Introduction
Peritoneal metastasis, a common feature of the
natural history of gastric cancer, is the most frequent cause of
death in patients with advanced gastric carcinoma (1–3).
Despite advances in therapeutic modalities for peritoneal diseases
such as combination chemotherapy and chemohyperthermia, the 5-year
survival rate of patients with peritoneal carcinomatosis is only 2%
(4). Thus, there is an urgent need
for further knowledge of the molecular mechanisms responsible for
peritoneal metastasis of gastric carcinoma and for identifying
potential novel therapeutic targets, which may facilitate the
development more effective treatment modalities.
It is now well established that peritoneal
metastasis is a multistep process involving the detachment of
malignant cells from the primary tumor, their migration into the
peritoneal cavity, their attachment to the peritoneum, and finally
their proliferation to form secondary tumor foci. Differences in
metastatic potential are expected to result from differences in
expression of a combination of genes related to cell adhesion,
apoptosis, signal transduction and other processes (5). Yet, details of the molecular basis of
peritoneal metastasis remain unclear.
DJ-1, a ubiquitously expressed and highly conserved
intracellular protein, was originally discovered as a novel
oncogene product that can transform mouse NIH3T3 cells in
combination with H-Ras or c-Myc (6). Subsequent studies demonstrated that
DJ-1 has multiple functions and is involved in diverse cellular
processes ranging from cellular transformation, transcriptional
regulation, antioxidative stress response to control of male
infertility, among several others (7–11).
Recently, accumulating evidence has shown that DJ-1 is
overexpressed in many types of malignant tumors, including breast
(12), primary lung cancer
(13), leukemia (14), prostate (15), cervical (16), papillary thyroid (17) and pancreatic cancer (18), indicating a possible role for DJ-1
in the occurrence and development of tumors. Moreover, of note,
several recent studies have also reported that DJ-1 may be related
to cancer metastasis. For example, Pardo et al (19) found that DJ-1 expression was
upregulated in uveal melanoma cells that had a high degree of
metastatic potential, when compared with that in corresponding
normal cells. Yuen et al (20) found that DJ-1 was amplified and
overexpressed in esophageal squamous cell carcinoma metastatic
lesions when compared with that in normal esophageal epithelium and
primary tumors. In addition, DJ-1 expression was found to be
significantly correlated with human non-small cell lung cancer
lymphatic metastasis (21). DJ-1
was also confirmed to promote in vitro and in vivo
invasion and metastasis of human glioma cells and pancreatic cancer
cells (22,23). These results suggest that high
expression of DJ-1 is a key alteration contributing to the
metastasis of tumor cells.
However, although considerable evidence has
accumulated suggesting that DJ-1 may play a major role in
tumorigenesis and metastasis, virtually nothing is known regarding
its involvement in the development of peritoneal metastasis by
gastric carcinoma. Therefore, the objective of this study was to
investigate the role of DJ-1 in peritoneal metastasis of gastric
carcinoma and to determine the underlining mechanisms of this
process.
In the present study, we present initial evidence
that DJ-1 is highly expressed in gastric cancer with peritoneal
metastasis. The existence of DJ-1 in gastric cancer promotes the
invasive and metastatic abilities of gastric cancer cells through
activation of the Akt pathway and consequent upregulation of MMP-2
and MMP-9 expression.
Materials and methods
Chemicals and reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS) and Lipofectamine 2000 transfection reagent were
purchased from Invitrogen (Carlsbad, CA, USA). API-2 (an inhibitor
of Akt) was purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Transwell inserts were purchased from Corning Incorporated
(Corning, NY, USA). Matrigel was purchased from BD Biosciences
(Bedford, MA, USA). An anti-DJ-1 antibody was obtained from Abcam
(Cambridge, MA, USA). Anti-phospho-Akt (Thr 308) and anti-Akt
antibodies were purchased from Cell Signaling Technology (Beverly,
MA, USA). MMP-2/MMP-9 inhibitor I, anti-MMP-2, anti-MMP-9 and
anti-β-actin primary antibodies were from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). All other chemicals were purchased from
Sigma Chemical, unless otherwise noted.
Cell lines and cell culture
Human gastric adenocarcinoma cell lines, SGC7901,
MKN45, MKN28 and BGC823, and the normal human gastric mucosal cell
line GES-1, were obtained from the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). Cells were cultured
in DMEM containing 10% heat-inactivated FBS, 100 U/ml penicillin
and 100 μg/ml streptomycin. All cultures were maintained in a
humidified 5% CO2 atmosphere at 37°C, and cells were
passaged by treating them with 0.02% EDTA in phosphate-buffered
saline (PBS) and 0.25% trypsin after they had achieved confluence.
Exponentially growing cells were used for the experiments.
Tissue samples
Tissue samples of primary tumors were obtained
during gastrectomy from untreated patients with gastric carcinomas.
Histological diagnosis was confirmed for each specimen. All
surgical samples were obtained from the Department of General
Surgery, First Affiliated Hospital, Nanchang University (Nanchang,
China). A written informed consent for molecular analysis of the
surgical sample was obtained from each patient.
Western blot analysis
Total proteins were extracted from the tumor tissues
or cells using a protein extraction kit (Pierce, Rockford, IL, USA)
according to the manufacturer’s protocols. The protein content was
determined by the Lowry method using a DC protein assay kit
(Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of
proteins were resolved by 10% SDS-PAGE and transferred to
polyvinylidene difluoride membranes. After blocking with 5% non-fat
milk, the blots were then probed with primary antibodies against
DJ-1 (dilution 1:2,000), Akt (1:1,000), phospho-Akt (1:1,000),
MMP-2 (1:500), MMP-9 (1:500) and β-actin (1:3,000). The bound
antibodies were detected using appropriate horseradish
peroxidase-conjugated secondary antibody and visualized using an
enhanced chemiluminescence system (Millipore, Billerica, MA, USA).
The levels of each protein were standardized to the loading
control, β-actin, and were quantified using an ImageJ 1.41 program
(National Institutes of Health, Bethesda, MD, USA).
Generation of DJ-1-knockdown SGC7901
cells
Three candidate human DJ-1 siRNA target sequences
and a negative control siRNA (NC siRNA) sequence were designed
using the siRNA Target Finder and Design Tool available at
www.ambion.com/techlib/misc/siRNA_finder.html. These
siRNA sequences were 5′-AAATGGAGACGGTCATCCCTG-3′ (DJ-1 siRNA-1),
5′-AAGACCCAGTACAGTGTAGCC-3′ (DJ-1 siRNA-2),
5′-AAGGTCACCGTTGCAGGCCTG-3′ (DJ-1 siRNA-3), and
5′-AAGACGCTGAAGACTCTTGGC-3′ (NC siRNA), respectively. Subsequently,
these siRNA duplexes were synthesized and were transiently
transfected into SGC7901 cells according to manufacturer’s
instructions. Their efficacies in extinguishing DJ-1 expression
were evaluated by western blot analysis. DJ-1 siRNA-3, above,
showing the highest efficacy, was selected for establishing
DJ-1-knockdown SGC7901 cell lines. Briefly, the template
oligonucleotides corresponding to DJ-1 siRNA-3 and NC siRNA were,
respectively, synthesized and ligated into BbsI-BamHI
sites of the pGPU6/GFP/Neo vector (Shanghai Genepharma, Shanghai,
China) to generate pGPU6/GFP/Neo-DJ-1-siRNA and
pGPU6/GFP/Neo-NC-siRNA recombinant plasmids. After being confirmed
by DNA sequencing, the pGPU6/GFP/Neo-DJ-1-siRNA and
pGPU6/GFP/Neo-NC-siRNA vectors were transfected into SGC7901 cells
using Lipofectamine 2000 reagent according to the manufacturer’s
protocol. Twenty-four hours after transfection, cells were split
into medium containing G418 (400 μg/ml) (Calbiochem, Nottingham,
UK) and maintained for 14 days, with regular replenishment of
medium and drug. At this time individual clones were selected,
expanded, and screened for expression of DJ-1 by western blot
analysis. Clones of the SGC7901 cells stably transfected with
either pGPU6/GFP/Neo-DJ-1-siRNA (designated as SGC7901/DJ-1-siRNA)
or pGPU6/GFP/Neo-NC-siRNA (designated as SGC7901/NC-siRNA) were
maintained in DMEM supplemented with 200 μg/ml G418.
Construction of the DJ-1 gene expression
vector
For rescue of DJ-1 expression levels, rat DJ-1,
containing two mismatches with respect to the human-specific DJ-1
siRNA-3, was introduced in SGC7901/DJ-1-siRNA cells. To obtain the
coding region of rat wild-type DJ-1 cDNA, oligonucleotide primers
complementary to the 5′ and 3′ ends of DJ-1 were designed that
incorporated the restriction sites EcoRI and KpnI:
Primer S, 5′-CCGGAATTCAATGGCATCCAAAAGAGC-3′; and Primer A,
5′-CGGGGTACCCTAGTCTTTGAGAACAAG CG-3′. PCR products were digested
with EcoRI and KpnI and analyzed by agarose gel
electrophoresis. Bands of the expected length were cut out and
ligated into the eukaryotic expression vector pFLAG-CMV-4 (Sigma),
which had been digested with EcoRI and KpnI.
Subsequently, the generated construct was confirmed by sequencing
and further referred to as pFLAG-rDJ-1.
Analysis of in vitro migration and
invasion
Cell migration and invasion assays were performed
using 8.0-μm pore size Transwell inserts as previously described
(24). For all migration assays, in
brief, the undersurface of the membrane was coated with fibronectin
(10 μg/ml) in PBS at 37°C for 2 h. The membrane was washed in PBS
to remove excess ligand, and the lower chamber was filled with 0.6
ml DMEM with 10% FBS. Cells were serum-starved overnight (0.5%
FBS), harvested with trypsin/EDTA and washed twice with serum-free
DMEM. Then, cells were resuspended in migration medium (DMEM with
0.5% FBS), and 1×105 cells in 0.1 ml were added to the
upper chamber. After 24 h at 37°C, the cells on the upper surface
of the membrane were removed using cotton swabs. The migrated cells
that had attached to the lower surface were fixed in methanol at
room temperature for 30 min and stained for 20 min with a solution
containing 0.5% crystal violet and 2% ethanol in 100 mM borate
buffer (pH 9.0). The number of cells that migrated to the lower
surface of the membrane were counted under a microscope in random
five fields at ×100 magnification. For the cell invasion assay, all
procedures were carried out as in the migration assay except that
Matrigel was used to coated beforehand the upper surface of the
chambers according to the manufacturer’s protocol.
In vivo peritoneal metastasis assay
Pathogen-free, 6-week-old, female BALB/c nude mice
were purchased from Hunan Slack King of Laboratory Animal Co., Ltd.
(Changsha, China). All animal experiments complied with the
Guidelines for the Care and Use of Laboratory Animals of Nanchang
University. The peritoneal metastasis model was conducted as
previously described (25,26). In brief, mice weighing 18–22 g were
injected intraperitoneally with 5×106 SGC7901 cells in 1
ml PBS. Mice were sacrificed 21 days after SGC7901 cell injection,
and the size and the number of metastatic nodules in the peritoneal
cavity were counted by the naked eye as previously described
(27).
Statistical analysis
The results are presented as means ± SD. The
unpaired t-test was used to compare the differences between groups.
Multiple group means were compared by ANOVA followed by LSD post
hoc test. Differences with a two-tailed P<0.05 were considered
to indicate statistically significant results.
Results
DJ-1 is overexpressed in gastric cancer
tissues with peritoneal metastasis and in gastric cancer cell lines
with higher metastatic potential
To investigate the relationship between the
expression of DJ-1 and peritoneal metastasis of gastric cancer, we
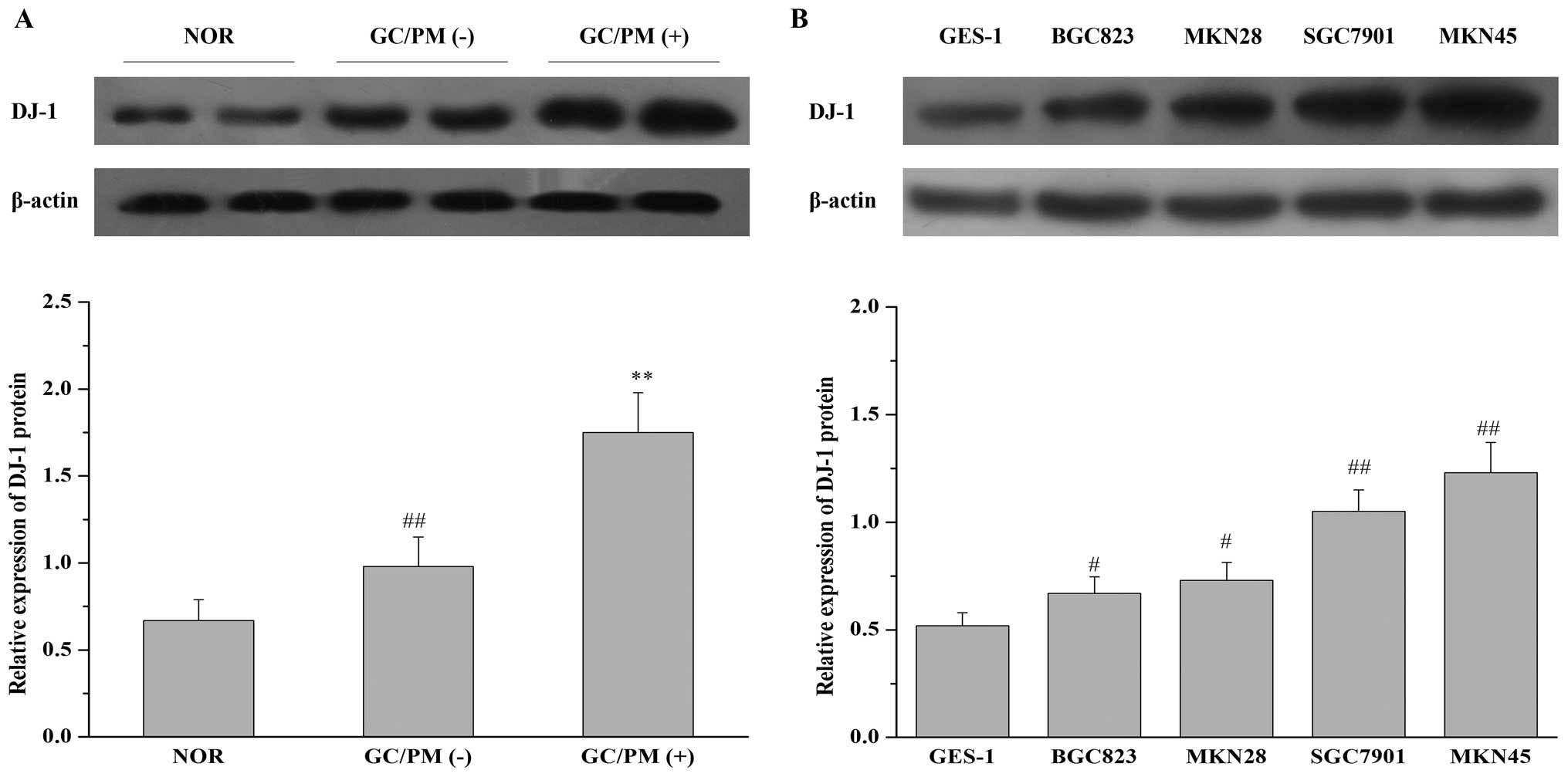
first detected the expression of DJ-1 protein in 37 specimens of
normal gastric mucosa, 40 specimens of gastric carcinoma without
peritoneal metastasis, and 28 specimens of gastric carcinoma with
peritoneal metastasis by western blot analysis. The relative values
for DJ-1 protein were determined as ratios to β-actin (Fig. 1A). The relative value for DJ-1
protein in normal gastric mucosa (37 cases, mean ± SD) was
0.67±0.12. In the primary tumors of gastric carcinoma without
peritoneal metastasis (40 cases), the value for DJ-1 was 0.98±0.17,
whereas in the primary tumors of gastric carcinoma with peritoneal
metastasis (28 cases), this value was 1.75±0.23. The results
revealed that the expression of DJ-1 was significantly upregulated
in gastric carcinoma with peritoneal metastasis when compared to
the expression in gastric carcinoma without peritoneal metastasis
or normal gastric mucosa. In addition, we also analyzed the
expression of DJ-1 protein in one normal human gastric mucosa cell
line GES-1 and four gastric cancer cell lines of different
metastatic potential (BGC823, MKN28, SGC7901 and MKN45), of which
SGC7901 and MKN45 were demonstrated to have higher metastatic
potential compared with BGC823 and MKN28 (28). As shown in Fig. 1B, DJ-1 expression in the four
gastric cancer cell lines was much higher than that in the GES-1
cell line. Furthermore, DJ-1 expression was higher in the SGC7901
and MKN45 cells when compared with that in the BGC823 and MKN28
cells. These results suggest that overexpression of DJ-1 may be
closely related to the development of peritoneal metastasis by
gastric carcinoma.
DJ-1 promotes in vitro invasion and
migration and in vivo peritoneal metastatic abilities of gastric
cancer cells
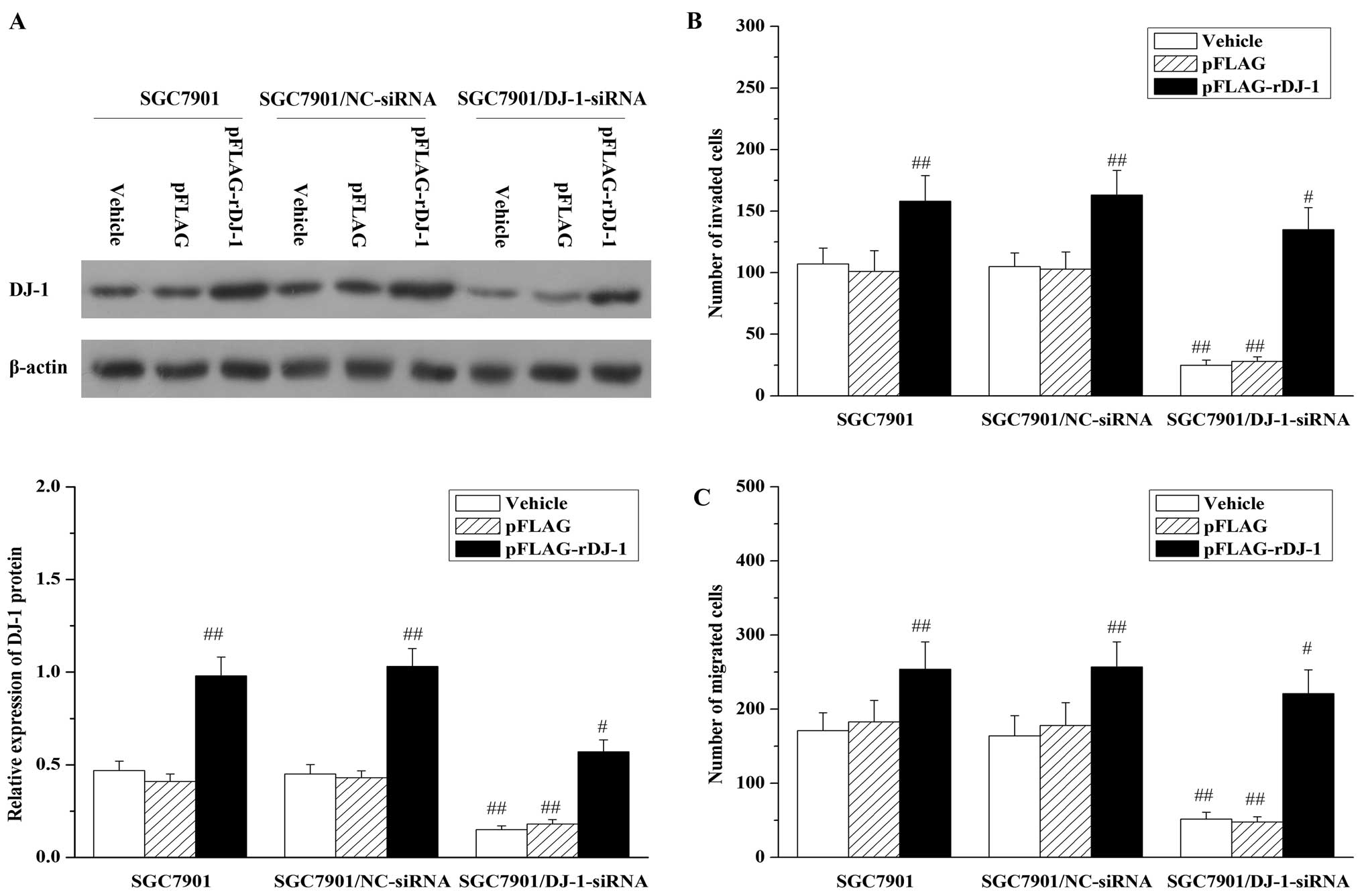
Next, to determine whether DJ-1 has a causal
function in gastric cancer peritoneal metastasis, we studied the
effects of DJ-1 knockdown on gastric cancer cell invasion and
migration using SGC7901/DJ-1-siRNA cells. This experiment revealed
that DJ-1 expression level in the SGC7901/DJ-1-siRNA cells was
significantly reduced (Fig. 2A).
More importantly, the knockdown of DJ-1 expression led to a
significant reduction in cell invasion (Fig. 2B) and migration (Fig. 2C), as compared to the control cells
(SGC7901 or SGC7901/NC-siRNA). To further confirm that the reduced
invasion and migration were caused by specific downregulation of
DJ-1 protein and were not due to off-target effects, we restored
DJ-1 expression in the SGC7901/DJ-1-siRNA cells by transfection of
the pFLAG-rDJ-1 vector. As shown in Fig. 2A, after transient transfection of
pFLAG-rDJ-1 for 24 h, DJ-1 expression in the SGC7901/DJ-1-siRNA
cells was restored to a level higher than the baseline level.
Notably, the restoration of DJ-1 expression was also accompanied by
the restoration of cell invasion (Fig.
2B) and migration (Fig. 2C) in
the SGC7901/DJ-1-siRNA cells. In contrast, transfection of
pFLAG-rDJ-1 markedly increased DJ-1 levels concomitant with an
obvious elevation in cell invasion and migration activities in the
parental SGC7901 and SGC7901/NC-siRNA cells.
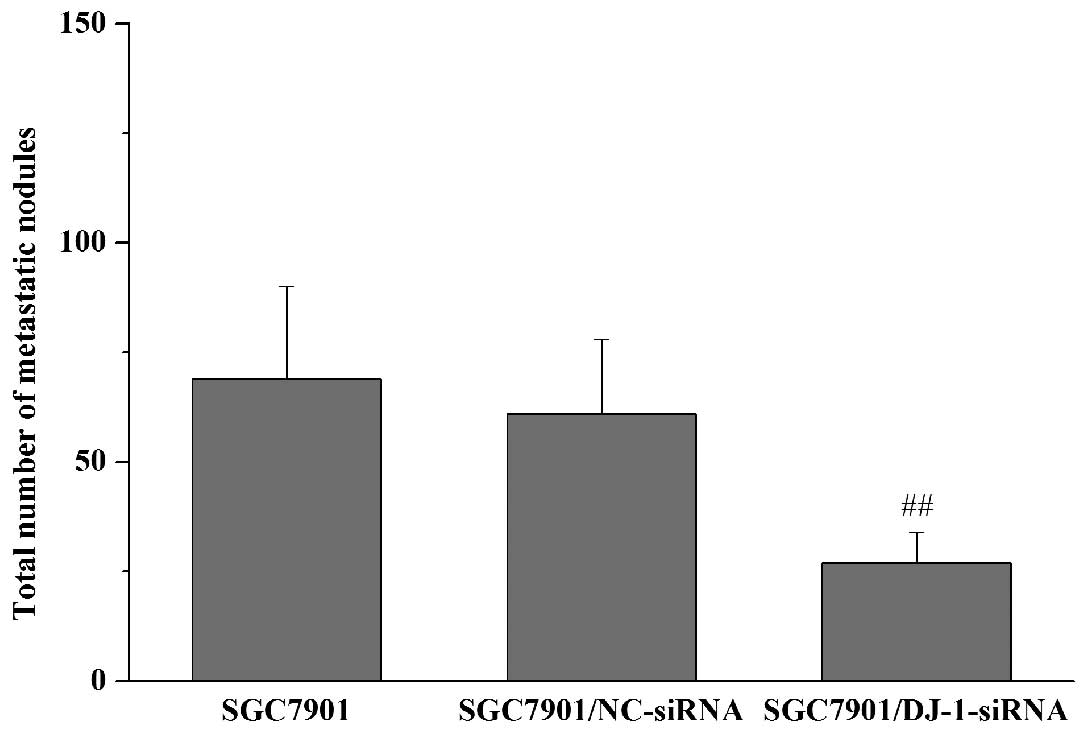
Subsequently, we examined whether DJ-1 knockdown
results in the inhibition of in vivo peritoneal
dissemination of gastric cancer cells. Nude mice received i.p.
injections of SGC7901, SGC7901/NC-siRNA or SGC7901/DJ-1-siRNA
cells. The macroscopic nodules of peritoneal dissemination were
then counted 3 weeks after tumor inoculation. The results showed
that metastatic nodules developed preferentially around the vessels
of the intestinal mesentery. Compared with the control cells
(SGC7901 and SGC7901/NC-siRNA cells), i.p. inoculation of
SGC7901/DJ-1-siRNA cells led to a significant decrease in the
number of metastatic nodules in the peritoneal cavity (Fig. 3). Taken together, these observations
suggest that DJ-1 has the potential to promote in vitro
invasion and migration and in vivo peritoneal metastasis of
gastric cancer cells.
Matrix metallopeptidase (MMP)-2 and MMP-9
are involved in the invasion and migration of gastric cancer cells
regulated by DJ-1
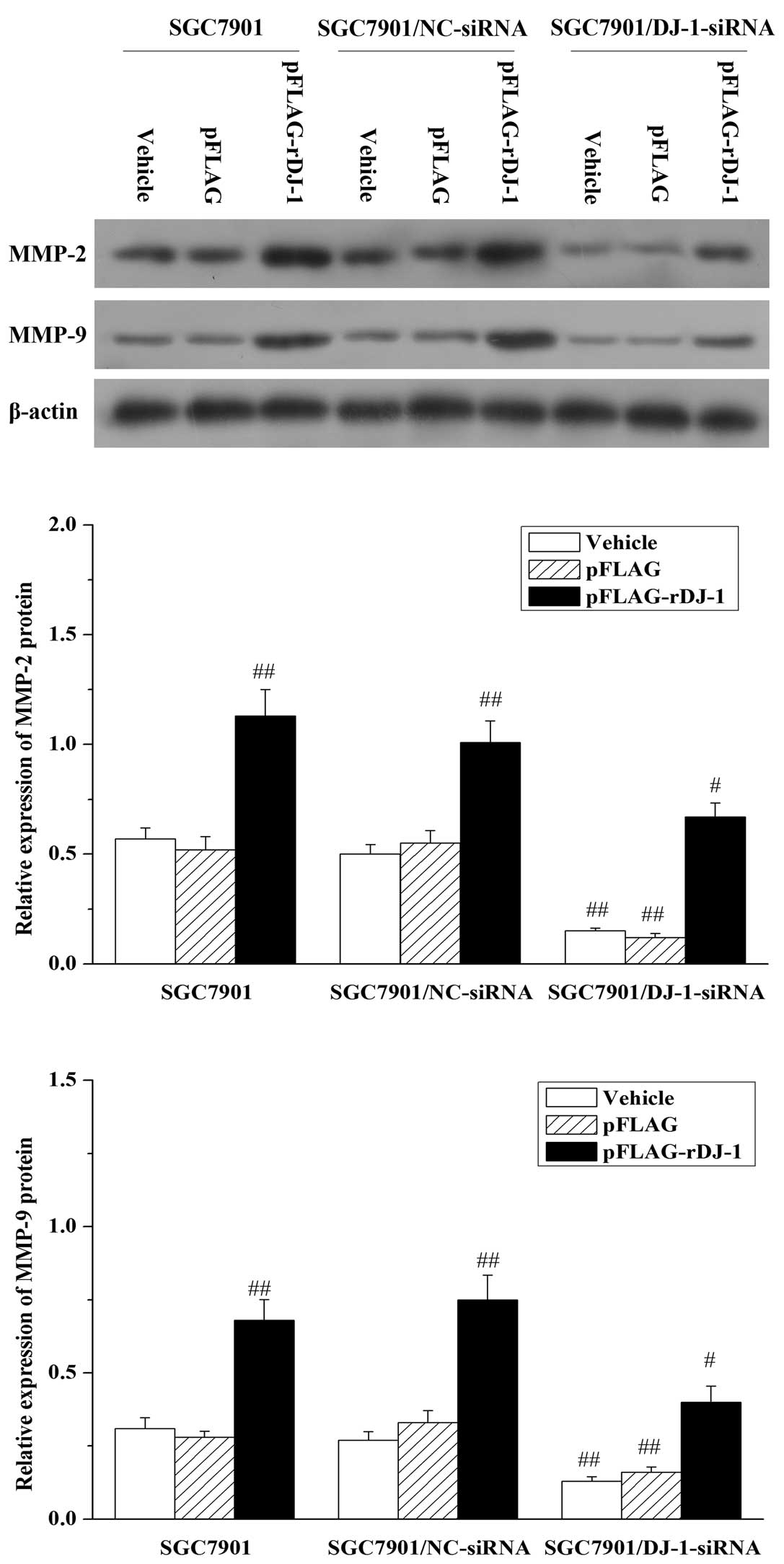
Extracellular matrix degradation is an essential
step in tumor invasion and metastasis, which is mainly mediated by
MMP-2 and MMP-9 (29). To
understand the mechanisms underlying DJ-1 regulation of cell
invasion and migration, we further analyzed MMP-2 and MMP-9
expression in the SGC7901/DJ-1-siRNA cells by western blot
analysis. As expected, silencing of DJ-1 resulted in reduction in
MMP-2 and MMP-9 protein expression in the SGC7901/DJ-1-siRNA cells
when compared to these levels in the parental SGC7901 and
SGC7901/NC-siRNA cells, which was reversed by restoration of DJ-1
expression (Fig. 4). Additionally,
the 24-h transfection of pFLAG-rDJ-1 markedly increased the
expression of MMP-2 and MMP-9 proteins in the parental SGC7901 and
SGC7901/NC-siRNA cells. In contrast, empty vector pFLAG did not
affect MMP-2 and MMP-9 expression. These data indicate that DJ-1
can cause the upregulation of MMP-2 and MMP-9 proteins.
Subsequently, to study the possible role of MMP-2 and MMP-9 in
DJ-1-mediated invasion and migration, SGC7901/DJ-1-siRNA and
control cells were treated with 20 μM of MMP-2/MMP-9 inhibitor I
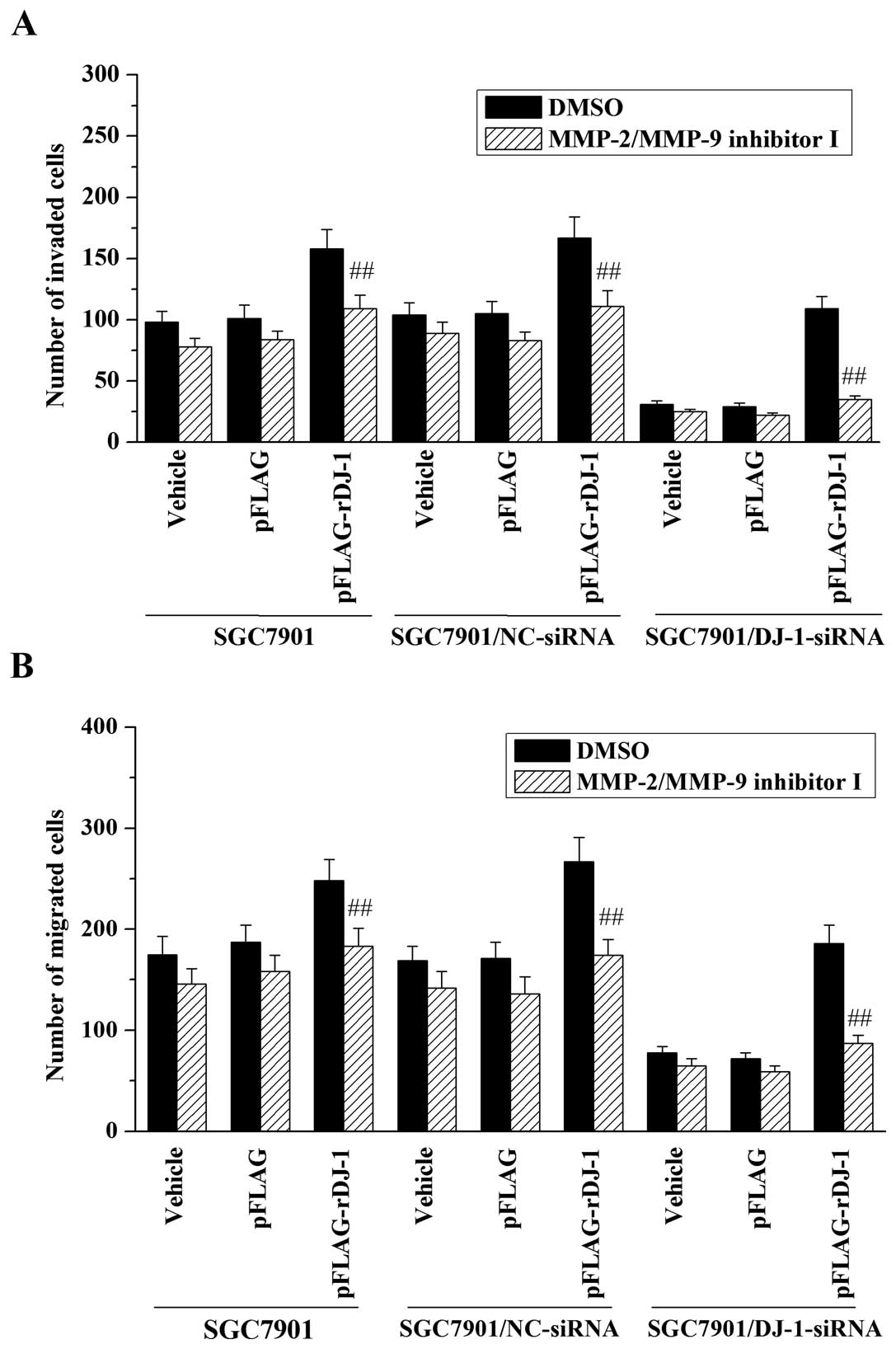
before performing invasion and migration assays. The results
demonstrated that treatment with MMP-2/MMP-9 inhibitor I inhibited
the promotive effect of DJ-1 on invasive and migrative abilities of
the SGC7901 cells (Fig. 5). In
addition, MMP-2/MMP-9 inhibitor I also reversed the effects of
restored DJ-1 on cell invasion (Fig.
5A) and migration (Fig. 5B) in
DJ-1-knockdown cells. Taken together, these data indicate that
MMP-2 and MMP-9 may be involved in the promotive effects of DJ-1 on
invasion and migration of gastric cancer cells.
The Akt pathway is involved in the
upregulation of MMP-2 and MMP-9 and the invasion and migration of
gastric cancer cells mediated by DJ-1
It has been reported that DJ-1 induces the Akt
pathway by negatively regulating the function of the
tumor-suppressor gene phosphatase and tensin homolog (PTEN)
(30), whereas the Akt pathway has
been shown to play a critical role in the migration and invasion of
many types of cancer cells (31,32).
Thus, we next examined whether the Akt pathway might also be
involved in DJ-1-related migration and invasion of gastric cancer
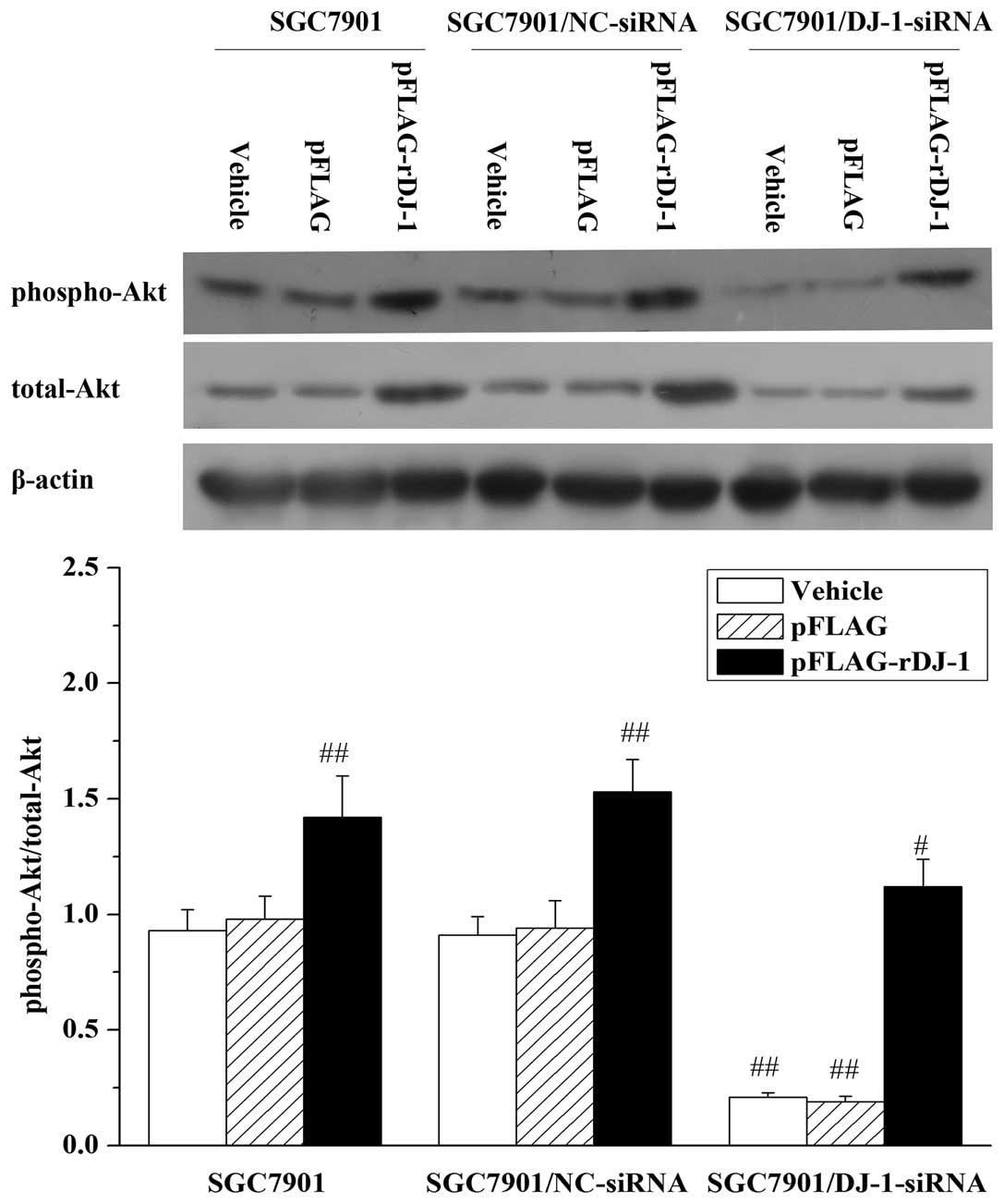
cells. Expectedly, in SGC7901/DJ-1-siRNA cells, knockdown of DJ-1
resulted in downregulation of phosphorylated Akt (Thr308).
Likewise, the effect was also reversed by restoration of DJ-1
expression and was increased further by exogenous expression of
DJ-1 in the parental SGC7901 and SGC7901/NC-siRNA cells (Fig. 6). To further determine whether the
promotive effects of DJ-1 on cell invasion and migration were
dependent on the Akt pathway, cell invasion and migration were
assessed following inhibition of Akt by API-2 in parental SGC7901
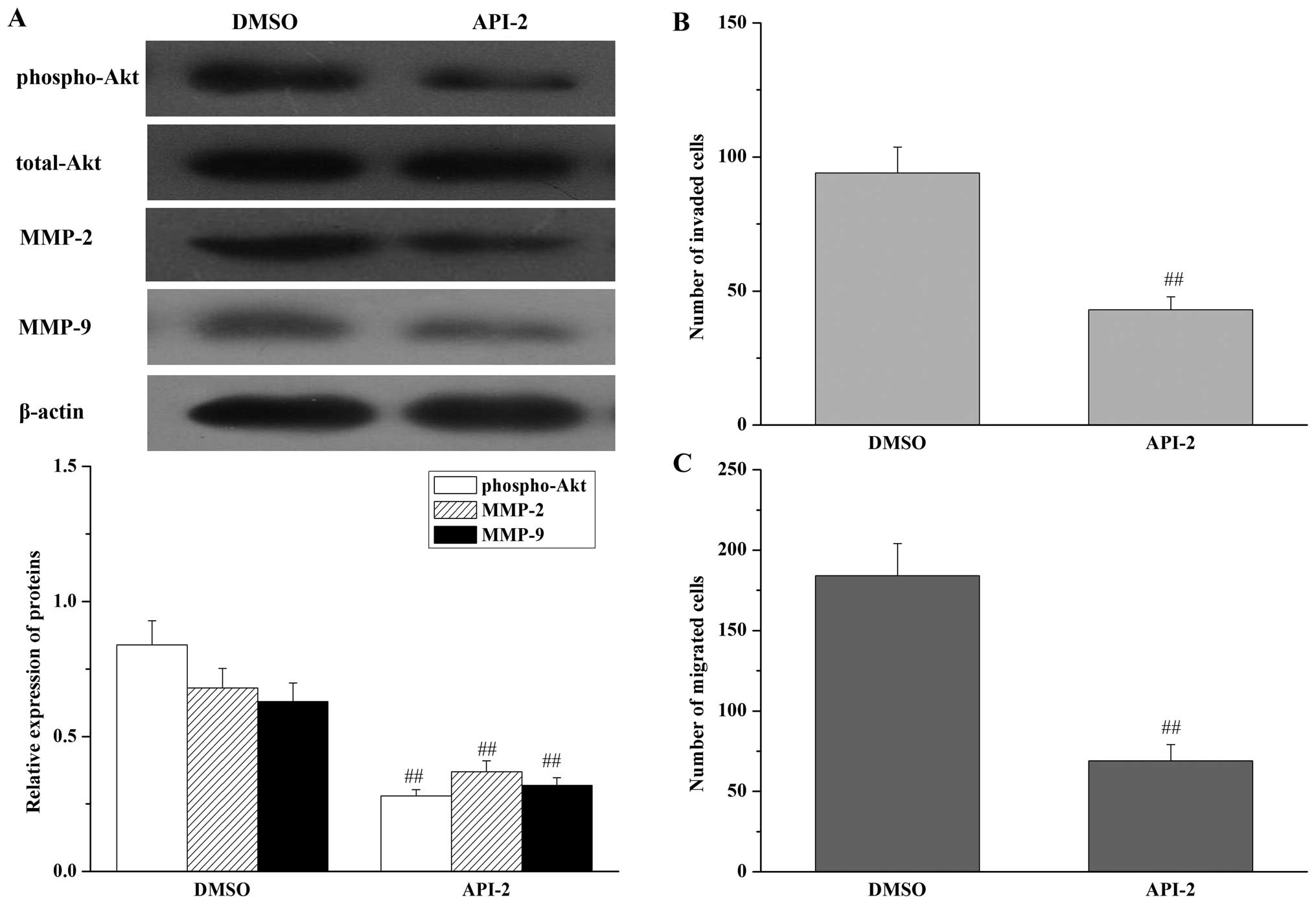
cells. As shown in Fig. 7,
inhibition of Akt had the same effects as knockdown of DJ-1, with a
decrease in MMP-2 and MMP-9 expression (Fig. 7A), a reduction in gastric cancer
cell invasion (Fig. 7B) and
migration potential (Fig. 7C) in
the parental SGC7901 cells. In addition, inhibition of Akt by API-2
reversed the effects of restored DJ-1 on MMP-2 and MMP-9
upregulation (Fig. 8A), invasion
(Fig. 8B) and cell migration
(Fig. 8C) in DJ-1 knockdown cells.
All of these results suggest that the Akt pathway is involved in
DJ-1-related invasion and migration, and is mediated by the
upregulation of MMP-2 and MMP-9 expression induced by DJ-1.
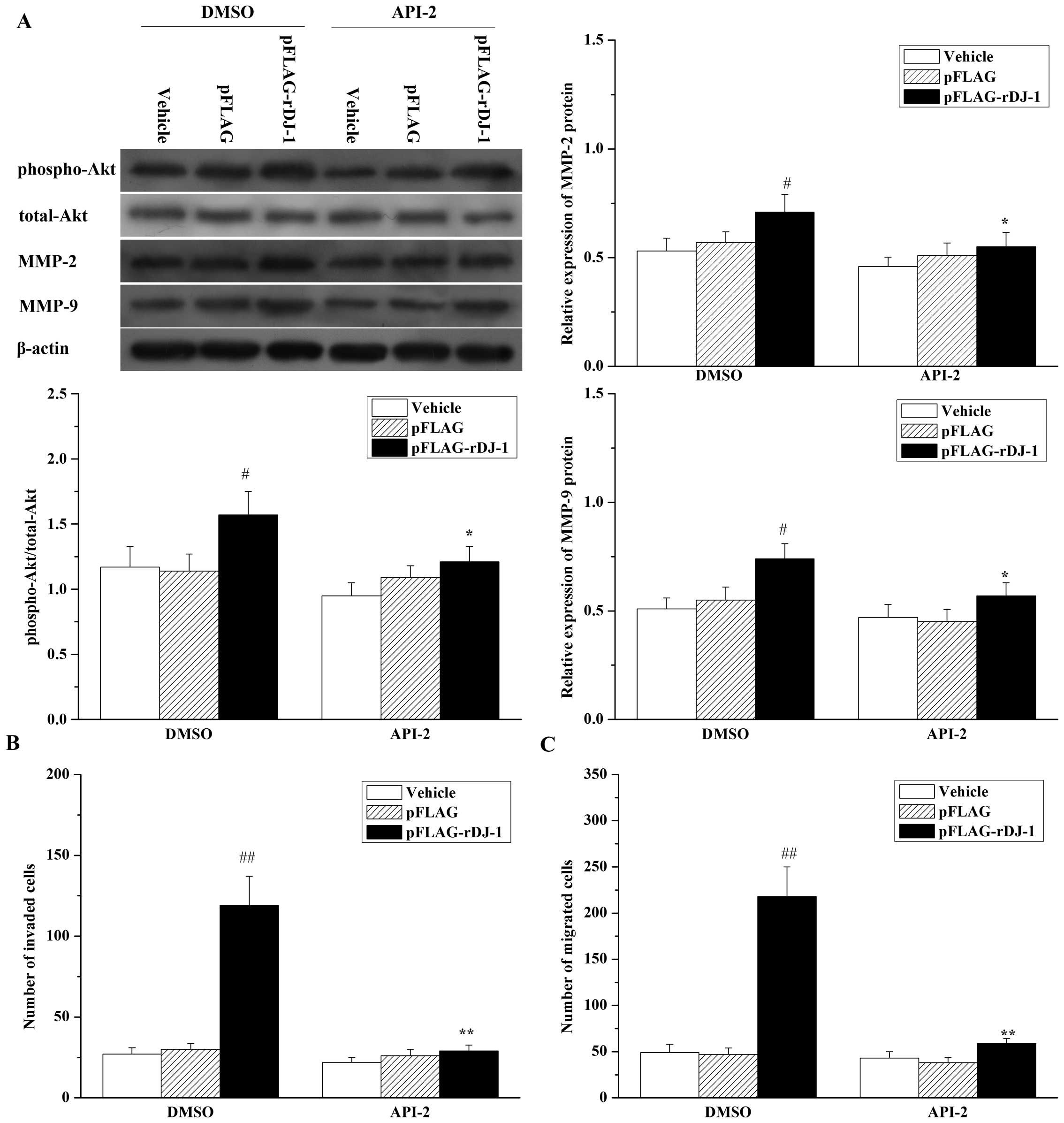 | Figure 8Inhibition of Akt reverses the
effects of restored DJ-1 on cell migration and invasion, and the
expression of MMP-2 and MMP-9 proteins in SGC7901/DJ-1-siRNA cells.
SGC7901/DJ-1-siRNA cells were transfected with or without pFLAG or
pFLAG-rDJ-1. Twenty-four hours later, cells were treated for 24 h
with 20 μM API-2 or 0.1% (v:v) DMSO as vehicle control, and (A)
expression of phospho-Akt, total-Akt, MMP-2 and MMP-9, and (B) cell
invasion and (C) migration were, respectively, analyzed, as
described in Materials and methods. Each value represents the mean
± SD of four independent experiments. #P<0.05,
##P<0.01 vs. DMSO + Vehicle *P<0.05,
**P<0.01 vs. DMSO + pFLAG-rDJ-1. |
Discussion
It is known that the majority of gastric cancer
deaths result from peritoneal metastases rather than from primary
tumors. Yet, the molecular mechanisms regulating peritoneal
metastasis of gastric cancer remain incompletely defined. DJ-1, a
ubiquitously expressed and highly conserved intracellular protein,
was discovered as a mitogen-dependent oncogene product by Nagakubo
et al (6). More recently,
DJ-1 has been reported to play an important role during the
invasion and metastasis of many types of carcinoma cells, including
uveal malignant melanoma (19),
esophageal squamous cell carcinoma (20), primary lung cancer (21), glioma (22) and pancreatic cancer (18,23).
However, to date, there are no reports concerning the role of DJ-1
in the peritoneal metastasis of gastric cancer.
In the present study, we presented initial evidence
that the expression of DJ-1 is significantly upregulated in gastric
cancers with peritoneal metastasis compared to that in cancers
without peritoneal metastasis or normal gastric mucosa. We also
found that DJ-1 was overexpressed in gastric cancer cells that had
a high degree of metastatic potential, as compared with
corresponding normal cells. These results indicate that the
overexpression of DJ-1 may be closely related to the development of
peritoneal metastasis by gastric carcinoma. However, whether there
is a causal relationship between DJ-1 expression and peritoneal
metastasis of gastric carcinoma deserves further exploration. Thus,
in the present study, we generated stable transfectants to
knockdown DJ-1 in SGC7901 cells and further tested the ability of
DJ-1-knockdown cells to form tumor nodules in the peritoneal cavity
using an in vivo model of peritoneal metastasis in nude
mice. The results showed that DJ-1-knockdown SGC7901 cells had a
decreased ability to form peritoneal metastatic nodules when
compared to the control cells (parental SGC7901 and
SGC7901/NC-siRNA cells). This suggests that DJ-1 is involved in the
development of peritoneal metastasis by gastric carcinoma.
The ability to migrate and invade the basement
membrane into surrounding tissues, blood and lymphatic vessels is
one of the essential hallmarks of cancer and is a prerequisite for
local tumor progression and metastatic spread (33). Accordingly, to identify the
mechanisms regulating the decreased ability of DJ-1-knockdown cells
to form peritoneal metastasis, the migration and invasion
capacities in vitro were also analyzed. As expected, we
observed that DJ-1-knockdown SGC7901 cells exhibited a marked
decrease in both cell invasion and migration, which was reversed by
restoration of DJ-1 expression, and was increased further by
ectopic expression of DJ-1 in parental SGC7901 cells. Our data
indicate that DJ-1 regulates gastric cancer cell migration and
invasion properties in cell culture. Extensive studies have shown
that DJ-1 plays an important role in cell proliferation and
survival (30,34,35);
however, our in vitro assay indicated that cell
proliferation and survival remained unchanged upon knockdown of
DJ-1 expression (data not shown). This suggests that the ability of
DJ-1 to promote peritoneal metastasis is mainly due to its effects
on the regulation of gastric cancer cell migration and invasion,
independent of cellular proliferation. The essential requirement
for DJ-1 in gastric cancer cell migration and invasion highlights
the potential for using DJ-1 as a target for blocking gastric
cancer metastasis.
Our studies also indicate the mechanisms by which
DJ-1 modulates cell migration and invasion. It is known that cancer
cell invasion and metastasis require controlled degradation of the
extracellular matrix. Matrix metalloproteinases (MMPs) are a family
of zinc-dependent endopeptidases which play an important role in
the proteolytic destruction of extracellular matrix and basement
membranes, thereby, they are essential for tumor invasion and
metastasis (36). MMPs,
particularly MMP-2 and MMP-9 have been implicated in cancer
invasion and metastasis (29).
Thus, it is necessary to investigate whether the involvement of
DJ-1 in invasion and migration is also correlated with MMP-2 and
MMP-9 in gastric cancer. Notably, we found that, in
SGC7901/DJ-1-siRNA cells, DJ-1 silencing resulted in reduction in
MMP-2 and MMP-9 expression, which was also reversed by restoration
of DJ-1 expression. Moreover, blockage of MMP-2 and MMP-9
activities by MMP-2/MMP-9 inhibitor I reversed the promotive effect
of DJ-1 on invasive and migrative abilities in gastric cancer
cells. These results indicate that MMP-2 and MMP-9 may be involved
in the promotion of cell invasion and migration by DJ-1.
In order to understand the connection between DJ-1
and gastric cancer cell invasion and migration in more detail,
identification of the signaling cascades by which DJ-1 regulates
invasion and migration is important. It has been reported that DJ-1
induces the Akt pathway by negatively regulating the function of
the tumor-suppressor gene PTEN (30). Moreover, the Akt pathway has been
shown to play a critical role in the migration and invasion of many
types of cancer cells by regulating the expression of multiple
metastasis-related genes, including MMP-2 and MMP-9 (31,32).
Thus, we speculated that the Akt pathway might also be responsible
for the DJ-1-mediated migration and invasion of gastric cancer
cells. To verify this hypothesis, we investigated the
phosphorylated status of Akt and its correlation with MMP-2 and
MMP-9 expression and cell migration and invasion in SGC7901 cells.
Our results demonstrated that downregulation of DJ-1 caused a
marked decrease in Akt phosphorylation, with concomitant
down-regulation of MMP-2 and MMP-9 expression and inhibition of
cell invasion and migration in SGC7901/DJ-1-siRNA cells. In
addition, we demonstrated that inhibition of the Akt pathway by
API-2 mimicked the effects of DJ-1 silencing in parental SGC7901
and reversed the effects of restored DJ-1 on MMP-2 and MMP-9
expression, cell migration and invasion. All of these results
suggest that the Akt pathway is specifically involved in
DJ-1-related invasion and migration, and mediates the upregulation
of MMP-2 and MMP-9 expression induced by DJ-1. However, in the
peritoneal metastatic of gastric cancer, the means by which DJ-1
activates the Akt pathway remains unknown and warrants further
study.
In summary, the present study provides unequivocal
evidence that DJ-1 is correlated with peritoneal metastatic of
gastric cancer and the promotion of cell invasion and migration.
The effects are at least partially mediated by activation of the
Akt pathway and consequent upregulation of MMP-2 and MMP-9
expression. This suggests that DJ-1 may be a potential therapeutic
target for blocking peritoneal carcinomatosis of gastric
carcinoma.
Acknowledgements
The present study was supported by the Natural
Scientific Foundation of China (no. 81160304), the Scientific
Research Foundation of Jiangxi Provincial Educational Department
(no. GJJ12062), and the Training Program for Young Scientists of
Jiangxi Province (no. 20133BCB23028).
References
|
1
|
Yonemura Y, Endou Y, Sasaki T, et al:
Surgical treatment for peritoneal carcinomatosis from gastric
cancer. Eur J Surg Oncol. 36:1131–1138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takahashi I, Matsusaka T, Onohara T, et
al: Clinicopathological features of long-term survivors of
scirrhous gastric cancer. Hepatogastroenterology. 47:1485–1488.
2000.PubMed/NCBI
|
|
3
|
Fujimura T, Ishii K, Oyama K, et al: A new
scoring system for peritoneal metastasis in gastric cancer. Gastric
Cancer. 6:146–152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bando E, Yonemura Y, Takeshita Y, et al:
Intraoperative lavage for cytological examination in 1,297 patients
with gastric carcinoma. Am J Surg. 178:256–262. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hippo Y, Yashiro M, Ishii M, et al:
Differential gene expression profiles of scirrhous gastric cancer
cells with high metastatic potential to peritoneum or lymph nodes.
Cancer Res. 61:889–895. 2001.PubMed/NCBI
|
|
6
|
Nagakubo D, Taira T, Kitaura H, et al:
DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in
cooperation with ras. Biochem Biophys Res Commun. 231:509–513.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishikawa S, Taira T, Takahashi-Niki K,
Niki T, Ariga H and Iguchi-Ariga SM: Human DJ-1-specific
transcriptional activation of tyrosine hydroxylase gene. J Biol
Chem. 285:39718–39731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shinbo Y, Taira T, Niki T, Iguchi-Ariga SM
and Ariga H: DJ-1 restores p53 transcription activity inhibited by
Topors/p53BP3. Int J Oncol. 26:641–648. 2005.PubMed/NCBI
|
|
9
|
Taira T, Saito Y, Niki T, Iguchi-Ariga SM,
Takahashi K and Ariga H: DJ-1 has a role in antioxidative stress to
prevent cell death. EMBO Rep. 5:213–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shendelman S, Jonason A, Martinat C, Leete
T and Abeliovich A: DJ-1 is a redox-dependent molecular chaperone
that inhibits α-synuclein aggregate formation. PLoS Biol.
2:e3622004.PubMed/NCBI
|
|
11
|
Honbou K, Suzuki NN, Horiuchi M, et al:
The crystal structure of DJ-1, a protein related to male fertility
and Parkinson’s disease. J Biol Chem. 278:31380–31384.
2003.PubMed/NCBI
|
|
12
|
Le Naour F, Misek DE, Krause MC, et al:
Proteomics-based identification of RS/DJ-1 as a novel circulating
tumor antigen in breast cancer. Clin Cancer Res. 7:3328–3335.
2001.PubMed/NCBI
|
|
13
|
MacKeigan JP, Clements CM, Lich JD, Pope
RM, Hod Y and Ting JP: Proteomic profiling drug-induced apoptosis
in non-small cell lung carcinoma: identification of RS/DJ-1 and
RhoGDIα. Cancer Res. 63:6928–6934. 2003.PubMed/NCBI
|
|
14
|
Liu H, Wang M, Li M, et al: Expression and
role of DJ-1 in leukemia. Biochem Biophys Res Commun. 375:477–483.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hod Y: Differential control of apoptosis
by DJ-1 in prostate benign and cancer cells. J Cell Biochem.
92:1221–1233. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arnouk H, Merkley MA, Podolsky RH, et al:
Characterization of molecular markers indicative of cervical cancer
progression. Proteomics Clin Appl. 3:516–527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giusti L, Iacconi P, Ciregia F, et al:
Fine-needle aspiration of thyroid nodules: proteomic analysis to
identify cancer biomarkers. J Proteome Res. 7:4079–4088. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tian M, Cui YZ, Song GH, et al: Proteomic
analysis identifies MMP-9, DJ-1 and A1BG as overexpressed proteins
in pancreatic juice from pancreatic ductal adenocarcinoma patients.
BMC Cancer. 8:2412008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pardo M, Garcia A, Thomas B, et al: The
characterization of the invasion phenotype of uveal melanoma tumour
cells shows the presence of MUC18 and HMG-1 metastasis markers and
leads to the identification of DJ-1 as a potential serum biomarker.
Int J Cancer. 119:1014–1022. 2006. View Article : Google Scholar
|
|
20
|
Yuen HF, Chan YP, Law S, et al: DJ-1 could
predict worse prognosis in esophageal squamous cell carcinoma.
Cancer Epidemiol Biomarkers Prev. 17:3593–3602. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bai J, Guo C, Sun W, et al: DJ-1 may
contribute to metastasis of non-small cell lung cancer. Mol Biol
Rep. 39:2697–2703. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fang M, Zhong XY, Du B, et al: Role of
DJ-1-induced PTEN down-regulation in migration and invasion of
human glioma cells. Chin J Cancer. 29:988–994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He X, Zheng Z, Li J, et al: DJ-1 promotes
invasion and metastasis of pancreatic cancer cells by activating
SRC/ERK/uPA. Carcinogenesis. 33:555–562. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang JW, Peng SY, Li JT, et al:
Identification of metastasis-associated proteins involved in
gallbladder carcinoma metastasis by proteomic analysis and
functional exploration of chloride intracellular channel 1. Cancer
Lett. 281:71–81. 2009. View Article : Google Scholar
|
|
25
|
Li Z, Zhan W, Wang Z, et al: Inhibition of
PRL-3 gene expression in gastric cancer cell line SGC7901 via
microRNA suppressed reduces peritoneal metastasis. Biochem Biophys
Res Commun. 348:229–237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yasumoto K, Koizumi K, Kawashima A, et al:
Role of the CXCL12/CXCR4 axis in peritoneal carcinomatosis of
gastric cancer. Cancer Res. 66:2181–2187. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sako A, Kitayama J, Koyama H, et al:
Transduction of soluble Flt-1 gene to peritoneal mesothelial cells
can effectively suppress peritoneal metastasis of gastric cancer.
Cancer Res. 64:3624–3628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pan Y, Zhao L, Liang J, et al: Cellular
prion protein promotes invasion and metastasis of gastric cancer.
FASEB J. 20:1886–1888. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khasigov PZ, Podobed OV, Gracheva TS,
Salbiev KD, Grachev SV and Berezov TT: Role of matrix
metalloproteinases and their inhibitors in tumor invasion and
metastasis. Biochemistry (Mosc). 68:711–717. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim RH, Peters M, Jang Y, et al: DJ-1, a
novel regulator of the tumor suppressor PTEN. Cancer Cell.
7:263–273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoeli-Lerner M and Toker A: Akt/PKB
signaling in cancer: a function in cell motility and invasion. Cell
Cycle. 5:603–605. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim D, Kim S, Koh H, et al: Akt/PKB
promotes cancer cell invasion via increased motility and
metalloproteinase production. FASEB J. 15:1953–1962. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Geiger TR and Peeper DS: Metastasis
mechanisms. Biochim Biophys Acta. 1796:293–308. 2009.PubMed/NCBI
|
|
34
|
Aron L, Klein P, Pham TT, Kramer ER, Wurst
W and Klein R: Pro-survival role for Parkinson’s associated gene
DJ-1 revealed in trophically impaired dopaminergic neurons. PLoS
Biol. 8:e10003492010.
|
|
35
|
Vasseur S, Afzal S, Tardivel-Lacombe J,
Park DS, Iovanna JL and Mak TW: DJ-1/PARK7 is an important mediator
of hypoxia-induced cellular responses. Proc Natl Acad Sci USA.
106:1111–1116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nabeshima K, Inoue T, Shimao Y and
Sameshima T: Matrix metalloproteinases in tumor invasion: role for
cell migration. Pathol Int. 52:255–264. 2002. View Article : Google Scholar : PubMed/NCBI
|