1. Introduction
WISP proteins [WNT1 (wingless-type MMTV integration
site family, member 1)-inducible signalling pathway proteins] are a
subfamily of the CCN family (1).
WNT1 is a member of a family of cysteine-rich, glycosylated
signalling proteins that mediate diverse developmental processes
(2). The CCN family of proteins is
a crucial group of signalling molecules found in eukaryotic
organisms, and its nomenclature is based on the first 3 members of
the family: cysteine-rich protein 61 (CYR61), connective tissue
growth factor (CTGF) and nephroblastoma overexpressed gene (NOV)
(3), which are now designated as
CCN1, CCN2 and CCN3; and 3 other family members WISP-1 WISP-2 and
WISP-3 are designated as CCN4, CCN5 and CCN6 (4).
rCop-1 (CCN5) was first identified as being
downregulated following transformation of rat embryo fibroblasts by
inactivation of p53 and concomitant activation of H-ras (5). At a similar time, WISP-1 and -2 were
identified as an indirect response to WNT1 but not WNT4 induction
in C57MG mouse mammary epithelial cells. After sequence alignment,
human WISP-1, -2 and -3 were found to be homologous and were cloned
in 1998 (1). These WISP proteins
exhibit the modular structure of the CCN family, characterised by 4
conserved cysteine-rich domains and are believed to be homologous
to the CTGF family of proteins of the CCN family. Another research
group analysed a human osteoblast cDNA library and identified an
EST that contained an IGF binding domain, and based on sequence
homology to the CCN family member CTGF, the authors named the
predicted gene product as CTGF-like protein or CTGF-L (6). CTGF-L (CCN5), encoding a 250-amino
acid single-chain polypeptide of 26 kDa, lacks the C-terminal
domain implicated in dimerisation and heparin binding. These early
discoveries led to further research into their roles in cell
signalling, proliferation, adhesion, invasion, wound healing,
fibrosis, skeletal development, implantation,
epithelial-mesenchymal transition and angiogenesis as well as in
cancers. WISP-2 has been particularly well investigated in human
cancers and is the main subject of the present review.
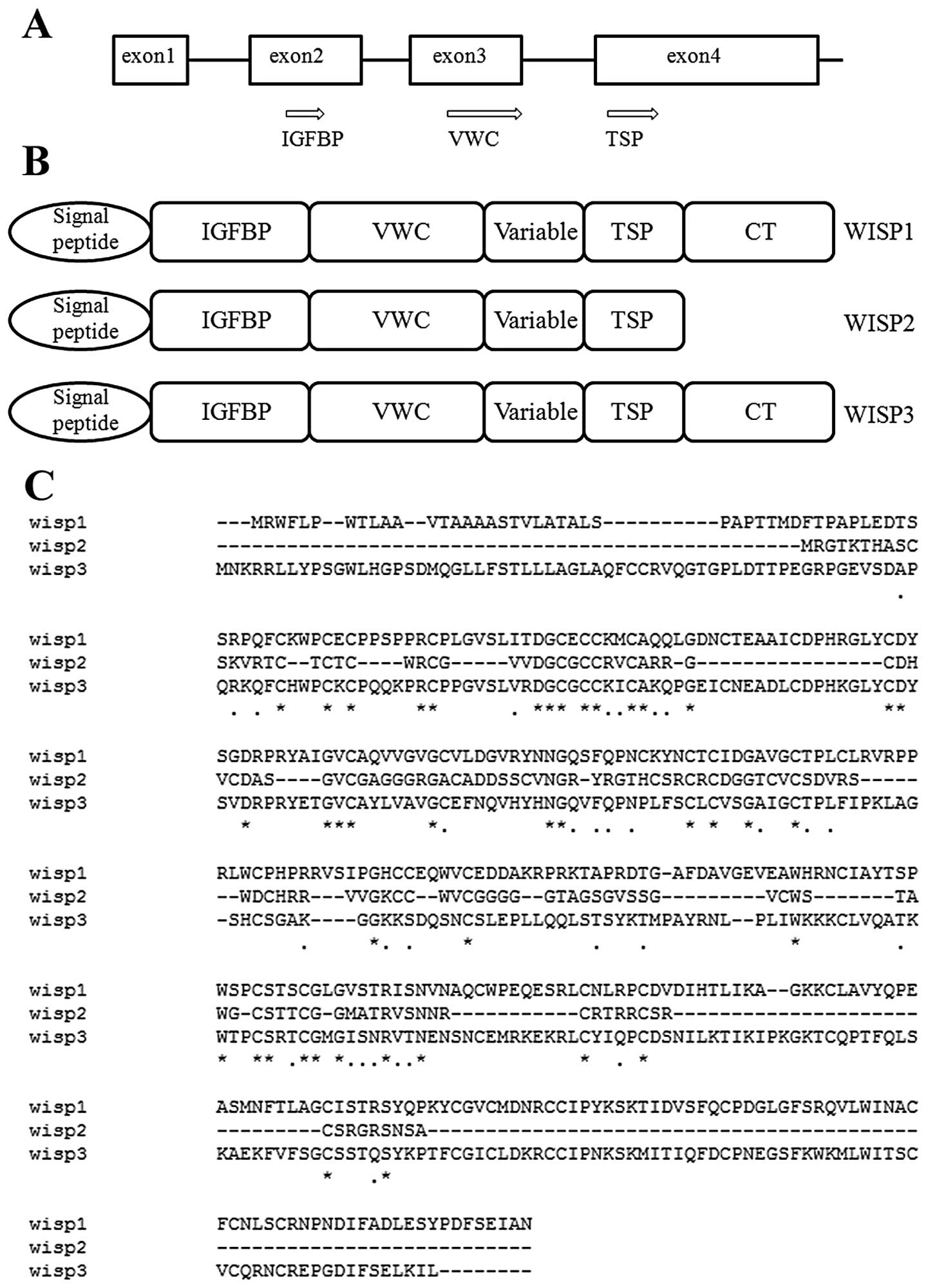
2. Structure of WISP-2
Human WISP-2 is located at chromosome
20q13.12 and consists of 3 exons. Full-length cDNA clones of human
WISP-2 are 1,293 bp in length and encode a protein of 250 amino
acids, with a predicted relative molecular mass of 26 kDa. Mouse
and human WISP-2 are 73% identical at the amino acid level and
homologous to the rat gene, rCop-1 (5). The nucleotide and protein sequence of
WISP-2 shares a 30–40% sequence homology with other family
members.
WISP proteins exhibit the modular architecture of
CCN family members that are characterised by 4 conserved and
discrete cysteine-rich domains that act both independently and in
concert; the N-terminal domain, which includes the first 12
cysteine residues, contains a consensus sequence (GCGCCXXC)
conserved in most insulin-like growth factor (IGF)-binding proteins
(BP) (IGFBPs). This sequence is conserved in WISP-2 and it has been
found that a truncated nov protein lacking the IGFBP domain
in chicken embryo fibroblasts was sufficient to induce their
malignant transformation (7). The
von Willebrand factor type C module (VWC) covers the next 10
cysteine residues, and is thought to participate in protein complex
formation and oligomerisation (8).
A short variable region closely following the VWC domain is highly
susceptible to proteolytic degradation by matrix metalloproteases
(MMPs) (9). It has been shown that
a wide variety of MMPs (MMP-1, -3, -7, -9 and -13) targets this
central linker region, and additional proteases such as elastase
and plasmin could attack linkers that connect domains 1 and 2 or
domains 3 and 4 (10). The third
domain, the thrombospondin domain (TSP), is implicated in binding
with sulfated glycoconjugates and contains 6 cysteine residues and
a conserved WSxCSxxCG motif first identified in thrombospondin
(11) and necessary for the
regulation of endothelial cell proliferation and promotion of cell
attachment (3,12). The C-terminal cystine knot-like (CT)
domain is present in all CCN family members described to date while
WISP-2-encoded protein lacks this domain which is implicated in
receptor binding and dimerisation (13). These receptors include heparin
(14), matrix molecules, integrins
and signalling molecules such as Notch-1 and LRP1 (15).
Due to the importance of the CT domain in receptor
binding, WISP-2 may bind its receptor through other domains, such
as the IGFBP domain (6).
Heparin-binding growth factor (HBGF) from pig uterine luminal
flushings was identified as a highly truncated form of CTGF and
showed that the N-terminal 2/3 of the CTGF primary translation
product is not required for mitogenic activity or heparin binding,
and mitogenic activity of the 10-kDa truncated form of CTGF is
heparin-dependent (14). Growth
factors, such as platelet-derived growth factor, TGF-β and nerve
growth factor, which contain a cystine knot motif, also exist as
dimers. This has led to speculation that WISP-1 and -3 may exist as
dimers, whereas WISP-2 exists as a monomer (1). This has further led to the hypothesis
that WISP-2 may act as a dominant-negative regulator of other CCN
family members (16). Furthermore,
the existence of a putative signal sequence in front of the
N-terminal IGFBP domain and the absence of a transmembrane domain
suggest that WISP-2 is a secreted protein (Fig. 1).
3. WISP-2 expression and clinical
significance in human cancers
Following the identification of WISP-2 as a Wnt1
inducible protein, a number of researchers have focused on the
roles and regulation for this molecule in human disorders,
particularly in cancers.
Clinical studies have shown different expression
profiles and roles of WISP-2 in cancers. The inconsistency between
the results in multiple cancers has raised uncertainty concerning
the role of WISP-2 in carcinogenesis. For example, induction of
WISP-2 by IGF-1 or EGF is required for the mitogenic action in
oestrogen receptor-positive non-invasive breast tumour cells
(17–19), while it acts as a growth
arrest-specific (gas) gene in vascular smooth muscle cells and
prostate cancer cells (20). In
addition, it is likely that WISP-2 plays a preventive role in the
progression of pancreatic cancer as it participates in
morphological alterations from mesenchymal to epithelial transition
(MET) of pancreatic adenocarcinoma (21) and breast cancer cells (22).
The first study concerning tumour cells was reported
in 2000. WISP-2 was found to be markedly increased in 17
β-estradiol-treated MCF-7 human breast cancer cells compared with
control cells and was directly regulated by the oestrogen receptor
(23). The induction of secreted
WISP-2 protein by oestrogen in the culture supernatant was
dose-dependent (24,25). It was therefore believed to be an
oestrogen response gene. A number of other studies have since
reported the relationship between WISP-2 and oestrogen, mostly in
breast cancer.
Breast cancer
More than 20 studies from several laboratories
suggest that elevated WISP-2 has a particular relevance to human
breast disease in vitro and in vivo (21,22,25–28),
and WISP-2 has been indicated as a useful indicator of breast
cancer progression (29). In these
studies, WISP-2 mRNA and protein levels were found to be elevated
in different human breast tumour-derived cell lines, such as MCF-7,
ZR-75, T-47D and SKBR2 (26,30),
in node-positive breast tumours with metastatic potential and in
breast tumours from patients with a poor prognosis (28). These studies also showed that WISP-2
was either undetectable or minimally detectable in normal breast
epithelial cells.
Similar reports from Banerjee et al showed
that WISP-2 was upregulated in non-invasive MCF-7 cells by
epithelial growth factor, and was believed to be linked to poor
prognosis in breast cancer (25).
Silencing of the function of the WISP-2 gene minimized
serum-induced breast tumour cell proliferation (17). Banerjee et al found that
WISP-2 expression in breast samples was biphasic; a marked increase
was noted in non-invasive breast lesions but a significant decrease
was found as cancers progressed from a non-invasive to an invasive
type (22). WISP-2 became almost
undetectable in poorly differentiated cancers when compared with
moderately or well-differentiated samples including testing with
microdissected sections (22). This
indicated a possible protective function of WISP-2 in non-invasive
breast tumour cells. In contrast, the same group also reported that
in hormone-related tumours, including breast cancer, the activation
of WISP-2 expression by oestrogen promoted cancer progression, and
disruption of WISP-2 signalling by use of antisense oligomers in
MCF-7 cells caused a significant reduction in tumour cell
proliferation (25).
Pancreatic adenocarcinoma
Pancreatic adenocarcinoma exhibits greatly decreased
levels of WISP-2 expression compared with adjacent normal
pancreatic tissue and chronic pancreatitis, and the loss of WISP-2
mRNA was associated with overexpression of p53 which was similar to
that in breast carcinoma. Dhar et al revealed a strong
correlation between the degree of differentiation and progression
of pancreatic adenocarcinoma and decreased expression of the WISP-2
signalling protein, which indicated that the development of
pancreatic adenocarcinoma was associated with the silencing of
WISP-2 signalling (21). Treatment
of the highly aggressive pancreatic cancer cell line, MIA-PaCa-2,
with recombinant WISP-2 protein reduced the expression of the
mesenchymal cell marker vimentin and altered the morphological
appearance of the cells to a cobble stoned, epithelial-like
phenotype. These results suggest that WISP-2 may have a role in
maintaining an epithelial-like phenotype in pancreatic
adenocarcinoma cells thereby decreasing their invasive
potential.
Colorectal cancer
Research has shown that WISP-2 is a potential
tumour-suppressor in colorectal cancer. WISP-2 DNA was amplified in
colon tumours, but its transcription was significantly reduced in
the majority of tumours when compared with that in paired normal
colonic mucosa (1). The gene for
human WISP-2 is localised to chromosome 20q13, in a region
frequently amplified and associated with poor prognosis in
node-negative breast cancer and colon cancers, suggesting the
existence of 1 or more oncogenes at this locus (31,32).
It is possible that the apparent amplification observed for WISP-2
may be caused by another gene in this amplicon (1). In another cohort which was comprised
of 94 human colorectal tumours and 80 normal colorectal tissues,
WISP-2 showed a significantly lower level of expression in
colorectal cancer cells when compared with that in normal cells.
Although no significant differences were found within the cancer
group when indices of a more aggressive tumour were compared with
the normal tissue, a significant reduction in expression was
associated with Dukes’ stage, poor differentiation, lower TNM stage
and node-positive disease (33).
Hepatocarcinoma
Research found that the WISP-2 transcript was not
expressed in the 4 hepatocellular carcinoma-derived cell lines
HepG2, HuH-6, HuH-7 and HA22T/VGH (34). However, overexpression of the
hepatitis C viral core protein in Huh-7 cells caused upregulation
of Wnt-1 and WISP-2 and increased proliferation of the cells
(35). As 1 of the 13 genes
activated by Wnt/β-catenin signalling pathway T-cell transcription
factor 4J isoform in HCC cells, WISP-2 was also upregulated in HCC
tumours when compared with that in adjacent peritumour tissues
(36).
Skin cancer
WISP-2 is one of the most abundantly expressed mRNAs
of the CCN family members in normal human skin. Following exposure
to UV irradiation, WISP-2 expression was found to be decreased by
50% at 24 h and returned to a basal level at 48 h (37).
Pituitary tumours
WISP-2 was found to be overexpressed in
adrenocorticotropic hormone (ACTH)-secreting pituitary tumours when
compared to its expression in normal pituitaries, non-secreting
pituitary tumours and growth hormone (GH)-secreting tumours
(38). There was otherwise no
reported association between WISP-2 and gender, age at diagnosis,
tumour size, altered visual field, remission of the disease, or
tumour progression in any subtype of pituitary tumours.
Gastric cancer
The expression of the 3 WISP molecules in a cohort
of 316 cases of human gastric cancers and normal gastric tissues
were analyzed using q-PCR and IHC, respectively, and were
correlated with the clinicopathological features and outcome of the
patients by our group (39).
Knockdown of WISP-2 in human gastric cancer cell lines HGC27 and
AGS was further carried out. The WISP family of proteins, in
particular WISP-2, was a significant independent prognostic
indicator for gastric cancer patients. WISP-2 knockdown resulted in
significant changes in the growth rate and in vitro
invasiveness, with little effect on the adhesive capability, when
compared with its transfection controls. This was found to be
linked to the MMP activities, mediated by the JNK pathway.
4. Signalling regulation of WISP-2 in
cancers
WISP-2 expression can be regulated by various
factors. For example, WNT1 was found to regulate WISP-2 in the
mouse mammary epithelial cell line C57MG (1) and WNT signalling-activated
s/αβ-catenin in synovial fibroblasts (40). In cancer cells, much effort has been
focused on the role of Wnt signalling, oestrogen signalling, serum
and hormones (41).
Wnt signalling
The wingless (wg)/Wnt family of secreted signalling
molecules and the downstream components of Wnt signal transduction
are highly conserved among animal species. Canonical and
non-canonical pathway transductions result in tissue-specific cell
fate decisions during embryogenesis and regulate cell
proliferation, proper alignment and bundling of actin filaments in
adult tissues (42). WISP-2 has
been found to be one of such downstream components. Expression of
HCV core protein by transient transfection in the human HCC-derived
cell line Huh-7 increased cell proliferation, DNA synthesis, and
cell cycle progression by upregulation of Wnt1 and WISP-2 (35). Wnt-PKA and Wnt-aPKC are both
non-canonical Wnt signalling pathways (43). Treatment with protein kinase A (PKA)
activators CT/IBMX induced WISP-2 mRNA expression in the MCF-7
human breast cancer cell line by a direct mechanism. Simultaneous
treatment with protein kinase C (PKC) activators,
12-O-tetradecanoylphorbol-13-acetate (TPA) and oestrogen E2
completely prevented WISP-2 induction by E2 (44). The Wnt/β-catenin signalling pathway
regulates genes involved in cell proliferation, survival, migration
and invasion through regulation by T-cell factor (TCF)-4
transcription factor proteins. WISP-2 was 1 of the 13 genes found
to be activated by T-cell transcription factor 4J isoform in HCC
HAK-1A cells, and it was also found to be upregulated in HCC
tumours when compared with adjacent peritumour tissues (36).
Oestrogen signalling
Several studies revealed that WISP-2 is oestrogen
inducible in human breast cancer MCF-7 cells and is implicated in
tumour cell proliferation (23–25).
Inadera et al found that WISP-2 induction was highly
specific for hormones that interact with the oestrogen receptor in
MCF-7 cells (24). The oestrogen
receptor α (ER-α) appears to be directly responsible for oestrogen
induction of WISP-2 expression, as cultured human mammary
epithelial cells that lack ER-α do not respond to oestrogen
stimulation. However, stable transfection of ER-α into these cells
rendered the ability of oestrogen to induce WISP-2 expression
(25). There is some evidence to
suggest that oestrogen may also function to stabilize WISP-2 mRNA.
Banerjee et al reported that WISP-2 was upregulated by
progesterone through a PR-dependent mechanism in MCF-7 cells,
although the induction of progesterone was rapid and transient.
When used in combination with oestradiol, progesterone acted as an
antagonist to inhibit the expression of WISP-2, indicating a dual
action of progesterone (25). In
addition, PLK1, a key regulator of cell division, was found to be
overexpressed in many types of human cancers, mediating
ER-regulated gene transcription by coactivating WISP-2 and
suggesting a mechanism for the tumour-suppressive role of PLK1 in
MCF7 cells as an interphase transcriptional regulator of WISP-2
(45).
Signalling pathway crosstalk within oestrogen/WISP-2
signalling has also been the subject of several investigations.
Treatment of MCF-7 cells with TPA completely blocked
oestrogen-induced WISP-2 mRNA transcription (44). Epidermal growth factor (EGF) has
been shown to induce expression of WISP-2 mRNA in MCF-7 cells in a
dose- and time-dependent manner and can act synergistically with
oestrogen to raise WISP-2 expression levels, possibly through the
PI3K and MAPK signalling pathways (17). A similar study was carried out by
the same group using IGF-1 and reported a similar result. IGF-1
induced WISP-2 mRNA expression in a dose- and time-dependent
manner, and knockdown of WISP-2 abrogated the ability of IGF-1 to
stimulate MCF-7 cell proliferation. The IGF-1 induction of CCN5
expression was blocked by a pure anti-oestrogen drug, but unlike
EGF the signalling crosstalk appeared to function through PI3K/AKT
signalling (18).
Other regulators: serum, hormone and
transcription factors
WISP-2 is serum-inducible during the process of
mitogen-induced tumour cell proliferation (26). WISP-2 was found to be overexpressed
in ACTH-secreting pituitary tumours when compared to that in normal
pituitaries, NS pituitary tumours and GH-secreting tumours
(38). However, there were no
differences in expression of genes in the canonical and
non-canonical Wnt pathways between all studied subtypes of
pituitary tumours and normal pituitaries. It has been suggested
that the elevated glucocorticoid levels observed in ACTH-secreting
pituitary tumours activate WISP-2 transcription since WISP-2 has a
glucocorticoid-responsive region in its promoter (46). The same phenomenon was found in
ER-negative breast cancer cells. MDA-MB-231 cells exposed to
glucocorticoids underwent morphological alterations, decreased
invasiveness and attenuated expression of mesenchymal markers.
These results thus indicate that the induction of the WISP-2 gene
promoter probably requires the agonist-activated glucocorticoid
receptor. Taken together, these results indicate that
glucocorticoid treatment of ER-negative breast cancer cells induces
high levels of WISP-2 expression and is accompanied by a more
differentiated and less invasive epithelial phenotype. These
findings propose a novel therapeutic strategy for high-risk breast
cancer patients (46). In addition,
Stiehl et al found that amphiregulin (AREG) and WISP-2
expression was strongly dependent on hypoxia inducible factor
(HIF)-2α and their promoters were particularly responsive to HIF-2α
in breast cancer. A strong correlation among HIF-2α/AREG/WISP-2
protein levels in breast cancer samples provides evidence that the
HIF-2α-specific transcriptional pathway could have an important
role in maintaining a non-invasive phenotype (47).
WISP-2 in other physiopathologic
processes
WISP-2 is also essential in other physiopathological
processes including apoptosis, anti-proliferation and osteogenic
differentiation. Retroviral overexpression of rCop-1 (WISP-2) was
found to induce apoptosis in transformed rat fibroblasts, but was
unable to affect normal fibroblasts (48). Cop-1 mRNA was expressed at high
levels in quiescent vascular smooth muscle cells (VSMCs) and in
heparin-treated VSMCs but was found at low levels in proliferating
VSMCs, indicating that COP-1 may play a role in the
anti-proliferative mechanism of action of heparin (48). Another report provided functional
evidence that WISP-2 is a growth arrest-specific gene that is
temporally and spatially expressed and can inhibit VSMC
proliferation, motility and invasiveness; however, adhesion and
apoptosis were unaffected by WISP-2 in VSMCs (20). In addition, WISP-2 was found to be
relevant to the low osteogenic differentiation capacity of
placental mesenchymal stromal cells when compared to mesenchymal
stromal cells from bone marrow (49). Large-scale analysis of transcripts
in non-familial, isolated ACTH-independent macronodular adrenal
hyperplasia (AIMAH) confirmed clinical heterogeneity and revealed
that WISP-2 can be used as a clinical index of GIP-dependent AIMAH
(50).
5. Roles of WISP-2 in proliferation,
motility, invasiveness, adhesion and epithelial-mesenchymal
transition
In vitro and in vivo studies have
indicated the potential roles of WISP-2 in regulating cell
proliferation, motility, invasiveness, adhesion and EMT.
Proliferation
Overexpression of WISP-2 has been shown to inhibit
serum-induced proliferation of highly invasive ER-negative breast
cancer cell line MDA-MB-231 (51).
However, in the less invasive ER-positive MCF-7 cell line, the
effect of WISP-2 is not consistent. Some have suggested an
inhibitory role in serum-induced proliferation of MCF-7 cells
(51). Others have suggested a
promoting role in MCF-7 cell proliferation (25) or no effect (22). Moreover, the ability of PMA, EGF or
IGF-1 alone to induce MCF-7 cell proliferation was blocked by
WISP-2 knockdown (17,19,21).
Knockdown of WISP-2 in MCF-7 cells was found to eliminate the
oestrogen-dependent growth requirement of these cells. More studies
are needed to clarify the biochemical and biological basis of the
contrasting role of WISP-2 in these cells.
Motility, invasiveness and
metastasis
Overexpression of WISP-2 was found to inhibit both
motility and invasiveness in the highly aggressive breast carcinoma
cell line, MDA-MB-231 (51). The
inhibitory effect of WISP-2 on motility was also observed in MCF-7
cells where knockdown of WISP-2 expression increased the
IGF-1-induced motility of MCF-7 cells. WISP-2 knockdown in MCF-7
cells also induced expression of pro-motility enzymes such as MMP-2
and -9 (22,51). Mutant p53 overexpression induced in
MCF-7 cells exhibiting increased invasiveness was inhibited by
treatment with recombinant WISP-2 protein (52).
Adhesion
Little is known concerning the role of WISP-2 in
cell adhesion. Kumar et al observed that 3 different
osteoblastic cell lines, primary human osteoblasts, osteosarcoma
MG63 cells, and rat osteoblast-like osteosarcoma Ros 17/2.8 cells,
attached to immobilized CCN5 in a dose-dependent manner (6). Recent data from our laboratory
revealed that WISP-2 knockdown in gastric cancer cells resulted in
little effect on the adhesive capability, compared with its
transfection controls (39).
EMT
Phenotypical alterations including EMT are a
hallmark of the progression of cancer and provide a new basis for
understanding the progression of cancer toward a more malignant
state. Mesenchymal cells are also implicated in the formation of
epithelial organs through mesenchymal-epithelial transition (MET).
Cellular plasticity, the ability to undergo EMT and subsequently
MET in the appropriate microenvironments are key features of a
successful metastatic cell (53).
The process of EMT plays an important role during foetal, postnatal
development, invasion and metastases and is regulated by
transcription factors such as Twist1, Snai1 and Slug, which inhibit
E-cadherin expression (54).
Current evidence suggests that WISP-2 may suppress
EMT in different cancers and that EMT in turn can suppress WISP-2
expression. Human pancreatic adenocarcinoma is associated with the
silencing of WISP-2/CCN5 signalling. Functional analysis studies
demonstrated that exposure of pancreatic cancer MIA-PaCa-2 cells to
WISP-2 recombinant protein for 48 h markedly altered the phenotype
of these cells from a spindle shape (mesenchymal type) to a
cobblestone-like shape (epithelial type) and also markedly reduced
the expression of vimentin, a mesenchymal marker, in these cells,
suggesting that WISP-2 may play a critical role in reversing EMT
(or inducing MET) (21). Although
mitogen-induced upregulation of WISP-2 participates in cell
proliferation events of ER-positive breast cancer cells, the basal
level of WISP-2 does not exhibit a mitogenic response in these
cells (17–19). Instead, it protects cells from
gaining invasive phenotypes. For example, silencing of WISP-2 in
MCF-7 non-invasive carcinoma cells significantly enhanced motility
and EMT, and it modulated the expression of several genes
associated with invasive phenotypes of cancer cells (22,51,52,55),
while ectopic Snail expression suppressed WISP-2 transcripts and
downregulated WISP-2 gene promoter expression in transfected cells
(55). In WISP-2-knockout
ER-α-positive breast cancer cells, IGF-1 and EGF lost their
mitogenic effect (17,18) but possibly gained aggressive
phenotypes. Sabbah et al showed that WISP-2 silencing
promoted EMT via activation of the TGF/β signalling cascade known
to promote EMT in breast cancer (56). Recently, Ferrand et al
discovered that glucocorticoid treatment of ER-negative breast
cancer cells induced high levels of WISP-2 expression and this was
accompanied by marked changes in the cellular morphology. Cells
were found to grow as groups of flattened cells consistent with a
normal epithelial cell phenotype. This morphological change was
correlated with a reduction in cell motility and invasion,
characteristic of a more differentiated and less invasive
epithelial phenotype. Meanwhile, WISP-2 expression repressed
cadherin 11, vimentin and ZEB1 expression (46).
6. Perspectives
WISP-2 is a unique member of the CCN family that
lacks the CT domain and exhibits different functions in multiple
cellular processes. However, similar to the other CCN family
members, WISP-2 is a protein with important roles in embryonic
development, normal cell function and disease, particularly in
cancers. The functions of WISP-2 in human cancers include effects
on cell proliferation, adhesion, motility, invasiveness, metastasis
and epithelial-mesenchymal transition (EMT); however these
functions are dependent upon the cell and tissue type and the
microenvironment. Several independent studies have shown the
expression pattern of WISP-2 and a link with patient clinical
course in breast cancer, pancreatic cancer, hepatocarcinoma,
colorectal and gastric cancer. However, the results are
inconsistent and somewhat conflicting in certain tumour types.
Further clinical research requires studies using larger clinical
cohorts and scientific investigation into the cellular functions in
more than 2 cell lines together, which would allow for more
powerful statistical conclusions and further insight into the
action of WISP-2. Studies to decipher the myth of its domain
binding in relation to the differential response in different cell
types to different stimuli would also be important. Together,
WISP-2 is a potential regulator and a novel therapeutic target in
cancer and warrants further investigation at the cellular and
clinical levels.
Acknowledgements
The authors wish to thank the Cancer Research Wales
and the Albert Hung Foundation for supporting their study. S.J. and
K.J. are recipients of the China Medical Scholarship of Cardiff
University.
References
|
1
|
Pennica D, Swanson TA, Welsh JW, et al:
WISP genes are members of the connective tissue growth
factor family that are up-regulated in wnt-1-transformed cells and
aberrantly expressed in human colon tumors. Proc Natl Acad Sci USA.
95:14717–14722. 1998. View Article : Google Scholar
|
|
2
|
Zhong N, Gersch RP and Hadjiargyrou M: Wnt
signalling activation during bone regeneration and the role of
Dishevelled in chondrocyte proliferation and differentiation. Bone.
39:5–16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bork P: The modular architecture of a new
family of growth regulators related to connective tissue growth
factor. FEBS Lett. 327:125–130. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brigstock DR: The CCN family: a new
stimulus package. J Endocrinol. 178:169–175. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang R, Averboukh L, Zhu W, et al:
Identification of rCop-1, a new member of the CCN protein family,
as a negative regulator for cell transformation. Mol Cell Biol.
18:6131–6141. 1998.PubMed/NCBI
|
|
6
|
Kumar S, Hand AT, Connor JR, et al:
Identification and cloning of a connective tissue growth
factor-like cDNA from human osteoblasts encoding a novel regulator
of osteoblast functions. J Biol Chem. 274:17123–17131. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Joliot V, Martinerie C, Dambrine G, et al:
Proviral rearrangements and overexpression of a new cellular gene
(nov) in myeloblastosis-associated virus type 1-induced
nephroblastomas. Mol Cell Biol. 12:10–21. 1992.PubMed/NCBI
|
|
8
|
Mancuso DJ, Tuley EA, Westfield LA, et al:
Structure of the gene for human von Willebrand factor. J Biol Chem.
264:19514–19527. 1989.PubMed/NCBI
|
|
9
|
Hashimoto G, Inoki I, Fujii Y, Aoki T,
Ikeda E and Okada Y: Matrix metalloproteinases cleave connective
tissue growth factor and reactivate angiogenic activity of vascular
endothelial growth factor 165. J Biol Chem. 277:36288–36295. 2002.
View Article : Google Scholar
|
|
10
|
de Winter P, Leoni P and Abraham D:
Connective tissue growth factor: structure-function relationships
of a mosaic, multifunctional protein. Growth Factors. 26:80–91.
2008.PubMed/NCBI
|
|
11
|
Holt GD, Pangburn MK and Ginsburg V:
Properdin binds to sulfatide [Gal(3-SO4)beta 1-1 Cer]
and has a sequence homology with other proteins that bind sulfated
glycoconjugates. J Biol Chem. 265:2852–2855. 1990.
|
|
12
|
Karagiannis ED and Popel AS: Peptides
derived from type I thrombospondin repeat-containing proteins of
the CCN family inhibit proliferation and migration of endothelial
cells. Int J Biochem Cell Biol. 39:2314–2323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Voorberg J, Fontijn R, Calafat J, Janssen
H, van Mourik JA and Pannekoek H: Assembly and routing of von
Willebrand factor variants: the requirements for disulfide-linked
dimerization reside within the carboxy-terminal 151 amino acids. J
Cell Biol. 113:195–205. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brigstock DR, Steffen CL, Kim GY, Vegunta
RK, Diehl JR and Harding PA: Purification and characterization of
novel heparin-binding growth factors in uterine secretory fluids.
Identification as heparin-regulated Mr 10,000 forms of connective
tissue growth factor. J Biol Chem. 272:20275–20282. 1997.
View Article : Google Scholar
|
|
15
|
Holbourn KP, Perbal B and Ravi Acharya K:
Proteins on the catwalk: modelling the structural domains of the
CCN family of proteins. J Cell Commun Signal. 3:25–41. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kubota S and Takigawa M: CCN family
proteins and angiogenesis: from embryo to adulthood. Angiogenesis.
10:1–11. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Banerjee S, Sengupta K, Saxena NK, Dhar K
and Banerjee SK: Epidermal growth factor induces WISP-2/CCN5
expression in estrogen receptor-α-positive breast tumor cells
through multiple molecular cross-talks. Mol Cancer Res. 3:151–162.
2005.
|
|
18
|
Dhar K, Banerjee S, Dhar G, Sengupta K and
Banerjee SK: Insulin-like growth factor-1 (IGF-1) induces
WISP-2/CCN5 via multiple molecular cross-talks and is essential for
mitogenic switch by IGF-1 axis in estrogen receptor-positive breast
tumor cells. Cancer Res. 67:1520–1526. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sengupta K, Banerjee S, Dhar K, et al:
WISP-2/CCN5 is involved as a novel signalling intermediate in
phorbol ester-protein kinase Cα-mediated breast tumor cell
proliferation. Biochemistry. 45:10698–10709. 2006.PubMed/NCBI
|
|
20
|
Lake AC, Bialik A, Walsh K and Castellot
JJ Jr: CCN5 is a growth arrest-specific gene that regulates smooth
muscle cell proliferation and motility. Am J Pathol. 162:219–231.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dhar G, Mehta S, Banerjee S, et al: Loss
of WISP-2/CCN5 signalling in human pancreatic cancer: a potential
mechanism for epithelial-mesenchymal-transition. Cancer Lett.
254:63–70. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Banerjee S, Dhar G, Haque I, et al:
CCN5/WISP-2 expression in breast adenocarcinoma is associated with
less frequent progression of the disease and suppresses the
invasive phenotypes of tumor cells. Cancer Res. 68:7606–7612. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Inadera H, Hashimoto S, Dong HY, et al:
WISP-2 as a novel estrogen-responsive gene in human breast cancer
cells. Biochem Biophys Res Commun. 275:108–114. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Inadera H, Dong HY and Matsushima K:
WISP-2 is a secreted protein and can be a marker of estrogen
exposure in MCF-7 cells. Biochem Biophys Res Commun. 294:602–608.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Banerjee S, Saxena N, Sengupta K, Tawfik
O, Mayo MS and Banerjee SK: WISP-2 gene in human breast cancer:
estrogen and progesterone inducible expression and regulation of
tumor cell proliferation. Neoplasia. 5:63–73. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zoubine MN, Banerjee S, Saxena NK,
Campbell DR and Banerjee SK: WISP-2: a serum-inducible gene
differentially expressed in human normal breast epithelial cells
and in MCF-7 breast tumor cells. Biochem Biophys Res Commun.
282:421–425. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ray G, Banerjee S, Saxena NK, Campbell DR,
Van Veldhuizen P and Banerjee SK: Stimulation of MCF-7 tumor
progression in athymic nude mice by 17β-estradiol induces
WISP-2/CCN5 expression in xenografts: A novel signalling molecule
in hormonal carcinogenesis. Oncol Rep. 13:445–448. 2005.PubMed/NCBI
|
|
28
|
Davies SR, Watkins G, Mansel RE and Jiang
WG: Differential expression and prognostic implications of the CCN
family members WISP-1, WISP-2, and WISP-3 in human breast cancer.
Ann Surg Oncol. 14:1909–1918. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Banerjee SK and Banerjee S: CCN5/WISP-2: a
micromanager of breast cancer progression. J Cell Commun Signal.
6:63–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saxena N, Banerjee S, Sengupta K, Zoubine
MN and Banerjee SK: Differential expression of WISP-1 and WISP-2
genes in normal and transformed human breast cell lines. Mol Cell
Biochem. 228:99–104. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tanner MM, Tirkkonen M, Kallioniemi A, et
al: Increased copy number at 20q13 in breast cancer: defining the
critical region and exclusion of candidate genes. Cancer Res.
54:4257–4260. 1994.PubMed/NCBI
|
|
32
|
Bischoff JR, Anderson L, Zhu Y, et al: A
homologue of Drosophila aurora kinase is oncogenic and
amplified in human colorectal cancers. EMBO J. 17:3052–3065.
1998.PubMed/NCBI
|
|
33
|
Davies SR, Davies ML, Sanders A, Parr C,
Torkington J and Jiang WG: Differential expression of the CCN
family members WISP-1, WISP-2 and WISP-3 in human colorectal cancer
and the prognostic implications. Int J Oncol. 36:1129–1136.
2010.PubMed/NCBI
|
|
34
|
Cervello M, Giannitrapani L, Labbozzetta
M, et al: Expression of WISPs and of their novel alternative
variants in human hepatocellular carcinoma cells. Ann NY Acad Sci.
1028:432–439. 2004. View Article : Google Scholar
|
|
35
|
Fukutomi T, Zhou Y, Kawai S, Eguchi H,
Wands JR and Li J: Hepatitis C virus core protein stimulates
hepatocyte growth: correlation with upregulation of wnt-1
expression. Hepatology. 41:1096–1105. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tomimaru Y, Koga H, Yano H, de la Monte S,
Wands JR and Kim M: Upregulation of T-cell factor-4
isoform-responsive target genes in hepatocellular carcinoma. Liver
Int. 33:1100–1112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Quan T, Shin S, Qin Z and Fisher GJ:
Expression of CCN family of genes in human skin in vivo and
alterations by solar-simulated ultraviolet irradiation. J Cell
Commun Signal. 3:19–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Colli LM, Saggioro F, Serafini LN, et al:
Components of the canonical and non-canonical Wnt pathways are not
mis-expressed in pituitary tumors. PLoS One. 8:e624242013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ji J, Jia S and Jiang WG: WISP2 is an
independent prognosis indicator of gastric cancer patients and
regulates the biological function of gastric cancer cells via the
JNK pathway. Eur J Cancer. 49:S5722013.
|
|
40
|
Tanaka I, Morikawa M, Okuse T, Shirakawa M
and Imai K: Expression and regulation of WISP2 in rheumatoid
arthritic synovium. Biochem Biophys Res Commun. 334:973–978. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Russo JW and Castellot JJ: CCN5: biology
and pathophysiology. J Cell Commun Signal. 4:119–130. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bejsovec A: Wnt pathway activation: new
relations and locations. Cell. 120:11–14. 2005.PubMed/NCBI
|
|
43
|
De A: Wnt/Ca2+ signalling
pathway: a brief overview. Acta Biochim Biophys Sin. 43:745–756.
2011.
|
|
44
|
Inadera H: Estrogen-induced genes, WISP-2
and pS2, respond divergently to protein kinase pathway. Biochem
Biophys Res Commun. 309:272–278. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wierer M, Verde G, Pisano P, et al: PLK1
signalling in breast cancer cells cooperates with estrogen
receptor-dependent gene transcription. Cell Rep. 3:2021–2032. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ferrand N, Stragier E, Redeuilh G and
Sabbah M: Glucocorticoids induce CCN5/WISP-2 expression and
attenuate invasion in oestrogen receptor-negative human breast
cancer cells. Biochem J. 447:71–79. 2012. View Article : Google Scholar
|
|
47
|
Stiehl DP, Bordoli MR, Abreu-Rodriguez I,
et al: Non-canonical HIF-2α function drives autonomous breast
cancer cell growth via an AREG-EGFR/ErbB4 autocrine loop. Oncogene.
31:2283–2297. 2012.PubMed/NCBI
|
|
48
|
Delmolino LM, Stearns NA and Castellot JJ
Jr: COP-1, a member of the CCN family, is a heparin-induced growth
arrest specific gene in vascular smooth muscle cells. J Cell
Physiol. 188:45–55. 2001. View Article : Google Scholar
|
|
49
|
Ulrich C, Rolauffs B, Abele H, et al: Low
osteogenic differentiation potential of placenta-derived
mesenchymal stromal cells correlates with low expression of the
transcription factors Runx2 and Twist2. Stem Cells Dev. Jul
20–2013.(Epub ahead of print).
|
|
50
|
Bourdeau I, Antonini SR, Lacroix A, et al:
Gene array analysis of macronodular adrenal hyperplasia confirms
clinical heterogeneity and identifies several candidate genes as
molecular mediators. Oncogene. 23:1575–1585. 2004. View Article : Google Scholar
|
|
51
|
Fritah A, Saucier C, De Wever O, et al:
Role of WISP-2/CCN5 in the maintenance of a differentiated and
noninvasive phenotype in human breast cancer cells. Mol Cell Biol.
28:1114–1123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dhar G, Banerjee S, Dhar K, et al: Gain of
oncogenic function of p53 mutants induces invasive phenotypes in
human breast cancer cells by silencing CCN5/WISP-2. Cancer Res.
68:4580–4587. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hugo H, Ackland ML, Blick T, et al:
Epithelial-mesenchymal and mesenchymal-epithelial transitions in
carcinoma progression. J Cell Physiol. 213:374–383. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ouelaa-Benslama R, De Wever O, Hendrix A,
et al: Identification of a GαGβγ, AKT and PKCα signalome associated
with invasive growth in two genetic models of human breast cancer
cell epithelial-to-mesenchymal transition. Int J Oncol. 41:189–200.
2012.
|
|
56
|
Sabbah M, Prunier C, Ferrand N, et al:
CCN5, a novel transcriptional repressor of the transforming growth
factor β signalling pathway. Mol Cell Biol. 31:1459–1469.
2011.PubMed/NCBI
|