Introduction
Longevity assurance homolog 2 of yeast LAG1
(Lass2) gene, also known as ceramide synthase 2, is a member
of the CerS family and is responsible for the generation of
long-chained ceramides (1,2). Under a physiological condition, mRNA
levels of Lass2 are significantly higher than those of the
other 5 CerSs, particularly abundant in the liver, kidney and brain
in mice (3,4). Since Lass2 was firstly reported
as a hepatocellular carcinoma (HCC)-suppressing gene (5,6), a
growing amount of research has provided experimental and clinical
evidence of the close association between Lass2 and some
types of cancer, including prostate (7), breast (8) and bladder cancer (9). We hypothesized that Lass2 may
be a potential protective gene in the process of carcinogen-induced
tumourigenesis. To test the hypothesis, we used a
hepatocyte-specific Lass2-knockout (Lass2 KO)
transgenic mouse model for assessment of diethylnitrosamine
(DEN)-induced liver tumourigenesis. The results demonstrated that
at week 23 following DEN exposure, all female and male Lass2
KO mice developed liver tumours whereas in the wild-type (WT)
group, only 28.6% of males and 14.3% of females developed tumours.
At week 40, although the majority of Lass2 KO and WT mice
carried tumours and no significant difference in tumour occurrence
was noted between the 2 genotypes, the tumours of livers from the
Lass2 KO mice were more numerous and larger in size. The
mRNA and protein levels of α-fetoprotein (AFP), a widely used liver
cancer marker, were markedly elevated in the liver (both in the
tumour region and in the non-tumour region) of Lass2 KO
mice. Our data indicate that the hepatocyte-specific deletion of
Lass2 in mice leads to a higher susceptibility to the
carcinogen DEN.
DEN, a chemical carcinogen, interacts with and
damages DNA inducing mutations and initiating neoplastic
alteration. The alterations in the genetic profile of liver tissues
in Lass2 KO mice when compared with WT mice may be involved
in the mechanism of the higher susceptibility to DEN. Plasminogen
activator inhibitor type 1 (PAI-1) was found to be one of
the markedly upregulated genes in the Lass2 KO liver
tissues. In previous studies, PAI-1, the inhibitor of both
tissue- and urokinase-type plasminogen activator (t-PA and u-PA,
respectively), was suggested to be a tumour-promotor gene, which
regulates cell proliferation, apoptosis, adhesion, migration,
invasion and angiogenesis during the process of carcinogenesis
(10,11). The retarded growth of implanted
tumours was observed in PAI-1-knockout mice (12,13).
In the present study, we compared the mRNA and protein levels of
PAI-1 in liver tissues (including the tumour region and the
non-tumour region) following exposure to DEN in Lass2 KO and
WT mice. We found that PAI-1 was markedly increased in the
Lass2 KO liver tissues when compared with that in the WT
specimens. We then examined the mRNA levels of TGF-β1 and
Smad-4 and -7, which are closely related with
PAI-1 (14,15). The data of the present study
demonstrated that alteration of the
TGF-β1-Smad4-PAI-1 axis is involved in the
mechanism of elevated susceptibility of Lass2 KO mice to
DEN.
Materials and methods
Ethics statement
All protocols for animal care and use were approved
by the Animal Care Committee of the Shanghai Cancer Institute and
were in accordance with regulations for the Administration of
Affairs Concerning Experimental Animals and National Institutes of
Health Guidelines. All of the mice were housed in pathogen-free
(SPF) animal facilities under a standard 12-h-light/12-h-dark
cycle. Animals received free access to water and commercial mouse
chow throughout the present study. Mice were sacrificed by cervical
dislocation.
Transgenic mice
Hepatocyte-specific Lass2 KO mice were
generated using the Cre/LoxP system for site-specific
excisional DNA recombination. The background of the transgenic mice
was C57BL/6J, and mice were obtained from the Jackson Laboratory
(Bar Harbor, ME, USA). Mice carrying the floxed second exon of
Lass2 (Lass2
LoxP+/LoxP−) were obtained from
the Shanghai Research Centre for Model Organisms. Albumin-Cre
transgenic mice expressing Cre recombinase under control of the
hepatocyte-specific albumin promoter were purchased from the
Jackson Laboratory. Lass2
LoxP+/LoxP− were crossbred with
Albumin-Cre transgenic mice to generate heterozygous transgenic
mice, and the siblings were crossbred with each other to generate
Lass2 LoxP+/LoxP+ and
Alb-Cre+ mice. The DNA of the mouse tail was
extracted using the QIAamp DNA Blood kit (Qiagen Inc., Germany),
and genotypes were identified by PCR assay. PCR was performed using
the Takara Taq system (Takara Bio, Inc., Otsu, Japan) using sense,
5′-GGCTTC GTGTTGGTCTTCTGA-3′ and antisense, 5′-ATTCCCTGGC
ATCCACCTTTC-3′ for Lass2 LoxP primer pairs; and sense,
5′-GCGGTCTGGCAGTAAAAACTATC-3′ and antisense,
5′-GTGAAACAGCATTGCTGTCACTT-3′ for Cre primer pairs. Lass2
LoxP+/LoxP+ and
Alb-Cre+ mice were used as Lass2 KO mice.
Wild-type control was Lass2
LoxP−/LoxP− and
Alb-Cre+ mice. When mice were sacrificed after
DEN treatment at week 23 or 40, the genotypes of the brain, liver
and kidney tissues were identified by PCR assay with the paired
primers as sense, 5′-TATGTAGCCAGAGTCCAAGGC-3′ and antisense,
5′-CACTATTGACCAGGCGAGGAG-3′, western blotting and anti-Lass2
immunohistology (IHC) in order to confirm the hepatocyte-specific
deletion of Lass2.
Hepatocarcinogen treatment
Groups of 14-day-old Lass2 KO and WT mice
were administered a single intraperitoneal injection of the
genotoxic hepatocarcinogen DEN (Sigma) dissolved in PBS at a dose
of 20 mg/kg body weight or saline (no DEN). Mice were sacrificed
respectively at week 23 and 40 following DEN or saline injections.
The livers were weighed, the diameter of tumours ≥1 mm on the
surface of the livers were enumerated and the sizes of tumours were
measured. Part of the liver tissues was fixed in AAF (100% alcohol
85 ml, acetic acid 5 ml, formalin 10 ml) for histological study,
and the remaining part was frozen at -80°C until use.
Histological study
The fixed liver, kidney and brain tissues were
embedded in paraffin, sectioned into 4-μm slices and anti-Lass2
immunohistochemical staining was performed to ascertain
hepatocyte-specific deletion of Lass2. The liver sections
were H&E-stained for examination of morphological alteration
after DEN treatment. Moreover, IHC staining of proliferating cell
nuclear antigen (PCNA) and 5-ethynyl-2′-deoxyuridine (EdU) staining
were performed for examining the proliferation, whereas terminal
deoxynucleotidyl-transferase-mediated dUTP-biotin nick end-labeling
(TUNEL) assay was carried out for assessment of apoptosis in the
DEN-treated livers.
Immunohistochemistry
For IHC staining, the standard protocol was
performed. Briefly, sections were incubated with 3%
H2O2 to eliminate the endogenous peroxidase
activity, then blocked with 5% bovine serum albumin (BSA), and then
incubated with rabbit anti-Lass2 polyclonal antibody (1:100)
(Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China) or
rabbit anti-PCNA polyclonal antibody (1:100) (ProSci, Inc., Poway,
CA, USA) at 4°C overnight, respectively. The sections were washed 3
times with PBS for 5 min each. For the Lass2 assay, the sections
were incubated with Alexa Fluor® 488-conjugated goat
anti-rabbit antibody (1:100) (Invitrogen Life Technologies,
Carlsbad, CA, USA). Images were captured using a Leica DM LB2
epifluorescence microscope. However, for PCNA detection, the
sections were incubated with HRP-conjugated mouse anti-rabbit IgG
(1:100) (ProSci, Inc.) for 1 h at 37°C. Color was developed with
DAB (KeyGen Biotech, Nanjing, China), and microscopic examination
was performed with a bright field microscope (Motic BA210; Xiamen,
China).
EdU assay
EdU assay was performed on mouse liver section using
the EdU DNA Imaging kit (RiboBio, Guangzhou, China) according to
the manufacturer’s instructions. Briefly, 5 mg/kg EdU was
intraperitoneally injected into mice 8 h before sacrifice. The mice
were sacrificed under ether anaesthesia, and the liver sections
were developed as indicated above. The slices were washed in a
shaker with glycine (2 mg/ml) for 10 min and then incubated with
0.5% Triton X-100 for 10 min. Following washing with PBS for 2
times, the sections were incubated with Apollo reaction buffer for
10–30 min. Finally, after being washed with 0.5% Triton X-100 for 3
times, methanol twice and PBS for 1 time in due order, the slices
were stained with 100 μl Hoechst 33342 (x1) for 30 min at room
temperature (RT). Images were obtained using a Leica DM LB2
epifluorescence microscope. The EdU labeling index of the liver
tissues was calculated as a percentage of EdU-positive cells to the
total of Hoechst 33342-stained cells in each field, counted in 5
random ×100 fields in 5 representative livers from each group.
TUNEL assay
Cell apoptosis in liver tissues was determined using
a TUNEL kit (Beyotime Institute of Biotechnology, Jiangsu, China)
according to the manufacturer’s recommendations. Briefly,
paraffin-embedded tissue sections were deparaffinized and
rehydrated as above. The slides were treated with protease K
(1:500, 20 μg/ml) for 15 min at 37°C, washed 3 times with PBS,
incubated in TUNEL reaction buffer (Tdt enzyme 2 μl, fluorescent
buffer 48 μl) for 60 min at 37°C while protecting from light, and
again washed 3 times with PBS, then stained with 100 μl Hoechst
33342 (x1) for 30 min at room temperature, and washed with PBS
twice. Slides were observed under a Leica DM LB2 epifluorescence
microscope. The apoptotic cell index was expressed as the average
of five random areas of a single ×100 field in a specific
individual liver tissue, with 5 mice/group and 1 section/mouse.
Western blotting
Tissues were lysed in RIPA lysis buffer containing
the protease inhibitor phenylmethanesulfonyl fluoride (both from
Beyotime Institute of Biotechnology). The cell extracts were
centrifuged at 12,000 × g for 20 min at 4°C, and the supernatants
were used for experiments. The protein concentrations were
determined with the BCA assay kit (Beyotime Institute of
Biotechnology). The equivalent tissue proteins (10 μg/lane) were
subjected to electrophoresis and transferred onto PVDF membranes
(Millipore, Bedford, MA, USA) via a semi-dry transfer system
(Bio-Rad, Hercules, CA, USA). The membranes were blocked with 5%
non-fat milk for 1 h at room temperature in TBST (50 mM pH 7.5
Tris, 0.9% NaCl and 0.1% Tween-20) and then incubated with a 1:200
dilution of the primary antibodies (anti-PAI-1, anti-AFP and
anti-Smad-4 were purchased from Bioworld Technology, Inc.;
anti-Lass2 was from Beijing Biosynthesis Biotechnology Co., Ltd;
and anti-β-actin from Santa Cruz Biotechnology, Inc.) overnight at
4°C. The membrane was washed then incubated with secondary antibody
for 1 h at room temperature and developed using BeyoECL Plus kit
(Beyotime Institute of Biotechnology) for 1 min, then scanned by
ChampChemi™ professional (Beijing Sage Creation Science Co., Ltd.,
Beijing, China).
RNA isolation and quantitative PCR (qPCR)
analysis
Total RNA from different liver tissues including the
non-DEN-treated, DEN-treated non-tumour region and tumour region
from the male Lass2 KO and WT mice (3 mice/group) at week 40
were, respectively, extracted using TRIzol (Invitrogen Life
Technologies) and subjected to reverse-transcription using the
miScript Reverse Transcription kit (Takara Bio, Inc.) to synthesize
cDNA, and comparative quantitative PCR (QT-PCR) was preformed using
SYBR-Green (Takara Bio, Inc.) chemistry analysis in quadruplicates.
Relative expression was calculated using the comparative threshold
cycle (Ct) method. The primers for AFP, PAI-1,
TGF-β1, Smad-4, Smad-7, GAPDH are shown
in Table I.
 | Table IPrimers for QT-PCR. |
Table I
Primers for QT-PCR.
| Gene symbol | Primer |
|---|
| AFP |
5′-TGCGCTCTCTACCAGACCTT-3′ |
|
5′-ACAGGGCTTGCTTCATTCC-3′ |
| PAI-1 |
5′-GCAACGGATAGACAGATCAAA-3′ |
|
5′-AGTCACCTACACTCTGAAATAAC-3′ |
| Smad7 |
5′-TCTTCAACAGCCGGTAGTC-3′ |
|
5′-GGAAAGGTTAGCAGCAAGT-3′ |
| Smad4 |
5′-CTTACCCACTGAAGGACATT-3′ |
|
5′-GTGGCGTTAGACTCTGC-3′ |
| TGF-β1 |
5′-GTGGAAATCAACGGGATCAG-3′ |
|
5′-TTCTCTGTGGAGCTGAAGCA-3′ |
| GAPDH |
5′-GCAAGGTCATCCCAGAG-3′ |
|
5′-AAGTCGCAGGAGACAAC-3′ |
Statistical analysis
All data are presented as means ± standard error of
the mean (SEM). Unpaired Student’s t-test was used for statistical
analysis. P≤0.05 was considered to indicate a statistically
significant result. All QT-PCR and western blot analyses were
repeated at least 3 times. All statistical analyses were conducted
using the GraphPad Prism 5.
Results
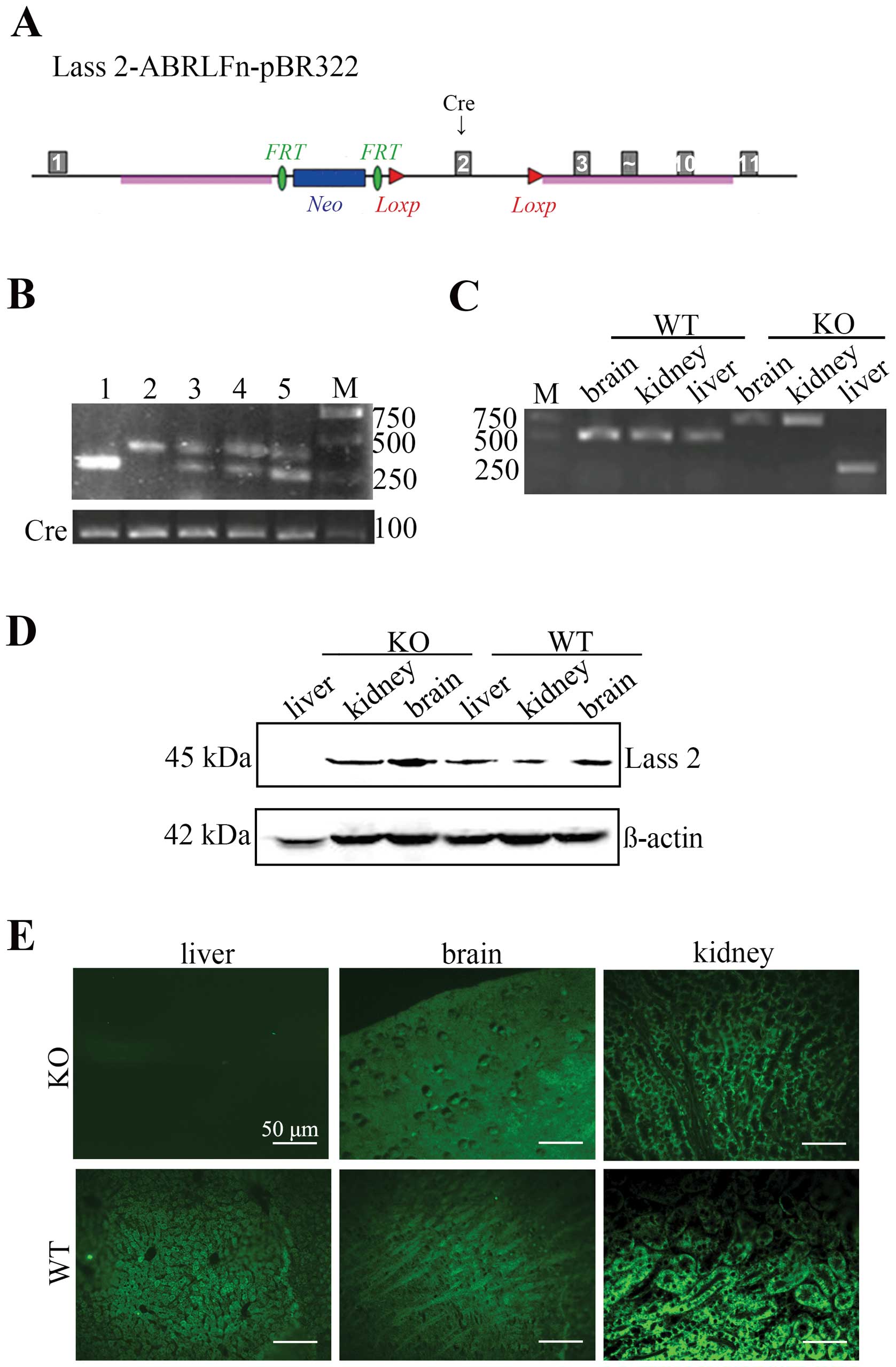
Establishment of hepatocyte-specific
Lass2-knockout transgenic mice
Hepatocyte-specific Lass2 KO mice were
produced using the Cre-LoxP system.
Lass2-ABRLFn-pBR322 vector carrying 2 LoxP cassettes
was inserted into the intron 1–2 and intron 2–3, respectively. The
excision of Lass2 was the result of the recombination event
by one Cre recombinase enzyme controlled by the upstream
Alb-promoter on the Lass2-ABRLFn-pBR322 which was able to
identify the sequence between 2 LoxP cassettes (Fig. 1A). Mice carrying a floxed
Lass2 allele were mated with Alb-Cre transgenic mice that
express the Cre recombinase in hepatocytes. The siblings that
carried both floxed Lass2 allele and Alb-Cre were selected
by the genotyping of tail DNA and were named as Lass2 KO
mice (Fig. 1B, lane 2). To confer
hepatocyte-specific Lass2 deletion, PCR assay and anti-Lass2
immunohistology of the liver, kidney and brain tissues were carried
out. In Lass2 KO mice, liver tissue displayed the
Lass2-knockout band whereas kidney and brain tissues did not
(Fig. 1C). The phenotype was
verified by western blotting, and the results showed the absence of
the Lass2 protein from the liver tissues of Lass2 KO mice
when compared with the control mice; no significant difference in
brain and kidney tissues was noted between the 2 groups (Fig. 1D). Immunohistology demonstrated that
Lass2 signals were undetectable in the livers from Lass2 KO
mice whereas they were visible in kidney and brain tissues
(Fig. 1E).
Enhancement of DEN-induced liver
carcinogenesis following hepatocyte-specific deletion of Lass2
The liver carcinogenesis of mice was induced using
the classical carcinogen DEN. In the well-established model of
DEN-induced liver carcinogenesis, the development of tumour is
usually monitored approximately 38–40 weeks following the treatment
of DEN. We hypothesized that the loss of Lass2 may
accelerate the formation of tumours. Therefore, the mice were
sacrificed at two time points: week 23 and 40, respectively, after
DEN or saline treatment. Following DEN injection, at week 23,
notably, all the KO mice, either male or female, developed tumours
in the livers, whereas only 2 out of 7 males and 1 out of 7 females
in the WT group developed tumours. The difference in the tumour
occurrence between the Lass2 KO and WT group was highly
statistically significant (P<0.001). At week 40 following DEN
treatment, although almost all mice from both groups developed
tumours and no significant difference in tumour occurrence was
noted between the 2 groups (Table
II), in Lass2 KO group, the indices of liver weight/body
weight, the numbers of tumours on the surface of livers and the
average sizes of tumours were higher than these values in the
control group (Fig. 2A and B). We
also found a greater elevation in the AFP level in the Lass2
KO group (both in the tumour and non-tumour regions) when compared
with its counterpart at week 40 (Fig.
2C). Histologic analysis of H&E-stained liver sections from
Lass2 KO and WT mice revealed that the tumours were
hepatocellular adenomas/carcinomas, showing basophilic foci
characterized by clearly defined margins, compression of the
surrounding parenchyma, thicker trabeculae extensive fatty
degeneration and Mallory bodies inside of the tumours (Fig. 2D). Unequivocal evidence of
metastases or vascular invasion was not found. The results
indicated that the deletion of Lass2 resulted in the earlier
occurrence of DEN-induced liver carcinogenesis and accelerated
tumour growth.
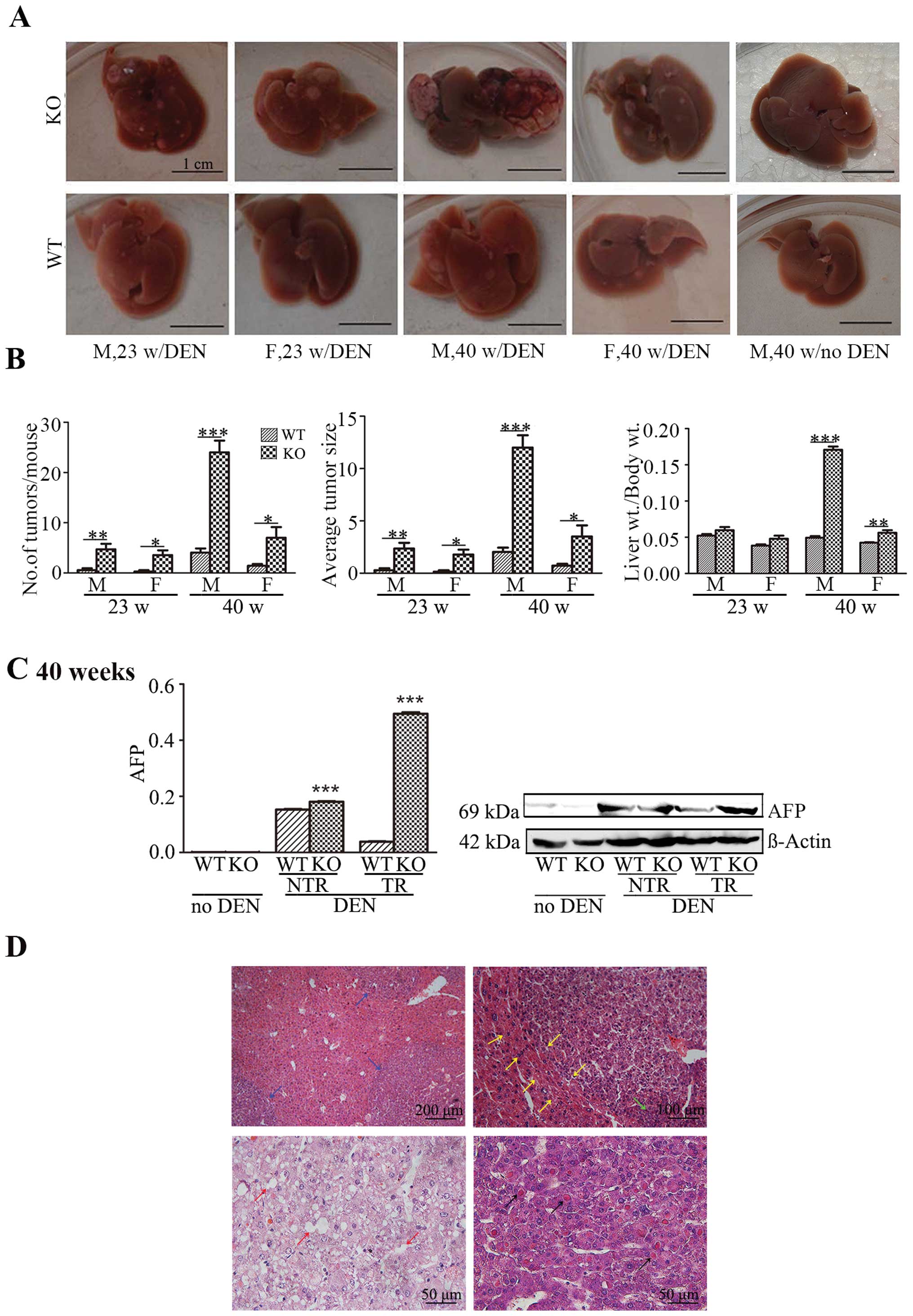 | Figure 2Hepatocyte-specific deletion of
Lass2 enhances DEN-induced liver carcinogenesis. (A) Images
are representative of livers carrying tumours, demonstrating the
visible nodules on the surface of livers from male and female
Lass2 KO and WT mouse at week 23 and 40 following
DEN-treatment, respectively. At week 23, the livers from the
Lass2 KO mice carried more small-sized nodules and at week
40 large-sized well-vascularized tumour nodules appeared with the
dark-coloured non-tumour liver tissue in the Lass2 KO mice,
whereas the WT and KO livers were visibly normal without
DEN-treatment. (B) The ratios of liver to body weight, numbers of
tumours and the average sizes of tumour nodules following
DEN-treatment are summarized. These indices in the Lass2 KO
mice were strikingly higher than those of the WT mice. The
increased indices at week 40 when compared to week 23 implied the
ongoing process of development of liver tumours. Moreover, the male
mouse indices were higher than those of the female mice, indicating
the higher susceptibility to DEN in males. (C) The expression of
AFP in Lass2 KO livers was measured using QT-PCR analysis
and western blotting. The level of AFP was barely detected in the
two groups without DEN-treatment. After DEN injection, the level of
AFP was significantly increased in the Lass2 KO livers,
particularly in the tumour region. The western blotting shows the
same trend as the results of QT-PCR analysis. (D) The examination
of H&E-stained paraffin-embedded sections of liver tissue
demonstrated basophilic foci characterized by clearly defined
margins (blue arrows), compression of the surrounding parenchyma
(yellow arrows), thicker trabeculae (green arrows), extensive fatty
degeneration (red arrows) and Mallory bodies (black arrows) inside
the tumours. DEN, diethylnitrosamine; Lass2 KO, Lass2
knockout; WT, wild-type. |
 | Table IComparison of DEN-induced liver
carcinogenesis between Lass2 KO and WT mice. |
Table I
Comparison of DEN-induced liver
carcinogenesis between Lass2 KO and WT mice.
| 23 weeks | 40 weeks |
|---|
|
|
|
|---|
| Genotype | Male ratio (%) | Female ratio
(%) | Male ratio (%) | Female ratio
(%) |
|---|
| DEN-treated |
| Lass2
KO | 7/7 (100) | 7/7 (100) | 7/7 (100) | 7/7 (100) |
| WT | 2/7 (28.6) | 1/7 (14.3) | 6/7 (85.7) | 5/7 (71.4) |
| Saline-treated |
| Lass2
KO | 0/7 (0.0) | 0/7 (0.0) | 0/7 (0.0) | 0/7 (0.0) |
| WT | 0/7 (0.0) | 0/7 (0.0) | 0/7 (0.0) | 0/7 (0.0) |
Promotion of proliferation and inhibition
of apoptosis in liver tumours following hepatocyte-specific
deletion of Lass2
In our previous study, we found that overexpression
of Lass2 in a human liver cancer cell line inhibited
proliferation of the cells in vitro (6). In the present study, we also found
that the average size of tumours in the Lass2 KO group was
larger than that of the control group. Therefore, we detected the
cell proliferation of liver tissues in male mice at week 23 with
EdU assay (Fig. 3A), the expression
of cell proliferation marker PCNA with IHC staining (Fig. 3B), and apoptosis with TUNEL assay
(Fig. 3C). Quantification revealed
an ~8-fold increase in the EdU-positive ratio (P<0.001) and an
~12-fold increase in the PCNA-positive ratio (P<0.001) in
Lass2 KO mice when compared with the control. Moreover,
TUNEL staining demonstrated that the average TUNEL-positive ratio
was dramatically decreased in the liver tissues of Lass2 KO
mice when compared with the control (P<0.001) (Fig. 3D). The results suggest that the
deletion of Lass2 promoted the proliferation and decreased
the apoptosis of the liver tissues.
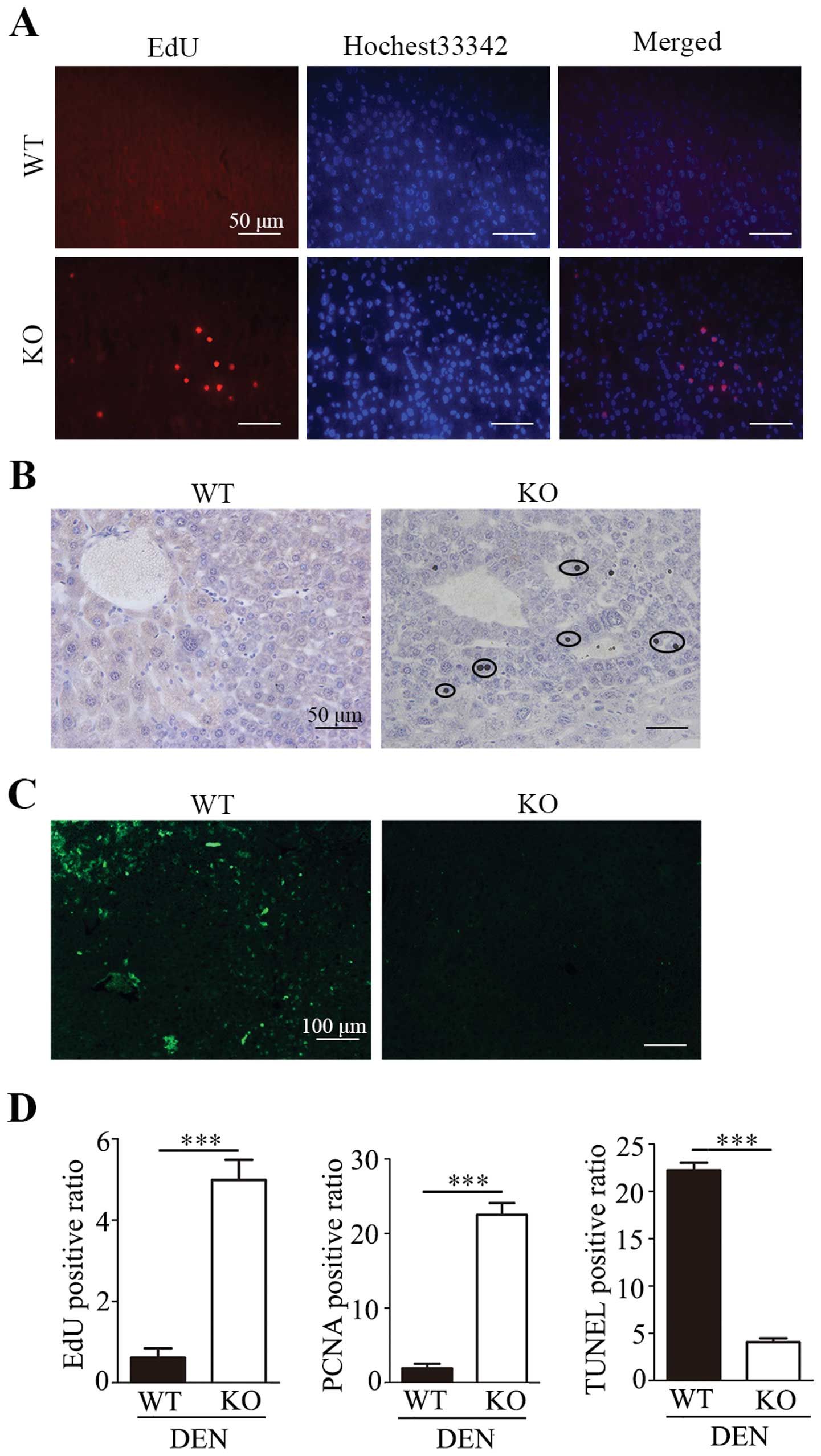 | Figure 3Lass2 KO livers after
DEN-treatment exhibit increased proliferation and inhibition of
apoptosis. The proliferation and apoptosis of liver tissue at week
23 after DEN treatment were detected with ethidium deoxyuridine
(EdU) cell proliferation assay, anti-PCNA immunochemistry and TUNEL
staining. (A) EdU cell proliferation staining of liver tissue from
Lass2 KO and WT mice. Left, EdU-positive nuclei (red);
middle, Hochest 33342-labeled nuclei (blue); right, overlay EdU-
and Hochest 33342-labeled nuclei. The number of EdU-positive cells
relative to the total number of cells was counted in each of 5
random ×100 fields of each section (5 mice/group, 1 section/mouse).
The histogram of the EdU-positive ratio (the number of EdU-positive
nuclei/the number of total nuclei/field) is shown in D, indicating
that the DEN-treated Lass2 KO mice underwent more active
proliferation in liver tissue than the control. (B) Anti-PCNA
immunochemistry of liver tissues from Lass2 KO and WT mice
shows PCNA-positive cells, indicated by circles. The histogram in D
shows the average PCNA-positive ratio (PCNA-positive cells/total
cells, counted in 5 random ×100 fields, 5 mice/group, 1
section/mouse). The number of PCNA-positive cells was higher in
DEN-treated Lass2 KO liver when compared with the WT
control, which is coincident with the previous result shown in A.
(C) TUNEL staining of the liver tissues from Lass2 KO and WT
mice (green). The histogram of average TUNEL-positive ratio
(TUNEL-positive cells/total cells, in 5 random ×100 field fields, 5
mice/group, 1 section/mouse) in D indicates that the number of
TUNEL-positive cells was significantly less in Lass2 KO
liver than the WT control, indicating that the deletion of
Lass2 inhibits apoptosis in liver tissue. (D) The histogram
of average EdU-positive ratio, PCNA-positive ratio and
TUNEL-positive ratio. ***P<0.001, Lass2 KO vs.
WT. DEN, diethylnitrosamine; Lass2 KO, Lass2
knockout; WT, wild-type. |
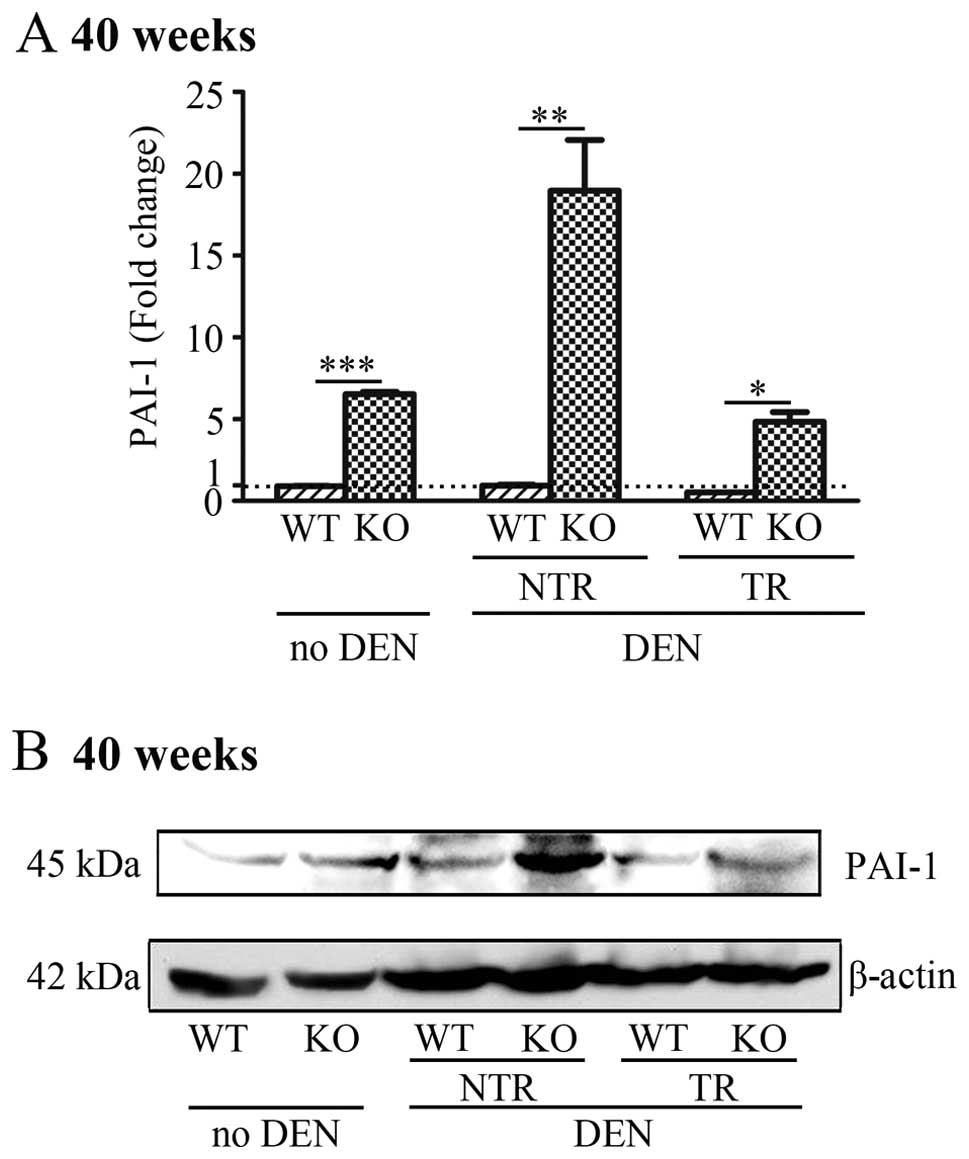
Hepatocyte-specific deletion of Lass2
upregulates the expression of PAI-1 in liver tissues
PAI-1 is a tumour-promoting gene
predominantly produced by hepatocytes in liver tissue. We used
QT-PCR, western blotting and IHC to compare the expression of PAI-1
in the liver tissues of the Lass2 KO and control mice at
week 40 with or without treatment of DEN, including respective
tumour regions (TR) and non-tumour regions (NTR). The results
indicated, in each paired liver tissues from Lass2 KO and WT
mice, markedly increased mRNA and protein levels of PAI-1 in the
Lass2 KO mice when compared with the WT counterparts.
Without DEN treatment, mRNA levels of PAI-1 increased
~7-fold. After DEN-treatment, before transformation (i.e. in NTR),
the mRNA level of PAI-1 in the Lass2 KO liver
increased even more than that in the WT counterpart, with an
increase of ~20-fold; whereas after transformation, i.e., in the
TR, this increase was decreased ~9-fold (Fig. 4A). The alteration of the protein
level of PAI-1 was in accordance with that of the mRNA level
(Fig. 4B).
Alterations in PAI-1-associated genes
TGF-β1 and Smad-4 and -7 in Lass2 KO liver tissues
We used QT-PCR to detect the mRNA levels of
TGF-β1 and Smad-4 and -7, genes reported to be
closely related with PAI-1, in Lass2 KO and WT liver
tissues. Smad-4 expression was also assessed by western blot
analysis. The results demonstrated that without DEN treatment, the
deletion of Lass2 caused increases in TGF-β1,
Smad-4 and -7, which were parallel to the levels of
PAI-1. Following DEN treatment, in each Lass2 KO and
WT paired specimen, mRNA levels of TGF-β1 and Smad-4
in the Lass2 KO specimen (including NTR and TR),
respectively, were increased when compared to the WT; whereas
regarding the mRNA level of Smad-7, a significant decrease
was noted in Lass2 KO NTR, and no significant difference was
noted in Lass2 KO TR, which exhibited a different pattern of
alteration to TGF-β1 and Smad-4 (Fig. 5A–C). Western blotting of Smad-4
showed a similar trend with the result of QT-PCR analysis (Fig. 5D). Taken together, in each pair of
Lass2 KO and WT specimens, the TGF-β1, Smad-4
and PAI-1 from the Lass2 KO group was consistently
and respectively significantly increased when compared to the WT
group, suggesting that upregulation of the
TGF-β1-Smad4-PAI-1 occurred in the
Lass2 KO mice. It was noted that mRNA levels of
TGF-β1, Smad-4 and -7, after DEN induction when
compared with these levels before DEN treatment, decreased in
several orders of magnitude, the significance of which is yet to be
known.
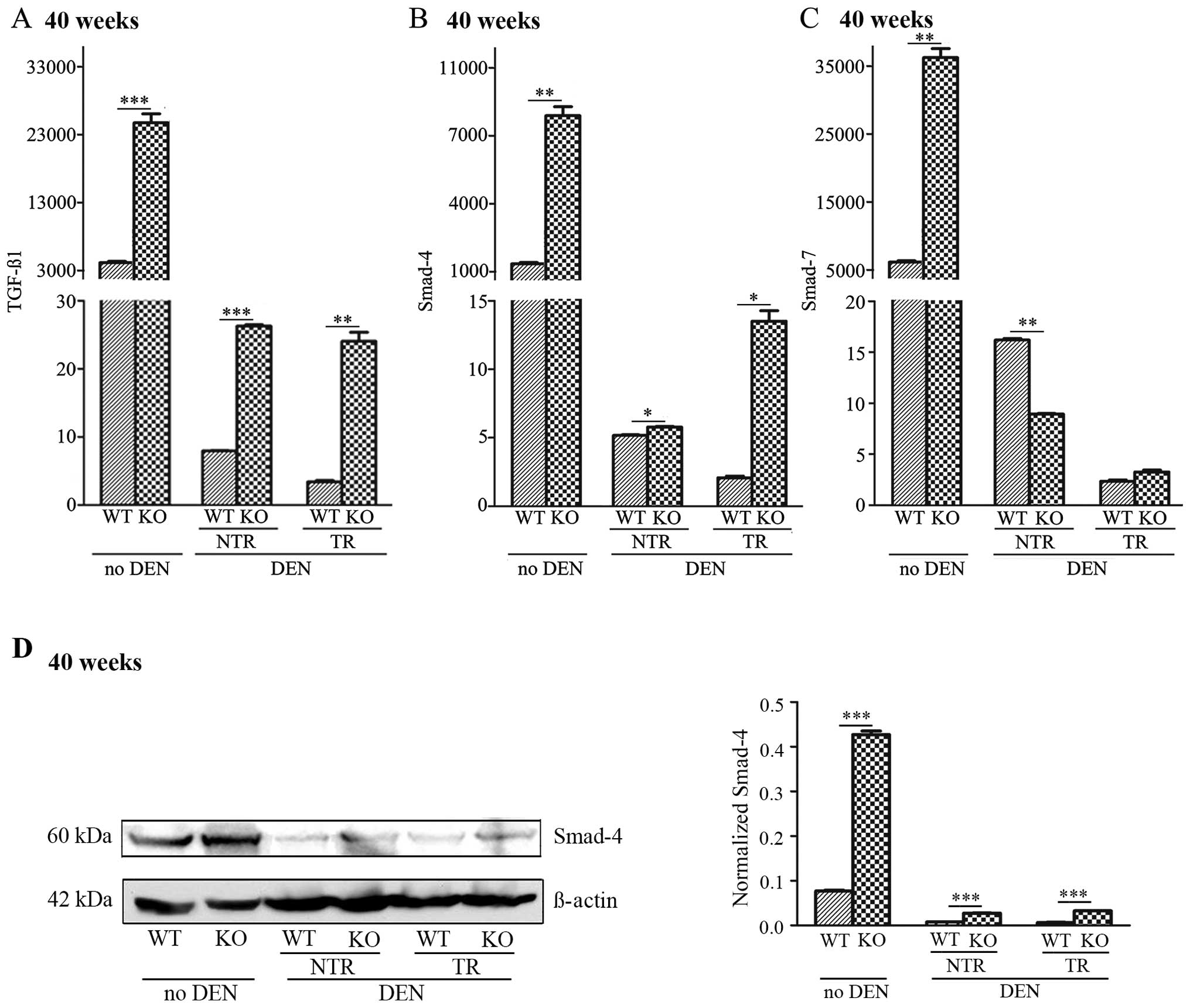 | Figure 5Increased expression of TGF-β1
and Smad-4 in Lass2 KO livers. The expression levels
of (A) TGF-β1, (B) Smad-4 and (C) Smad-7 in
Lass2 KO livers with or without DEN-treatment at week 40
were respectively detected using QT-PCR analysis. (D) Smad-4
expression was also assessed by western blot analysis. The results
demonstrated that without DEN treatment, the deletion of
Lass2 caused an increase in TGF-β1 (~7-fold,
***P<0.001), Smad-4 (~4-fold,
**P<0.01) and Smad-7 (~6-fold,
**P<0.01), which was parallel to the evaluated level
of PAI-1. Following DEN treatment, for each Lass2 KO
and WT paired specimen, mRNA levels of TGF-β1, mRNA and
protein levels of Smad-4 in Lass2 KO specimen (including NTR
and TR) respectively increased when compared with the WT; whereas
for the mRNA level of Smad-7, a significant decrease in
Lass2 KO NTR was observed and no significant difference in
Lass2 KO TR was noted, which exhibited a different pattern
of alteration to TGF-β1 or Smad-4. Lass2 KO,
Lass2 knockout; DEN, diethylnitrosamine; NTR, non-tumour
regions; TR, tumour regions; WT, wild-type. |
Discussion
Lass2 is a novel gene isolated from a human
liver cDNA library by our laboratory and is a human homologue of
the yeast longevity assurance gene Saccharomyces cerevisiae
longevity assurance gene (LAG1). The deletion of
Lass2 in mice caused hepatocarcinomas after they became
adults (5). Our previous study
found that overexpression of Lass2 inhibited the cell growth
of the human hepatocellular carcinoma (HCC) cell line SMMC-7721
(6). Recently, Lass2 has
attracted the interest of many researchers as more and more
evidence suggests that Lass2 may be a tumour-suppressor
gene. Previously reported experimental data revealed that
Lass2 inhibited the growth of HCC (5,6),
suppressed invasion of a prostate cancer cell line (7) and elevated drug-sensitivity in breast
cancer (8). Clinical investigation
showed that the deletion of Lass2 is positively associated
with clinical stage of progression, depth of tumour invasion and
recurrence of bladder carcinoma implying that Lass2 may be a
prognostic indicator for this type of carcinoma (9). Comprehensive laboratory and clinical
data strongly support that Lass2 is an effective
tumour-suppressor gene, and inhibits the proliferation and
metastasis of several types of cancer.
In the present study, to the best of our knowledge,
we first produced hepatocyte-specific Lass2-deficient mice.
The results of the EdU assay, PCNA histochemistry and TUNEL
staining demonstrated the increased proliferation and attenuated
apoptosis in Lass2 KO liver tissue when compared with the
control. We treated the Lass2 KO mice and WT mice with the
carcinogen DEN and found that Lass2 KO mice developed liver
tumours earlier than the control group. At week 23 following
treatment of DEN, 100% Lass2 KO mice developed obvious liver
tumours whereas only 21.4% of mice in the control group developed
tumours, for which the occurrence between the 2 genotypes was of
marked difference. The deletion of Lass2 not only promoted
the occurrence of liver tumours, but also accelerated the
development of liver tumours. At week 40 after treatment of DEN, in
the Lass2 KO mice, the number of tumours on the liver were
more numerous (P<0.001) and the average size of liver tumours
were larger than in the WT mice (P<0.001). Our data provide
obvious evidence that Lass2 is a protective gene against
DEN-induced carcinogenesis.
The mechanism by which Lass2 suppresses
tumours may rely upon several factors. One mechanism is related to
the ceramids that are produced by Lass2. Lass2, a ceramid
synthesase, is responsible for generating a long-chain spectrum of
ceramids, which act as secondary messengers and regulate cell
proliferation and apoptosis (16,17).
Another mechanism by which Lass2 suppresses tumours is via
the interaction with the c subunit of vacuolar ATPase (V-ATPase)
(7,8). V-ATPase, a proton pump, plays an
important role in carcinogenesis by regulation of intracellular and
extracellular pH, promoting carcinogenesis (18,19).
Lass2 interferes with the proton secretion of tumour cells
to produce an unfavorable environment for the malignancy of tumour
cells. The present study elucidated another novel pathway
associated with the promotion of development of hepatocellular
cancer in Lass2 KO mice.
Our results demonstrated a significant alteration in
PAI-1 mRNA and protein levels in Lass2 KO liver when
compared with the control mice. According to previous reports,
PAI-1 plays an important role in several malignant
processes, such as the promotion of tumour cell proliferation
(21,25), inhibition of apoptosis (22,23),
suppression of invasion and metastasis (24–26)
and enhancement of angiogenesis (27,28)
and in the clinic, high PAI-1 levels are associated with
poor prognosis for several different types of tumours (29–32).
Yet, there are also controversial reports in regards to the
association between PAI-1 and the metastasis of tumours. For
example, the transfection of PAI-1 was found to inhibit the
liver metastasis of pancreatic cancer by preventing angiogenesis
(33). Moreover, the
PAI-1-related pathway was found to be different in primary
cancers and metastases (34). In
the present study, the mRNA and protein levels of PAI-1 in liver
tissue from the non-DEN-treated Lass2 KO and WT mice were
detected and compared, which were determined as basal levels. The
basal level of PAI-1 was markedly increase in Lass2
KO when compared to the WT mice (~8-fold increase; P<0.001).
Following DEN treatment, in both the TR and NTR, the PAI-1 levels
in the Lass2 KO livers were significantly higher than levels
in the WT mouse tissues (P<0.05). After DEN treatment, before
transformation (i.e. in NTR), the mRNA level of PAI-1 in the
Lass2 KO liver increased even more than that of the WT
counterpart, with a fold increase of ~20-fold; whereas after
transformation, i.e. in the TR this fold increase was decreased
~9-fold. One of the possible explanations may be the different role
that Lass2-regulated PAI-1 plays before and after
transformation. Taken into consideration the treatments or regions,
in each paired liver tissue from the Lass2 KO and WT mice,
the mRNA and protein levels of PAI-1 in the Lass2 KO liver
were markedly increased when compared to the WT mouse liver
tissues.
TGF-β1 is an effective inducer of
PAI-1 expression and the Smad pathway mediates the
induction of PAI-1 by TGF-β (35,36).
Therefore, we detected the level of TGF-β1 in Lass2
KO livers and WT control. The results demonstrated that the
deletion of Lass2 caused significantly increased
TGF-β1, Smad-4 and -7 levels, which were parallel to
PAI-1 in the condition of non-DEN treatment (basal level).
Following DEN treatment, the mRNA levels of TGF-β1,
Smad-4 and -7 were markedly decreased when compared to basal
levels, and the significance of this is yet to be revealed.
However, for each paired specimen of Lass2 KO and WT tissue,
mRNA levels of TGF-β1 and Smad-4 in the Lass2
KO livers were significantly higher than those of the WT
counterparts (both in TR and NTR, respectively), whereas no
significant difference in mRNA levels of Smad-7 was observed
in the TR and these levels were significantly decreased in NTR in
the Lass2 KO group. The data revealed that the mRNA levels
of TGF-β1, Smad-4 and PAI-1 were significantly
increased in the Lass2 KO livers when compared with the WT
livers despite of the different treatments or regions, parallel to
the increase in PAI-1. These results suggest that the
elevated levels of PAI-1 in Lass2 KO liver may be the
result of upregulation of the
TGF-β1-Smad4-PAI-1 axis. This warrants further
exploration for confirmation. Although previous reports indicate
that the elevated level of TGF-β1-PAI-1 is associated
with metastasis (37–39), metastasis of HCC in both groups was
not observed in the present study.
In summary, the present study demonstrated that
Lass2 is a protective gene against DEN-induced liver
carcinogenesis, and deletion of Lass2 in hepatocytes results
in the earlier occurrence of DEN-induced tumours and more rapid
tumour growth, coincident with elevated levels of the
TGF-β1-Smad4-PAI-1 axis, which may be a
Lass2-associated pathway and the contributor of the
accelerated carcinogenesis in Lass2 KO mice.
Acknowledgements
This work was supported by grant from the State Key
Laboratory of Oncogenes and Related Genes (No. 90-10-02, to X.L.)
and the Shanghai Municipal Program of International Cooperation in
Science and Technology (No. 12410709800, to W.Q.). The authors
thank the Shanghai Research Centre for Model Organisms for their
support in the establishment of the Lass2 KO transgenic mice
used in the experiment.
References
|
1
|
Pewzner-Jung Y, Ben-Dor S and Futerman AH:
When do Lasses (longevity assurance genes) become CerS (ceramide
synthases)?: insights into the regulation of ceramide synthesis. J
Biol Chem. 281:25001–25005. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Teufel A, Maass T, Galle PR and Malik N:
The longevity assurance homologue of yeast lag1 (Lass) gene
family (Review). Int J Mol Med. 23:135–140. 2009.PubMed/NCBI
|
|
3
|
Laviad EL, Albee L, Pankova-Kholmyansky I,
et al: Characterization of ceramide synthase 2: tissue
distribution, substrate specificity, and inhibition by sphingosine
1-phosphate. J Biol Chem. 283:5677–5684. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Becker I, Wang-Eckhardt L, Yaghootfam A,
Gieselmann V and Eckhardt M: Differential expression of
(dihydro)ceramide synthases in mouse brain:
oligodendrocyte-specific expression of CerS2/Lass2.
Histochem Cell Biol. 129:233–241. 2008. View Article : Google Scholar
|
|
5
|
Imgrund S, Hartmann D, Farwanah H, et al:
Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin
sheath defects, cerebellar degeneration, and hepatocarcinomas. J
Biol Chem. 284:33549–33560. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang N, Jin J, Deng Y, et al: Lass2
interacts with V-ATPase and inhibits cell growth of hepatocellular
carcinoma. Sheng Li Xue Bao. 62:196–202. 2010.(In Chinese).
|
|
7
|
Xu X, You J and Pei F: Silencing of a
novel tumor metastasis suppressor gene Lass2/TMSG1 promotes
invasion of prostate cancer cell in vitro through increase of
vacuolar ATPase activity. J Cell Biochem. 113:2356–2363.
2012.PubMed/NCBI
|
|
8
|
Fan S, Niu Y, Tan N, et al: Lass2
enhances chemosensitivity of breast cancer by counteracting acidic
tumor microenvironment through inhibiting activity of V-ATPase
proton pump. Oncogene. 32:1682–1690. 2012. View Article : Google Scholar
|
|
9
|
Wang H, Wang J, Zuo Y, et al: Expression
and prognostic significance of a new tumor metastasis suppressor
gene Lass2 in human bladder carcinoma. Med Oncol.
29:1921–1927. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Binder BR, Christ G, Gruber F, et al:
Plasminogen activator inhibitor 1: physiological and
pathophysiological roles. News Physiol Sci. 17:56–61.
2002.PubMed/NCBI
|
|
11
|
Jing Y, Kovacs K, Kurisetty V, Jiang Z,
Tsinoremas N and Merchan JR: Role of plasminogen activator
inhibitor-1 in urokinase’s paradoxical in vivo tumor suppressing or
promoting effects. Mol Cancer Res. 10:1271–1281. 2012.
|
|
12
|
Bajou K, Noël A, Gerard RD, et al: Absence
of host plasminogen activator inhibitor 1 prevents cancer invasion
and vascularization. Nat Med. 4:923–928. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gutierrez LS, Schulman A, Brito-Robinson
T, Noria F, Ploplis VA and Castellino FJ: Tumor development is
retarded in mice lacking the gene for urokinase-type plasminogen
activator or its inhibitor, plasminogen activator inhibitor-1.
Cancer Res. 60:5839–5847. 2000.PubMed/NCBI
|
|
14
|
Kocic J, Bugarski D and Santibanez JF:
SMAD3 is essential for transforming growth factor-β1-induced
urokinase type plasminogen activator expression and migration in
transformed keratinocytes. Eur J Cancer. 48:1550–1557. 2012.
|
|
15
|
Konrad L, Scheiber JA, Schwarz L, Schrader
AJ and Hofmann R: TGF-β1 and TGF-β2 strongly enhance the secretion
of plasminogen activator inhibitor-1 and matrix metalloproteinase-9
of the human prostate cancer cell line PC-3. Regul Pept. 155:28–32.
2009.
|
|
16
|
Morad SA and Cabot MC:
Ceramide-orchestrated signalling in cancer cells. Nat Rev Cancer.
13:51–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ponnusamy S, Meyers-Needham M, Senkal CE,
et al: Sphingolipids and cancer: ceramide and
sphingosine-1-phosphate in the regulation of cell death and drug
resistance. Future Oncol. 6:1603–1624. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fais S, De Milito A, You H and Qin W:
Targeting vacuolar H+-ATPases as a new strategy against
cancer. Cancer Res. 67:10627–10630. 2007.PubMed/NCBI
|
|
19
|
Lu X, Qin W, Li J, et al: The growth and
metastasis of human hepatocellular carcinoma xenografts are
inhibited by small interfering RNA targeting to the subunit ATP6L
of proton pump. Cancer Res. 65:6843–6849. 2005. View Article : Google Scholar
|
|
20
|
Nishioka N, Matsuoka T, Yashiro M,
Hirakawa K, Olden K and Roberts JD: Plasminogen activator inhibitor
1 RNAi suppresses gastric cancer metastasis in vivo. Cancer Sci.
103:228–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choi JW, Lee JH, Park HS and Kim YS:
PAI-1 expression and its regulation by promoter 4G/5G
polymorphism in clear cell renal cell carcinoma. J Clin Pathol.
64:893–897. 2011. View Article : Google Scholar
|
|
22
|
Fang H, Placencio VR and DeClerck YA:
Protumorigenic activity of plasminogen activator inhibitor-1
through an antiapoptotic function. J Natl Cancer Inst.
104:1470–1484. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen SC, Henry DO, Reczek PR and Wong MK:
Plasminogen activator inhibitor-1 inhibits prostate tumor growth
through endothelial apoptosis. Mol Cancer Ther. 7:1227–1236. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maillard CM, Bouquet C, Petitjean MM, et
al: Reduction of brain metastases in plasminogen activator
inhibitor-1-deficient mice with transgenic ocular tumors.
Carcinogenesis. 29:2236–2242. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Klein RM, Bernstein D, Higgins SP, Higgins
CE and Higgins PJ: SERPINE1 expression discriminates site-specific
metastasis in human melanoma. Exp Dermatol. 21:551–554. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang L and Han X: The urokinase
plasminogen activator system in breast cancer invasion and
metastasis. Biomed Pharmacother. 67:179–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zubac DP, Wentzel-Larsen T, Seidal T and
Bostad L: Type 1 plasminogen activator inhibitor (PAI-1) in
clear cell renal cell carcinoma (CCRCC) and its impact on
angiogenesis, progression and patient survival after radical
nephrectomy. BMC Urol. 10:202010.
|
|
28
|
Jung SY, Song HS, Park SY, Chung SH and
Kim YJ: Pyruvate promotes tumor angiogenesis through
HIF-1-dependent PAI-1 expression. Int J Oncol. 38:571–576.
2011.PubMed/NCBI
|
|
29
|
Allott EH, Morine MJ, Lysaght J, et al:
Elevated tumor expression of PAI-1 and SNAI2 in obese
esophageal adenocarcinoma patients and impact on prognosis. Clin
Transl Gastroenterol. 3:e122012.
|
|
30
|
Zhang W, Ling D, Tan J, Zhang J and Li L:
Expression of urokinase plasminogen activator and plasminogen
activator inhibitor type-1 in ovarian cancer and its clinical
significance. Oncol Rep. 29:637–645. 2013.PubMed/NCBI
|
|
31
|
Foekens JA, Peters HA, Look MP, et al: The
urokinase system of plasminogen activation and prognosis in 2780
breast cancer patients. Cancer Res. 60:636–643. 2000.PubMed/NCBI
|
|
32
|
Schrohl AS, Christensen IJ, Pedersen AN,
et al: Tumor tissue concentrations of the proteinase inhibitors
tissue inhibitor of metalloproteinases-1 (TIMP-1) and plasminogen
activator inhibitor type 1 (PAI-1) are complementary in
determining prognosis in primary breast cancer. Mol Cell
Proteomics. 2:164–172. 2003. View Article : Google Scholar
|
|
33
|
Inoue M, Sawada T, Uchima Y, et al:
Plasminogen activator inhibitor-1 (PAI-1) gene transfection
inhibits the liver metastasis of pancreatic cancer by preventing
angiogenesis. Oncol Rep. 14:1445–1451. 2005.
|
|
34
|
Malinowsky K, Wolff C, Berg D, et al: uPA
and PAI-1 related signalling pathways differ between primary
breast cancers and lymph node metastases. Transl Oncol. 5:98–104.
2012.
|
|
35
|
Chen C, Sun MZ, Liu S, et al: Smad4
mediates malignant behaviors of human ovarian carcinoma cell
through the effect on expressions of E-cadherin, plasminogen
activator inhibitor-1 and VEGF. BMB Rep. 43:554–560. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu Y, Yin WL, Ba YF, et al: Transforming
growth factor-1 promotes the transcriptional activation of
plasminogen activator inhibitor type 1 in carcinoma-associated
fibroblasts. Mol Med Rep. 6:1001–1005. 2012.PubMed/NCBI
|
|
37
|
Kutz SM, Hordines J, McKeown-Longo PJ and
Higgins PJ: TGF-β1-induced PAI-1 gene expression requires
MEK activity and cell-to-substrate adhesion. J Cell Sci.
114:3905–3914. 2001.
|
|
38
|
Humbert L and Lebrun JJ: TGF-beta inhibits
human cutaneous melanoma cell migration and invasion through
regulation of the plasminogen activator system. Cell Signal.
25:490–500. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wilkins-Port CE, Ye Q, Mazurkiewicz JE and
Higgins PJ: TGF-β1 + EGF-initiated invasive potential in
transformed human keratinocytes is coupled to a
plasmin/MMP-10/MMP-1-dependent collagen remodeling axis: role for
PAI-1. Cancer Res. 69:4081–4091. 2009.
|