Introduction
Esophageal squamous cell carcinoma (SCC) is a fatal
disease that frequently occurs in Asia and South America (1). Cancer stem cells (CSCs) exhibit
several characteristics that make cancerous tumors fatal. CSCs
produce several types of proliferative progenies, are resistant to
several drugs and radiation and are tumorigenic. Due to CSCs, tumor
tissue often regenerates after therapy. CSCs have been detected in
several types of tumor tissues such as breast cancer and pancreas
adenocarcinoma (2,3). Thus, CSCs are thought to have critical
roles in therapy resistance, relapse and metastasis as well.
Recently, some CSCs in SCC were detected. CD75, CD44, CD90 and side
population cells were reported to be CSC markers (4–7).
Moreover, in our previous study, JARID1B was used as a CSC marker
(8). However, therapies for
esophageal SCC that target CSCs remain relatively unknown.
In the present study, we used a fluorescent vector
that can detect CSCs or therapy-resistant cells in some tumor
tissues (9–13). The vector is a fluorescein ZsGreen
fused to the carboxyl-terminal degron of ornithine decarboxylase
(cODC). The cells infected with the vector become ZsGreen-positive
when the protein is not degradated and become ZsGreen-negative when
the protein is lysed. On the basis of this mechanism, this protein
is believed to be able to detect the function of protein
degradation machineries, such as proteasomes. In the present study,
we investigated the use of the above-mentioned fluorescent vector
for detection of CSCs or therapy-resistant cells in esophageal
SCC.
Materials and methods
Cell culture
The human esophageal SCC cell lines TE4 and TE8 were
cultured in RPMI-1640 medium supplemented with 10% fetal bovine
serum (FBS; HyClone, Logan, UT, USA) at 5% CO2 and 37°C.
The Platinum-A Retroviral Packaging Cell Line (Plat-A) was
purchased from Cell Biolabs (San Diego, CA, USA). Plat-A was
cultured in DMEM supplemented with 10% FBS, 1 μg/ml puromycin
(Sigma-Aldrich, St. Louis, MO, USA), 10 μg/ml blasticidin
(Sigma-Aldrich), 100 U/ml penicillin and streptomycin (Life
Technologies, Inc., Gaithersburg, MD, USA). StemPro Accutase (Life
Technologies) was used for detachment of all the cells. A
retroviral vector known as pQCXIN-ZsGreen-cODC consisting of a
fluorescein ZsGreen fused to cODC was kindly provided by Dr Shinji
Tanaka. The retroviral vector was transfected into Plat-A to
generate retrovirus. The virus collected from the supernatant of
the Plat-A was used to induct cells. The cells stably expressing
fluorescein ZsGreen fused to cODC were selected by adding 500 μg/ml
of G418 (Life technologies) and purified by single-cell
cloning.
Sphere formation
The ReproStem medium (ReproCELL, Inc., Kanagawa,
Japan) supplemented with 5 ng/ml fibroblast growth factor 2
(ReproCELL) was used to suspend 1×103 cells.
Subsequently, 1×103 cells were seeded in ultra-low
attachment 6-well plates (Corning Incorporated, Corning, NY, USA).
Following incubation for ~2 weeks, formed spheres >100 μm in
size were counted.
Clonogenic survival assay
Appropriate numbers of cells were seeded in 10-cm
dishes and exposed to radiation at 0, 2, 4 and 6 Gy. Following
incubation for ~2 weeks, colonies stained with the Diff-Quick
solution (Sysmex, Kobe, Japan) were counted.
Drug screening
Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto,
Japan), in which 2×103 cells/100 μl were seeded into
each well of a 96-well plate, was used to determine cell viability.
Following incubation for 24 h, the cells were exposed to drugs or
radiation. Then, following incubation for 72 h, 10 μl of Cell
Counting Kit-8 solution was added to each well followed by further
incubation for 2 h. Cell viability was determined by reading the
optical density (Bio-Rad Laboratories, Hercules, CA, USA) in each
well at 450 nm.
Animal experiments
Portions containing 1×103 cells, mixed
with BD Matrigel (Becton-Dickinson, Franklin Lakes, NJ, USA) at a
1:1 ratio, were subcutaneously injected into NOD/SCID mice. These
mice were examined for 51 days and sacrificed when the tumors
reached a maximum diameter of ~15 mm. The animal studies were
approved by the Animal Experiments Committee of Osaka University
(Suita, Japan).
Results
Establishment of visualized CSC-like
cells of human esophagus
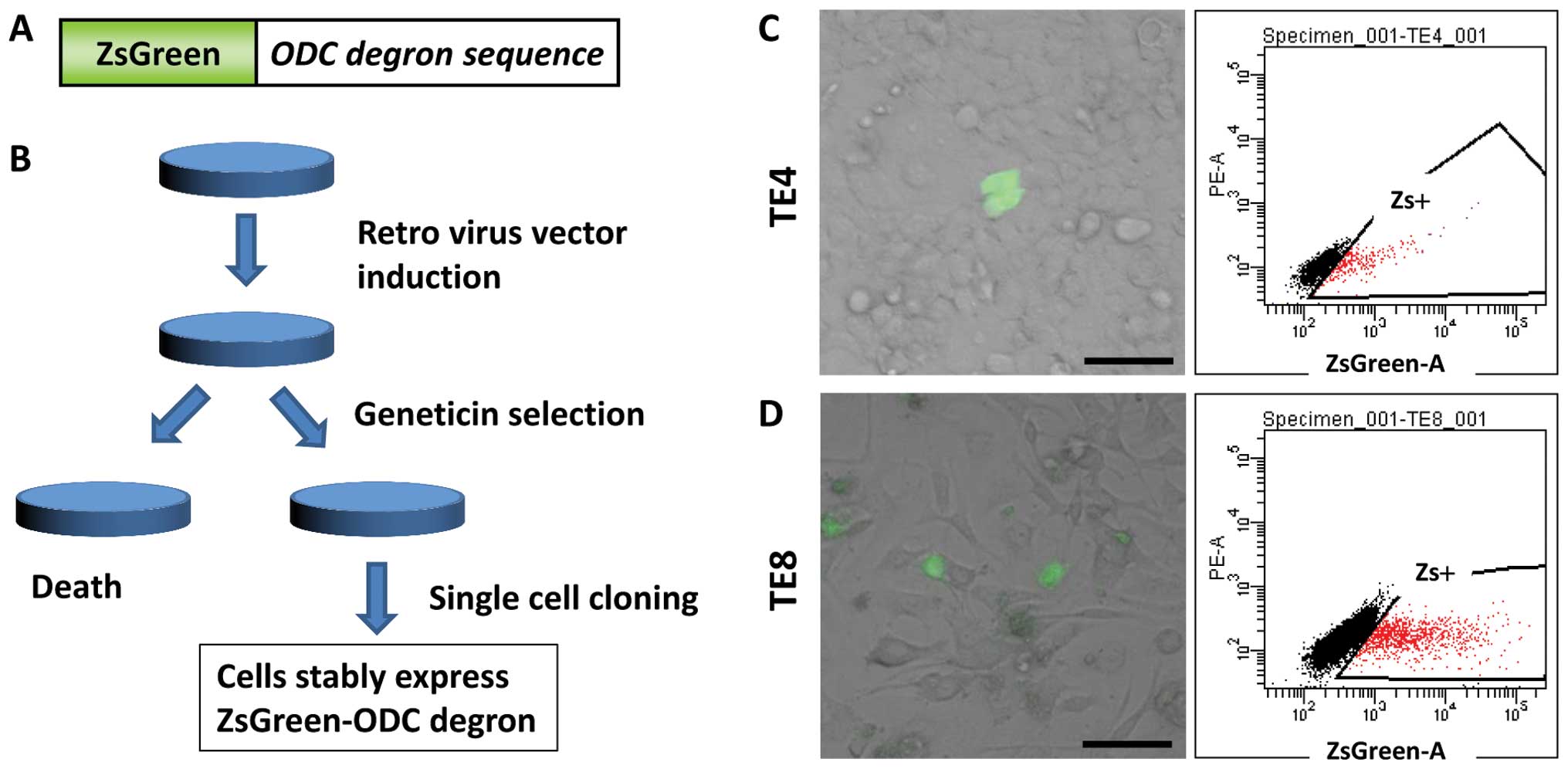
Two esophageal SCC cell lines, TE4 and TE8, were
infected with a retroviral vector containing fluorescein ZsGreen
fused to cODC (the fusion protein is presented in Fig. 1A). Following Geneticin®
selection and single-cell cloning, the cells stably expressing
fluorescein ZsGreen fused to cODC were generated (Fig. 1B). The ZsGreen-positive cells were
detected by microscopy and FACS analysis in both cell lines.
Approximately 15% of the population was detected as
ZsGreen-positive by FACS analysis, but only ~0.1% of the population
was detected as ZsGreen-positive by microscopy in both the cell
lines (Fig. 1C and D).
Self-renewal study
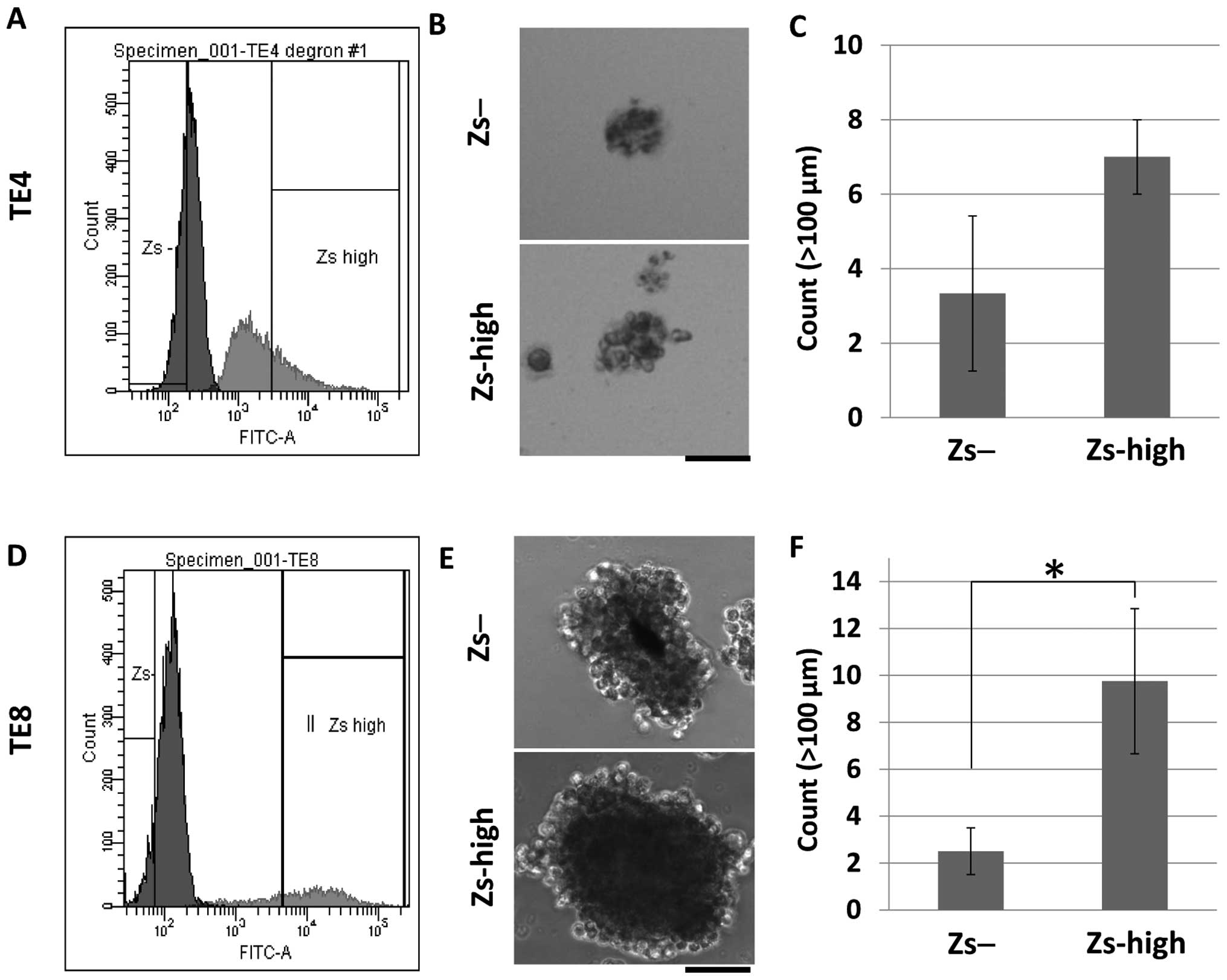
To investigate the characteristics of the
ZsGreen-high cells and the ZsGreen-negative cells, a sphere-forming
assay was performed. A sphere-forming assay is often performed to
investigate the self-renewal capacity of cells, which is one of the
characteristics of CSCs. An FACS sorter was used to isolate the
ZsGreen-high and ZsGreen-negative cells, and 1×103
cells/well were seeded into low-attachment 6-well plates. Following
incubation for ~2 weeks, formed spheres >100 μm in size were
counted. As a result, both the ZsGreen-high and ZsGreen-negative
cells formed in TE4 (Fig. 2A and B)
and TE8 (Fig. 2D and E), but
differences were observed in the number of formed spheres between
these two populations. More spheres were formed by the ZsGreen-high
cells than by the ZsGreen-negative cells in TE4 (Fig. 2C) and TE8 (Fig. 2F) cells.
Sensitivity to chemotherapy agents
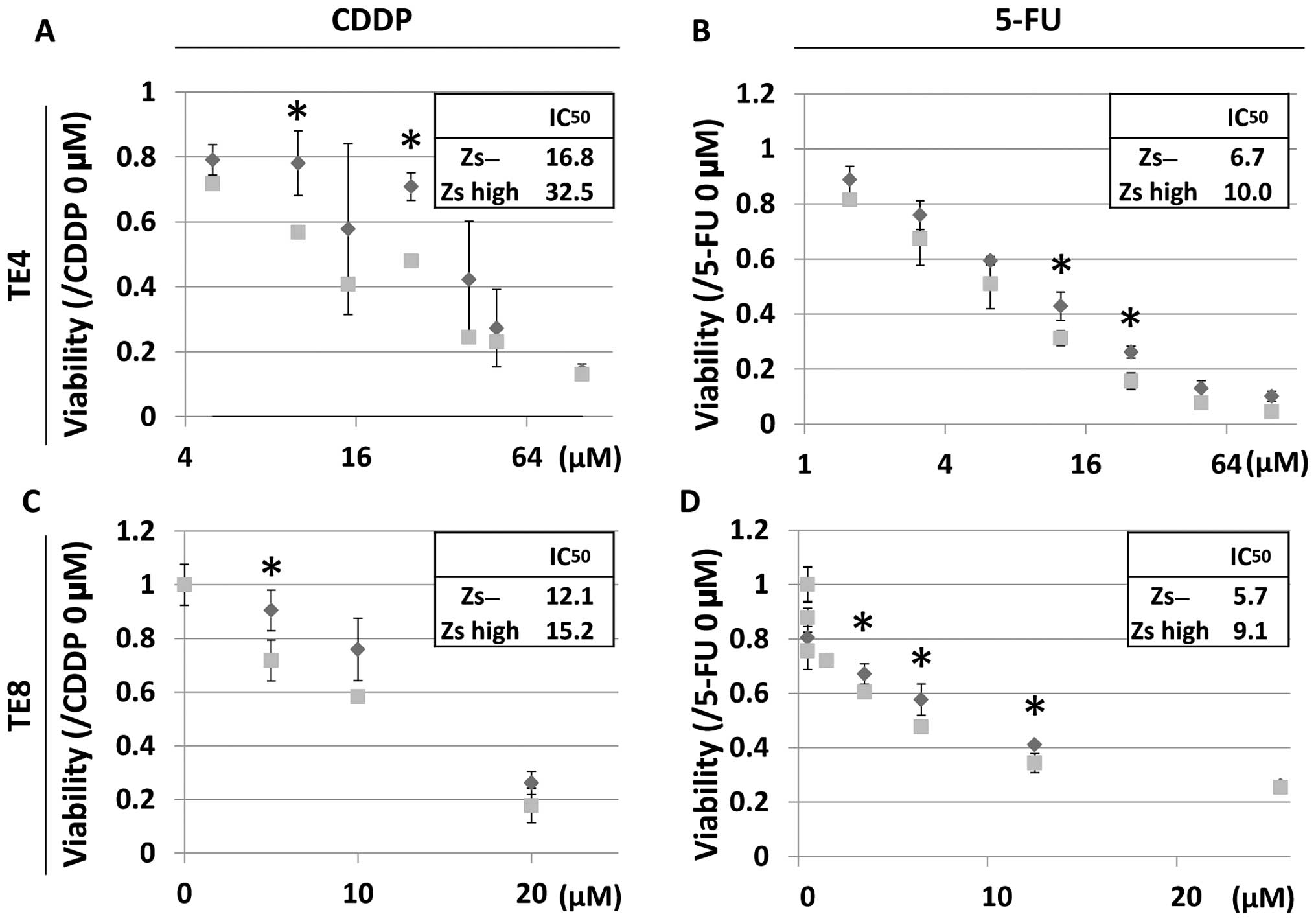
To further investigate the characteristics of the
ZsGreen-high and ZsGreen-negative cells, the drug sensitivities of
these two populations were analyzed. In the present study,
cisplatin (CDDP) and fluorouracil (5-FU) were used. These drugs are
often used to treat esophageal SCCs. As a result, the
IC50 values of the two drugs were higher for the
ZsGreen-high cells compared with those for the ZsGreen-negative
cells (Fig. 3).
Sensitivity to ionized radiation
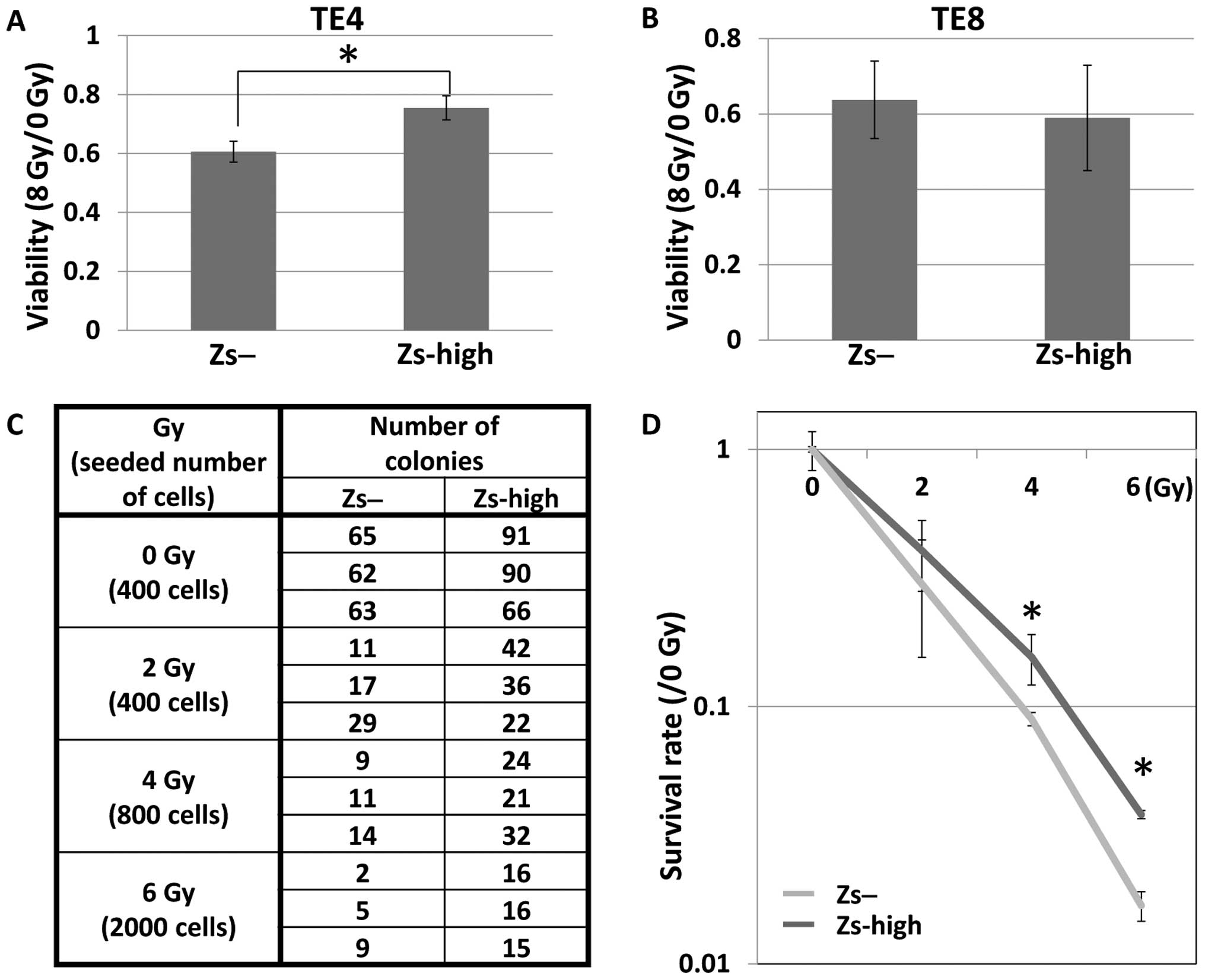
Since radiotherapy is often used to treat esophageal
SCCs as well, the radiation sensitivities of the ZsGreen-high and
ZsGreen-negative cells were analyzed. First, the viabilities of
these two populations after exposure to radiation were analyzed.
The FACS sorter was used to isolate these two populations, which
were then seeded into 96-well plates and exposed to radiation at 0
and 8 Gy. Following incubation for 120 h, the viabilities were
analyzed. A higher number of living ZsGreen-high cells than
ZsGreen-negative cells were observed in TE4 cells (Fig. 4A), but both cell populations were
similarly viable in TE8 cells (Fig.
4B).
To further investigate the radiation sensitivities
of the ZsGreen-high cells and ZsGreen-negative cells in TE8 cells,
clonogenic survival assays were performed. The two populations
isolated in the FACS sorter were seeded in 10-cm dishes and exposed
to radiation at 0, 2, 4 and 6 Gy. Following incubation for ~2
weeks, the colonies were counted and the survival rates were
analyzed. The number of viable ZsGreen-high cells was more elevated
compared with that of the ZsGreen-negative cells in TE8 cells
(Fig. 4C and D).
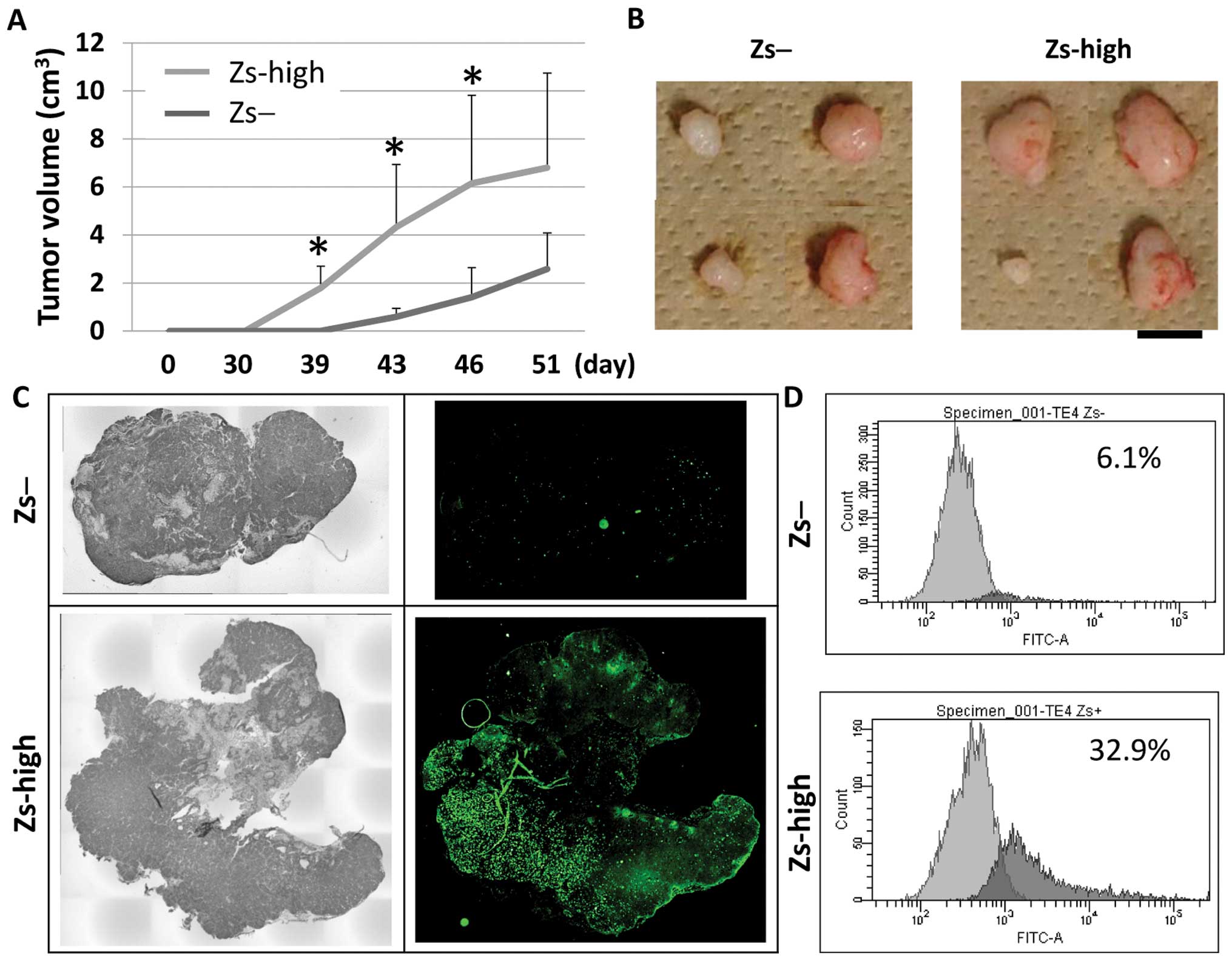
Tumorigenicity study
To investigate the tumorigenicity of the
ZsGreen-high and ZsGreen-negative cells, 1×103 TE4 cells
from the two isolated populations were subcutaneously injected into
the NOD/SCID mice (n=4, each). At day 39, four xenografts derived
from the ZsGreen-high cells were detected in the mice, but no
xenografts derived from the ZsGreen-negative cells were detected.
However, four xenografts derived from the ZsGreen-negative cells
were detected at day 43 as well (Fig.
5A).
Subsequently, these mice were sacrificed and
xenografts were collected (Fig.
5B). Investigation of the sliced sections of xenografts by
microscopy and FACS analysis showed that there were several
ZsGreen-positive cells in the xenografts derived from the
ZsGreen-high cells, but there were few ZsGreen-positive cells in
the xenografts derived from the ZsGreen-negative cells (Fig. 5C and D).
The same study should have been performed in TE8,
but it was not performed. TE8 was much less tumorigenic than TE4 in
NOD/SCID mice. Approximately 500,000 TE8 cells were required to
generate xenografts in the mice. In TE8, there were not enough
ZsGreen-high cells to provide a sufficient number of fluorescent
cells. This was because tumorigenicity studies of these
ZsGreen-high and ZsGreen-negative cells in TE8 were not
performed.
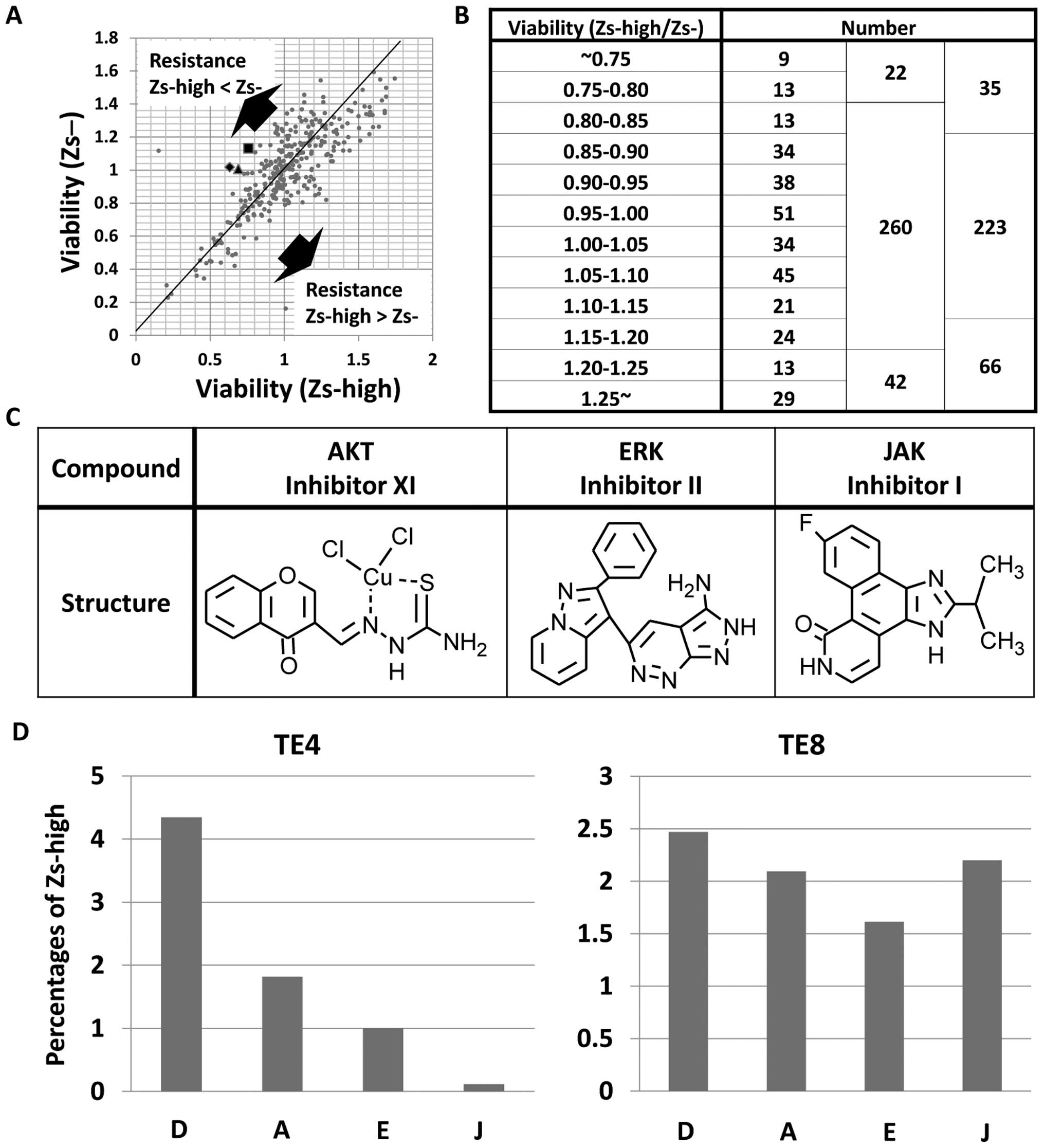
Drug screening against visualized
CSC-like cells
To detect the drugs that can specifically treat the
ZsGreen-high cells, drug screening of 324 types of drugs in TE4
cells was performed. After exposure to 10-μM concentrations of the
drugs for 72 h, the viabilities were analyzed. Consequently, the
ZsGreen-high cells were found to exhibit resistance to more drugs
compared with that exhibited by the ZsGreen-negative cells
(Fig. 6A and B). As a result of the
screening, three drugs were selected: AKT inhibitor XI, ERK
inhibitor II and JAK inhibitor I (Fig.
6C). To validate the effects of these three drugs, the rate of
the ZsGreen-high cells in TE4 and TE8 was analyzed after exposure
to each of the drugs with FACS analysis. The drugs were found to
kill the ZsGreen-high cells (Fig.
6D).
Discussion
The present study revealed that a fluorescent vector
consisting of fluorescein ZsGreen fused to cODC could be used to
detect CSCs or therapy-resistant cancer cells in esophageal SCC.
Transfection into cancer cells with a retroviral vector allowed the
populations of ZsGreen-positive and ZsGreen-negative cells to be
distinguished. The use of FACS sorting made it simple to isolate
the populations of ZsGreen-high and ZsGreen-negative cells.
Investigation of the characteristics of these two populations
indicated that the ZsGreen-high cells were more malignant compared
with the ZsGreen-negative cells. The ZsGreen-high cells exhibited
higher resistance to CDDP and 5-FU, sphere-forming capacity, and
tumorigenicity compared with those exhibited by the
ZsGreen-negative cells. In addition, the ZsGreen-high cells
survived and proliferated faster (up to 120 h post-irradiation
incubation) compared with the ZsGreen-negative cells in TE4 but not
in TE8. The clonogenic survival assay revealed that the
ZsGreen-high cells survived longer compared with the
ZsGreen-negative cells in TE8. These data suggest that the
ZsGreen-high cells are more resistant to radiation compared with
the ZsGreen-negative cells.
To detect the drugs targeting the malignant
ZsGreen-high cells, drug screening of 324 drugs was performed. AKT
inhibitor XI, ERK inhibitor II and JAK inhibitor I were identified
as novel drugs targeting the malignant ZsGreen-high cells. AKT is
well known as part of the PI3K/AKT pathway, ERK is well known as
part of the MEK/ERK pathway and JAK is well known as part of the
JAK/STAT pathway. These three pathways are involved in several
important phenotypes such as proliferation, differentiation and
survival. In the CSCs of several tumor tissues, the PI3K/AKT,
MEK/ERK and JAK/STAT pathways are enhanced, and inhibition of the
pathways can kill the CSCs in several tumor tissues (14–16).
Even in the CSC population of esophageal SCC, the PI3K/AKT pathway
was enhanced and inhibition of PI3K or AKT led to reduction in the
CSC population (5). These data
support the fact that our selected drugs were beneficial in the
management of tumor tissues.
In conclusion, a fluorescent vector consisting of
fluorescein ZsGreen fused to cODC was used to detect CSCs or
therapy-resistant cancer cells. In addition, therapy-resistant
cells were killed by AKT inhibitor XI, ERK inhibitor II and JAK
inhibitor I. These drugs can be used as novel methods in the
management of esophageal SCC.
Acknowledgements
The present study was supported in part by a
grant-in-aid for Scientific Research from the Ministry of
Education, Culture, Sports, Science and Technology; a grant-in-aid
from the Third Comprehensive 10-year Strategy for Cancer Control,
Ministry of Health, Labor and Welfare; a grant from the Kobayashi
Cancer Research Foundation; a grant from the Princess Takamatsu
Cancer Research Fund, Japan; and a grant from the National
Institute of Biomedical Innovation. H.I. and M.K. received partial
support from Chugai Co., Ltd. and Yakult Honsha Co., Ltd. through
institutional endowments.
References
|
1
|
Cheng KK and Day NE: Nutrition and
esophageal cancer. Cancer Causes Control. 7:33–40. 1996. View Article : Google Scholar
|
|
2
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
et al: Prospective identification of tumorigenic breast cancer
cells. Proc Natl Acad Sci USA. 100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dembinski JL and Krauss S:
Characterization and functional analysis of a slow cycling stem
cell-like subpopulation in pancreas adenocarcinoma. Clin Exp
Metastasis. 26:611–623. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang KH, Dai YD, Tong M, et al: A
CD90+ tumor-initiating cell population with an
aggressive signature and metastatic capacity in esophageal cancer.
Cancer Res. 73:2322–2332. 2013.PubMed/NCBI
|
|
5
|
Li H, Gao Q, Guo L and Lu SH: The
PTEN/PI3K/Akt pathway regulates stem-like cells in primary
esophageal carcinoma cells. Cancer Biol Ther. 11:950–958. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao JS, Li WJ, Ge D, et al: Tumor
initiating cells in esophageal squamous cell carcinomas express
high levels of CD44. PLoS One. 6:e214192011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang SD, Yuan Y, Liu XH, et al:
Self-renewal and chemotherapy resistance of p75NTR positive cells
in esophageal squamous cell carcinomas. BMC Cancer. 9:92009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kano Y, Konno M, Ohta K, et al:
Jumonji/Arid1b (Jarid1b) protein modulates human esophageal cancer
cell growth. Mol Clin Oncol. 1:753–757. 2013.PubMed/NCBI
|
|
9
|
Vlashi E, Kim K, Lagadec C, et al: In vivo
imaging, tracking, and targeting of cancer stem cells. J Natl
Cancer Inst. 101:350–359. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan J, Zhang Q, Wang Y, et al: 26S
proteasome activity is down-regulated in lung cancer stem-like
cells propagated in vitro. PLoS One. 5:e132982010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adikrisna R, Tanaka S, Muramatsu S, et al:
Identification of pancreatic cancer stem cells and selective
toxicity of chemotherapeutic agents. Gastroenterology.
143:234–245.e7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Della Donna L, Lagadec C and Pajonk F:
Radioresistance of prostate cancer cells with low proteasome
activity. Prostate. 72:868–874. 2012.PubMed/NCBI
|
|
13
|
Muramatsu S, Tanaka S, Mogushi K, et al:
Visualization of stem cell features in human hepatocellular
carcinoma reveals in vivo significance of tumor-host
interaction and clinical course. Hepatology. 58:218–228. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang YK, Zhu YL, Qiu FM, et al: Activation
of Akt and MAPK pathways enhances the tumorigenicity of
CD133+ primary colon cancer cells. Carcinogenesis.
31:1376–1380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iwanaga R, Wang CA, Micalizzi DS, et al:
Expression of Six1 in luminal breast cancers predicts poor
prognosis and promotes increases in tumor initiating cells by
activation of extracellular signal-regulated kinase and
transforming growth factor-beta signaling pathways. Breast Cancer
Res. 14:R1002012. View
Article : Google Scholar
|
|
16
|
Hernandez-Vargas H, Ouzounova M, Le
Calvez-Kelm F, et al: Methylome analysis reveals Jak-STAT pathway
deregulation in putative breast cancer stem cells. Epigenetics.
6:428–439. 2011. View Article : Google Scholar : PubMed/NCBI
|