Introduction
Colorectal cancer (CRC) is one of the most common
types of cancer (the third and fourth most common cancer in women
and men, respectively) in the world and the second leading cause of
cancer-related mortality in developed countries. More than 1.2
million cases are diagnosed globally each year, with ~600,000
deaths (1,2). In order to find new diagnostic and
therapeutic tools to reduce CRC-related morbidity, it is key to
understand the etiology and biology of CRC. Progression to CRC is
caused by an accumulation of various genetic and epigenetic
alterations, leading to transformation from a normal tissue to a
malignant, and potentially metastatic, tumor. CRC develops through
two main genetic pathways that are characterized by different forms
of genomic instability, the chromosomal instability (CIN, 85%) and
microsatellite instability (MSI, 15%) pathways (3–5). Many
tumor-suppressor genes and oncogenes have been described, and the
discovery of new tumor markers, including those for CRC, continues
at a rapid pace.
A new group of biomarkers, microRNAs (miRs), has
recently been established. miRs are 20–25-nucleotide non-coding
RNAs that negatively regulate gene transcription at the
transcriptional or post-transcriptional level by interacting with
the 3′ untranslated regions (UTRs) of specific messenger RNAs
(mRNAs) and are key modulators in the control of biological
processes, such as cell development, differentiation, proliferation
and apoptosis (6–9). Estimates based on bioinformatics and
microarray analyses suggest that >30% of all genes are regulated
by multiple miRs (10). miR-34s
form an evolutionarily conserved miR family, with miR-34a,
miR-34b and miR-34c occurring in vertebrates. The
miR-34a and miR-34b/c loci are regulated directly by
interaction with TP53, which induces apoptosis, cell cycle arrest
and senescence (11–13). In addition, reduced miR-34a
expression is a frequent feature of both pancreatic tumors and
neuroblastomas (14,15) and reduced miR-34b/c
expression has been observed in non-small cell lung cancer
(16). CpG methylation of
miR-34b/c has been found in CRC (2,17),
oral squamous cell carcinoma (18),
and malignant melanoma, where it correlated with metastatic
potential (19).
The downregulation of miR-34 family members in
cancer suggests that these miRs function as tumor-suppressor genes,
suggesting a possible role as prognostic markers (20). Several mechanisms regulating miR
expression, including gene amplification, deletion, epigenetic
alterations and single-nucleotide substitution, have been
implicated, but not demonstrated (20,21).
Although single nucleotide polymorphisms (SNPs) in miRs are not
considered functionally important, nucleotide variations in primary
(pri)- or precursor (pre)-miRs may affect miR processing and modify
miR expression (22). Recently,
studies reported that a potentially functional SNP, rs4938723, in
the promoter region of pri-miR-34b/c may contribute to
susceptibility to hepatocellular carcinoma (23), CRC (24), endometrial cancer (25) and survival in breast cancer
(26). However, there are few
reports on the relationship between SNPs in the
miR-34b/c promoter and risk and prognostic
significance in CRC patients.
In the present study, we investigated whether the
SNPs rs4938723 (T>C) in the promoter region of miR-34b/c
and Arg72Pro (G>C) in codon 72 of TP53 are independently
or complementarily associated with the risk and clinical outcomes
of CRC and whether the combined effect of these SNPs and metabolic
risks (diabetes and hypertension) is related to progression of CRC,
which is known to be associated with metabolic disease (27,28) in
the Korean population.
Materials and methods
Patients and clinical samples
From June 2000 to January 2009, blood samples were
collected from 545 patients diagnosed with CRC at CHA Bundang
Medical Center of CHA University in South Korea. We retrospectively
obtained information concerning the age; gender; underlying
conditions [hypertension (HTN), diabetes mellitus (DM), body mass
index (BMI), smoking and alcohol consumption]; tumor size, stage
and site; time to progression; and time to mortality. We estimated
homocysteine and folic acid levels. The American Joint Committee on
Cancer: Classification and Stage Groupings, 7th edition was used
for tumor assessment. The cancer-free control group consisted of
428 individuals who were randomly selected from participants in a
health-screening program to exclude those with a history of cancer
and other medical diseases. All study subjects provided written
consent and all were ethnic Koreans. The subjects’ recruitment was
approved by the Institutional Review Board of CHA Bundang Medical
Center.
Genotyping
DNA was extracted from leukocytes using a G-DEX™ II
Genomic DNA Extraction kit (iNtRON Biotechnology, Seongnam, Korea),
according to the manufacturer’s instructions. The SNPs
miR-34b/c rs4938723 and TP53 Arg72Pro rs1042522 were
genotyped by polymerase chain reaction-restriction fragment length
polymorphism (PCR-RFLP) assays. Primer sequences used for
amplification of rs4938723 were: (forward) 5′-CCT CTG GGA ACC TTC
TTT GAC CAA T-3′ and (reverse) 5′-TGA GAT CAA GGC CAT ACC ATT CAA
GA-3′. Primer sequences used for amplification of TP53
Arg72Pro were: (forward) 5′-TTG CCG TCC CAA GCA ATG GAT GA-3′ and
(reverse) 5′-TCT GGG AAG GGA CAG AAG ATG AC-3′. For each of the
polymorphisms, 30% of the PCR assays were randomly chosen and
repeated, followed by DNA sequencing to validate the RFLP findings.
Sequencing was performed using an ABI 3730xl DNA Analyzer (Applied
Biosystems, Foster City, CA, USA). The concordance of the quality
control samples was 100%.
Statistical analysis
The genotypes for each SNP were analyzed as a
three-group categorical variable (reference model) and were also
grouped according to the dominant and recessive model. Odds ratios
(ORs), adjusted odds ratios (AORs) and 95% confidence intervals
(CIs) were used to calculate the strength of association. To
analyze baseline characteristics, we used a Chi-square test for
categorical data when comparing patient and control baseline data.
Multivariate analysis was performed to select independent risk
factors for CRC among genotypes and clinical variables using
logistic regression analysis. The overall survival was compared
using the Kaplan-Meier method and potential variables were verified
by multivariate analysis using a Cox regression model. All tests
were two-tailed, and a P-value <0.05 was deemed to indicate a
statistically significant difference. Analyses were performed using
GraphPad Prism 4.0 (GraphPad Software, San Diego, CA, USA) and
MedCalc version 11.1.1.0 (MedCalc Software, Ostend, Belgium). The
distribution of allele frequencies for the rs4938723 (T>C) and
TP53 Arg72Pro (G>C) gene polymorphisms were calculated by
Chi-square test to determine whether the observed genotype
distributions conformed to the expected Hardy-Weinberg equilibrium
(29).
Results
Population
Baseline characteristics of patients with CRC and
controls are shown in Table I. The
mean age was 62 years in CRC patients and 61 years in controls. Of
the CRC patients, 302 (55.4%) were male. Approximately 61.5% of CRC
cases had HTN and 33.6% had DM; these were significantly higher
values than observed among controls (34.1 and 6.1%, respectively)
(P<0.001). However, the number of smokers in the control group
exceeded that for CRC patients. Tumors occurred most frequently in
the rectum and proximal colon. With regard to tumor-node-metastasis
(TNM) staging, the majority of patients (80.7%) had stage II–III
disease.
 | Table IBaseline characteristics of CRC
patients and controls. |
Table I
Baseline characteristics of CRC
patients and controls.
| Variable n (%) | Controls
(n=428) | CRC patients
(n=545) | OR (95% CI) | P-valuea |
|---|
| Age (years, mean ±
SD) | 60.75±11.73 | 62.07±12.15 | 1.008
(0.998–1.019) | 0.134 |
| Gender (male) | 172 (40.2) | 302 (55.4) | 1.379
(1.100–1.729) | 0.005 |
| Hypertension | 146 (34.1) | 335 (61.5) | 1.802
(1.429–2.272) | <0.0001 |
| Diabetes
mellitus | 26 (6.1) | 183 (33.6) | 10.120
(6.657–15.380) | <0.0001 |
| Body mass index
≥25.0 kg/m2 | 96 (22.4) | 143 (26.2) | 1.170
(0.877–1.560) | 0.285 |
| Smoking | 140 (32.7) | 125 (22.9) | 0.701
(0.534–0.921) | 0.010 |
| Tumor size ≥5
cm | NA | 319 (58.5) | | |
| Tumor site |
| Proximal
colon | NA | 180 (33.0) | | |
| Distal colon | NA | 123 (22.6)) | | |
| Mixed colon | NA | 7 (1.3) | | |
| Rectum | NA | 225 (41.3) | | |
| TNM stage |
| I | NA | 55 (10.1) | | |
| II | NA | 228 (41.8) | | |
| III | NA | 212 (38.9) | | |
| IV | NA | 49 (9.0) | | |
Variant genotypes of TP53 codon 72 are
related to reduced CRC risk
Genotype frequencies of miR-34b/c rs4938723
and TP53 Arg72Pro rs1042522 in CRC patients and controls are
shown in Table II. In multivariate
analysis, the variant genotypes of TP53 Arg72Pro GC and
GC/CC were associated with a significantly decreased risk of CRC
compared with the wild-type GG genotype (AOR = 0.727, 95% CI =
0.550–0.960 for GC; AOR = 0.735, 95% CI = 0.565–0.958 for GC/CC).
However, no overall association was observed between
miR-34b/c rs4938723 and CRC risk in our study
population. The observed genotype frequencies for miR-34b/c
rs4938723 (T>C) and TP53 Arg72Pro (G>C) polymorphisms
in the cases and controls were all as expected for Hardy-Weinberg
equilibrium (P>0.05).
 | Table IIGenotype frequencies of
miR-34b/c rs4938723 and TP53 Arg72Pro in CRC patients
and controls. |
Table II
Genotype frequencies of
miR-34b/c rs4938723 and TP53 Arg72Pro in CRC patients
and controls.
| Characteristics n
(%) | Controls
(n=428) | CRC patients
(n=545) | AOR (95%
CI)a | P-valueb | P-valuec | Colon (n=310) | AOR (95%
CI)a | P-valueb | Rectum (n=230) | AOR (95%
CI)a | P-valueb |
|---|
| miR-34b/c
rs4938723 |
| TT | 216 (50.5) | 272 (49.9) | 1.000 | | | 158 (51.0) | 1.000 | | 114 (49.6) | 1.000 | |
| TC | 171 (40.0) | 233 (42.8) | 1.083
(0.830–1.414) | 0.557 | 0.557 | 131 (42.3) | 1.045
(0.769–1.420) | 0.777 | 98 (42.6) | 1.101
(0.785–1.542) | 0.578 |
| CC | 41 (9.5) | 40 (7.3) | 0.780
(0.487–1.250) | 0.302 | 0.302 | 21 (6.7) | 0.700
(0.398–1.232) | 0.216 | 18 (7.8) | 0.859
(0.471–1.569) | 0.621 |
| Dominant model (TT
vs. TC + CC) | | | 1.025
(0.796–1.321) | 0.848 | 0.848 | | 0.978
(0.730–1.311) | 0.883 | | 1.052
(0.763–1.451) | 0.758 |
| Recessive model
(TT + TC vs. CC) | | | 0.754
(0.478–1.190) | 0.225 | 0.450 | | 0.687
(0.397–1.188) | 0.179 | | 0.821
(0.459–1.467) | 0.505 |
| HWE P | 0.402 | 0.301 | | | | 0.376 | | | 0.628 | | |
| TP53
Arg72Pro |
| GG | 145 (33.9) | 222 (40.7) | 1.000 | | | 130 (41.9) | 1.000 | | 88 (38.3) | 1.000 | |
| GC | 218 (50.9) | 247 (45.3) | 0.727
(0.550–0.960) | 0.025 | 0.050 | 135 (43.5) | 0.675
(0.489–0.931) | 0.016 | 111 (48.3) | 0.827
(0.582–1.175) | 0.289 |
| CC | 65 (15.2) | 76 (14.0) | 0.757
(0.511–1.120) | 0.164 | 0.302 | 45 (14.6) | 0.769
(0.491–1.204) | 0.251 | 31 (13.4) | 0.762
(0.459–1.265) | 0.294 |
| Dominant model (GG
vs. GC + CC) | | | 0.735
(0.565–0.958) | 0.023 | 0.046 | | 0.699
(0.516–0.946) | 0.020 | | 0.815
(0.583–1.138) | 0.229 |
| Recessive model
(GG + GC vs. CC) | | | 0.903
(0.631–1.293) | 0.577 | 0.577 | | 0.947
(0.627–1.431) | 0.797 | | 0.860
(0.542–1.366) | 0.523 |
| HWE P | 0.250 | 0.583 | | | | 0.305 | | | 0.667 | | |
Combined genotype effects of rs4938723
and TP53 (TT/GC and CC/GG) are associated with decreased CRC risk
in colon cancer patients
Combined genotype analyses were conducted to
evaluate the combined effects of the two polymorphisms on the risk
of CRC (Table III). Nine combined
genotypes were estimated from the two polymorphisms in CRC
patients. In the multivariate analysis, combined genotypes TT/GC
and CC/GG were associated with significantly decreased CRC risk
when compared with the wild-type TT/GG genotype (AOR = 0.628, 95%
CI = 0.422–0.934 for TT/GC; AOR = 0.381, 95% CI = 0.183–0.793 for
CC/GG, respectively). This association was observed only in
patients with colon, not rectal, cancer and, moreover, was observed
only in proximal colon cancer patients (data not shown).
 | Table IIICombined genotype frequencies of
miR-34b/c rs4938723 and TP53 Arg72Pro in CRC patients
and controls. |
Table III
Combined genotype frequencies of
miR-34b/c rs4938723 and TP53 Arg72Pro in CRC patients
and controls.
| Characteristics n
(%) | Controls
(n=428) | CRC patients
(n=545) | AOR (95%
CI)a | P-valueb | P-valuec | Colon (n=310) | AOR (95%
CI)a | P-valueb | Rectum (n=230) | AOR (95%
CI)a | P-valueb |
|---|
|
rs4938723-TP53 Arg72Pro | | | | | | | | | | | |
| TT-GG | 69 (16.1) | 115 (21.1) | 1.000 | | | 74 (23.9) | 1.000 | | 41 (17.8) | 1.000 | |
| TT-GC | 113 (26.4) | 122 (22.4) | 0.628
(0.422–0.934) | 0.022 | 0.088 | 61 (19.7) | 0.494
(0.313–0.780) | 0.002 | 61 (26.5) | 0.883
(0.536–1.457) | 0.627 |
| TT-CC | 34 (7.9) | 35 (6.4) | 0.627
(0.358–1.100) | 0.103 | 0.206 | 23 (7.4) | 0.658
(0.351–1.233) | 0.192 | 12 (5.2) | 0.554
(0.254–1.206) | 0.137 |
| TC-GG | 54 (12.6) | 93 (17.1) | 1.017
(0.648–1.596) | 0.942 | 0.942 | 50 (16.1) | 0.850
(0.512–1.413) | 0.531 | 40 (17.4) | 1.262
(0.716–2.224) | 0.421 |
| TC-GC | 92 (21.5) | 105 (19.3) | 0.685
(0.454–1.033) | 0.071 | 0.189 | 62 (20.0) | 0.634
(0.399–1.007) | 0.053 | 42 (18.3) | 0.764
(0.448–1.300) | 0.321 |
| TC-CC | 25 (5.8) | 35 (6.4) | 0.837
(0.462–1.516) | 0.557 | 0.743 | 19 (6.1) | 0.711
(0.360–1.405) | 0.326 | 16 (7.0) | 1.086
(0.517–2.279) | 0.828 |
| CC-GG | 22 (5.1) | 14 (2.6) | 0.381
(0.183–0.793) | 0.010 | 0.080 | 6 (1.9) | 0.255
(0.097–0.665) | 0.005 | 7 (3.0) | 0.533
(0.208–1.365) | 0.190 |
| CC-GC | 13 (3.0) | 20 (3.7) | 0.923
(0.432–1.972) | 0.835 | 0.942 | 12 (3.9) | 0.867
(0.369–2.037) | 0.743 | 8 (3.5) | 1.056
(0.402–2.775) | 0.912 |
| CC-CC | 6 (1.6) | 6 (1.0) | 0.601
(0.186–1.938) | 0.394 | 0.630 | 3 (1.0) | 0.467
(0.112–1.940) | 0.294 | 3 (1.3) | 0.891
(0.209–3.799) | 0.876 |
SNP rs4938723 with DM is associated with
increased CRC risk
Table IV shows CRC
risk by combined genetic-environmental effects (HTN, DM,
homocysteine and folic acid). TP53 Arg72Pro GG and all
genotypes of rs4938723 with HTN were significantly associated with
increased risk of CRC. All DM patients, in particular, showed
strong positive association with CRC, regardless of genotype. The
polymorphism rs4938723 with DM was associated with a significantly
increased CRC risk compared with wild-type TT and TP53
Arg72Pro CC with DM significantly decreased the risk when compared
with wild-type GG. No differences were observed between
homocysteine and folic acid levels for any genotype (data not
shown). A homocysteine level >11.7 μmol/l was associated with
increased risk of CRC in rs4938723 TC and TC/CC, but with decreased
risk for TP53 Arg72Pro SNPs. Folic acid level <4.45 ng/ml
was associated with an increased CRC risk, but the rs4938723 and
TP53 Arg72Pro polymorphisms decreased the risk of CRC when
compared with wild-type (Table
IV).
 | Table IVColorectal cancer risk by combined
gene-environmental effects. |
Table IV
Colorectal cancer risk by combined
gene-environmental effects.
| Genotypes | Without HTN AOR
(95% CI)a | With HTN AOR (95%
CI)a | Without DM AOR (95%
CI)a | With DM AOR (95%
CI)a |
|---|
| miR-34b/c
rs4938723 TT | 1.000 | 2.985
(1.962–4.542)b | 1.000 | 5.737
(3.152–10.442)b |
| miR-34b/c
rs4938723 TC | 1.021
(0.678–1.538) | 3.570
(2.252–5.661)b | 1.045
(0.760–1.436) | 6.545
(3.520–12.171)b |
| miR-34b/c
rs4938723 CC | 0.574
(0.264–1.249) | 3.098
(1.427–6.725)b | 0.752
(0.429–1.319) | 5.246
(1.130–24.359)b |
| miR-34b/c
rs4938723 TC + CC | 0.980
(0.679–1.415) | 3.964
(2.643–5.945)b | 1.009
(0.758–1.343) | 8.323
(4.498–15.402)b |
| TP53
Arg72Pro GG | 1.000 | 2.007
(1.215–3.315)b | 1.000 | 7.183
(3.428–15.052)b |
| TP53
Arg72Pro GC | 0.562
(0.359–0.880)b | 2.362
(1.426–3.913) | 0.798
(0.573–1.111) | 5.699
(3.019–10.760)b |
| TP53
Arg72Pro CC | 0.846
(0.475–1.510) | 1.734
(0.855–3.515) | 0.938
(0.589–1.494) | 2.605
(1.116–6.082)b |
| TP53
Arg72Pro GC + CC | 0.704
(0.476–1.041) | 2.595
(1.656–4.066)b | 0.780
(0.579–1.051) | 5.398
(3.091–9.429)b |
|
| Hcy ≤11.70 μmol/l
AOR (95% CI)a | 11.70< Hcy
μmol/l AOR (95% CI)a | 4.45< FA ng/ml
AOR (95% CI)a | FA ≤4.45 ng/ml AOR
(95% CI)a |
|
| miR-34b/c
rs4938723 TT | 1.000 | 1.375
(0.885–2.134) | 1.000 | 3.406
(2.127–5.453)b |
| miR-34b/c
rs4938723 TC | 1.008
(0.731–1.389) | 1.996
(1.236–3.223)b | 1.238
(0.898–1.709) | 2.490
(1.528–4.056)b |
| miR-34b/c
rs4938723 CC | 0.637
(0.362–1.121) | 2.447
(0.750–7.979) | 0.715
(0.396–1.294) | 2.430
(0.890–6.637) |
| miR-34b/c
rs4938723 TC + CC | 0.929
(0.685–1.261) | 2.055
(1.302–3.243)b | 1.136
(0.836–1.545) | 2.483
(1.577–3.912)b |
| TP53
Arg72Pro GG | 1.000 | 2.904
(1.641–5.138)b | 1.000 | 3.438
(1.922–6.148)b |
| TP53
Arg72Pro GC | 0.814
(0.581–1.140) | 1.076
(0.682–1.699) | 0.726
(0.518–1.019) | 1.809
(1.139–2.876)b |
| TP53
Arg72Pro CC | 0.926
(0.588–1.460) | 1.389
(0.596–3.234) | 0.847
(0.534–1.346) | 2.218
(0.972–5.064) |
| TP53
Arg72Pro GC + CC | 0.842
(0.614–1.155) | 1.143
(0.745–1.753) | 0.755
(0.549–1.037) | 1.895
(1.234–2.909)b |
Polymorphisms rs4938723 and TP53 Arg72Pro
show a trend toward, but are not significantly associated with,
improved survival
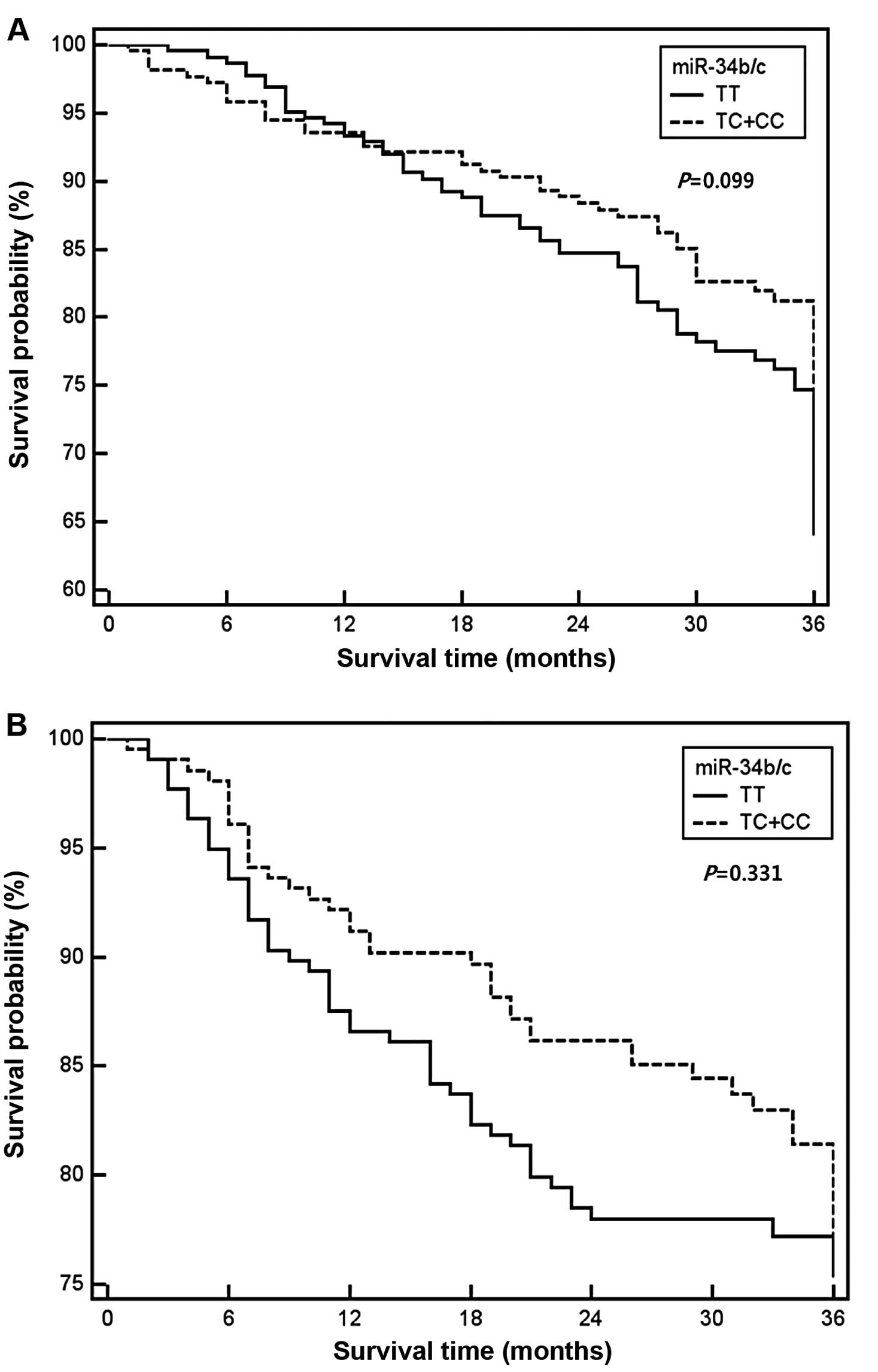
The 3-year survival rate was estimated in each
miR-34b/c rs4938723 and TP53 Arg72Pro patient
group (Table V). In the
multivariate analysis, overall survival of the variant
miR-34b/c rs4938723 and TP53 Arg72Pro genotypes was
more evident in subjects carrying polymorphisms than in wild-type.
However, we did not find a significant association between
miR-34b/c rs4938723 and TP53 Arg72Pro genotypes and
survival of CRC (Fig. 1).
Stratified analyses (Table VI)
showed that the risk reduction effect of the variant TP53
Arg72Pro GC/CC genotypes was significant in subjects without HTN
(AOR = 0.635, 95% CI = 0.418–0.966). However, no significant
interactions were observed between miR-34b/c rs4938723 TC/CC
and other clinical features.
 | Table VMultivariate survival analysis
according to miR-34b/c rs4938723 and TP53 Arg72Pro
polymorphisms. |
Table V
Multivariate survival analysis
according to miR-34b/c rs4938723 and TP53 Arg72Pro
polymorphisms.
| | Overall
survival | Relapse-free
survival |
|---|
| |
|
|
|---|
| Characteristics n
(%) | CRC patients
(n=545) | Mortality
(n=112) | Adjusted HR (95%
CI) | P-value | Relapse (n=75) | Adjusted HR (95 %
CI) | P-value |
|---|
| miR-34b/c
rs4938723 |
| TT | 272 (49.9) | 64 (57.1) | 1.000 | | 42 (56.0) | 1.000 | |
| TC | 233 (42.8) | 43 (38.4) | 0.716
(0.476–1.077) | 0.111 | 30 (40.0) | 0.711
(0.456–1.108) | 0.134 |
| CC | 40 (7.3) | 5 (4.5) | 0.582
(0.233–1.451) | 0.248 | 3 (4.0) | 0.804
(0.315–2.050) | 0.649 |
| Dominant model (TT
vs. TC + CC) | | | 0.704
(0.475–1.043) | 0.082 | | 0.729
(0.477–1.116) | 0.148 |
| Recessive model
(TT+TC vs. CC) | | | 0.863
(0.338–2.208) | 0.760 | | 0.899
(0.365–2.217) | 0.818 |
| TP53
Arg72Pro |
| GG | 222 (40.7) | 50 (44.6) | 1.000 | | 34 (45.3) | 1.000 | |
| GC | 247 (45.3) | 53 (47.3) | 0.862
(0.577–1.286) | 0.469 | 35 (46.7) | 0.813
(0.524–1.260) | 0.356 |
| CC | 76 (14.0) | 9 (8.1) | 0.565
(0.274–1.165) | 0.124 | 6 (8.0) | 0.653
(0.310–1.374) | 0.264 |
| Dominant model (GG
vs. GC + CC) | | | 0.809
(0.550–1.190) | 0.285 | | 0.782
(0.515–1.189) | 0.253 |
| Recessive model
(GG + GC vs. CC) | | | 0.630
(0.318–1.249) | 0.188 | | 0.752
(0.375–1.510) | 0.426 |
 | Table VIStratified effect of miR-34b/c
rs4938723 and TP53 polymorphisms on colorectal cancer
risk. |
Table VI
Stratified effect of miR-34b/c
rs4938723 and TP53 polymorphisms on colorectal cancer
risk.
| miR-34b/c
rs4938723 TC + CC | TP53
Arg72Pro GC + CC |
|---|
|
|
|
|---|
| Variables | AOR (95%
CI)a | P-valueb | P-valuec | AOR (95%
CI)a | P-valueb | P-valuec |
|---|
| Age (years) |
| <62 | 0.986
(0.661–1.470) | 0.943 | 0.965 | 0.819
(0.546–1.228) | 0.334 | 0.904 |
| ≥62 | 1.009
(0.675–1.508) | 0.965 | 0.965 | 0.847
(0.550–1.306) | 0.452 | 0.904 |
| Gender |
| Male | 1.182
(0.794–1.760) | 0.410 | 0.605 | 0.965
(0.635–1.465) | 0.866 | 0.866 |
| Female | 0.856
(0.570–1.285) | 0.454 | 0.605 | 0.695
(0.457–1.058) | 0.090 | 0.360 |
| Hypertension |
| Yes | 1.087
(0.722–1.637) | 0.689 | 0.747 | 1.086
(0.716–1.645) | 0.698 | 0.747 |
| No | 0.938
(0.633–1.388) | 0.747 | 0.747 | 0.635
(0.418–0.966) | 0.034 | 0.136 |
| Diabetes
mellitus |
| Yes | 1.150
(0.524–2.524) | 0.728 | 0.943 | 0.715
(0.300–1.704) | 0.449 | 0.898 |
| No | 0.989
(0.731–1.338) | 0.943 | 0.943 | 0.839
(0.613–1.149) | 0.274 | 0.898 |
| Tumor site |
| Colon | 0.989
(0.714–1.369) | 0.946 | 0.999 | 0.756
(0.540–1.060) | 0.105 | 0.420 |
| Rectum | 1.057
(0.737–1.515) | 0.765 | 0.999 | 1.000
(0.684–1.462) | 0.999 | 0.999 |
| TNM stage |
| I+II | 1.117
(0.798–1.565) | 0.519 | 0.643 | 0.849
(0.598–1.205) | 0.359 | 0.643 |
| III+IV | 0.922
(0.655–1.298) | 0.643 | 0.643 | 0.824
(0.577–1.176) | 0.286 | 0.643 |
Discussion
In the present study on CRC in a Korean population,
we investigated the correlation between the SNPs rs4938723, in the
promoter region of pri-miR-34b/c and TP53 Arg72Pro
and the risk of CRC. We found that the TP53 Arg72Pro
polymorphism was significantly associated with a decreased risk of
CRC. The combined genotypes rs4938723 CC and TP53 GG had a
tendency to protect individuals against CRC. This finding suggests
that SNPs in the promoter regions of miRs may have important roles
in the etiology of CRC, providing novel biomarkers for
malignancies. This tendency was shown for cancer of the colon,
particularly the proximal colon, but not for rectal cancer. Further
investigation of the relationship between the site of cancer and
genotype is required. Recently, studies reported that TP53
influences the expression of miR-34b/c in a one-way
relationship. However, rs4938723 TT and TP53 Arg72Pro GC
were also associated with decreased CRC risk in the present study,
which suggests that TP53 and miR-34b/c may interact
bidirectional and supports the concept that CRC is a complex
disease involving multiple genes.
We also found that HTN or DM was associated with a
significantly increased risk of CRC, regardless of genotype
(Table IV). SNP rs4938723 with DM
was associated with increased CRC risk when compared with
wild-type, but TP53 Arg72Pro CC with DM was associated with
decreased risk when compared with wild-type. Moreover, decreased
levels of homocysteine and folic acid showed a positive correlation
with the risk of CRC (Table VII).
At folic acid levels of <4.45 ng/ml, both the rs4938723 and
TP53 codon 72 SNPs were related to a decreased risk of CRC.
At homocysteine levels >11.70 μmol/l, rs4938723 was positively
correlated with CRC risk, but the TP53 codon 72 SNP was
inversely correlated. These findings suggest that genetic factors
and environmental factors, such as metabolic diseases, influence
each other in CRC and may implicate miRs as pathophysiologic
linkers/modulators between metabolic syndrome and cancer.
 | Table VIIHomocysteine and folic acid levels in
CRC patients and controls. |
Table VII
Homocysteine and folic acid levels in
CRC patients and controls.
| Homocysteine
level | Controls
(n=423) | CRC patients
(n=463) | OR (95% CI) | P-value |
|---|
| Hcy ≤7.26 | 104 (24.6) | 118 (25.5) | 1.000 | |
| 7.26< Hcy
≤8.29 | 130 (30.7) | 93 (20.1) | 0.631
(0.433–0.917) | 0.016 |
| 8.29< Hcy
≤11.70 | 107 (25.3) | 113 (24.4) | 0.931
(0.641–1.352) | 0.707 |
| 11.70< Hcy | 82 (19.4) | 139 (30.0) | 1.494
(1.022–2.184) | 0.038 |
|
| Folate level | Controls
(n=421) | CRC patients
(n=462) | OR (95% CI) | P-value |
|
| FA ≤4.45 | 66 (15.7) | 156 (33.8) | 1.000 | |
| 4.45< FA
≤6.39 | 121 (28.7) | 99 (21.4) | 0.346
(0.234–0.512) | <0.0001 |
| 6.39< FA
≤9.36 | 117 (27.8) | 104 (22.5) | 0.376
(0.254–0.556) | <0.0001 |
| 9.36< FA | 117 (27.8) | 103 (22.3) | 0.373
(0.252–0.551) | <0.0001 |
Rs4938723 is located within the CpG island of
pri-miR-34b/c (423-bp upstream of the transcription
start site), making it a potential binding site for GATA-X
transcription factors. According to the web-based SNP analysis tool
TFSEARCH 1.3, GATA family members bind to promoters of many genes
and directly activate or suppress expression of target genes and
may be involved in carcinogenesis (30). Therefore, SNP rs4938723 (T>C) may
affect the expression level of miR-34b/c. Ectopic
miR-34b/c caused cell cycle arrest in the G1 phase (31) and miR-34b/c inhibited
proliferation and colony formation in soft agar (11). The relationship between miR
expression in tumors and prognosis, with regard to both the
treatment response rate and survival, is of increasing interest.
This polymorphism has been reported to be associated with
susceptibility to hepatocellular carcinoma (23), CRC (24), survival of breast cancer (26), renal cell carcinoma (32), non-small cell lung (33) and oral cancer (18).
Arg72Pro is the most widely investigated of the
variations in the TP53 gene. The 72Arg allele induces
apoptosis more efficiently than the 72Pro allele (34). It has been reported that
homozygosity for Pro of TP53 Arg72Pro is potentially a risk
factor for cancer of the lung, esophagus, stomach, breast,
nasopharynx, urothelium and prostate (35–37).
In CRC, meta-analysis was performed to estimate the effect of the
TP53 Arg72Pro polymorphism and CRC risk, and no significant
association was identified (38).
In contrast to that result, we found a negative relationship
between the TP53 Arg72Pro polymorphism and CRC risk in the
Korean population.
Our study has several limitations. First, the
enrolled patients were selected at a single institution in Korea
and the sample size may limit the statistical power, especially for
evaluating the connection between SNPs and CRC risk and survival in
detailed genotype subgroups. Second, as the study was
hospital-based, the cases and controls could not be representative
of the general population. This possible selection bias could not
be avoided. Finally, the mechanisms underlying the effects of
genetic polymorphisms on the levels of pre/mature miRs and the
identity of the miR target genes remain unknown and should be
determined in future studies.
In conclusion, although we identified no significant
prognostic value of SNPs for CRC, we found that SNPs rs4938723 and
TP53 Arg72Pro show a trend toward improved survival and that
the TP53 Arg72Pro CC genotype and dominant model (GC + CC)
significantly decrease the risk of CRC. Additionally, the combined
genotype rs4938723 and TP53 Arg72Pro (TT/GC and CC/GG) was
significantly associated with decreased CRC risk in the Korean
population. Also, SNP rs4938723 with DM was more closely related to
increased CRC risk than the wild-type genotype. Future studies in
multiethnic populations are warranted to validate our results and
to define the functional effects of these SNPs on CRC.
Acknowledgements
This study was partially supported by a National
Research Foundation (NRF) of Korea Grant funded by the Korean
Government (2009-0075784 and 2012R1A1A2007033), and a Priority
Research Centers Program Grant, administered by the NRF and funded
by the Ministry of Education (2009-0093821), Republic of Korea.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Cin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Hrašovec S and Glavač D: MicroRNAs as
novel biomarkers in colorectal cancer. Front Genet. 3:1802012.
|
|
3
|
Cunningham JM, Christensen ER, Tester DJ,
et al: Hypermethylation of the hMLH1 promoter in colon
cancer with microsatellite instability. Cancer Res. 58:3455–3460.
1998.PubMed/NCBI
|
|
4
|
Toyota M, Ahuja N, Ohe-Toyota M, Herman
JG, Baylin SB and Issa JP: CpG island methylator phenotype in
colorectal cancer. Proc Natl Acad Sci USA. 96:8681–8686. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shen L, Toyota M, Kondo Y, et al:
Integrated genetic and epigenetic analysis identifies three
different subclasses of colon cancer. Proc Natl Acad Sci USA.
104:18654–18659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fazi F and Nervi C: MicroRNA: basic
mechanisms and transcriptional regulatory networks for cell fate
determination. Cardiovasc Res. 79:553–561. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meister G and Tuschl T: Mechanisms of gene
silencing by double-stranded RNA. Nature. 431:343–349. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pillai RS, Bhattacharyya SN and Filipowicz
W: Repression of protein synthesis by miRNAs: how many mechanisms?
Trends Cell Biol. 17:118–126. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lim LP, Lau NC, Garrett-Engele P, et al:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Corney DC, Flesken-Nikitin A, Godwin AK,
Wang W and Nikitin AY: MicroRNA-34b and MicroRNA-34c
are targets of p53 and cooperate in control of cell proliferation
and adhesion-independent growth. Cancer Res. 67:8433–8438. 2007.
View Article : Google Scholar
|
|
12
|
He L, He X, Lim LP, et al: A microRNA
component of the p53 tumour suppressor network. Nature.
447:1130–1134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tarasov V, Jung P, Verdoodt B, et al:
Differential regulation of microRNAs by p53 revealed by massively
parallel sequencing: miR-34a is a p53 target that induces apoptosis
and G1-arrest. Cell Cycle. 6:1586–1593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang TC, Wentzel EA, Kent OA, et al:
Transactivation of miR-34a by p53 broadly influences gene
expression and promotes apoptosis. Mol Cell. 26:745–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Welch C, Chen Y and Stallings RL:
MicroRNA-34a functions as a potential tumor suppressor by inducing
apoptosis in neuroblastoma cells. Oncogene. 26:5017–5022. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bommer GT, Gerin I, Feng Y, et al:
p53-mediated activation of miRNA34 candidate tumor-suppressor
genes. Curr Biol. 17:1298–1307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Toyota M, Suzuki H, Sasaki Y, Maruyama R,
Imai K, Shinomura Y and Tokino T: Epigenetic silencing of
microRNA-34b/c and B-cell translocation gene 4 is
associated with CpG island methylation in colorectal cancer. Cancer
Res. 68:4123–4132. 2008.PubMed/NCBI
|
|
18
|
Kozaki K, Imoto I, Mogi S, Omura K and
Inazawa J: Exploration of tumor-suppressive microRNAs silenced by
DNA hypermethylation in oral cancer. Cancer Res. 68:2094–2105.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lujambio A, Calin GA, Villanueva A, et al:
A microRNA DNA methylation signature for human cancer metastasis.
Proc Natl Acad Sci USA. 105:13556–13561. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kong YW, Ferland-McCollough D, Jackson TJ
and Bushell M: microRNAs in cancer management. Lancet Ooncol.
13:e249–e258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lujambio A, Ropero S, Ballestar E, et al:
Genetic unmasking of an epigenetically silenced microRNA in human
cancer cells. Cancer Res. 67:1424–1429. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iwai N and Naraba H: Polymorphisms in
human pre-miRNAs. Biochem Biophys Res Commun. 331:1439–1444. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu Y, Liu L, Liu J, et al: A potentially
functional polymorphism in the promoter region of miR-34b/c is
associated with an increased risk for primary hepatocellular
carcinoma. Int J Cancer. 128:412–417. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao LB, Li LJ, Pan XM, Li ZH, Liang WB,
Bai P, Zhu YH and Zhang L: A genetic variant in the promoter region
of miR-34b/c is associated with a reduced risk of colorectal
cancer. Biol Chem. 394:415–420. 2013.PubMed/NCBI
|
|
25
|
Hiroki E, Suzuki F, Akahira J, Nagase S,
Ito K, Sugawara J, Miki Y, Suzuki T, Sasano H and Yaegashi N:
MicroRNA-34b functions as a potential tumor suppressor in
endometrial serous adenocarcinoma. Int J Cancer. 131:E395–E404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bensen JT, Tse CK, Nyante SJ,
Barnholtz-Sloan JS, Cole SR and Millikan RC: Association of
germline microRNA SNPs in pre-miRNA flanking region and breast
cancer risk and survival: the Carolina Breast Cancer Study. Cancer
Causes Control. 24:1099–1109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ahmed RL, Schmitz KH, Anderson KE,
Rosamond WD and Folsom AR: The metabolic syndrome and risk of
incident colorectal cancer. Cancer. 107:28–36. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moghaddam AA, Woodward M and Huxley R:
Obesity and risk of colorectal cancer: a meta-analysis of 31
studies with 70,000 events. Cancer Epidemiol Biomarkers Prev.
16:2533–2547. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wittke-Thompson JK, Pluzhnikov A and Cox
NJ: Rational inferences about departures from Hardy-Weinberg
equilibrium. Am J Hum Genet. 76:967–986. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chou J, Provot S and Werb Z: GATA3 in
development and cancer differentiation: cells GATA have it! J Cell
Physiol. 222:42–49. 2010.
|
|
31
|
Xiang Y, Fan S, Cao J, Huang S and Zhang
LP: Association of the microRNA-499 variants with susceptibility to
hepatocellular carcinoma in a Chinese population. Mol Biol Rep.
39:7019–7023. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin J, Horikawa Y, Tamboli P, Clague J,
Wood CG and Wu X: Genetic variations in microRNA-related genes are
associated with survival and recurrence in patients with renal cell
carcinoma. Carcinogenesis. 31:1805–1812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu Z, Chen J, Tian T, et al: Genetic
variants of miRNA sequences and non-small cell lung cancer
survival. J Clin Invest. 118:2600–2608. 2008.PubMed/NCBI
|
|
34
|
Dumont P, Leu JI, Della Pietra AC III,
George DL and Murphy M: The codon 72 polymorphic variants of p53
have markedly different apoptotic potential. Nat Genet. 33:357–365.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Irarrázabal CE, Rojas C, Aracena R,
Márquez C and Gil L: Chilean pilot study on the risk of lung cancer
associated with codon 72 polymorphism in the gene of protein p53.
Toxicol Lett. 144:69–76. 2003.PubMed/NCBI
|
|
36
|
Kuroda Y, Tsukino H, Nakao H, Imai H and
Katoh T: p53 codon 72 polymorphism and urothelial cancer
risk. Cancer Lett. 189:77–83. 2003. View Article : Google Scholar
|
|
37
|
Suzuki K, Matsui H, Ohtake N, et al: A p53
codon 72 polymorphism associated with prostate cancer development
and progression in Japanese. J Biomed Sci. 10:430–435. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dahabreh IJ, Linardou H, Bouzika P,
Varvarigou V and Murray S: TP53 Arg72Pro polymorphism and
colorectal cancer risk: a systematic review and meta-analysis.
Cancer Epidemiol Biomarkers Prev. 19:1840–1847. 2010. View Article : Google Scholar
|