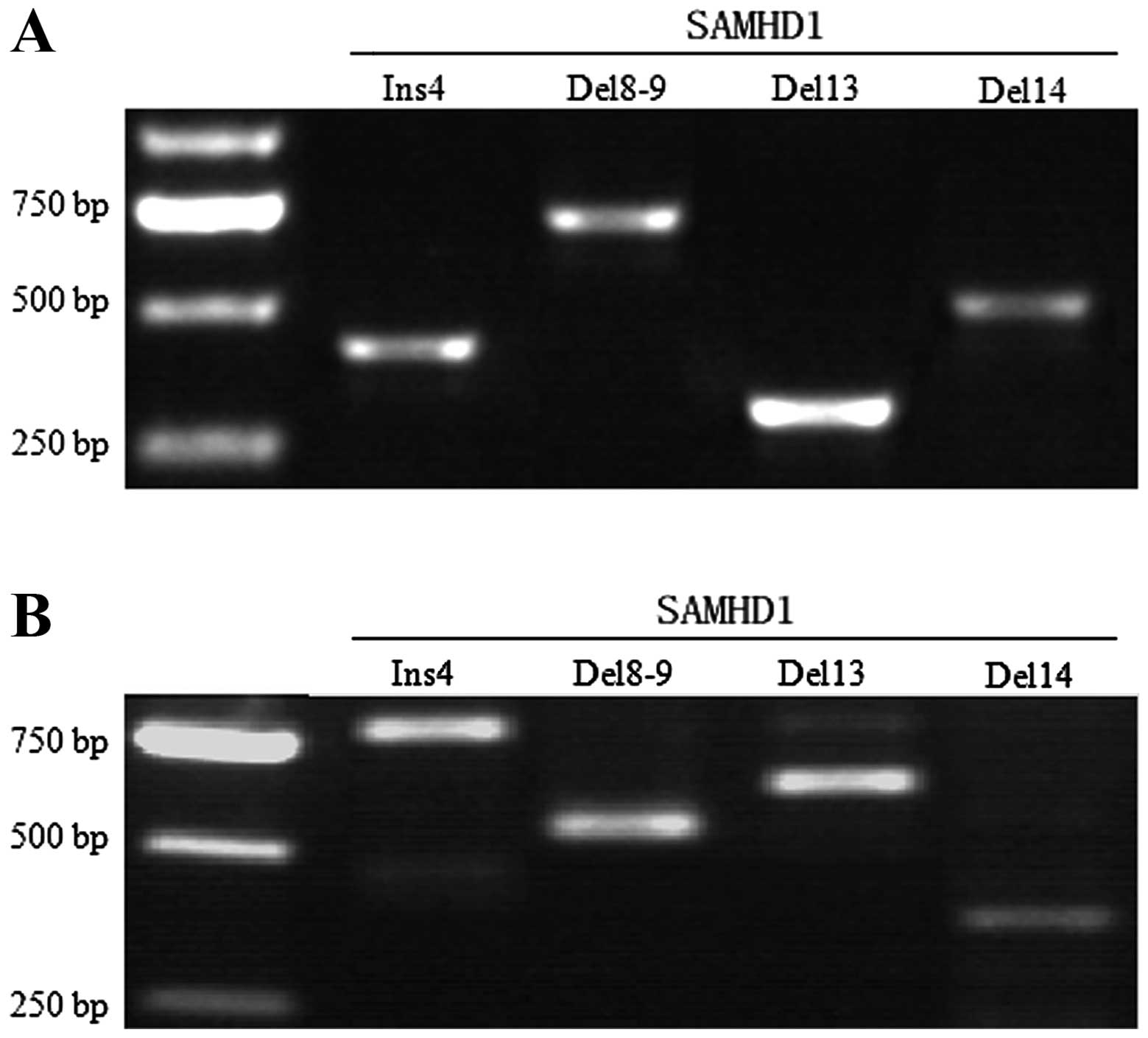
Confirmation of the expression profile of
SAMHD1 splice variants
In order to investigate whether the natural variants
also occur at the genomic level, we used the genomic DNAs extracted
from the positive samples to test the expression of the
corresponding mutations. As shown in Fig. 2, the above mutations occurred at the
transcriptional level, but not at the genomic level.
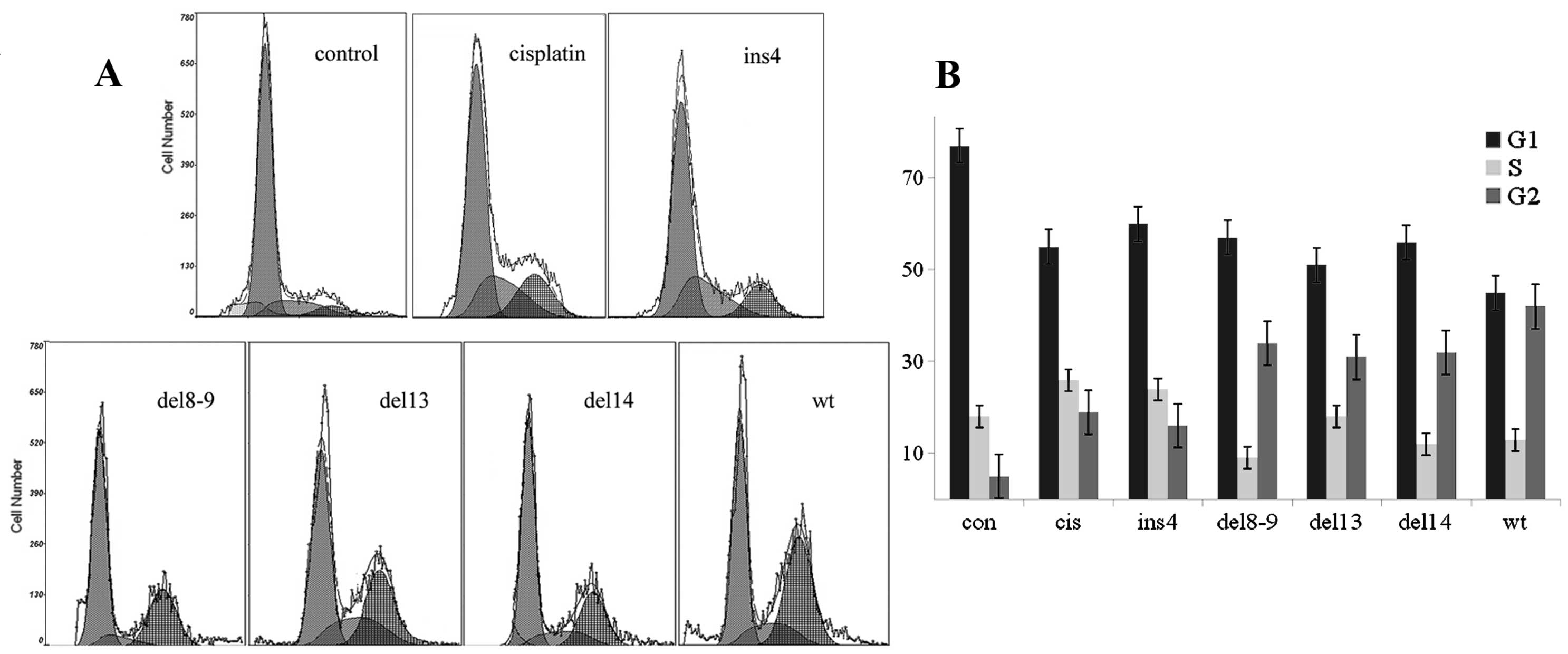
Investigation of the different roles of
SAMHD1 splice variants in regulating cell sensitivity to
cisplatin
To investigate the effect of splice variants in HCC,
we selected pcDNA3.1(−)/Myc-His to construct plasmids of the
different SAMHD1 mutants (pcDNA-His-SAMHD1 WT, ins4, del8-9, del13
and del14) and used pCMV-N-His to construct a pCMV-His-Vpx vector.
Next, we co-transfected HepG2 with pCMV-His-Vpx and vectors of the
SAMHD1 mutants, respectively, as shown in Fig. 3. Following an 18-h transfection with
1 μM cisplatin (15), we examined
the effects of SAMHD1 splice variants in regulating cell
sensitivity to cisplatin. The results indicated that the WT SAMHD1
had the strongest ability to increase cell sensitivity to the drug,
while the ability of del8-9, del13 and del14 was relatively weaker.
As shown in Fig. 4, there was no
obvious difference between the ins4 and the
single-cisplatin-treated group.
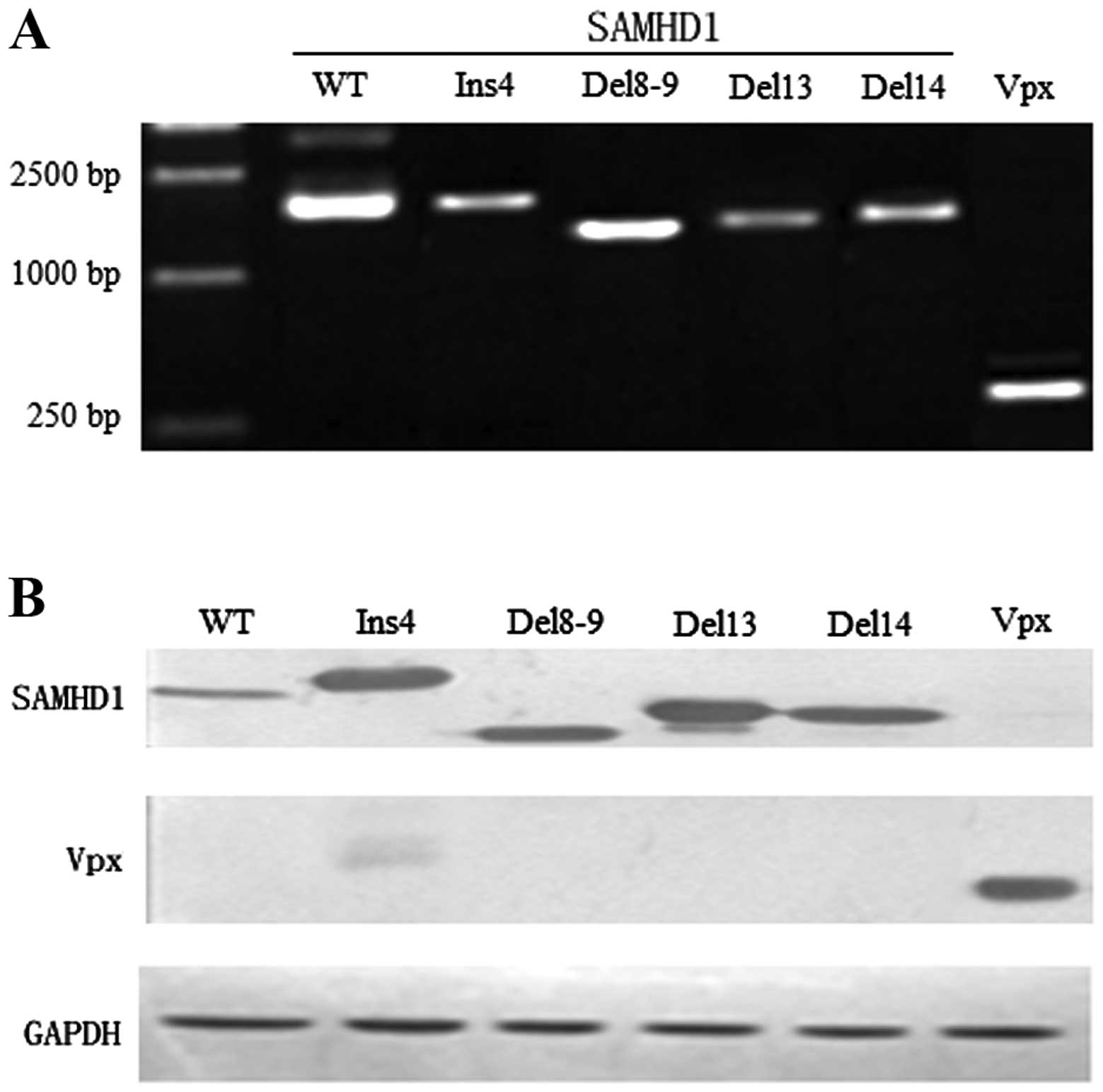 | Figure 3Construction of vectors bearing the
mutations. (A) PCR products of HepG2 cells that were co-transfected
with pcDNA-SAMHD1 WT, ins4, del8-9, del13, del14 and Vpx
respectively (WT, 1,869 bp; ins4, 1,932 bp; del8-9, 1,659 bp;
del13, 1,776 bp; del14, 1,764 bp; Vpx: 339 bp). (B) Whole-cell
extracts were prepared at 48 h following transfection and separated
on 10% SDS-PAGE. Immunoblot analysis was performed with Anti-His
monoclonal antibodies specific to SAMHD1, Vpx and GAPDH (WT, 75
kDa; ins4, 78 kDa; del8-9, 68 kDa; del13, 72 kDa; del14, 71 kDa;
Vpx, 16 kDa; GAPDH, 37 kDa). |
Discussion
Previous studies have shown that SAMHD1, along with
other members of the same family such as TREX1 and RNase H2, which
are characterized by a 3′–5′ exonuclease activity, have effects on
autoimmune diseases, auch as AGS and SLE. This is related to
mutations in these proteins that lead to nucleic acid accumulation
and genomic instability (3,15). In the present study, we identified
several natural slice variants of SAMHD1 from 44 patients with HCC
and 10 healthy controls, including variants with an insertion in
exon4 and deletions of exon8-9, 13 and 14. It was further confirmed
that these mutations occurred at the transcriptional level and not
at the genomic level.
The deletion of exon13 has been shown to cause
cerebral vasculopathy and early onset of cerebrovascular accidents
(13). The exon8-9 and 14 deletion
variants have been reported to be expressed along with the
full-length SAMHD1 in a variety of cell types (14). In the present study, we found that
del8-9, del13 and del14 were generally present in both patients and
HC, whereas the exon4 insertion showed a high prevalence in
virus-infected HCC. A total of 14 samples out of 44 HCC patients
had the exon4 insertion, resulting in a frequency of 36.4% in the
HBV-infected group, 30% in the HCV-infected group, 25% in the
non-virus-infected group and 10% in HC, respectively. There was a
statistically significant difference between the virus-infected
groups compared to the non-virus-infected group and the HC
(P<0.01). These results indicate a potential relationship
between the SAMHD1 exon4 insertion and the occurrence of
virus-induced HCC.
Differences in activities among SAMHD1 splice
variants were also investigated, so as to understand their roles in
cancer. In addition to the complete SAMHD1 gene composed of 16
exons, we constructed eukaryotic expression vectors by conjugating
pcDNA3.1(−)/Myc-His with ins4, del8-9, del13 and del14
respectively. To exclude the influence of endogenous SAMHD1 on the
results, pCMV-His-Vpx was used to degrade the intracellular SAMHD1.
After co-transfecting HepG2 with the above expression vectors, the
typical antineoplastic drug cisplatin was added into each pore to
inspect the different effects of SAMHD1 slice variants. The result
showed that the WT SAMHD1 vector induced G2 arrest (Fig. 4B) and had the strongest ability to
increase the cell sensitivity to cisplatin, while del8-9, 13 and 14
were relatively weaker. Ins4 and the single-cisplatin-treated group
presented the weakest activity, and showed no obvious differences
between them. By sequence alignments, a stop codon (TAG) was
identified in ins4, leading to a translation-terminating mutation.
This might explain the similarity between the ins4 and the
single-cisplatin-treated group with regards to G2 arrest.
The different activities of SAMHD1 slice variants in
regulating cell sensitivity to medicinal treatment suggest that
SAMHD1 is not only an effector in the innate immune response, but
might also be a negative regulator during the occurrence of HCC. We
presumed that a full-length SAMHD1 might act as an antitumor factor
by increasing the cell sensitivity to chemotherapy drugs. However
in the presence of other splice variants, the antitumor activity of
SAMHD1 might be gradually weakened, leading to a high risk of liver
cancer. Although the splice variants of the gene SAMHD1 are
naturally present in vivo, they displayed different
abilities to increase cell susceptibility to cisplatin. Because of
its high frequency in the virus-infected groups, the exon4
insertion, which led to an abnormal SAMHD1 translation termination,
appears to correlate with the risk of liver cancer. Although the
results indicated that this insertion might be an indicator of
hepatocarcinogenesis, the precise mechanism behind the occurrence
of this insertion still needs to be studied in detail.
Acknowledgements
We are very grateful to Dr Ding, Dr Zhang and Dr Yu
for the sample collection and the helpful clinical information
support. This study was supported by grants from the Special Funded
Project of Jilin Provincial Finance Department (no. 2012Z001).
References
|
1
|
Li N, Weiping Z and Cao X: Identification
of human homologue of mouse IFN-γ induced protein from human
dendritic cells. Immunol Lett. 74:221–224. 2000.
|
|
2
|
Crow MK, Kirou KA and Wohlgemuth J:
Microarray analysis of interferon-regulated genes in SLE.
Autoimmunity. 36:481–490. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rice GI, Bond J, Asipu A, Brunette RL,
Manfield IW, Carr IM, Fuller JC, et al: Mutations involved in
Aicardi-Goutières syndrome implicate SAMHD1 as regulator of the
innate immune response. Nat Genet. 41:829–832. 2009.
|
|
4
|
Ramantani G, Kohlhase J, Hertzberg C,
Innes AM, Engel K, Hunger S, Borozdin W, et al: Expanding the
phenotypic spectrum of lupus erythematosus in Aicardi-Goutières
syndrome. Arthritis Rheum. 62:1469–1477. 2010.PubMed/NCBI
|
|
5
|
Powell RD, Holland PJ, Hollis T and
Perrino FW: Aicardi-Goutières syndrome gene and HIV-1 restriction
factor SAMHD1 is a dGTP-regulated deoxynucleotide
triphosphohydrolase. J Biol Chem. 286:43596–43600. 2011.
|
|
6
|
Goldstone DC, Ennis-Adeniran V, Hedden JJ,
Groom HC, Rice GI, Christodoulou E, Walker PA, et al: HIV-1
restriction factor SAMHD1 is a deoxynucleoside triphosphate
triphosphohydrolase. Nature. 480:379–382. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lahouassa H, Daddacha W, Hofmann H, Ayinde
D, Logue EC, Dragin L, Bloch N, et al: SAMHD1 restricts the
replication of human immunodeficiency virus type 1 by depleting the
intracellular pool of deoxynucleoside triphosphates. Nat Immunol.
13:223–228. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kao JH and Chen DS: Global control of
hepatitis B virus infection. Lancet Infect Dis. 2:395–403. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Daga PR, Duan J and Doerksen RJ:
Computational model of hepatitis B virus DNA polymerase: Molecular
dynamics and docking to understand resistant mutations. Protein
Sci. 19:796–807. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lindenbach BD and Rice CM: Unravelling
hepatitis C virus replication from genome to function. Nature.
436:933–938. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alter MJ, Kruszon-Moran D, Nainan OV,
McQuillan GM, Gao F, Moyer LA, Kaslow RA, et al: The prevalence of
hepatitis C virus infection in the United States, 1988 through
1994. N Engl J Med. 341:556–562. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murayama A, Weng L, Date T, Akazawa D,
Tian X, Suzuki T, Kato T, et al: RNA polymerase activity and
specific RNA structure are required for efficient HCV replication
in cultured cells. PLoS Pathog. 6:e10008852010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramesh V, Bernardi B, Stafa A, Garone C,
Franzoni E, Abinun M, Mitchell P, et al: Intracerebral large artery
disease in Aicardi-Goutières syndrome implicates SAMHD1 in vascular
homeostasis. Dev Med Child Neurol. 52:725–732. 2010.PubMed/NCBI
|
|
14
|
Welbourn S, Miyagi E, White TE,
Diaz-Griffero F and Strebel K: Identification and characterization
of naturally occurring splice variants of SAMHD1. Retrovirology.
9:862012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He G, Kuang J, Khokhar AR and Siddik ZH:
The impact of S- and G2-checkpoint response on the fidelity of
G1-arrest by cisplatin and its comparison to a non-cross-resistant
platinum (IV) analog. Gynecol Oncol. 122:402–409. 2011. View Article : Google Scholar : PubMed/NCBI
|