Introduction
Osteosarcoma (OS) is the most common primary bone
malignancy mainly affecting children and adolescents with an
extremely high propensity for local invasion and distant metastasis
(1,2). Chemotherapy has become a cornerstone
for the primary treatment of osteosarcoma. Multimodal treatment
regimens usually involve neoadjuvant chemotherapy with high-dose
methotrexate, doxorubicin, cisplain and more recently ifosfamide.
This regimen has improved patient survival from 20% with surgical
resection alone to 70% for localized disease (3). However, despite intensive efforts in
both surgical and medical management, the survival rate has not
improved over the last 30 years, and 40% of OS patients succumb to
the disease (4). Multidrug
resistance (MDR) is a formidable barrier to the success of cancer
chemotherapy (5,6). Therefore, researchers are working
intensely to discover new anticancer drugs as therapeutic regimens
against OS.
Natural products, including plants, animals and
microorganisms, have played a major role in new drug discovery for
centuries, with over 74.9% of approved anticancer agents being of
natural origin (7,8). Traditional Chinese medicine (TCM) uses
natural products guided by TCM theories, to treat various diseases.
TCM has been confirmed to possess effective anticancer drugs
against cancers including OS (9–11), and
even against drug-resistant cells (12–14).
Pien Tze Huang (PZH) is a well-known traditional Chinese formula
that was first prescribed 450 years ago in the Ming Dynasty. The
main ingredients of PZH include Moschus, Calculus
bovis, snake gall and Radix Notoginseng. The main
efficacy of PZH is heat-clearing, detoxification, promotion of
blood circulation, reduction in blood stasis and swelling (15). According to TCM theories, the
pathogenesis of cancer is related to accumulation of toxic dampness
and heat and stagnation of blood stasis, thus PZH has been used to
treat various types of cancers (16–19).
Our previous studies revealed that PZH is able to inhibit OS growth
in vivo and in vitro via induction of apoptosis and
inhibition of migratory and invasive abilities (20–23).
However, little is known regarding the effects of PZH on
chemotherapy-resistant OS cell lines.
OS cells employ a host of different mechanisms
against resistant to one or more chemotherapeutic drugs. Abnormal
expression of apoptosis-related proteins is closely related to
chemotherapeutic drug resistance. Previous reports indicate that
Bcl-2 family proteins are expressed at a high level in OS (24). Apoptosis is regulated by the balance
of pro- and anti-apoptotic members of the Bcl-2 family proteins.
Anti-apoptotic Bcl-2 protein is a key protein that blocks
apoptosis; overexpression of anti-apoptotic Bcl-2 protein is
closely related to evasion of apoptosis and increased chemotherapy
resistance. In contrast, pro-apoptotic Bax proteins can improve the
sensitivity of malignant cells to apoptosis, thereby overcoming
drug resistance (25,26). Survivin protein which belongs to the
inhibitor of apoptosis (IAP) family has two known functions:
regulation of cell division and inhibition of apoptosis. It has
been widely demonstrated that overexpression of survivin causes
resistance to various chemotherapeutic agents (27). P-glycoprotein (P-gp), a
transmembrane glycoprotein, that functions as a drug efflux pump,
reduces the intercellular accumulation and toxicity of numerous
anticancer drugs, including doxorubicin, paclitaxel, and the vinca
alkaloids. The overexpression of P-gp is one of the most studied
mechanisms of drug resistance (28,29).
In addition, P-gp also plays a special role in the
caspase-dependent apoptosis pathway in drug-resistant cancer cells
(30).
In order to extend the clinical observations of the
potential anticancer effect of PZH and help to elucidate the
mechanism of its anticancer activity, in the present study, the
cellular effects of PZH on multidrug-resistant OS U2OS/ADM cells
were investigated, and the changes in apoptosis and
drug-resistance-related factors were also examined. We found that
PZH significantly inhibited the growth of U2OS/ADM cells through
arrest in the G2/M phase of the cell cycle and promoted
apoptosis of U2OS/ADM cells by downregulation of the expression of
Bcl-2 and survivin and upregulation of the expression of Bax; at
the same time P-gp expression was inhibited. These data indicate
that PZH is a valuable agent that may be useful for treating OS
patients with drug resistance.
Materials and methods
Materials and reagents
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), Hoechst 33258, TRIzol reagent,
penicillin-streptomycin were obtained from Invitrogen Inc. (Grand
Island, NY, USA). Trypsin was purchased from HyClone Laboratories
Inc. (Logan, UT, USA). Rhodamine was purchased from Sigma (St.
Louis, MO, USA). Cycle Test Plus DNA Reagent kit and an apoptosis
assay (FITC Annexin V-FITC Apoptosis Detection Kit II) were
provided by Becton-Dickinson (San Jose, CA, USA). The Bcl-2, Bax,
survivin and GAPDH primers were purchased from Sangon Biotech Co.,
Ltd. (Shanghai, China). Bcl-2, Bax, survivin and P-gp antibodies,
horseradish peroxidase (HRP)-conjugated secondary antibodies and
the antibody against β-actin were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA).
Preparation of PZH
PZH was obtained from and authenticated by the sole
manufacturer Zhangzhou Pien Tze Huang Pharmaceutical Co., Ltd.
China (Chinese FDA approval no: Z35020242). Stock solutions were
prepared by dissolving PZH power in 10% dimethyl sulfoxide (DMSO)
to a concentration of 30 mg/ml. The working concentrations of PZH
were obtained by diluting the stock solution in the culture medium.
The final concentrations of DMSO in the medium were <1%.
Cell lines and cell culture
The human OS cell line U2OS was obtained from the
Institute of Biochemistry and Cell Biology, Chinese Academy of
Sciences (Shanghai, China). The multidrug-resistant OS cell line
U2OS/ADM, which overexpresses multidrug resistance protein 1 (MDR1,
also known as P-gp) and multidrug resistance-associated protein
(MRP1), was selected in a step-wise manner by exposing
drug-sensitive parental cells to increasing doses of adriamycin
(ADM) (31). Both cell lines were
grown as adherent monolayers in a flask with DMEM culture medium
containing 10% FBS and 100 U/ml penicillin and 100 μg/ml
streptomycin at 37°C in a humidified atmosphere of 5%
CO2. To maintain drug resistance, 1 μg/ml ADM was
supplemented at a regular interval of 2 days, but was omitted 2
weeks before any of the experiments. Logarithmically growing cells
were used for all experiments.
Cell viability studies
The effects of PZH on the proliferation of U2OS/ADM
and U2OS cells were measured by MTT colorimetric assay. Cells were
seeded at 1×104 cells/well in 96-well plates (Corning
Costar Corporation, Corning, NY, USA). After 24 h of incubation
with fresh medium, various concentrations of PZH were added to the
plates. The number of viable cells was determined at daily
intervals (24, 48 and 72 h). At the end of the treatment, 100 μl
MTT [0.5 mg/ml in phosphate-buffered saline (PBS)] was added to
each well, and the samples were incubated for an additional 4 h at
37°C. The purple-blue MTT formazan precipitate was dissolved in 100
μl DMSO. The absorbance was measured at 570 nm using an ELx808™
absorbance microplate reader (BioTek Instruments Inc., Winooski,
VT, USA). The relative cell viability was expressed as the ratio
(%) of the absorbance in the experimental wells to that of the
control wells (normal culture medium with 1% DMSO). The 50%
inhibitory concentration (IC50) was calculated.
Cell cycle assays
Cell cycle analysis was carried out by a flow
cytometry assay and the Cycle Test Plus DNA Reagent kit. Briefly,
U2OS/ADM cells were treated with PZH for 48 h at concentrations of
0.4, 0.8 and 1.6 mg/ml. Control cells were treated with normal
culture medium with 1% DMSO. After incubation, cells were harvested
by trypsinization and washed twice with ice cold PBS. PI staining
was performed according to the Cycle Test Plus DNA Reagent kit
manufacturer's recommendations. Finally, the cell cycle
distribution was determined using fluorescence-activated cell
sorting (FACSCalibur; Becton-Dickinson, San Jose, CA, USA). The
proportion of DNA in different phases was analyzed using ModFit LT
version 3.0 (Verity Software House, Topsham, ME, USA).
Hoechst 33258 staining assay
U2OS/ADM cells were seeded into 6-well plates at a
density of 2.0×105 cells/well and incubated for 24 h to
allow cell attachment. Different concentrations of PZH were then
added to each well and incubated for an additional 48 h, and the
cells were then washed with ice cold PBS twice and fixed with 4%
paraformaldehyde for 15 min at room temperature. The cells were
incubated in 1 ml PBS containing 10 μmol/l Hoechst 33258 at 37°C
for 30 min. Fluorescence microscopy (Olympus, Japan) was used to
observe the apoptotic characteristics of nuclear condensation.
Annexin V/propidium iodide staining
analysis by flow cytometry
Following incubation with various doses of PZH for
48 h, apoptosis of U2OS/ADM cells was determined by flow cytometric
(FCM) analysis using fluorescence-activated cell sorting
(FACSCalibur; Becton-Dickinson) and the Annexin V-FITC Apoptosis
Detection Kit II. Staining was performed according to the
manufacturer's instructions. The percentage of cells in early
apoptosis was calculated by Annexin V-positivity and PI-negativity,
while the percentage of cells in late apoptosis was calculated by
Annexin V-positivity and PI-positivity.
Rhodamine 123 accumulation assay
Rhodamine 123 was used to evaluate the transport
function of P-gp in U2OS/ADM cells by flow cytometric analysis.
Cells (2.0×105 cells/well in 6-well plates) were treated
with different concentrations of PZH for 48 h, followed by addition
of Rhodamine 123 (5 μg/ml). After incubating at 37°C for 30 min,
the cells were harvested and washed twice with ice-cold PBS and
subsequently analyzed by flow cytometry. The values are expressed
as the mean fluorescence intensity of Rhodamine 123.
RNA extraction and RT-PCR analysis
U2OS/ADM cells were seeded into 25-cm2
culture flasks at a density of 1×105 cells/ml in 4 ml of
medium and treated with various doses of PZH for 48 h. Total RNA
from U2OS/ADM cells was isolated with TRIzol reagent (Invitrogen).
Oligo(dT)-primed RNA (1 μg) was reverse-transcribed with
SuperScript II reverse transcriptase (Promega, Madison, WI, USA)
according to the manufacturer's instructions. The obtained cDNA was
used to determine the mRNA amount of Bcl-2, Bax and survivin by PCR
with Taq DNA polymerase (Fermentas). GAPDH was used as an
internal control. The primers and the annealing temperature (°C)
used for amplification of Bcl-2, Bax, survivin and GAPDH
transcripts are as follows: Bcl-2 forward, 5′-CAG CTG CAC CTG ACG
CCC TT-3 and reverse, 5′-GCC TCC GTT ATC CTG GAT CC-3′, 55°C; Bax
forward, 5′-TGC TTC AGG GTT TCA TCC AGG-3′ and reverse, 5′-TGG CAA
AGT AGA AAA GGG CGA-3′, 55°C; survivin forward, 5′-ACC ACC GCA TCT
CTA CAT TC-3′ and reverse, 5′-GTT CCT CTA TGG GGT CGT C-3′, 55°C;
P-gp forward, 5′-TAG AAA ACT TCC GAA CCG TTG T-3′ and reverse,
5′-TAG CTG TCA ATC AAA GGG GTT T-3′, 55°C; GAPDH forward, 5′-AGA
AGG CTG GGG CTC ATT TG-3′ and reverse, 5′-AGG GGC CAT CCA CAG TCT
TC-3′, 55°C. PCR products were visualized on a 1.5% agarose gel.
The DNA bands were examined using a Gel Documentation System (Model
Gel Doc 2000; Bio-Rad Laboratories, Hercules, CA, USA). Intensities
of the mRNA levels were normalized to those of the GAPDH products
as ratios to produce arbitrary units of relative abundance.
Western blot analysis
U2OS/ADM cells were treated with various doses of
PZH for 48 h. The cells were harvested; protein lysates from the
cells were generated through the mammalian cell lysis buffer
containing protease and phosphatase inhibitor cocktails. The
quantification of the protein content was performed with the
bicinchoninic acid (BCA) protein assay kit. Equal aliquots of
protein lysate were separated by 12% SDS-PAGE, followed by
electrophoresis and transferred to polyvinylidence difluoride
(PVDF) membranes. Membranes were blocked for 1 h in 5% nonfat dry
milk in TBS with Tween-20 (TBST) and probed overnight with
appropriate dilutions of primary antibodies against Bcl-2
(1:1,000), Bax (1:500), survivin (1:1,000), P-gp (1:1,000) and
β-actin (1:1,000) at 4°C. Three consecutive washes were performed
for 10 min with TBST; the membranes were incubated with the
secondary HRP-conjugated antibodies at a dilution of 1:2,000 for 1
h at room temperature. Finally, the membranes were washed again in
TBST. Antibody-bound protein band detection was performed with the
ECL Detection VersaDoc™ Imaging System (Bio-Rad Laboratories).
Statistical analysis
All of the data were confirmed by at least 3
independent experiments. Statistical analysis of data was carried
out using the statistical software SPSS 13.0. Data are expressed in
terms of means ± SD. The statistical analysis of the results was
performed by Student's t-test for paired samples. Differences
between concentrations were analyzed statistically with ANOVA. A
P-value <0.05 was considered to indicate a statistically
significant result.
Results
PZH inhibits U2OS/ADM cell
proliferation
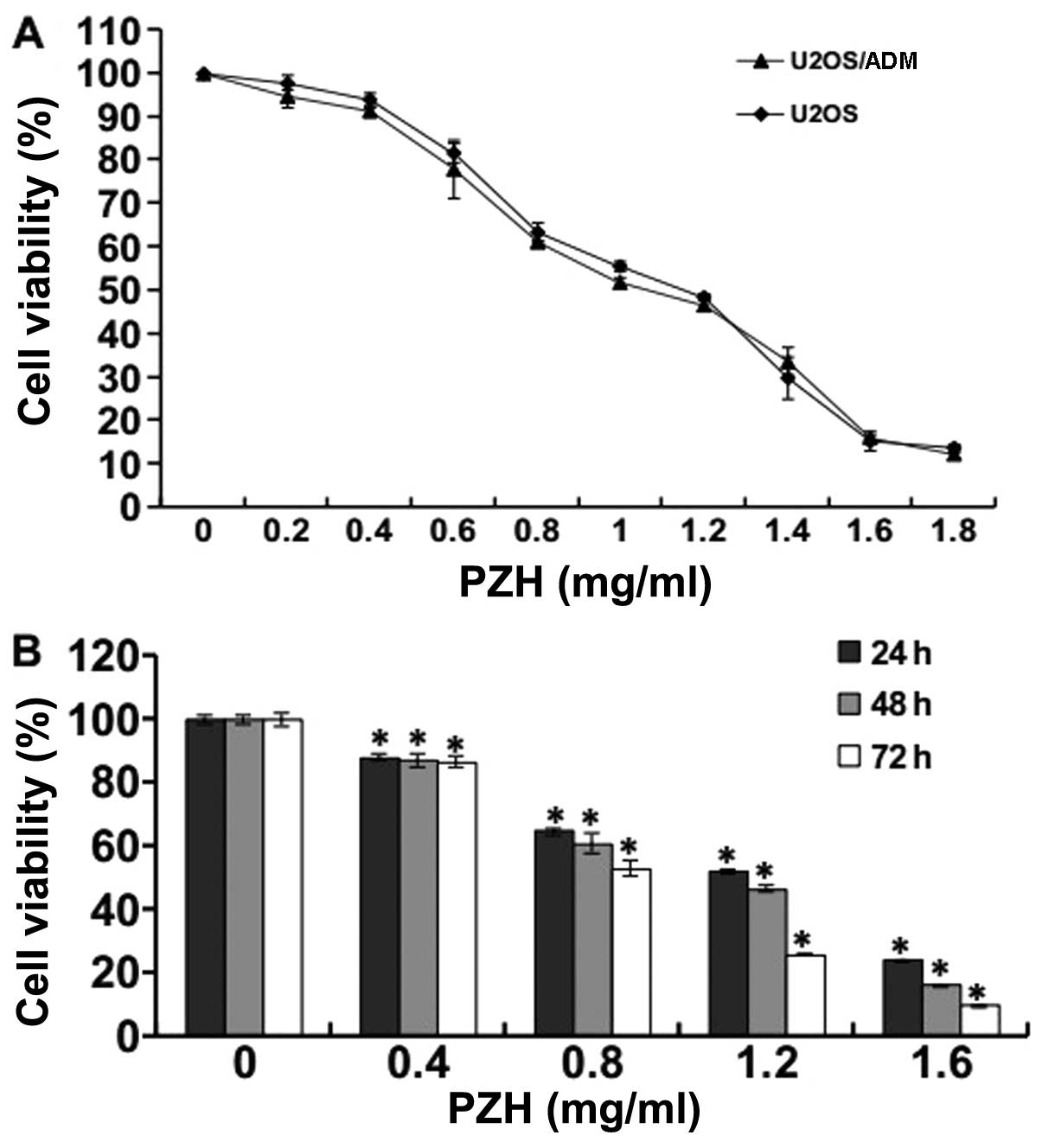
To investigate the effects of PZH on the viability
of U2OS/ADM cells, an MTT assay was performed. As shown in Fig. 1A, U2OS/ADM cells and their parental
U2OS cells were exposed to different PZH concentrations for 48 h,
and PZH induced cell death in a dose-dependent manner. The
half-inhibitory concentration (IC50) of PZH at 48 h in
U2OS/ADM and U2OS cells was ~1.06 and 1.14 mg/ml, respectively,
suggesting that PZH has similar inhibitory effects on cell
proliferation in both resistant and parental OS cells. Thus,
drug-resistant OS cells are sensitive to PZH. Fig. 1B shows that the treatment of
U2OS/ADM cells with PZH resulted in a significant inhibition of
cell growth in a time-dependent manner (P<0.05, vs. control
group).
PZH induces U2OS/ADM cell cycle arrest at
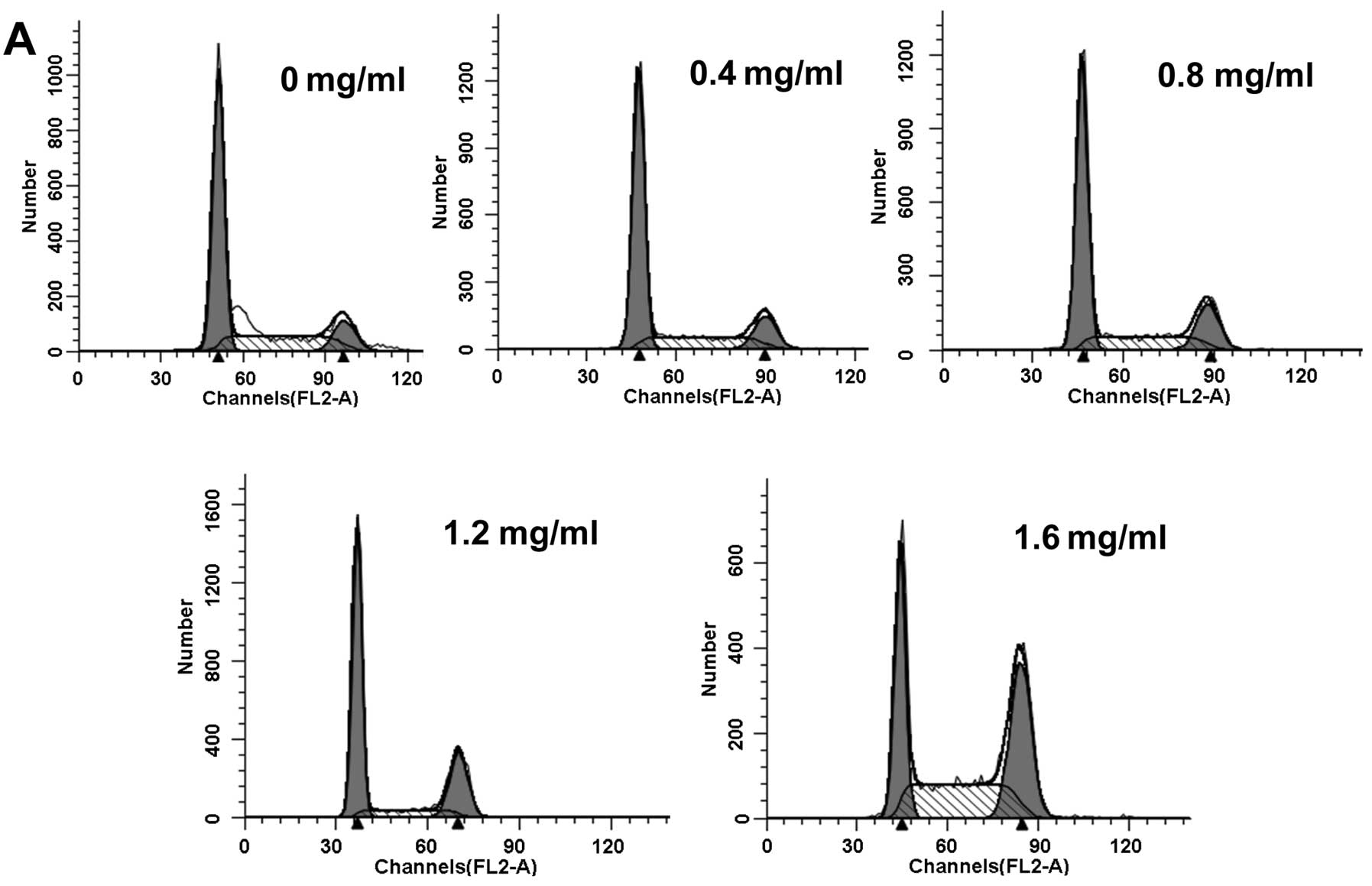
the G2/M phase
The cell cycle is a crucial regulator of cell
proliferation. When the cell cycle is disturbed, the rate of cell
proliferation is reduced or apoptosis is induced. We aimed to
determine whether PZH causes cell cycle arrest. Cell cycle
distribution was evaluated by flow cytometry after U2OS/ADM cells
were exposed to PZH at various concentrations for 48 h. As shown in
Fig. 2A and B, the percentage of
accumulated cells in the G2/M phase increased from 12.6%
in the control group to 14.03, 17.27, 23.45 and 35.11% in cells
treated with 0.4, 0.8, 1.2 and 1.6 mg/ml of PZH for 48 h,
respectively. These results indicate that the inhibitory effect of
PZH on U2OS/ADM cell proliferation was associated with
G2/M phase cell cycle arrest.
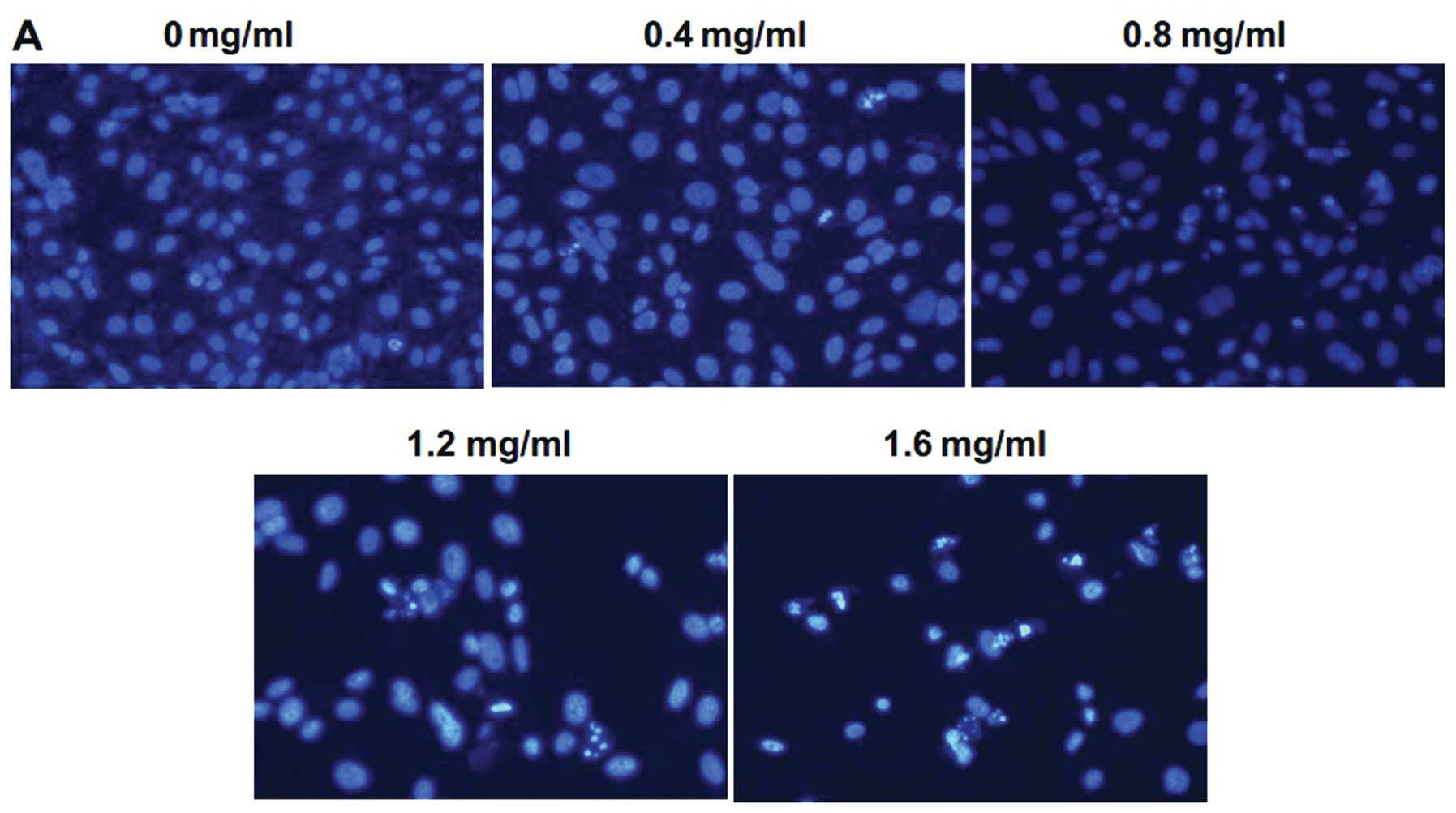
PZH induces apoptosis in U2OS/ADM
cells
To determine whether the inhibition of cell growth
by PZH resulted from the induction of apoptosis, Hoechst 33258
staining was performed to observe changes in cell apoptosis induced
by PZH. Following treatment with different concentrations of PZH
for 48 h, the cells were analyzed by fluorescence microscopy. As
shown in Fig. 3A, control cells
showed round and homogeneous nuclei, while PZH-treated cells
exhibited typical apoptotic morphologic changes including condensed
and fragmented nuclei in a dose-dependent manner. To further study
the apoptosis induced by PZH, the cells undergoing apoptosis or
necrosis were detected by FACS analysis after staining with Annexin
V-FITC and PI. The percentage of apoptotic cells included both
early apoptotic cells (lower right) and late apoptotic cells along
with the necrotic fractions (upper right). As shown in Fig. 3B and C, the percentage of cells
undergoing apoptosis following treatment with 0.4, 0.8, 1.2 and 1.6
mg/ml of PZH (including early and late apoptotic cells) was
8.32±0.93, 20.28±1.39, 35.54±1.65 and 53.86±1.71%, respectively
(P<0.05, vs. control group). These results indicate that PZH
treatment induces U2OS/ADM cell apoptosis in a dose-dependent
manner.
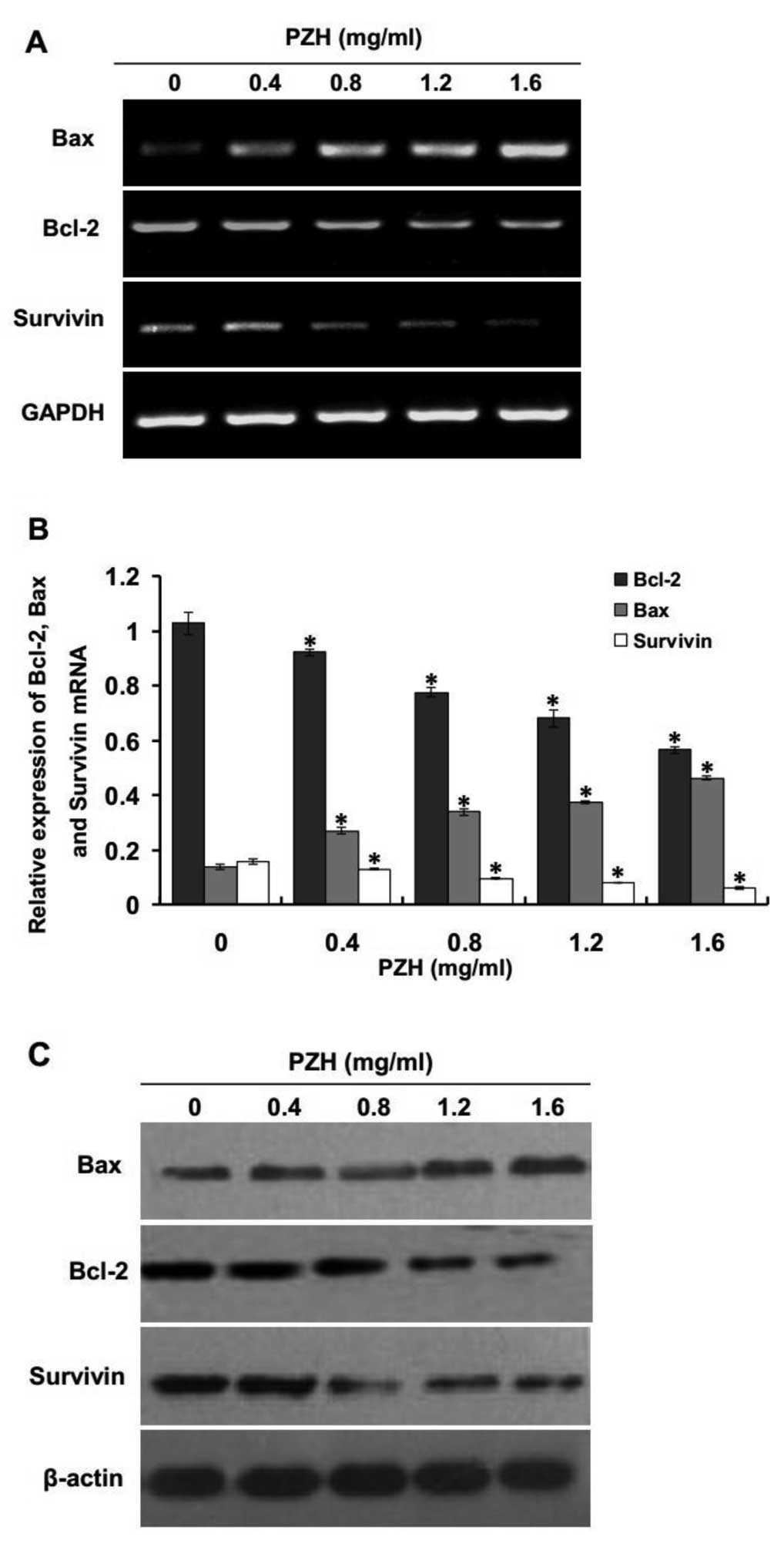
PZH upregulates the expression of Bax and
downregulates the expression of Bcl-2 and survivin
It is well established that anti-apoptotic proteins
Bcl-2 and survivin play an important role in preventing apoptosis
in cancer cells while pro-apoptotic protein Bax has a reverse
effect. To further study the mechanism of the induction of
apoptosis by PZH activity, we performed RT-PCR and western blot
analysis to examine the mRNA and protein expression of Bcl-2,
survivin and Bax in PZH-treated U2OS/ADM cells. The results of the
RT-PCR assay showed that the pro-apoptotic Bax was significantly
upregulated and the anti-apoptotic Bcl-2 and survivin were
significantly decreased; both effects occurred in a dose-dependent
manner (Fig. 4A and B; P<0.05,
vs. control group), and the pattern of protein expression of Bax,
Bcl-2 and survivin was similar to their respective mRNA levels
(Fig. 4C). These results indicate
that PZH induced apoptosis via the intrinsic pathway by
upregulating expression of pro-apoptotic Bax and downregulating
anti-apoptotic Bcl-2 and survivin in U2OS/ADM cells.
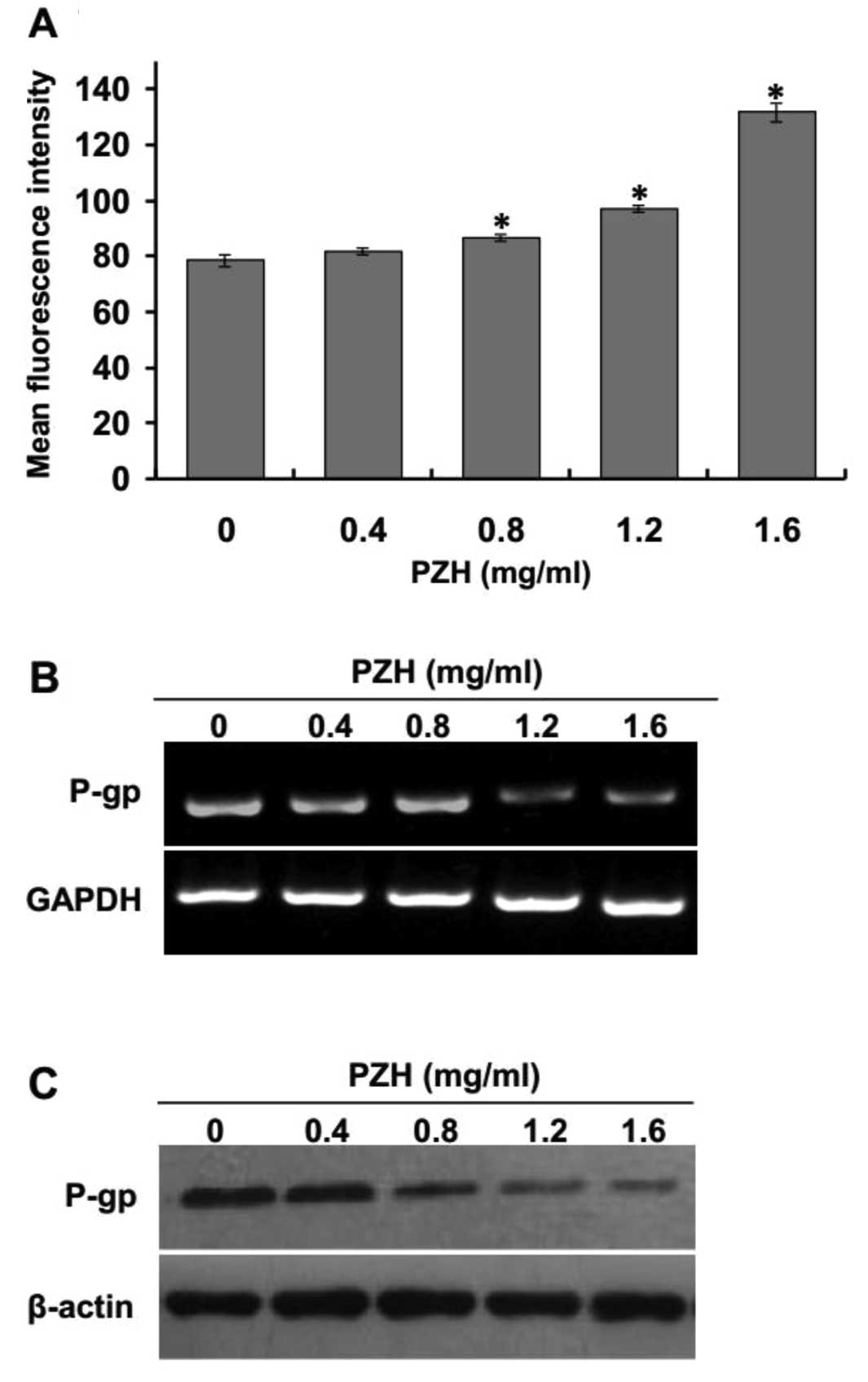
PZH decreases P-gp expression
Overexpression of P-gp, which reduces the
intercellular accumulation and toxicity of many anticancer drugs,
is one of the most studied mechanisms of drug resistance. U2OS/ADM
cells have been shown to express high levels of P-gp (31). To investigate whether PZH may
modulate P-gp expression, the intracellular accumulation of
Rhodamine 123 was examined by flow cytometry. As shown in Fig. 5A, the intracellular accumulation of
Rhodamine 123 in U2OS/ADM cells was dose-dependently increased when
compared to the control group after treatment with PZH (P<0.05,
vs. control group). In addition, the expression of P-gp was also
determined by RT-PCR and western blotting after treatment with
various concentrations of PZH. The results indicated that PZH
treatment profoundly and dose-dependently reduced the expression of
P-gp (Fig. 5B and C).
Discussion
Drug resistance to chemotherapeutic agents is a
major obstacle to the treatment of human osteosarcoma. Despite the
numerous studies that have attempted to develop and discover
effective chemotherapeutic drugs or reversal agents, the successful
modulation of clinical drug resistance has not been achieved
(32,33). Thus, it is imperative to develop
less toxic and more efficient therapeutic anticancer agents. TCM
has recently been recognized as a new source of anticancer drugs
and new chemotherapy adjuvants to enhance the efficacy of
chemotherapy and to diminish side-effects and the resistance of
cancer chemotherapies (34). PZH, a
well-known traditional Chinese formula which was first prescribed
450 years ago in the Ming Dynasty, has been used to treat various
types of cancers (16–19). Our previous studies revealed that
PZH significantly inhibited the proliferation of U2OS parental
cells, arrested the cell cycle in the G2/M phase and
promoted apoptosis (20,22). However, the effects of PZH on
chemotherapy-resistant osteosarcoma cells are still largely
unknown. Therefore, in order for PZH to be further developed as an
anticancer agent, its inhibition of chemotherapy-resistant OS cell
proliferation and the underlying mechanisms must be elucidated.
We first tested PZH for cytotoxicity against
chemotherapy-resistant OS U2OS/ADM cells and the parental cells
in vitro. We found that PZH had a marked inhibitory effect
on the cell proliferation in both the resistant and sensitive OS
cell lines. The IC50 of U2OS/ADM cells was quite similar
to the U2OS cells, indicating that PZH has no cross-resistance to
adriamycin and other classic anticancer drugs. The observations of
the morphological changes and Annexin V/PI double staining analysis
indicated that the percentage of apoptotic cells increased in a
dose-dependent manner following incubation with different
concentrations of PZH for 48 h. These results suggest that PZH may
play an important role in the treatment of patients with
drug-resistant OS.
Cell cycle control is one of the major regulatory
mechanisms of cancer cell division and duplication. When suffering
drug-toxicity, cancer cells may activate cell cycle checkpoints to
block cell cycle progression, which enhances damage repair and
leads to a resistance phenotype. Many anticancer agents have been
reported to arrest the cell cycle at a specific checkpoint
(11,35,36).
Therefore, inhibiting the specific checkpoint of the cell cycle is
one of the key approaches for the development of anticancer agents.
To determine whether PZH inhibited cell proliferation via cell
cycle arrest, flow cytometric analyses of the cell cycle were
performed. We found that the effect of PZH in resistant U2OS/ADM
cells was associated with cell cycle arrest at the G2/M
phase in a dose-dependent manner, indicating that PZH inhibited
U2OS/ADM cell proliferation by blocking the G2 to M
progression, which may partly explain its mechanisms of antitumor
activity.
Apoptosis, a type of programmed cell death, is a
major mechanism of cell death following many types of
chemotherapeutic agents. It is also closely related to
chemotherapeutic drug resistance. Apoptosis occurs via two main
routes, including the extrinsic and the intrinsic pathways. The
intrinsic pathway is the major route for chemotherapy-induced
apoptosis, and perturbation of this pathway may lead to
considerable alterations in the response to chemotherapy (37). It has been confirmed that
overexpression of Bcl-2 protein is correlated with chemotherapy
resistance, and lentivirus-mediated Bcl-2 knockdown was found to
sensitize human drug-resistant OS MG63 cells to doxorubicin
(26). Similarly, the correlation
of Bax expression levels with response to chemotherapy has
generated conflicting reports (25). Our previous studies found that the
expression of survivin in U2OS/ADM cells was significantly higher
than that in the parental cells (31). Therefore, the upregulation of Bcl-2
and survivin and the downregulation of Bax are related to the
reduction in sensitivity to apoptosis in cancer cells. In the
present study, an decrease in Bcl-2 and survivin and an increase in
Bax were observed in U2OS/ADM cells following treatment with PZH,
suggesting that PZH may induce U2OS/ADM cell apoptosis.
Recent studies have demonstrated that P-gp does not
only function as an energy-dependent drug pump to reduce
intracellular chemical concentrations, but also leads to
drug-resistance through inhibiting the activation of caspase-3 and
−8 (38). As P-gp plays a key role
in inhibiting apoptosis in drug-resistant cancer cells, effective
inhibition of P-gp may be critical for providing a targeted site
for cancer treatment for patients with drug resistance. The present
study showed that the treatment of U2OS/ADM cells with PZH led to a
dose-dependent decrease in P-gp mRNA and protein, accompanied by
elevated Rhodamine 123 accumulation, which may result in apoptosis
and cytotoxic effects in U2OS/ADM cells.
In conclusion, our data for the first time
demonstrate that PZH inhibits U2OS/ADM cell proliferation via cell
cycle G2/M arrest and enhanced apoptosis via the
downregulation of the expression of Bcl-2, survivin and P-gp and
upregulation of Bax. These data suggest that PZH has potential as a
therapeutic agent against multidrug-resistant osteosarcoma and
warrants further in vivo investigation.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 30901916 and
81373659).
Abbreviations:
|
PZH
|
Pien Tze Huang
|
|
OS
|
osteosarcoma
|
|
P-gp
|
P-glycoprotein
|
|
TCM
|
Traditional Chinese medicine
|
|
IAP
|
inhibitor of apoptosis
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
ADM
|
adriamycin
|
|
IC50
|
50% inhibitory concentration
|
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
3
|
Jaffe N, Puri A and Gelderblom H:
Osteosarcoma: evolution of treatment paradigms. Sarcoma.
2013:2035312013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rajani R and Gibbs CP: Treatment of bone
tumors. Surg Pathol Clin. 5:301–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schwartz CL, Gorlick R, Teot L, et al:
Multiple drug resistance in osteogenic sarcoma: INT0133 from the
Children's Oncology Group. J Clin Oncol. 25:2057–2062. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chou AJ and Gorlick R: Chemotherapy
resistance in osteosarcoma: current challenges and future
directions. Expert Rev Anticancer Ther. 6:1075–1085. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saha SK and Khuda-Bukhsh AR: Molecular
approaches towards development of purified natural products and
their structurally known derivatives as efficient anti-cancer
drugs: current trends. Eur J Pharmacol. 714:239–248. 2013.
View Article : Google Scholar
|
|
8
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the 30 years from 1981 to 2010. J Nat
Prod. 75:311–335. 2012.PubMed/NCBI
|
|
9
|
Meng QX, Roubin RH and Hanrahan JR:
Ethnopharmacological and bioactivity guided investigation of five
TCM anticancer herbs. J Ethnopharmacol. 148:229–238. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jia L, Ma S, Hou X, et al: The synergistic
effects of traditional Chinese herbs and radiotherapy for cancer
treatment. Oncol Lett. 5:1439–1447. 2013.PubMed/NCBI
|
|
11
|
Wu G, Chu J, Huang Z, et al: Xiao Jin
Wan, a traditional Chinese herbal formula, inhibits
proliferation via arresting cell cycle progression at the G2/M
phase and promoting apoptosis via activating the
mitochondrial-dependent pathway in U-2OS human osteosarcoma cells.
Int J Oncol. 42:1070–1080. 2013.
|
|
12
|
Deng S, Hu B, An HM, et al:
Teng-Long-Bu-Zhong-Tang, a Chinese herbal formula, enhances
anticancer effects of 5-fluorouracil in CT26 colon carcinoma. BMC
Complement Altern Med. 13:1282013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu M, Sheng LH, Zhu XH, Zeng SB and Zhang
GJ: Reversal effect of Stephania tetrandra-containing Chinese herb
formula SENL on multidrug resistance in lung cancer cell line
SW1573/2R120. Am J Chin Med. 38:401–413. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang L, Wei DD, Chen Z, Wang JS and Kong
LY: Reversal effects of traditional Chinese herbs on multidrug
resistance in cancer cells. Nat Prod Res. 25:1885–1889. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chinese Pharmacopoeia Commission.
Pharmacopoeia of the Peoples Republic of China. Chinese Med Sci
Technol Press; 1. pp. 573–575. 2010
|
|
16
|
Zhao SL and Pan J: A clinical trial of
combined use of Pien Tze Huang and chemotherapy in the treatment of
primary liver cancer. Med World. 9:40–51. 2006.
|
|
17
|
Xu YY and Yu EX: Clinical analysis of the
effect of Pien Tze Huang in treatment of 42 patients with moderate
or advanced liver cancer. Shanghai J Tradit Chin Med. 12:4–5.
1994.
|
|
18
|
Zhuang Q, Hong F, Shen A, et al: Pien Tze
Huang inhibits tumor cell proliferation and promotes apoptosis via
suppressing the STAT3 pathway in a colorectal cancer mouse model.
Int J Oncol. 40:1569–1574. 2012.PubMed/NCBI
|
|
19
|
Shen AL, Hong F, Liu LY, Lin JM, Zhuang
QC, Hong ZF and Peng J: Effects of Pien Tze Huang on angiogenesis
in vivo and in vitro. Chin J Integr Med. 18:431–436. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Yu B and Lin JH: Apoptosis
induction of traditional Chinese herb Pianzihuang in human
osteosarcoma U-2OS cells. Zhongguo Gu Shang. 22:265–268. 2009.(In
Chinese).
|
|
21
|
Zhang L, Zeng QQ and Lin JH: Inhibitory
effect of human P27KIP1 gene AVV virus combining with Chinese herb
Pien Tze Huang on human osteosarcoma transplant mice model. China J
TCM Pharm. 24:511–514. 2009.
|
|
22
|
Zhang Y, Wang QH, Zhang L, Niu SS and Liu
Y: Effects of Pien Tze Huang on the cell cycle of human osteocarcom
U2OS cells. J Jiangxi Univ TCM. 24:19–21. 2012.
|
|
23
|
Fu Y, Yang DH and Zhang L: Effects of Pien
Tze Huang on the migration and invasion of osteosarcoma MG63 cell.
China J TCM Pharm. 28:1577–1580. 2013.
|
|
24
|
Wu X, Cai ZD, Lou LM and Zhu YB:
Expressions of p53, c-myc, Bcl-2 and apoptotic index in human
osteosarcoma and their correlations with prognosis of patients.
Cancer Epidemiol. 36:212–216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wesarg E, Hoffarth S, Wiewrodt R, Kröll M,
Biesterfeld S, Huber C and Schuler M: Targeting BCL-2 family
proteins to overcome drug resistance in non-small cell lung cancer.
Int J Cancer. 121:2387–2394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao Y, Zhang GL, Zeng BF, Wu XS, Gao TT
and Oda Y: Enhanced chemosensitivity of drug-resistant osteosarcoma
cells by lentivirus-mediated Bcl-2 silencing. Biochem
Biophys Res Commun. 390:642–647. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shoeneman JK, Ehrhart EJ III, Eickhoff JC,
Charles JB, Powers BE and Thamm DH: Expression and function of
survivin in canine osteosarcoma. Cancer Res. 72:249–259. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Serra M, Scotlandi K, Reverter-Branchat G,
et al: Value of P-glycoprotein and clinicopathologic factors as the
basis for new treatment strategies in high-grade osteosarcoma of
the extremities. J Clin Oncol. 21:536–542. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gomes CM, van Paassen H, Romeo S, et al:
Multidrug resistance mediated by ABC transporters in osteosarcoma
cell lines: mRNA analysis and functional radiotracer studies. Nucl
Med Biol. 33:831–840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Johnstone RW, Ruefli AA, Tainton KM and
Smyth MJ: A role for P-glycoprotein in regulating cell death. Leuk
Lymphoma. 38:1–11. 2000.PubMed/NCBI
|
|
31
|
Zhang Y, Liu JN, Wang QH, Liu Y and Zhang
L: Comparison of characteristics of two adriamycin resistant human
osteosarcoma U2OS cell lines. Acta Acad Med Weifang. 34:336–339.
2012.
|
|
32
|
Shen M, Chan TH and Dou QP: Targeting
tumor ubiquitin-proteasome pathway with polyphenols for
chemosensitization. Anticancer Agents Med Chem. 12:891–901. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Milane L, Ganesh S, Shah S, Duan ZF and
Amiji M: Multi-modal strategies for overcoming tumor drug
resistance: hypoxia, the Warburg effect, stem cells, and
multifunctional nanotechnology. J Control Release. 155:237–247.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu Y and Li XJ: Multi-target therapeutics
and new drug discovery. Yao Xue Xue Bao. 44:226–230. 2009.(In
Chinese).
|
|
35
|
Ji T, Lin C, Krill LS, Eskander R, Guo Y,
Zi X and Hoang BH: Flavokawain B, a kava chalcone, inhibits growth
of human osteosarcoma cells through G2/M cell cycle
arrest and apoptosis. Mol Cancer. 12:552013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng SE, Xiong S, Lin F, et al:
Pirarubicin inhibits multidrug-resistant osteosarcoma cell
proliferation through induction of G2/M phase cell cycle arrest.
Acta Pharmacol Sin. 33:832–838. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mellor HR and Callaghan R: Resistance to
chemotherapy in cancer: a complex and integrated cellular response.
Pharmacology. 81:275–300. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Friedrich K, Wieder T, Von Haefen C, et
al: Overexpression of caspase-3 restores sensitivity for
drug-induced apoptosis in breast cancer cell lines with acquired
drug resistance. Oncogene. 20:2749–2760. 2001. View Article : Google Scholar : PubMed/NCBI
|