Introduction
Colorectal cancer is one of the most common types of
cancer and is responsible for 10% of all cancer-related mortality
worldwide (1). The liver is the
most common site of distant metastasis in colorectal cancer.
Approximately 15% of patients have liver metastases when the
primary tumor is diagnosed (2), and
half of patients ultimately develop liver metastases during the
course of colorectal cancer. Colorectal cancer is unique among
solid tumors in that the surgical resection of distant metastases,
such as those in the liver and lung, can offer long-term survival
in selected patients (3). Indeed,
hepatic resection is the only curative treatment and is the
standard therapy for liver metastases of colorectal cancer.
However, only 25% of patients with liver metastases are candidates
for liver resection based on tumor number, size and location
(4), and one-third of patients
treated with curative liver resection experience a recurrence in
the liver (5,6). Systemic chemotherapy is the treatment
of choice for patients with unresectable liver metastases (7). Although long-term survival is rare
among patients treated only with chemotherapy (8), recent advances in systemic
chemotherapy have prolonged the survival of patients with
unresectable metastases (9). The
development of new agents and the shift from monotherapy to
combination chemotherapy have been key in improving the clinical
outcome for patients with unresectable metastases.
Green tea, a common beverage worldwide, has been
studied extensively for its health benefits, including its
anticancer effects (10).
Epidemiological studies have shown that green tea has a potential
preventive effect against colorectal cancer (11,12).
The antiproliferative effect of green tea has been attributed to
the biological activities of its polyphenol components. The
principal polyphenols in green tea include (−)-epicatechin (EC),
(−)-epicatechin-3-gallate (ECG), (−)-epigallocatechin (EGC) and
(−)-epigallocatechin-3-gallate (EGCG). EGCG is the most abundant
and accounts for half of tea polyphenols (13); it is also the major biologically
active component that inhibits cell proliferation and induces
apoptosis in colorectal cancer cells (14). Recent studies have revealed that
EGCG exerts its antiproliferative effect, at least in part, by
modulating the activities of various receptor tyrosine kinases and
their multiple downstream signaling pathways, including the Akt
signaling pathway, which controls the expression of the multiple
target genes involved in cell proliferation and apoptosis (15). Additionally, EGCG activates stress
signals, such as c-Jun N-terminal kinase (JNK) and p38
mitogen-activated protein kinase (MAPK), and induces apoptosis in
colorectal cancer cell lines (16).
EGCG has also been reported to inhibit the growth of human
colorectal cancer cells in subcutaneous xenograft models (17–19).
However, no previous studies have reported the ability of EGCG to
suppress liver metastases of human colorectal cancer. The aim of
the present study was to extend the investigation of the potential
use of EGCG to treat liver metastases of human colorectal cancer.
We examined the effect of EGCG on cell proliferation and apoptosis
in the human colorectal cancer cell lines, RKO and HCT116.
Subsequently, we investigated the effect of EGCG on liver
metastases of RKO tumors in vivo.
Materials and methods
EGCG
EGCG was obtained from Sigma (St. Louis, MO, USA).
For use in cell culture, EGCG was dissolved in sterile water at a
concentration of 1 mg/ml and stored at −20°C. For use in
intraperitoneal (i.p.) injections, EGCG was dissolved in saline at
a concentration of 6 mg/ml and stored at 4°C.
Cell culture
The human colorectal cancer cell lines, RKO and
HCT116, were tested for mycoplasma prior to use. All cancer cells
were aliquoted and frozen in liquid nitrogen immediately upon
receipt and used for the present experiments within 6 months of
thawing. All cancer cells were cultured in Dulbecco’s modified
Eagle’s medium (DMEM) (Wako Pure Chemical Industries, Ltd., Osaka,
Japan) supplemented with 10% fetal bovine serum (FBS) (HyClone,
Logan, UT, USA) and 1% penicillin and streptomycin (Invitrogen,
Grand Island, NY, USA). All cultures were maintained at 37°C in a
humidified atmosphere containing 5% CO2.
Cell viability assay
To measure the cytotoxicity of EGCG against these
cancer cells, a total of 3×103 cells in 100 μl of DMEM
supplemented with 10% FBS were seeded into each well of a 96-well
plate. Following overnight culture, EGCG was added at the specified
concentrations. After 24 h of incubation, cell viability was
measured by the level of mitochondrial activity in reducing
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-
(2,4-disulfophenyl)-2H-tetrazolium monosodium salt (WST-8) to
formazan using a Cell Counting kit-8 (CCK-8) (Dojindo Laboratories,
Kumamoto, Japan). The cells were incubated with the reagent as
specified by the manufacturer’s instructions. The plates were read
at A450 on a spectrometer.
Cell proliferation assay
To measure the effect of EGCG on the proliferative
activity of these cancer cells, a total of 3×103 cells
in 100 μl of DMEM supplemented with 10% FBS were seeded in each
well of a 96-well plate. Following overnight culture, EGCG was
added at the specified concentrations. After 24 h of incubation,
cell proliferation was measured with a Cell Proliferation ELISA,
BrdU (colorimetric) (Roche Diagnostics, Penzberg, Germany). The
cells were incubated with the reagent as specified by the
manufacturer and the plates were read at A350 on a
spectrometer.
Apoptosis assay
To detect apoptosis in these cancer cells, a total
of 6×104 cells in 1 ml of DMEM supplemented with 10% FBS
were seeded in each well of a Lab-Tek II Chamber Slide (Nalge Nunc
International, Tokyo, Japan). Following overnight culture, EGCG was
added at the specified concentrations, and the cells were incubated
for a further 4 h. Apoptosis in treated cells and paraffin-embedded
liver samples was evaluated with a DeadEnd™ Colorimetric TUNEL
System (Promega, Madison, WI, USA) according to the manufacturer’s
recommendations.
Western blot analysis
For western blot analysis of these cancer cells, a
total of 3×105 cells in 5 ml of DMEM supplemented with
10% FBS were seeded into each well of a 6-cm dish. After 48 h of
culture, EGCG was added at the specified concentrations, and the
cells were harvested 12 h later. Cell lysates were subjected to 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred to a nitrocellulose membrane (Millipore, Bedford, MA,
USA). The following antibodies were used as primary antibodies: Akt
(9272), phospho-Akt (9271), p38 (9212) and phospho-p38 (9211) (Cell
Signaling Technology, Beverly, MA, USA). β-actin (4970) (Cell
Signaling Technology) was used as the endogenous control. An
anti-rabbit IgG horseradish peroxidase-conjugated antibody (7074)
(Cell Signaling Technology) was used as the secondary antibody.
Immunoblots were analyzed by enhanced chemiluminescence.
Total RNA extraction
For reverse transcription-polymerase chain reaction
(RT-PCR), a total of 3×105 cells in 5 ml of DMEM
supplemented with 10% FBS were seeded into each well of a 6-cm dish
and cultured for 48 h. EGCG was added at the specified
concentrations, and after 2 h of incubation, total RNA was isolated
from whole cells using a NucleoSpin® RNA II kit
(Macherey-Nagel, Düren, Germany) according to the manufacturer’s
instructions. RNA concentrations were determined by measuring the
absorbance at 260/280 nm with a NanoDrop Spectrophotometer
(NanoDrop Technologies, Wilmington, DE, USA). The synthesis of
complementary DNA was performed using AMV reverse transcriptase
(Promega) and random primers (Takara Bio Inc., Shiga, Japan).
Briefly, a mixture of 1 mM dNTPs (Fermentas Life Sciences,
Burlingame, ON, Canada), 0.025 μg/ml random primers, 0.25 U/ml
reverse transcriptase, and 500 ng of total RNA was incubated at
30°C for 10 min, 37°C for 60 min, 95°C for 5 min and at 4°C before
storage at −80°C.
Quantitative real-time RT-PCR
Primers for RT-PCR were designed using Primer
Express® software for Real-Time PCR ver. 3.0 (Applied
Biosystems, Foster City, CA, USA) based on sequences available in
GenBank. Primers were purchased from Hokkaido System Science
(Hokkaido, Japan). We examined 4 receptor tyrosine kinases:
vascular endothelial growth factor receptor 2 (VEGFR2), epidermal
growth factor receptor (EGFR), human EGFR-related 2 (HER2) and
c-Met. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as
an endogenous control. RT-PCR was performed using SYBR-Green
real-time PCR Master Mix-Plus (Toyobo, Osaka, Japan) and an Applied
Biosystems 7300 real-time PCR system (Applied Biosystems) as
recommended by the manufacturers. The following primers were used
for amplification: VEGFR2, 5′-TTGCCCTTGTTCTGTCCTTTTT-3′ and
5′-GTCATTGTTCCCAGCATTTCAC-3′; EGFR, 5′-GGCTGCCTCCTGGACTATGT-3′ and
5′-AGTTCATGCCCTTTGCATCT-3′; HER2, 5′-TCCCCCAAAGCCAACAAAG-3′ and
5′-CCGTGGATGTCAGGCAGAT-3′; c-Met, 5′-CTCTCTGCCCCACCCTTTG-3′ and
5′-TGTCCCGCTCAGGCATTC-3′; and GAPDH, 5′-GGAGTCCACTGGCGTCTTCA-3′ and
5′-TTCACACCCATGACGAACATG-3′.
Animal model of liver metastasis
All animal experiments were conducted in a humane
manner in accordance with the Regulation for Animal Experiments of
the University and Fundamental Guidelines for Proper Conduct of
Animal Experiments and Related Activities in Academic Research
Institutions under the jurisdiction of the Ministry of Education,
Culture, Sports, Science and Technology of Japan, and were approved
by the Institutional Animal Experiment Committee of the University
of Tsukuba. All surgery was performed under isoflurane anesthesia
and all efforts were made to minimize suffering.
Eight-week-old male severe combined immunodeficiency
(SCID) mice weighing 21–26 g (CLEA Japan, Tokyo, Japan) were
utilized in all experiments. The mice were housed in a
temperature-controlled room with a 12-h light-dark cycle. They had
free access to water and standard chow throughout the experiment.
After an acclimation period of at least 7 days, laparotomy was
performed with midline incision, and hepatic ischemia was induced
by clamping the portal triad (hepatic artery, portal vein and bile
duct) with a microclip (Aesculap, Tuttlingen, Germany) for 1 min.
At 1 min after reperfusion of the liver, 2×106 RKO cells
were injected into the lower splenic pole with a 27-gauge needle,
and splenectomy was performed to prevent peritoneal dissemination
from the spleen tumor. The mice were separated into two groups; the
control group remained untreated (n=12), and the EGCG group was
treated with EGCG (n=12). In the EGCG group, we administered EGCG
(30 mg/kg body weight) by i.p. injection every other day over a
2-week period beginning 1 week after inoculation. At 21 days after
inoculation, the mice were sacrificed and their livers were removed
for examination.
Histological examination
To assess the liver metastatic area, all lobes of
the liver were divided into 2 pieces, fixed with 10% neutral
buffered formaldehyde. The tissues were embedded in paraffin and
cut into 4 μm sections. They were stained with hematoxylin-eosin,
and the percentage of liver metastatic area was calculated using
the image-processing software WinROOF (Mitani Co., Fukui,
Japan).
Immunohistological examination
The paraffin-embedded tissues were deparaffinized
with xylene, rehydrated with ethanol, immersed in 0.03% hydrogen
peroxidase to block endogenous peroxidase activity, and then
blocked with 3% bovine serum albumin (BSA) in phosphate-buffered
saline to reduce background staining. An anti-CD31 antibody
(LifeSpan Biosciences, Seattle, WA, USA) diluted 1:100 in 3% BSA
was added to the sections and incubated for 1 h at room
temperature. They were then incubated with a peroxidase-labeled
polymer conjugated to goat anti-rabbit immunoglobulins (Dako,
Tokyo, Japan) for 30 min at room temperature, followed by
diaminobenzidine chromogen (Dako) for 5 min. The sections were
counterstained with hematoxylin, dehydrated with ethanol, made
transparent with xylene and mounted with Multi Mount 480 (Matsunami
Glass, Osaka, Japan).
Serum parameters
Blood was collected from the retro-orbital sinuses
of the mice before sacrifice. The blood was centrifuged at 1,200 ×
g for 10 min at 4°C. Supernatants were collected and stored at
−20°C until testing using a FUJI DRI-CHEM serum multiple
biochemical analyzer (Fujifilm, Tokyo, Japan) to measure liver
function, including aspartate aminotransferase (AST) and alanine
aminotransferase (ALT).
Statistical analysis
All data are expressed as the means ± standard
deviation of samples. Unpaired t-test was used to compare two
groups. Comparisons among more than three groups were performed
using one-way analysis of variance with the Bonferroni post-test.
In all cases, P<0.05 was considered to indicate a statistically
significant difference.
Results
Inhibition of colorectal cancer cell
proliferation by EGCG
We initially determined whether EGCG treatment led
to the inhibition of colorectal cancer cell proliferation.
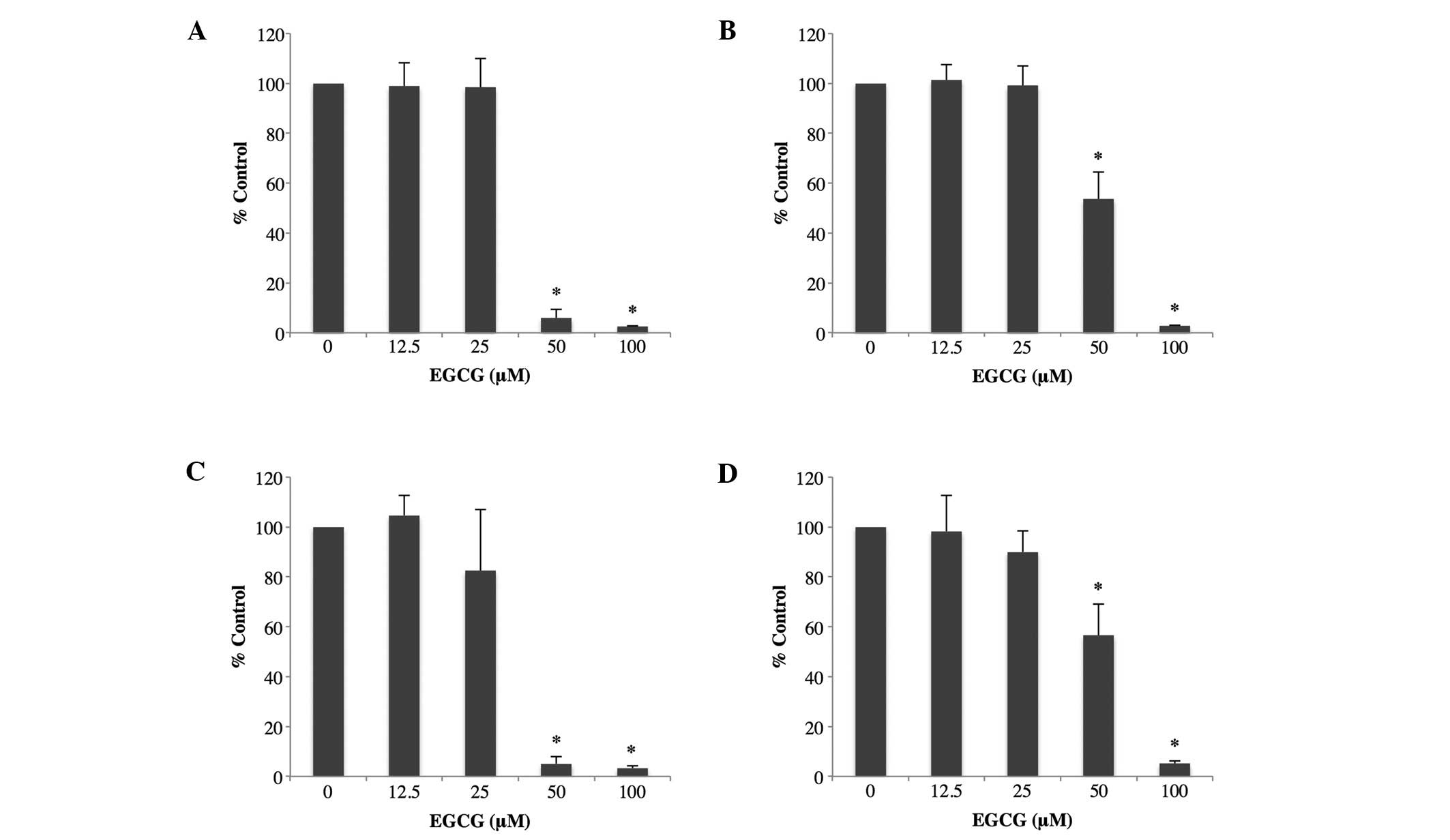
Colorectal cancer cells were treated with various doses of EGCG for
24 h. Cell viability was assayed using the CCK-8 assay (Fig. 1A and B), and DNA synthesis was
assessed using the BrdU assay (Fig. 1C
and D). As shown in Fig. 1,
EGCG at 50 and 100 μM inhibited the proliferation of both
colorectal cancer cells significantly compared to no EGCG treatment
(P<0.01).
Induction of apoptosis by EGCG
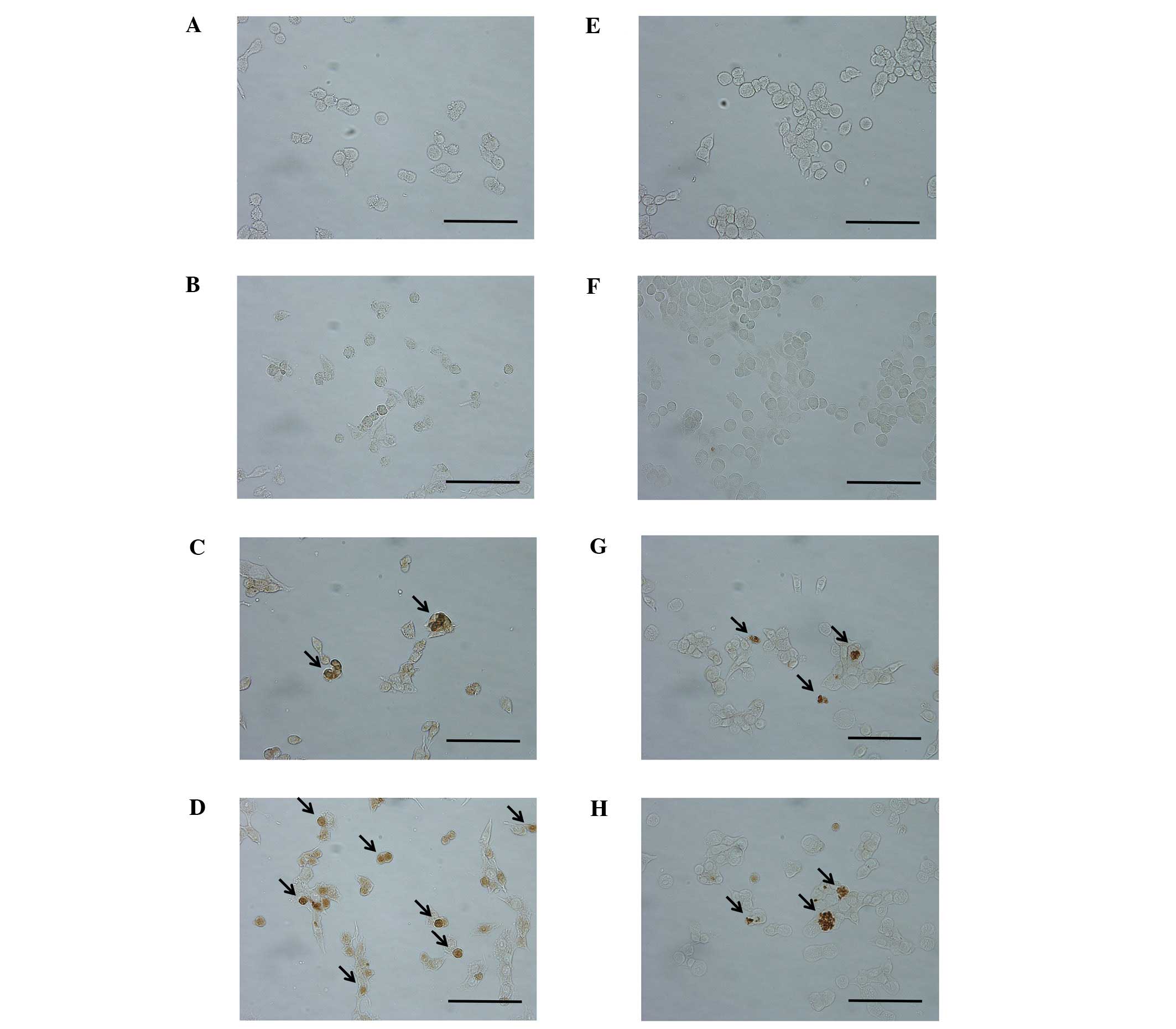
The cancer cells were treated with various doses of
EGCG for 4 h, and cell apoptosis was detected by TUNEL staining. As
shown in Fig. 2, TUNEL staining
revealed that EGCG induced apoptosis in both colorectal cancer
cells at 50 and 100 μM.
The dephosphorylation of Akt and the
phosphorylation of p38 by EGCG
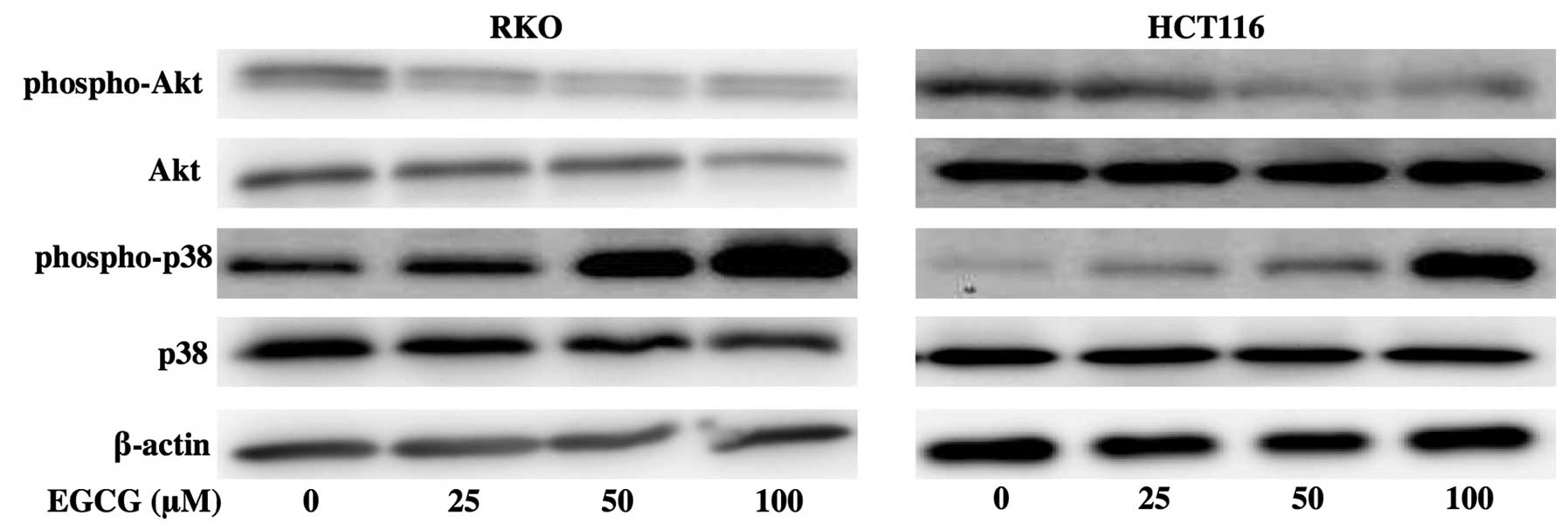
In subsequent experiments, we examined the effects
of EGCG on the expression of the signal transduction pathways in
colorectal cancer cells. When RKO and HCT116 cells were treated
with 25, 50 or 100 μM EGCG, there was the dephosphorylation of
constitutive phosphorylation of Akt. In RKO cells, there was a
marked increase in phospho-p38 at 50 and 100 μM EGCG. In HCT116
cells, the activation of p38 occurred at 100 μM EGCG (Fig. 3).
Decrease of VEGFR2 mRNA by EGCG
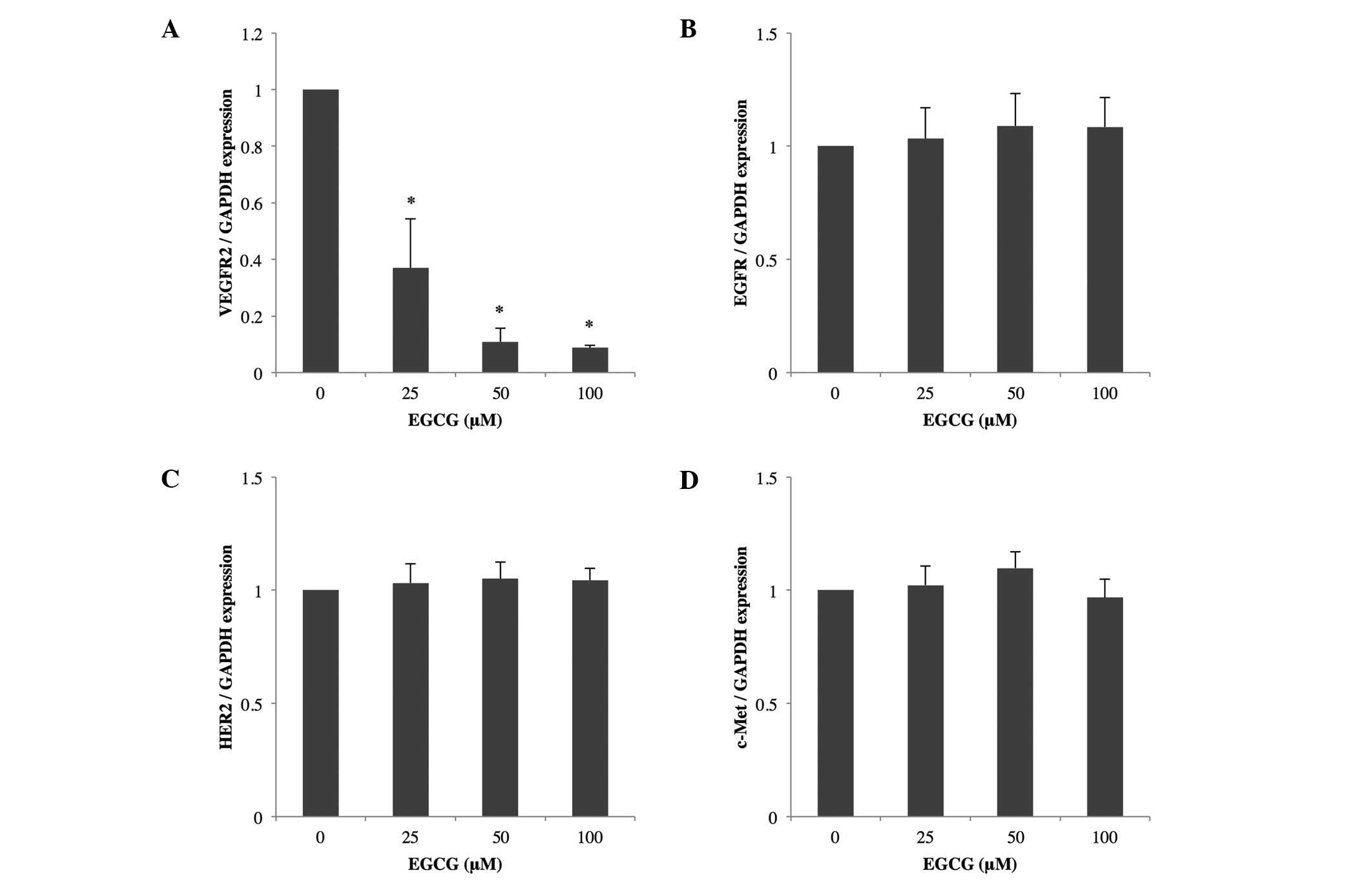
We examined the effects of EGCG on the expression of
VEGFR2, EGFR, HER2 and c-Met mRNAs in RKO cells. Quantitative
real-time RT-PCR indicated that treatment with 25, 50 or 100 μM
EGCG reduced the level of VEGFR2 mRNA significantly compared to no
treatment; EGFR, HER2 and c-Met mRNAs were not affected by EGCG
treatment (Fig. 4).
Suppression of liver metastases of human
colorectal cancer by EGCG
To evaluate the effect of EGCG on liver metastases,
we administered EGCG (30 mg/kg body weight) by i.p. injection every
other day over a 2-week period beginning 1 week after RKO cell
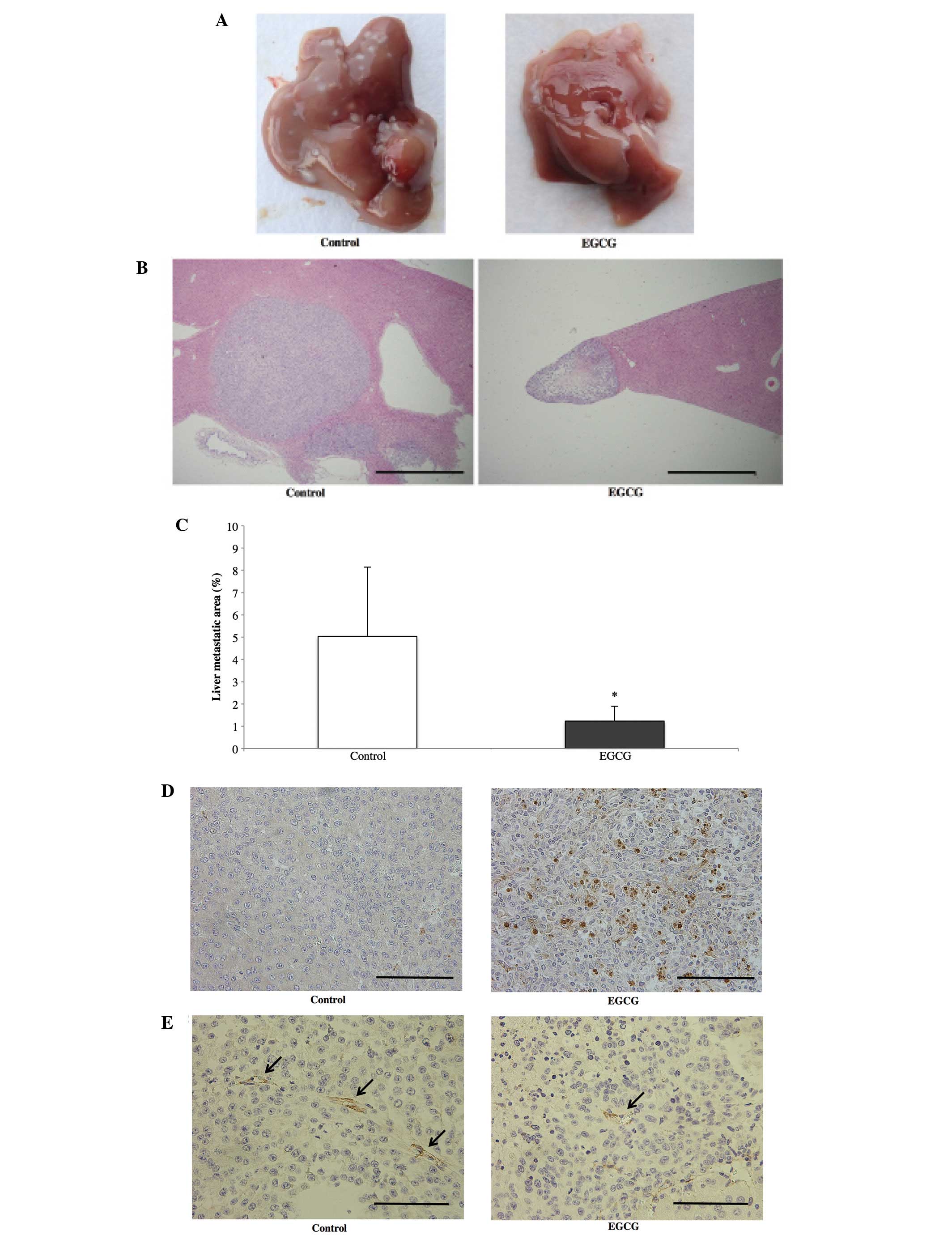
inoculation. Fig. 5A and B shows
macroscopic findings and histological cross-sections of the livers
removed from representative control and EGCG mice. A reduction in
the growth of liver metastases was observed in the EGCG group.
Fig. 5C shows the tumor areas
throughout the liver in both groups. Tumor growth was significantly
reduced by EGCG administration. Fig.
5D shows the TUNEL staining of liver sections in the control
and EGCG groups. In the EGCG group, apoptotic tumor cells were
observed within the liver metastases. To further investigate
whether EGCG inhibited angiogenesis, we used an anti-CD31 antibody
to stain liver metastases. As shown in Fig. 5E, fewer tumor blood vessels were
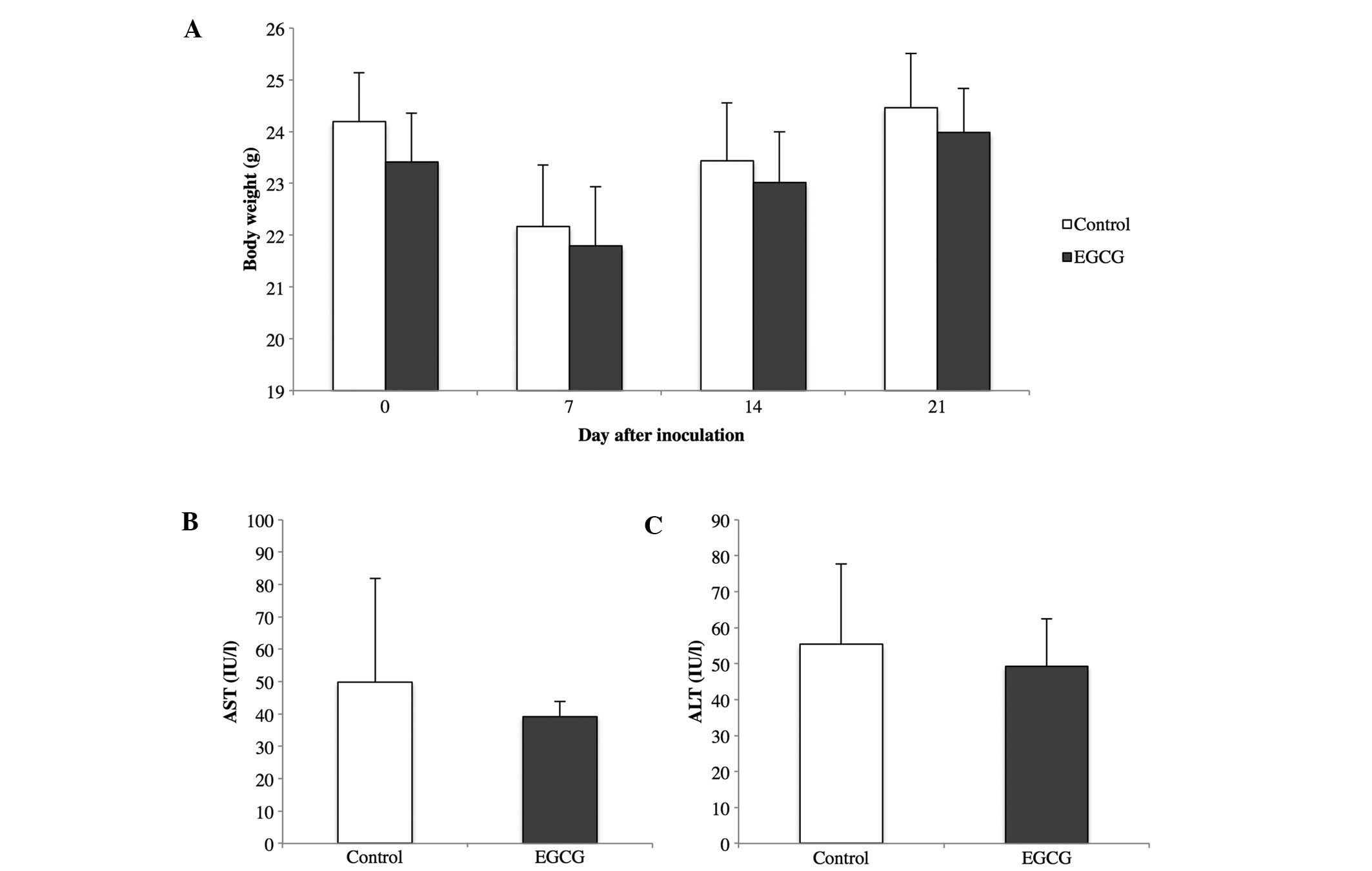
observed in the EGCG group than in the control group. No signs of
toxicity, such as weight loss or elevated AST and ALT, were
observed in mice treated with EGCG compared to the control mice
(Fig. 6).
Discussion
The most common site of distant metastases in
colorectal cancer is the liver. We extended the investigation of
EGCG for the treatment of liver metastases of human colorectal
cancer. In the present study, we revealed that EGCG inhibits cell
proliferation and induces apoptosis in human colorectal cancer
cells in vitro, and suppresses the growth of liver
metastases of human colorectal cancer in vivo. Several
studies of EGCG as a treatment of ectopic cancer models,
particularly subcutaneous models, using various cancer cell lines,
including colorectal cancer, have been reported (17–19).
The major advantage of the ectopic model is that it allows the
rapid screening of new cytotoxic agents. A variety of host organ
environment factors have profound influences on the biological
behavior of tumor cells, including the production of degradative
enzymes, angiogenesis, the induction of differentiation and the
transcriptional properties of genes. Studies using ectopic
inoculation do not account for the organ-specific factors that
influence the growth of tumor cells, and it is necessary to further
examine the antitumor activity of agents in the appropriate
organ-specific environment (20).
This is the first study on inhibiting liver metastases of human
colorectal cancer by administering EGCG.
Angiogenesis is the process of generating new blood
vessels, and it plays a critical role in the growth and metastasis
of solid tumors by supplying nutrients and oxygen and removing
waste products from the tumor (21). The inhibition of angiogenesis using
chemotherapeutic agents, such as bevacizumab, is an attractive
strategy for antitumor treatment (22). VEGFR2 activates various downstream
signal transduction proteins, including Akt (23), which is a critical regulator of cell
survival, proliferation, migration and angiogenesis (24). The interruption of VEGFR2 signaling
is considered necessary for inhibition of tumor angiogenesis and
tumor growth. In the present study, we demonstrated that EGCG
inhibited the expression of VEGFR2 and the phosphorylation of Akt
in vitro and that it suppressed angiogenesis and the growth
of liver metastases in vivo.
p38 is a member of the MAPK family that is activated
in response to a variety of cellular stresses including osmotic
shock, inflammatory cytokines, lipopolysaccharide and UV light
(25). Once phosphorylated, p38
interacts with a wide range of substrates and acts on inflammatory
responses, cell differentiation, cell cycle arrest, senescence,
apoptosis and other cell pathways (26). Anticancer drugs, such as irinotecan
and oxaliplatin, have been reported to activate p38 in colon cancer
cells (27,28). EGCG can induce apoptosis in human
colon adenocarcinoma cells by activating JNK and p38 MAPK (16). In the present study, we demonstrated
that EGCG promoted the phosphorylation of p38 and induced
apoptosis.
Cases of hepatotoxicity have been associated with
the consumption of high doses of dietary supplements containing
green tea (29), and a high oral
dose of EGCG has been reported to induce hepatotoxicity in mice
(30). In the present study, EGCG
administration did not induce hepatotoxicity and weight loss. EGCG
preferentially inhibits the growth of human colorectal and liver
cancer cells in comparison to normal colon epithelial cells and
hepatocytes (31,32). Taken together, the administration of
an appropriate dose of EGCG has little influence on normal cells
and effectively controls liver metastases of colorectal cancer with
few side-effects.
In the present study, EGCG inhibited liver
metastases of human colorectal cancer in a mouse model. EGCG was
recently reported to have a synergistic antitumor effect in
combination with existing anticancer drugs, such as 5-fluorouracil
(5-FU), capecitabine and oxaliplatin (33–35).
Both 5-FU and oxaliplatin are key drugs for treating patients with
liver metastases of colorectal cancer (9), and EGCG treatment in combination with
these drugs has the potential to prolong survival in patients with
liver metastases of colorectal cancer. Green tea, which contains
abundant EGCG, is a commonly consumed beverage worldwide, and the
oral intake of EGCG is reported to inhibit the growth of colorectal
cancer in vivo (17). In a
pilot study, supplementation with green tea extract prevented the
development of metachronous colorectal adenomas (36). Collectively, EGCG may be suitable
not only for chemotherapy for patients with liver metastases of
colorectal cancer in combination with existing anticancer drugs,
but also for oral adjuvant therapy for patients after colorectal
cancer surgery.
In conclusion, EGCG inhibits cell proliferation and
induces apoptosis in human colorectal cancer through Akt, p38 and
VEGFR2, and it suppresses angiogenesis and liver metastases of
colorectal cancer. EGCG may be useful in the treatment of liver
metastases of human colorectal cancer.
Acknowledgements
The present study was supported in part by grants
from the Ministry of Education, Culture, Sports, Science and
Technology of Japan (MEXT) and Public Trust Surgery Research Fund.
The authors thank Ako Takahashi for providing technical
assistance.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Manfredi S, Lepage C, Hatem C, Coatmeur O,
Faivre J and Bouvier AM: Epidemiology and management of liver
metastases from colorectal cancer. Ann Surg. 244:254–259. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hughes KS, Rosenstein RB, Songhorabodi S,
et al: Resection of the liver for colorectal carcinoma metastases.
A multi-institutional study of long-term survivors. Dis Colon
Rectum. 31:1–4. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scheele J, Stang R, Altendorf-Hofmann A
and Paul M: Resection of colorectal liver metastases. World J Surg.
19:59–71. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kato T, Yasui K, Hirai T, et al:
Therapeutic results for hepatic metastasis of colorectal cancer
with special reference to effectiveness of hepatectomy: analysis of
prognostic factors for 763 cases recorded at 18 institutions. Dis
Colon Rectum. 46:S22–S31. 2003.
|
|
6
|
Yamada H, Katoh H, Kondo S, Okushiba S and
Morikawa T: Repeat hepatectomy for recurrent hepatic metastases
from colorectal cancer. Hepatogastroenterology. 48:828–830.
2001.PubMed/NCBI
|
|
7
|
Scheer MG, Sloots CE, van der Wilt GJ and
Ruers TJ: Management of patients with asymptomatic colorectal
cancer and synchronous irresectable metastases. Ann Oncol.
19:1829–1835. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dy GK, Hobday TJ, Nelson G, et al:
Long-term survivors of metastatic colorectal cancer treated with
systemic chemotherapy alone: north central cancer treatment group
review of 3811 patients, n0144. Clin Colorectal Cancer. 8:88–93.
2009. View Article : Google Scholar
|
|
9
|
Venook A: Critical evaluation of current
treatments in metastatic colorectal cancer. Oncologist. 10:250–261.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cabrera C, Artacho R and Gimenez R:
Beneficial effects of green tea: a review. J Am Coll Nutr.
25:79–99. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ji BT, Chow WH, Hsing AW, et al: Green tea
consumption and the risk of pancreatic and colorectal cancers. Int
J Cancer. 70:255–258. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang G, Shu XO, Li H, et al: Prospective
cohort study of green tea consumption and colorectal cancer risk in
women. Cancer Epidemiol Biomarkers Prev. 16:1219–1223. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cabrera C, Gimenez R and Lopez MC:
Determination of tea components with antioxidant activity. J Agric
Food Chem. 51:4427–4435. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Du GJ, Zhang Z, Wen XD, et al:
Epigallocatechin gallate (EGCG) is the most effective cancer
chemopreventive polyphenol in green tea. Nutrients. 4:1679–1691.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shimizu M, Adachi S, Masuda M, Kozawa O
and Moriwaki H: Cancer chemoprevention with green tea catechins by
targeting receptor tyrosine kinases. Mol Nutr Food Res. 55:832–843.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen C, Shen G, Hebbar V, Hu R, Owuor ED
and Kong AN: Epigallocatechin-3-gallate-induced stress signals in
HT-29 human colon adenocarcinoma cells. Carcinogenesis.
24:1369–1378. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shimizu M, Shirakami Y, Sakai H, et al:
(−)-Epigallocatechin gallate inhibits growth and activation of the
VEGF/VEGFR axis in human colorectal cancer cells. Chem Biol
Interact. 185:247–252. 2010.
|
|
18
|
Tran PL, Kim SA, Choi HS, Yoon JH and Ahn
SG: Epigallocatechin-3-gallate suppresses the expression of HSP70
and HSP90 and exhibits anti-tumor activity in vitro and
in vivo. BMC Cancer. 10:2762010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin H, Gong W, Zhang C and Wang S:
Epigallocatechin gallate inhibits the proliferation of colorectal
cancer cells by regulating Notch signaling. Onco Targets Ther.
6:145–153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hoffman RM: Orthotopic metastatic mouse
models for anticancer drug discovery and evaluation: a bridge to
the clinic. Invest New Drugs. 17:343–359. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishida N, Yano H, Nishida T, Kamura T and
Kojiro M: Angiogenesis in cancer. Vasc Health Risk Manag.
2:213–219. 2006. View Article : Google Scholar
|
|
22
|
Shih T and Lindley C: Bevacizumab: an
angiogenesis inhibitor for the treatment of solid malignancies.
Clin Ther. 28:1779–1802. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang BH and Liu LZ: AKT signaling in
regulating angiogenesis. Curr Cancer Drug Targets. 8:19–26. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Manning BD and Cantley LC: AKT/PKB
signaling: navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zarubin T and Han J: Activation and
signaling of the p38 MAP kinase pathway. Cell Res. 15:11–18. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi Y and Gaestel M: In the cellular
garden of forking paths: how p38 MAPKs signal for downstream
assistance. Biol Chem. 383:1519–1536. 2002.PubMed/NCBI
|
|
27
|
Rudolf E, Kralova V, Rudolf K and John S:
The role of p38 in irinotecan-induced DNA damage and apoptosis of
colon cancer cells. Mutat Res. 741–742:27–34. 2013.PubMed/NCBI
|
|
28
|
Liu HF, Hu HC and Chao JI: Oxaliplatin
down-regulates survivin by p38 MAP kinase and proteasome in human
colon cancer cells. Chem Biol Interact. 188:535–545. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mazzanti G, Menniti-Ippolito F, Moro PA,
et al: Hepatotoxicity from green tea: a review of the literature
and two unpublished cases. Eur J Clin Pharmacol. 65:331–341. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lambert JD, Kennett MJ, Sang S, Reuhl KR,
Ju J and Yang CS: Hepatotoxicity of high oral dose
(−)-epigallocatechin-3-gallate in mice. Food Chem Toxicol.
48:409–416. 2010.
|
|
31
|
Shimizu M, Deguchi A, Lim JT, Moriwaki H,
Kopelovich L and Weinstein IB: (−)-Epigallocatechin gallate and
polyphenon E inhibit growth and activation of the epidermal growth
factor receptor and human epidermal growth factor receptor-2
signaling pathways in human colon cancer cells. Clin Cancer Res.
11:2735–2746. 2005.
|
|
32
|
Shimizu M, Shirakami Y, Sakai H, et al:
EGCG inhibits activation of the insulin-like growth factor
(IGF)/IGF-1 receptor axis in human hepatocellular carcinoma cells.
Cancer Lett. 262:10–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yunos NM, Beale P, Yu JQ and Huq F:
Synergism from the combination of oxaliplatin with selected
phytochemicals in human ovarian cancer cell lines. Anticancer Res.
31:4283–4289. 2011.PubMed/NCBI
|
|
34
|
Yang XW, Wang XL, Cao LQ, et al: Green tea
polyphenol epigallocatechin-3-gallate enhances
5-fluorouracil-induced cell growth inhibition of hepatocellular
carcinoma cells. Hepatol Res. 42:494–501. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu H, Xin Y, Xu C and Xiao Y: Capecitabine
combined with (−)-epigallocatechin-3-gallate inhibits angiogenesis
and tumor growth in nude mice with gastric cancer xenografts. Exp
Ther Med. 3:650–654. 2012.
|
|
36
|
Shimizu M, Fukutomi Y, Ninomiya M, et al:
Green tea extracts for the prevention of metachronous colorectal
adenomas: a pilot study. Cancer Epidemiol Biomarkers Prev.
17:3020–3025. 2008. View Article : Google Scholar : PubMed/NCBI
|