Introduction
The tumor microenvironment plays a fundamental role
in promoting tumor progression and metastasis (1). One of the primary forces of the
metabolic tumor microenvironment guiding an invasive and metastatic
phenotype is the low extracellular pH (pHe) produced by tumor cells
(2,3). Studies using in vitro
cell-culture and in situ tumor spectroscopy have revealed
that, while having an alkaline intracellular pH (pHi), tumors can
reach an acidic interstitial pHe 0.5–1 units lower than normal
tissue (tumor pHe of 6.5–7 vs. a normal tissue pHe of 7.4)
(4). This acidic pHe was previously
demonstrated to stimulate in vitro invasion (5) and in vivo metastasis (6), but the mechanism(s) underlying acidic
pHe-induced effects are only recently being defined. According to
the ‘acid-mediate invasion hypothesis’ (7), acidic conditions promote extracellular
release and activity of key proteases such as cathepsin B and
matrix metalloproteinase-2 and -9 (MMP-2, -9) (8), which break down both extracellular
matrix (ECM) and basement membrane, thereby promoting migration,
invasion and metastasis (9).
Therefore, we initiated studies to elucidate the nature of the
molecular pathways and mechanisms that regulate these events.
Aggressive tumor cells from multiple malignancies
localize and concentrate a series of different proteases including
the secreted MMP-2 and -9 and the transmembrane proteases (such as
MT1-MMP) at actin-rich invasive protrusions called invadopodia that
precisely regulate the directed proteolysis of the ECM and
facilitate invasion (10). A wide
variety of actin-interacting proteins, scaffolding proteins,
signaling proteins and ion transporters are involved in invadopodia
formation and functioning (11). In
particular, the plasma membrane Na+/H+
exchanger 1 (NHE1) is localized at cancer cell invadopodia, where
it plays an integrated role in both invadopodia formation and
proteolytic activity (12). Taken
together, these data suggest that there exists at invadopodia a
concordance between NHE1 localization, extracellular acidification,
protease activity on the cell surface at invadopodia and the
cytoskeleton reorganization necessary for invadopodial maturation
in human malignant breast carcinoma cells. There is currently no
report on the effect of NHE1-induced acid pHe on protease activity
at the most important sites of ECM digestion by cancer cells, the
invadopodia. This is due to the fact that, although biochemical
studies can determine net protease activity in the tumor
extracellular medium, they do not provide any information if the
localization and activation of proteases may occur at invadopodia.
Here, by using in situ invadopodial zymography, we measured
the individual activity of the different proteases and related this
invadopodial-localized protease expression and activity to NHE1 and
pHe at invadopodia of the human metastatic breast cancer cell line,
MDA-MB-231.
Materials and methods
Reagents
Porcine skin gelatin, type A, was from Sigma.
Matrigel growth factor reduced w/o phenol red from BD Biosciences.
DQ™Green BSA, DQ™Red BSA and DQ™pig skin gelatin fluorescein
conjugated from Molecular Probes. Primary antibodies: monoclonal
[4E9] anti-NHE1 (Abcam), monoclonal anti-Cortactin (p80/85) clone
4F1 (Millipore), polyclonal anti-MMP-2 and anti-MMP-9 (Cell
Signaling Technology, Inc.), polyclonal anti-cathepsin B
(Fitzgerald), monoclonal anti-β actin (Sigma). Rhodamine B,
isothiocyanate, mixed isomers (TRITC) was from Sigma.
Immunofluorescence: polyclonal anti-NHE1 (Alpha Diagnostic).
Secondary antibodies: anti-mouse (Sigma) and anti-rabbit (Cell
Signaling Technology, Inc.) HRP linked antibodies, goat anti-mouse
and anti-rabbit Alexa Fluor 488- and Alexa Fluor 568-linked
(Molecular Probes, Inc., Eugene, OR, USA).
Cell culture and preparation of different
pHe in growth medium and protease inhibitors
MDA-MB-231 cells were grown in Dulbecco’s modified
Eagle’s medium (DMEM) high glucose (4,500 mg/l) supplemented with
NaHCO3 (3,700 mg/l), 10% (v/v) heat-inactivated fetal
bovine serum, L-glutamine (2 mM), sodium-pyruvate (1 mg/ml) and
penicillin (100 units)/streptomycin (100 mg/ml) in a 5%
CO2/95% air humidified incubator at 37°C.
Protease expression levels and in situ
protease zymography (i.e. invadopodia-dependent ECM digestion) were
measured at neutral (pHe 7.4), acidic (pHe 6.7) or basic (pHe 7.8)
growth mediums, in the absence or presence of the following
protease inhibitors: cathespin B inhibitor CA-074 (5 μM),
Calbiochem (cat. no. 205030), MMP-9 inhibitor 1 (7.5 nM) Calbiochem
(cat. no. 444278) or MMP-2 inhibitor 1 (15 μM) Calbiochem (cat. no.
444244), Batimastat (Sigma) and Cariporide (Sanofi-Aventis).
Degradation assay and in situ protease
zymography
The activity of each protease was assayed at
invadopodia by measuring the quantitative levels of focal and
pericellular ECM digestion via in situ zymography in cells
plated on Matrigel containing quenched DQ-BSA, as previously
described (12), in the absence and
presence of the specific inhibitor of each protease.
Invadopodia cell fractionation
Cytosol, membrane and invadopodia fractions were
obtained from cells grown on gelatin, as previously described
(12).
Preparation of conditioned media, cell
lysates and western blotting
These procedures were performed as previously
described (13).
Image acquisition and analysis
Images were acquired and analyzed as previously
described (14) with a ×60 oil
objective using a Nikon Eclipse TE 2000S epifluorescence microscope
or a laser scanning confocal microscope (LSCM) (C1/TE2000-U; Nikon
Instruments S.p.A., Sesto Fiorentino-FI, Italy). Confocal images
were analyzed using ImageJ (http://rsb.info.nih.gov/ij/).
Duolink proximity ligation assay
(PLA)
Cells were plated for the invadopodial matrix
degradation assay, fixed, permeabilized and stained with primary
antibodies (NHE1, MMP-2, MMP-9 and cathepsin B) at the recommended
immunofluorescence dilution (or 1:400 if no dilution was given).
Proximity ligation was performed according to the manufacturer’s
protocol using the Duolink Detection kit with PLA PLUS and MINUS
probes for mouse and rabbit (Olink Biosciences; ref. 43). Samples
were analyzed with a confocal microscope (LSM 510 Meta; Carl Zeiss
Inc.) under a ×63 oil objective.
Statistical analysis
Student’s t-test was applied to analyze the
statistical significance between treatments and p<0.05 was
considered to indicate a statistically significant difference,
assuming equal variances on all experimental data sets. All
comparisons were performed with InStat (GraphPad Software, San
Diego, CA, USA).
Results
Acidic pHe increases MMP-2, -9 and
cathepsin B secretion
A correlation between tumor progression and
metastasis and elevated levels of the zinc-dependent matrix
metalloproteases MMP-2 and -9 (15)
and/or the acidic cysteine proteases (16) has been reported in several
experimental and clinical studies. Furthermore, the acidic
microenvironment has been shown to be essential for promoting
expression and activity of these proteases in both cancer and
stromal cells during tumor invasion, angiogenesis and metastasis
(6,17). Therefore, we focused on MMP-2, -9
and cathepsin B to first assess how shifting the pHe of culture
medium of breast cancer cells towards acidic or alkaline values
changes their cellular levels and extracellular secretion. We
incubated metastatic breast cancer cells, MDA-MB-231, with media at
various pH (pH 6.7, pH 7.4 and pH 7.8) for 8 h and measured the
expression levels of MMP-2, -9 and cathepsin B by western blot
analysis in both the total cell homogenates and in the collected
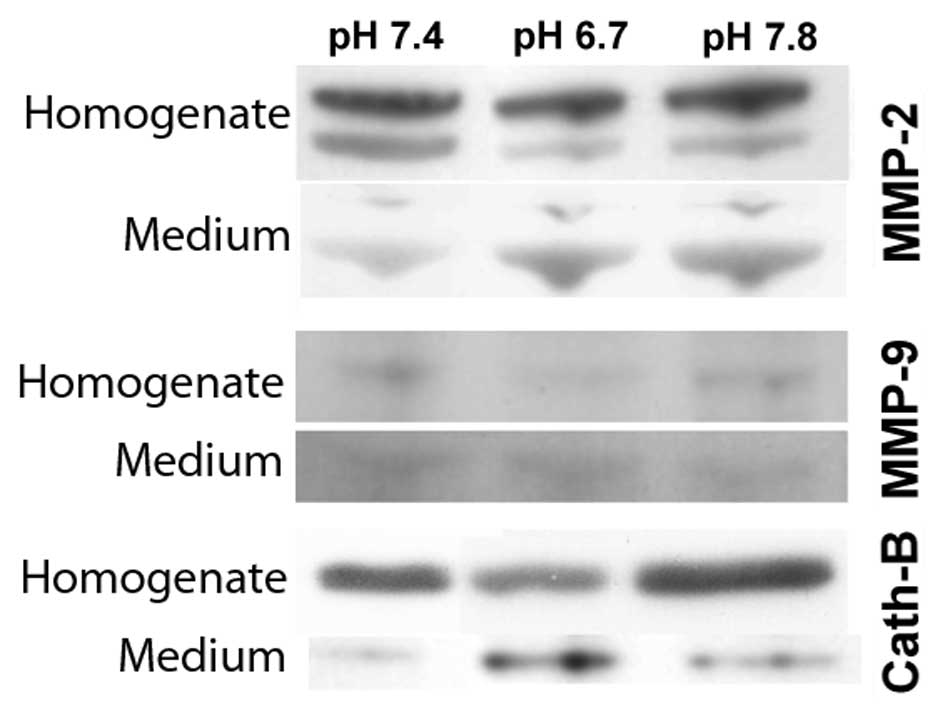
tumor conditioned medium. As seen in Fig. 1, cells incubated with acidic medium
(pH 6.7) in comparison to neutral medium (pH 7.4), exhibited a
decrease of the MMP-2 (72 kDa) and MMP-9 (92 kDa) inactive
pro-forms in the total cell homogenates and a marked increase of
their mature forms (64 and 84 kDa respectively) released into the
tumor conditioned medium. Similarly, extracellular acidosis (pH
6.7) reduced cathepsin B expression in the cellular homogenate
while highly inducing its secretion into the tumor extracellular
medium. On the contrary, when cells were incubated with alkaline
medium (pH 7.8), we found that MMP-9 and cathepsin B did not change
their expression in homogenates or secretion into conditioned media
when compared to cells cultured at neutral pH, while MMP-2
continued to show decreased levels in the cellular homogenates and
an increased secretion into the tumor conditioned medium. The data
presented here confirm that extracellular acidic conditions
increase the secretion of the activated forms of MMP-2, MMP-9 and
cathepsin B. However, it remains unknown whether this increased
secretion occurs globally for the cell or preferentially at
invadopodia, known to be centers of enrichment for a variety of
proteases.
Cathepsin B, MMP-2 and -9 directly
interact with NHE1 at matrix-degrading invadopodia
As we recently demonstrated that invadopodia are
focal hotspots of very acidic pHe driven by high levels of NHE1
activity (12) and that this NHE1
activity is necessary for the digestive activity of the
invadopodia, we explored whether the lowered peri-invadopodia pHe
driving focal ECM degradation is through the regulation of the
expression and/or activity of proteases at invadopodia. We first
determined the presence of the three major proteases, cathepsin B,
MMP-2 and -9, in invadopodia by fractionating cells plated on
cross-linked porcine gelatin into cytosol, cell membrane or
invadopodia fractions (12). The
invadopodia compartment was identified on the basis of a strong
cortactin overexpression when compared to the membrane fraction
(12,18,19).
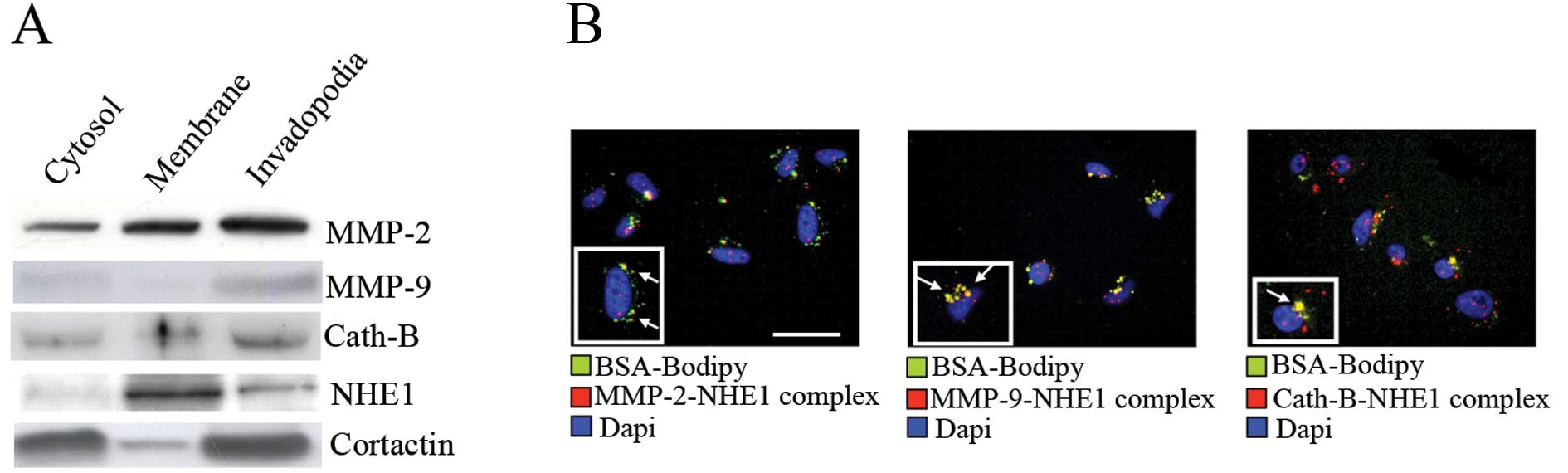
As can be seen in Fig. 2A, all
three proteases were enriched in the invadopodia although with
different relative distributions. Furthermore, as previously
reported (12), NHE1 was also
expressed in the invadopodia compartment. To further analyze the
potential association among NHE1, MMP-2, -9 and cathepsin B at
proteolytically active invadopodia, a PLA, which can detect
endogenous protein-protein interactions that occur within 40 nm
(20), was combined with a Matrigel
degradation assay, in which cells are plated on a mixture of
Matrigel containing DQ Green BSA-Bodipy and a green fluorescent
emission staining is indicative of invadopodia driven-ECM digestion
(12). The advantages of PLA are
that this technique provides a fluorescent signal (red) only when
two target proteins are colocalized, allowing improved sensitivity
for establishing endogenous protein-protein interactions and giving
in situ information whether these colocalizations occur in
specific intracellular compartments. As shown by the red
fluorescent staining reported in Fig.
2B, NHE1 associated with all three proteases at the level of
matrix-degrading invadopodia (shown in green, while the merged area
in yellow are indicated with arrows). These results demonstrate
that some sub-populations of MMP-2, -9 and cathepsin B may reside
at the level of functionally active invadopodia where they interact
with NHE1.
Protease activity suppression increases
relative invadopodial NHE1 expression, while NHE1 inhibition
increases acid-induced protease secretion
As both NHE1 and MMPs are involved in the
invadopodia mediated-ECM degradation, and having demonstrated in
Fig. 2A that breast cancer cells
have an enrichment of NHE1 and protease expression at invadopodia
when compared to cellular bodies, we investigated if proteases and
NHE1 exert a reciprocal transmodulation of their expression and/or
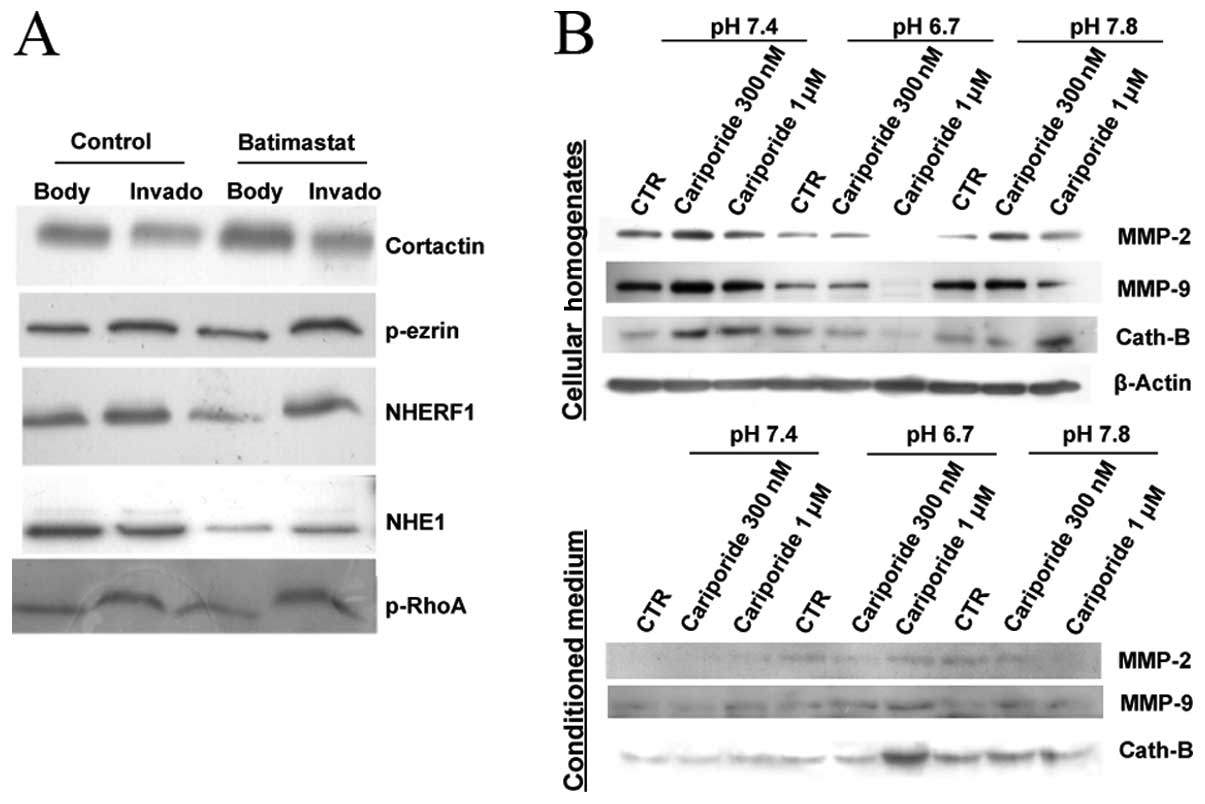
activities. We first evaluated the role of MMP activity on NHE1
compartmentalization in invadopodia by using a general MMP
inhibitor, batimastat. As can be observed in the invadopodia
fractionation experiments reported in Fig. 3A, treatment with 5 μM batimastat for
6 h significantly redistributed NHE1 from cellular bodies to
invadopodia while having no effect on the distribution of a series
of other invadopodia located proteins, suggesting a feed-back role
of these enzymes on invadopodia proteolytic activity in breast
cancer cells by controlling NHE1 expression and localization. These
data are consistent with evidence that: (i) inhibiting NHE1
activity reduced MMP-2 and -9 activation (21) and cathepsin B activation (5) in the extracellular medium, and (ii)
that the overexpression of a non-transporting NHE1 mutant reduced
MMP-9 expression and activity (22).
To determine whether inhibition of NHE1 by different
doses of cariporide affects protease release and if there is a
pH-dependence of this process, we examined the effect of cariporide
on MMP-2, -9 and cathepsin B expression at the same pHe values
utilized in the previous experiments shown in Fig. 1. As shown in Fig. 3B, when NHE1 activity was inhibited
by 300 nM cariporide in cells grown at physiological pH (pH 7.4),
we observed an intracellular accumulation of the three proteases
and a corresponding reduction of their secretion in the tumor
conditioned medium. As previously, we found that acidic pHe
increased the release of MMP-2, -9 and cathepsin B and inhibition
of NHE1 by cariporide (especially at 1 μM) strongly stimulated the
release of the proteases almost completely emptying the cells of
all three proteases resulting in peaks of their levels in the
conditioned medium. The use of cariporide concentrations near its
IC50 value (~280 nM) and at a near maximal concentration
(1 μM) for inhibiting NHE1 activity (12), demonstrated that the release of the
proteases have somewhat different interaction kinetics with NHE1
activity.
Acidic pHe-dependent protease activity is
localized to the invadopodia
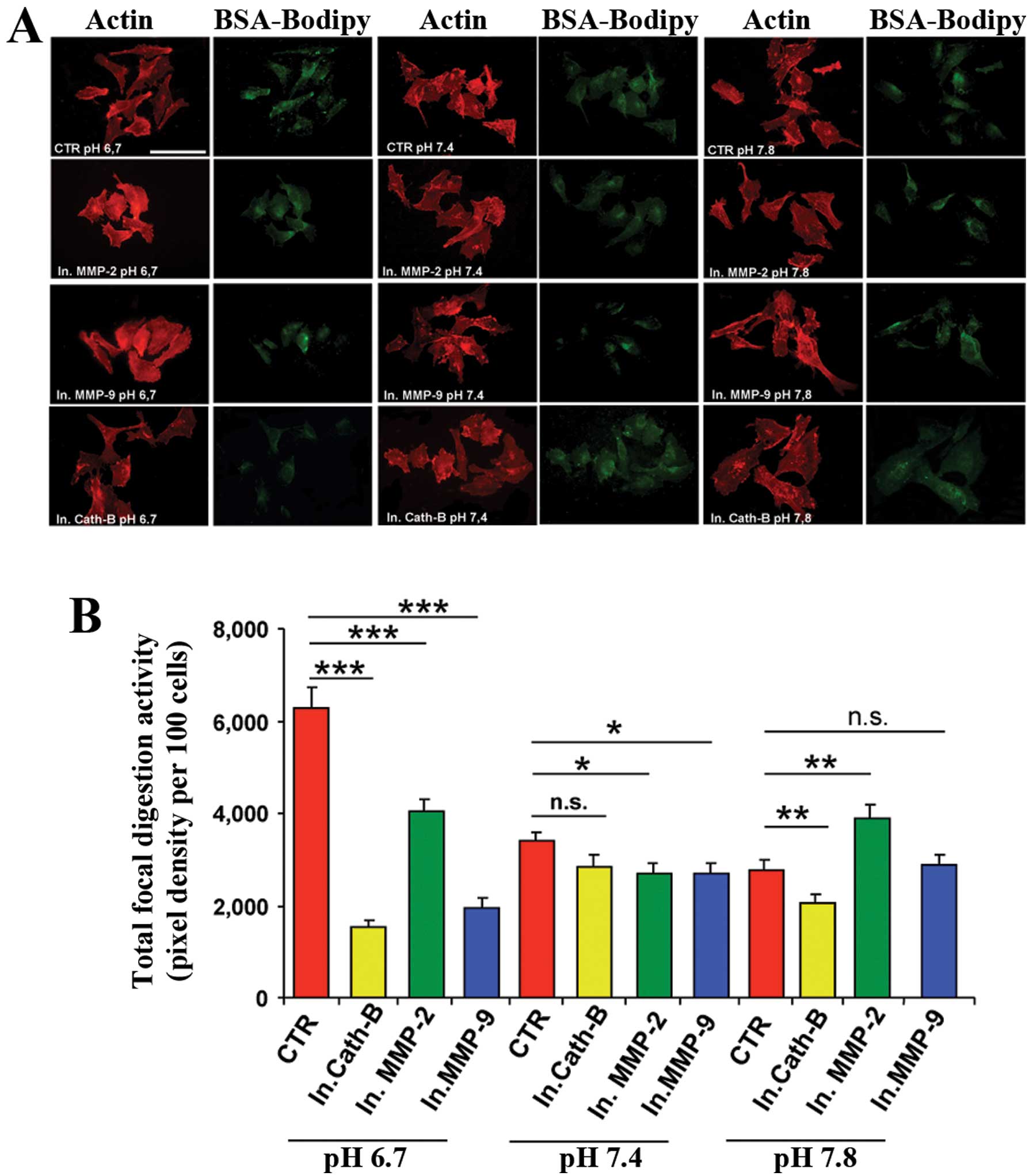
We finally determined the activity and role of the
three proteases at the level of invadopodia. Similar to studies of
experimental extracellular acidification in determining
osteoclastic proteolytic programs (23,24),
we examined whether exposure to an experimental acidosis may
increase focal, invadopodia-dependent ECM degradation in MDA-MB-231
cells plated on Matrigel containing DQ Green BSA-Bodipy and if this
increase was due, at least in part, to a direct increase in
protease activity. To determine the pH-dependence of the activity
of the specific proteases MMP-2, -9 and cathepsin B at the
invadopodia, we performed focal digestion experiments via in
situ zymography in Matrigel by incubating the cells in acidic
(pHe 6.7), neutral (pHe 7.4) or basic (pHe 7.8) growth mediums, in
the absence or presence of the following small-molecule inhibitors
to pharmacologically knock-out protease function: cathepsin B
inhibitor CA-074 (5 μM), MMP-9 inhibitor 1 (7.5 nM) or MMP-2
inhibitor 1 (15 μM). Proteolysis of individual cells was followed
as previously described (12) with
invadopodia-dependent proteolysis being defined as the pixel
density of focal zones of digestion for each cell. Fig. 4A shows confocal immunofluorescence
images, using this in situ zymographic assay, of the actin
cytoskeleton (red) and digestion (green) from typical cells of the
different pHe treatments, while Fig.
4B shows the histogram of the cumulative data of total
invadopodia (focal) ECM proteolysis from four independent
experiments. Exposure to extracellular acidification produced a
significant increase in both invadopodia expression/number (percent
of proteolytically active cells, data not shown) and total
invadopodial activity in control cells (red bars in Fig. 4B) when compared to cells treated at
neutral or basic pHe. These results confirm data from previous
studies showing the pH dependence of ECM digestion at invadopodia
(12). Notably, the ability of all
three protease inhibitors to block the invadopodia-localized
proteolysis was much greater in the acidic medium and again,
declined with increasing pHe demonstrating that, indeed, MMP-2
(green bars), MMP-9 (blue bars) and cathepsin B (yellow bars) are
more active at acidic pHe.
Collectively, these data show that, as in
osteoclasts, an experimental acidification increases tumor cell
proteolytic activity by turning on intrinsic invadopodial programs
and that an important future direction will be to identify the
molecular components of these specific invasive programs.
Discussion
Matrix metalloproteases (i.e. MMP-2, -9 and MT1-MMP)
and cysteine proteases such as cathepsin B are key invasion markers
due to their critical role in digesting both extracellular matrix
(ECM) and basement membrane proteins (25). However, except for the membrane
anchored MT1-MMP, there is some controversy concerning the
respective importance of each protease in ECM digestion, their
interaction and the role of extracellular pH (pHe) in driving or
regulating protease activity and action at the most important sites
of ECM digestion by cancer cells, the invadopodia.
While a large series of observations demonstrate
that NHE1-dependent acidification of the extracellular space
functions together with extracellular proteases to digest the ECM
in a controlled manner, the experiments were performed with whole
conditioned medium; therefore, the structural and functional
mechanisms/determinants linking the extracellular acidification to
the ECM proteolysis remain unknown. In this context, data from
various groups has shown that NHE1-dependent proton extrusion at
the invadopodia site is a crucial event for localized ECM digestion
(12,26,27).
However, while it has been demonstrated for MT1-MMP that its
accumulation at mature-ECM digesting invadopodia is a pH
dependent-event (21), the
presence, pH dynamics and interaction with NHE1 of the other
proteases at invadopodia are unknown. The goal of the present study
was to elucidate other proteases present at or surrounding the
invadopodia and the dynamics of both their regulation by pHe and
their relationship with NHE1.
To highlight these three key processes, we applied
the following experimental strategies; first, we measured the
cellular expression and secretion of cathepsin B, MMP-2 and -9 in
cells incubated in neutral (pHe 7.4), in acidic (pHe 6.7) or in
basic (pHe 7.8) growth medium and these experiments confirmed that
all three proteases are secreted at higher levels at lower pHe
(Fig. 1). Furthermore, we reported
for the first time in a single study by invadopodia fractionation
experiments that all these proteases compartmentalize with NHE1 at
invadopodia (Fig. 2A). In this
respect, an important novel observation of the present study was
that, in ECM-digesting invadopodia, NHE1 was very closely
associated with the three proteases, such that we observed a
positive interaction signal in an in situ assay (proximity
ligation assay) that measures interactions between two proteins in
close proximity (under 40 nm) with high spatial resolution
(28) (Fig. 2B). This suggests that a tight
physical and functional association between the major enzyme
responsible for extra-invadopodial acidification, NHE1, and the
acidic-driven proteases in that microspace exists in invadopodia.
Also, as NHE1 is associated with MMP-2/-9 and cathepsin B at
invadopodia, we investigated whether NHE1 activity is important for
the secretion of the three proteases into the peri-invadopodial
space and if protease activity may possibly reciprocally regulate
NHE1 expression at the invadopodia. We observed a dose-dependent
stimulation of the release of cathepsin B, MMP-2 and -9 by NHE1
inhibitor, cariporide (Fig. 3B) at
acidic pHe, indicating a strict control of NHE1 over the protease
secretion pathway. Lastly, as a direct measure of a pH-dependent
regulation of protease activity at the invadopodia site of ECM
digestion is still lacking, we performed a series of in situ
zymogram experiments in the absence and presence of specific
inhibitor of each protease, confirming that the major site of
protease digestion of the ECM occurs at invadopodia (Fig. 4A and B). These data indicate that
more than one protease is functioning to digest the ECM at
invadopodia and that the proteases have a functional pHe optimum
that is acidic not only in vitro but also in situ.
Indeed, our use of small-molecule inhibitors to pharmacologically
knock-out protease function revealed that each of the proteases
plays important roles in invadopodia proteolysis of the ECM and,
therefore, presumably in proteolysis-dependent functions such as
tumor growth, tumor vascularity and invasion.
In conclusion, our data demonstrate for the first
time that proton extrusion at the invadopodial site is a crucial
event for proteolytic ECM digestion in that at the invadopodial
sites of ECM proteolysis, more than one protease is functioning to
digest the ECM and these proteases have a functional pHe optimum
that is acidic. These data provide a structural basis for the well
known role of the NHE1 in tumor cell invasion and the regulation of
protease activity localized at invadopodia.
Acknowledgements
This study was supported by ‘Associazione Italiana
per la Ricerca sul Cancro’ (AIRC) grant no. 11348 and PRIN Grant
2009 no. 1341 to S.J.R. The SJR laboratory is part of the Italian
network ‘Istituto Nazionale Biostrutture e Biosistemi’ (INBB), and
the ‘Centro di Eccellenza di Genomica in Campo Biomedico ed
Agrario’ of the University of Bari and the project ‘BioBoP’ of the
Region Puglia.
References
|
1
|
Spano D and Zollo M: Tumor
microenvironment: a main actor in the metastasis process. Clin Exp
Metastasis. 29:381–395. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cardone RA, Casavola V and Reshkin SJ: The
role of disturbed pH dynamics and the Na+/H+
exchanger in metastasis. Nat Rev Cancer. 5:786–795. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bailey KM, Wojtkowiak JW, Hashim AI and
Gillies RJ: Targeting the metabolic microenvironment of tumors. Adv
Pharmacol. 65:63–107. 2012. View Article : Google Scholar
|
|
4
|
Martin NK, Robey IF, Gaffney EA, Gillies
RJ, Gatenby RA and Maini PK: Predicting the safety and efficacy of
buffer therapy to raise tumour pHe: an integrative modelling study.
Br J Cancer. 106:1280–1287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bourguignon LY, Singleton PA, Diedrich F,
Stern R and Gilad E: CD44 interaction with
Na+-H+ exchanger (NHE1) creates acidic
microenvironments leading to hyaluronidase-2 and cathepsin B
activation and breast tumor cell invasion. J Biol Chem.
279:26991–27007. 2004.PubMed/NCBI
|
|
6
|
Rofstad EK, Mathiesen B, Kindem K and
Galappathi K: Acidic extracellular pH promotes experimental
metastasis of human melanoma cells in athymic nude mice. Cancer
Res. 66:6699–6707. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Estrella V, Chen T, Lloyd M, Wojtkowiak J,
Cornnell HH, Ibrahim-Hashim A, et al: Acidity generated by the
tumor microenvironment drives local invasion. Cancer Res.
73:1524–1535. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reshkin SJ, Cardone RA and Harguindey S:
Na+-H+ exchanger, pH regulation and cancer.
Recent Pat Anticancer Drug Discov. 8:85–99. 2013.
|
|
9
|
Mason SD and Joyce JA: Proteolytic
networks in cancer. Trends Cell Biol. 21:228–237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eckert MA and Yang J: Targeting
invadopodia to block breast cancer metastasis. Oncotarget.
2:562–568. 2011.PubMed/NCBI
|
|
11
|
Yamaguchi H: Pathological roles of
invadopodia in cancer invasion and metastasis. Eur J Cell Biol.
91:902–907. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Busco G, Cardone RA, Greco MR, Bellizzi A,
Colella M, Antelmi E, et al: NHE1 promotes invadopodial ECM
proteolysis through acidification of the peri-invadopodial space.
FASEB J. 24:3903–3915. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cardone RA, Greco MR, Capulli M, Weinman
EJ, Busco G, Bellizzi A, et al: NHERF1 acts as a molecular switch
to program metastatic behavior and organotropism via its PDZ
domains. Mol Biol Cell. 23:2028–2040. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cardone RA, Bagorda A, Bellizzi A, Busco
G, Guerra L, Paradiso A, et al: Protein kinase A gating of a
pseudopodial-located RhoA/ROCK/p38/NHE1 signal module regulates
invasion in breast cancer cell lines. Mol Biol Cell. 16:3117–3127.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roomi MW, Monterrey JC, Kalinovsky T, Rath
M and Niedzwiecki A: Distinct patterns of matrix
metalloproteinase-2 and -9 expression in normal human cell lines.
Oncol Rep. 21:821–826. 2009.PubMed/NCBI
|
|
16
|
Matarrese P, Ascione B, Ciarlo L, Vona R,
Leonetti C, Scarsella M, et al: Cathepsin B inhibition interferes
with metastatic potential of human melanoma: an in vitro and in
vivo study. Mol Cancer. 9:2072010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brisson L, Gillet L, Calaghan S, Besson P,
Le Guennec JY, Roger S and Gore J: NaV1.5 enhances
breast cancer cell invasiveness by increasing NHE1-dependent
H+ efflux in caveolae. Oncogene. 30:2070–2076. 2011.
|
|
18
|
Bowden ET, Barth M, Thomas D, Glazer RI
and Mueller SC: An invasion-related complex of cortactin, paxillin
and PKCμ associates with invadopodia at sites of extracellular
matrix degradation. Oncogene. 18:4440–4449. 1999.PubMed/NCBI
|
|
19
|
Caldieri G, Ayala I, Attanasio F and
Buccione R: Cell and molecular biology of invadopodia. Int Rev Cell
Mol Biol. 275:1–34. 2009. View Article : Google Scholar
|
|
20
|
Söderberg O, Gullberg M, Jarvius M,
Ridderstråle K, Leuchowius KJ, Jarvius J, et al: Direct observation
of individual endogenous protein complexes in situ by proximity
ligation. Nat Methods. 3:995–1000. 2006.PubMed/NCBI
|
|
21
|
Lin Y, Chang G, Wang J, Jin W, Wang L, Li
H, et al: NHE1 mediates MDA-MB-231 cells invasion through the
regulation of MT1-MMP. Exp Cell Res. 317:2031–2040. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Putney LK and Barber DL: Expression
profile of genes regulated by activity of the Na-H exchanger NHE1.
BMC Genomics. 5:462004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Komarova SV, Pereverzev A, Shum JW, Sims
SM and Dixon SJ: Convergent signaling by acidosis and receptor
activator of NF-κB ligand (RANKL) on the calcium/calcineurin/NFAT
pathway in osteoclasts. Proc Natl Acad Sci USA. 102:2643–2648.
2005.PubMed/NCBI
|
|
24
|
Pereverzev A, Komarova SV, Korcok J,
Armstrong S, Tremblay GB, Dixon SJ and Sims SM: Extracellular
acidification enhances osteoclast survival through an
NFAT-independent, protein kinase C-dependent pathway. Bone.
42:150–161. 2008. View Article : Google Scholar
|
|
25
|
Monet M, Lehen’kyi V, Gackiere F, Firlej
V, Vandenberghe M, Roudbaraki M, et al: Role of cationic channel
TRPV2 in promoting prostate cancer migration and progression to
androgen resistance. Cancer Res. 70:1225–1235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lucien F, Brochu-Gaudreau K, Arsenault D,
Harper K and Dubois CM: Hypoxia-induced invadopodia formation
involves activation of NHE-1 by the p90 ribosomal S6 kinase
(p90RSK). PLoS One. 6:e288512011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Magalhaes MA, Larson DR, Mader CC,
Bravo-Cordero JJ, Gil-Henn H, Oser M, et al: Cortactin
phosphorylation regulates cell invasion through a pH-dependent
pathway. J Cell Biol. 195:903–920. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fredriksson S, Gullberg M, Jarvius J,
Olsson C, Pietras K, Gústafsdóttir SM, et al: Protein detection
using proximity-dependent DNA ligation assays. Nat Biotechnol.
20:473–477. 2002. View Article : Google Scholar : PubMed/NCBI
|